Abstract
High-resolution single nucleotide polymorphism genomic microarray (SNP-chip) is a useful tool to define gene dosage levels over the whole genome, allowing precise detection of deletions and duplications/amplifications of chromosomes in cancer cells. We found that this new technology can also identify breakpoints of chromosomes involved in unbalanced translocations, leading to identification of fusion genes. Using this technique, we found that the PAX5 gene was rearranged to a variety of partner genes including ETV6, FOXP1, AUTS2, and C20orf112 in pediatric acute lymphoblastic leukemia (ALL). The 3′ end of the PAX5 gene was replaced by the partner gene. The PAX5 fusion products bound to PAX5 recognition sequences as strongly as wild-type PAX5 and suppressed its transcriptional activity in a dominant-negative fashion. In human B cell leukemia cells, binding of wild-type PAX5 to a regulatory region of BLK, one of the direct downstream target genes of PAX5, was diminished by expression of the PAX5-fusion protein, leading to repression of BLK. Expression of PAX5-fusion genes in murine bone marrow cells blocked development of mature B cells. PAX5-fusion proteins may contribute to leukemogenesis by blocking differentiation of hematopoietic cells into mature B cells. SNP-chip is a powerful tool to identify fusion genes in human cancers.
Keywords: chromatin immunoprecipitation, dominant negative, fusion gene, PAX5, SNP-chip
Pediatric acute lymphoblastic leukemia (ALL) is the most common malignant disease in children (1–3). It is a genetic abnormality resulting from accumulation of mutations in tumor suppressor genes and oncogenes (1–3). Fusion genes including ETV6/RUNX1 and E2A/PBX1 are frequently detected in pediatric ALL (1). Deletion of the INK4A/ARF gene (9p21) is also a common abnormality in ALL (1). However, other genetic changes remain to be elucidated in this disease.
Identification of mutated genes in ALL has evolved with improvements in technology. A very recent approach is single nucleotide polymorphism (SNP) analysis using an array based technology (4–6) that allows identification of amplifications, deletions, and allelic imbalances, such as uniparental disomy (represents doubling of the abnormal allele due to somatic recombination or duplication, and loss of the other normal allele) (7, 8). However, SNP-chip analysis is only able to detect changes of gene dosage and is unable to identify balanced translocations, which commonly occur in ALL.
Previously, we analyzed 399 pediatric ALL cases by SNP-chip analysis and found a number of genomic abnormalities, in addition to well known common alterations (9). This technique is sensitive enough to identify genes involved in start sites of deletions/duplications. Indeed, this method allowed us to identify that the PBX1 gene was involved in start sites of duplication of 1q23 generated by der(19)t(1;19)(q23;p13) (9). Furthermore, correlation analysis of the individual genomic abnormalities suggested the presence of der(12)t(12;21)(p13;q22) and der(21)t(12;21)(p13;q22), as well as dic(9;20)(p13;q11) (9).
In this study, we found that this new technology permitted us to identify genes involved in well known unbalanced translocations including ETV6/RUNX1. Further, we found previously undetected fusion genes between PAX5 and a number of other partner genes by using this technique.
Results
Genes Involved in Unbalanced Translocations Were Identified by SNP-Chip Analysis.
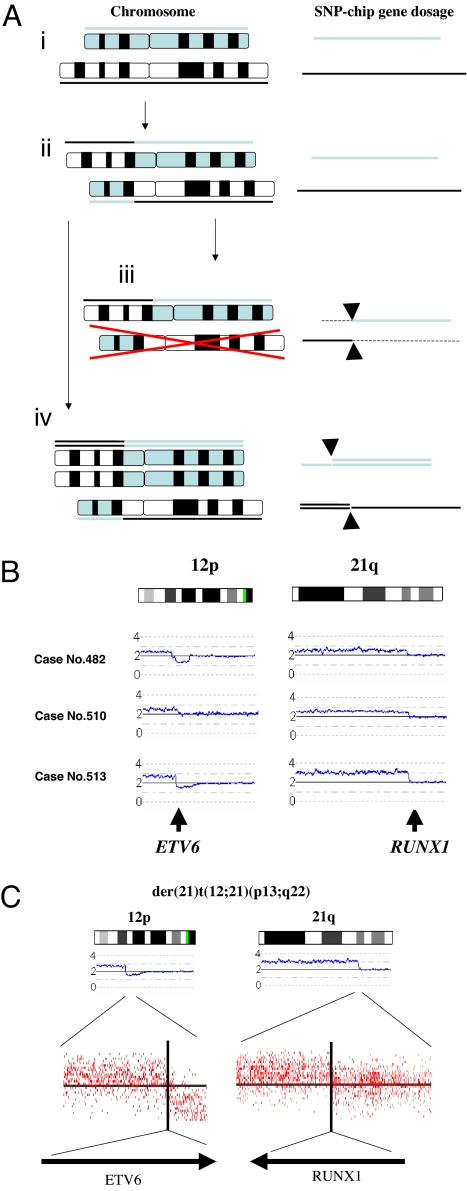
Because SNP-chip analysis can only detect changes of gene dosage including deletions, duplications, and amplifications (Fig. 1A), this technique is unable to identify balanced translocations (Figs. 1Aii). However, when one of a pair of reciprocally translocated chromosomes is lost, SNP-chip analysis can detect this abnormality as partial deletions of involved chromosomes (Fig. 1Aiii). Similarly, when one of a pair of reciprocally translocated chromosomes becomes duplicated, SNP-chip can also detect this abnormality as partial duplication of the involved chromosomes (Fig. 1Aiv). Furthermore, high resolution SNP-chip analysis allows us to identify the genes involved in these unbalanced translocations.
Fig. 1.
SNP-chip analysis detected genes involved in unbalanced translocations. (A) SNP-chip analysis can identify breakpoints of translocations when one of the paired translocated chromosomes is either lost or duplicated/amplified. (Left) Chromosomal status. Gene dosages are indicated either above or beneath the chromosomes. (Right) Results of SNP-chip analysis. (Ai) Normal chromosomes; gene dosage is normal. (Aii) Reciprocal translocation; gene dosage is normal. (Aiii) One of the paired translocated chromosomes is lost; gene dosage is lower than normal on the left side of the upper chromosome and the right side of the lower chromosome. Arrow heads indicate the breakpoint of the translocation in each chromosome. (Aiv) One of the paired translocated chromosomes is duplicated; gene dosage is higher than normal on the right side of the upper chromosome and the left side of the lower chromosome. Arrow heads indicate the breakpoint of this translocation in each chromosome. (B) Representative cases with unbalanced translocation of der(21)t(12;21)(p13;q22). (Left) Start sites of duplication at 12p13 involving the ETV6 gene. (Right) Start sites of duplication at 21q22 involving the RUNX1 gene. SNP-chip data of representative cases with dup(12)(p13) and dup(21)(q22) are shown. These abnormalities were validated by FISH and/or RT-PCR (data not shown). Results of SNP-chip data were visualized by CNAG software. Lines above each chromosome show total gene dosage; level 2 indicates diploid (2N) amount of DNA, which is normal. (C) Magnified view of SNP-chip data. (Upper) Start sites of duplications at 12p13 and 21q22 are magnified. Signals of individual probe signals are shown. Vertical lines indicated the positions of start sites of duplications. (Lower) Genes involved in the start sites of duplications.
To prove that SNP-chip analysis can detect unbalanced translocations and the genes involved in these translocations, we analyzed cases having extra copies of ETV6/RUNX1 fusion genes generated by der(21)t(12;21)(p13;q22) (Fig. 1B), which were initially identified by FISH and/or RT-PCR (data not shown). SNP-chip was clearly able to identify this abnormality as duplications involving chromosome 12 and 21 (Fig. 1B). Further, the result of high-resolution (250k) SNP-chip clearly identified ETV6 (12p13) and RUNX1 (21q22) as the target genes involved in this unbalanced translocation (Fig. 1C).
PAX5 Gene Is Frequently Fused to Partner Genes.
Our previous data showed the presence of dic(9;20)(p13;q11) in 11 cases of ALL (9), 5 of which had deletion 9p13.2-pter. These 5 cases had start sites of this deletion at 9p13.2 mapping to the PAX5 gene (Fig. 2A and data not shown). This prompted us to reexamine all cases of B-ALL that had deletion of 9p [supporting information (SI) Table S1]. We found a total of 9 cases with similar start sites (9p13.2), mapping to the PAX5 gene (Fig. 2A and data not shown). In 2 of these cases, simple abnormalities were detected by SNP-chip: case 514 had only del9p13.2-pter and del7q11.2-pter; case 458 had only del9p13.2-pter and dup3p13-pter (Table S1 and Fig. 2A). Three cases (536, 543, 572) had complex abnormalities including del9p13.2-pter and del20q11.21-qter, all with the C20ORF112 gene within the start site of del20q (Table S1 and Fig. 2A). The other 2 cases (659, 767) had complex abnormalities that included ETV6 on 12p13 (Table S1 and Fig. 2A).
Fig. 2.
PAX5 gene is fused to partner genes. (A) Start sites of deletion at 9p13.2 involving the PAX5 gene. (Left) SNP-chip data of representative cases with 9p13.2 deletions. A vertical arrow indicates the start sites of 9p deletion that involves the PAX5 gene. A horizontal arrow shows the direction of transcription of the PAX5 gene. (Right) Chromosomal abnormalities of partner chromosomes. Arrow heads indicate the start sites of duplication or deletions. Genes involved in the start sites are shown. (B) Result of RT-PCR. The ALL samples suggesting the presence of PAX5 fusion genes by SNP-chip analysis were examined by RT-PCR using the primers of PAX5 and the respective partner genes. (C) Fusion sequences of the PAX5 and partner genes. Joining sequences of fused transcripts are shown from the indicated exon of the fused gene. (D) Schematic structure of wild-type and mutant PAX5. Amino acid positions (aa) of each protein are indicated. PAX5/FOXP1 fusion construct has an early termination codon caused by a frame-shift. PD, paired domain; TA, transcription activation domain; O, octapeptide H, homeodomain-like; I, inhibitory domain. (E) Subcellular fractionation of PAX5-fusion proteins. pcDNA vector encoding wild-type PAX5, PAX5-ETV6, PAX5-FOXP1, PAX5-C20ORF112S, or PAX5-C20ORF112L was transfected into 293T cells. Nuclear and cytoplasmic proteins were separated and electrophoresed in the gel. Localization of PAX5-fusion proteins was examined by PAX5 N-terminal specific antibody. Purity of cytoplasmic protein was examined with anti-GAPDH antibody and purity of nuclear proteins with the anti-PARP antibody. C, cytoplasmic fraction; N, nuclear fraction.
Thus, we found four candidate partner genes fused to PAX5 in seven cases by SNP-chip analysis; ETV6 on 12p13 (two cases) (12), C20orf112 on 20q11.1 (three cases), AUTS2 on 7q11.1 (one case) and FOXP1 on 3p13 (one case) (Fig. 2A). Because these translocations could lead to fusion transcripts between PAX5 and different partner genes, the presence of the predicted fusion transcript was examined by RT-PCR using the mapping information from the SNP-chip data. RT-PCR and nucleotide sequencing data of the PCR products confirmed that the PAX5 gene was fused to either the ETV6 (two cases), C20orf112 (three cases), AUTS2 (one case), or FOXP1 (one case) gene and transcribed into aberrant fusion messages (Fig. 2 B and C). Each fusion gene was mutually and exclusively detected in the samples studied. In one case with dic(9;20), exon 5 of PAX5 was fused to exon 8 of C20orf112, and in two cases with dic(9;20), exon 8 of PAX5 was fused to exon 3 of C20orf112. PAX5/ETV6 involved exon 4 of PAX5 and exon 3 of ETV6.
Cellular Localization and DNA Binding Affinity of PAX5 Fusion Products.
In the PAX5/FOXP1 fusion transcript, the amino acid coding frame of the FOXP1 gene was not identical to that of PAX5, leading to a frame-shift and an early termination codon after the fusion point of these two genes (Fig. 2D). However, all other fusion genes were in frame and were predicted to encode chimeric proteins. Two proteins (a short and long form) with different breakpoints were predicted from the PAX5/C20orf112 fusion genes (Fig. 2D).
To confirm cellular localization of PAX5-fusion proteins, we transfected vectors encoding wild-type PAX5 and PAX5 fusion genes (PAX5-ETV6, PAX5-FOXP1, PAX5-C20ORF112S, and PAX5-C20ORF112L) into 293T cells, fractionated the cytoplasmic and nuclear proteins, and examined the wild-type PAX5 and PAX5-fusion proteins by Western blot analysis (Fig. 2E). PAX5-ETV6 protein was detected in both the cytoplasm and nucleus; PAX5-FOXP1 and PAX5-C20ORF112L proteins were predominantly localized in the nucleus; and 20% and 80% of PAX5-C20ORF112S proteins were localized in the cytoplasm and the nucleus, respectively (Fig. 2E). Localization of the fusion proteins was also confirmed by immunohistochemical staining (data not shown).
Because PAX5-fusion proteins were localized in the nucleus, we analyzed DNA binding affinity of these PAX5-fusion proteins in vitro. DNA binding affinity of the PAX5 wild-type and fusion proteins expressed in 293T cells was analyzed by electrophoretic mobility shift assay (EMSA), and signals of probes bound to the proteins were plotted graphically (Fig. 3A). Binding activity of each protein in the absence of cold competitor oligonucleotide probe was regarded as 1.0, and the binding activity in the presence of cold competitor oligonucleotide probes was measured. All PAX5-fusion proteins showed similar binding activity to the PAX5 recognition sequences as the wild-type PAX5 (Fig. 3A).
Fig. 3.
PAX5-fusion proteins suppress transcriptional activity of PAX5 in a dominant-negative fashion and block the growth of B cells. (A) Result of EMSA: Wild-type PAX5 and PAX5 fusion were expressed in 293T cells, and nuclear proteins were purified. The purified nuclear proteins were mixed with radioisotope labeled double-strand oligonucleotide DNA, in either the presence or absence of cold competitor oligonucleotides (5-, 25-, and 50-fold cold competitor probes). Intensity of each shifted band indicating DNA–protein complex was measured and plotted graphically. Intensity of shifted bands in the absence of cold competitor probes was regarded as 1.0. (B) Reporter gene assay. Wild-type and mutant PAX5 were mixed at a various ratios (1:0, 1:0.3, 1:1, 1:3, respectively, 1 = 500 ng of construct) and transfected. Forty-eight hours later, relative activity of firefly luciferase was measured and plotted. Results represent the mean values of the three experiments. CD19, PAX5 luciferase reporter construct having PAX5 binding region of CD19 promoter; PAX5, wild-type PAX5; PAX5/ETV6, PAX5/ETV6 fusion; PAX5/C20L, long form of PAX5/C20orf112 fusion in which PAX5 exon 8 is fused to C20orf112 exon 3; PAX5/C20S, short form of PAX5/C20orf112 fusion in which PAX5 exon 5 is fused to C20orf112 exon 8; PAX5/FOXP1, PAX5/FOXP1 fusion with an early termination codon caused by a frame-shift after the site of fusion. (C) Results of expression of wild-type PAX5 and PAX5-fusion proteins. After cotransfection of equal amounts of vector encoding either wild-type or fusion PAX5 genes into 293T cells, the expression of respective proteins was examined by Western blot. Levels of expression of wild-type PAX5 protein were minimally affected by coexpression of the PAX5-fusion proteins. (D) Semiquantitative RT-PCR of downstream target genes of PAX5. Expression of PAX5 downstream target genes was examined by semiquantitative RT-PCR. Nalm 6, a human B cell ALL cell line expressing endogenous PAX5, was transfected with pMSCV-GFP (Empty), pMSCV-GFP-PAX5-C20orf112S (PAX5-C20S), or pMSCV-GFP-PAX5-C20orf112L (PAX5-C20L). GFP-positive cells were sorted and subject to semiquantitative RT-PCR. Optimal cycle numbers to semiquantify the expression of respective genes are as follows; BLK: 25 cycles; Nedd5; 25 cycles; TCF7L2: 25 cycles; ATP1B1: 25 cycles; β-actin: 22 cycles; CCR2: 25 cycles; CCR8: 30 cycles; IRF8: 30 cycles. (E) Structure of human BLK gene. Structure of BLK and primers used for ChIP assay within the 5′ regulatory region (Promoter-PCR) and 3′ end (Control-PCR) of the BLK gene is schematically shown. PAX5 binding site in the promoter region is indicated. (F) ChIP analysis of the PAX5 binding site in the BLK gene promoter. pMSCV-GFP (empty vector) or pMSCV-GFP-PAX5-C20S (PAX5-C20S) was transfected into human Nalm 6 B cell leukemia cells expressing endogenous PAX5. GFP-positive cells were subject to ChIP assay. The cells were fixed in formaldehyde solution and sonicated by ultrasound. DNA-protein complex was incubated with wild-type PAX5 specific antibody, which detected the C-terminal region of PAX5 but not the PAX5-C20orf112S protein (Upper). As a control, the DNA-protein complex was reacted with isotype nonspecific antibody (Lower). Immnoprecipitated DNA was subjected to PCR to amplify either the BLK promoter region containing PAX5 binding sequence (P) or, as an internal control, the 3′ end of the BLK gene (C). (G) Retrovirus infection experiments. Murine bone marrow cells were collected at 5 days after injection of 5FU. The hematopoietic cells were infected by retrovirus containing pMSCV-GFP empty vector (GFP), pMSCV-GFP-C20orf112L (PAX5/C20L), or pMSCV-GFP-C20orf112S (PAX5/C20S). GFP-positive murine hematopoietic cells were sorted and plated at 5 × 104 cells per plate in methylcellulose containing mSCF, mIL7, and hFL. At 8 days after the plating, the colony numbers were counted (Left; results represent means and SD of three experiments). Cell surface antigens on the GFP-positive cells infected with pMSCV-GFP (GFP) at Day 11 were examined by FACS using antibodies against CD43 and B220 (Upper Right), c-kit and CD11b (Lower Right) antibodies, to confirm the development of B cells.
PAX5 Fusion Products Suppressed Transcriptional Activity of Wild-Type PAX5 in a Dominant Negative Fashion, Leading to Inhibition of B-Cell Development.
To examine the effect of PAX5-fusion proteins on transcriptional activity of wild-type PAX5, we performed a reporter gene assay using 293T cells. Cotransfection reporter gene assays using wild-type and fusion PAX5 expression vectors along with a reporter gene driven by the murine CD19 promoter (which contains three repeats of PAX5 binding sequences) showed that the PAX5 fusion products suppressed transcriptional activity of PAX5 in a dominant-negative fashion (Fig. 3B). Expression of wild-type PAX5 proteins was minimally affected by coexpression of PAX5-fusion proteins (Fig. 3C), suggesting that PAX5-fusion proteins competed with wild-type PAX5 for the PAX5 binding sequences on the reporter gene.
Further, we transfected vectors encoding either PAX5-C20orf112S or PAX5-C20orf112L, each coexpressing the GFP marker, into Nalm 6 cells (a human B cell ALL cell line, which expresses endogenous PAX5) (data not shown). After transfection, GFP-positive cells were sorted by FACS and expression of PAX5-downstream genes was examined by semiquantitative RT-PCR (Fig. 3D and data not shown). We examined 10 downstream target genes (seven positively regulated direct target genes and three negatively regulated genes) of PAX5 (10–12) and found that four, including ATP1B1, BLK, NEDD5 and TCF7L2, were down-regulated by induction of either PAX5-C20orf112S or PAX5-C20orf112L protein. However, expression of other reported PAX5 downstream target genes, including three positively regulated direct target genes (IRF8, BST1, CD19) and three negatively regulated genes (CCR2, CCR5, NOTCH1) were not affected by the induction of expression of the fusion proteins in these cells.
To examine the effect of PAX5 fusion protein on binding of wild-type PAX5 to the direct target gene BLK in the leukemic cells, we performed chromatin-immunoprecipitation (ChIP) assay using Nalm 6 cells transfected with either an empty vector or a construct encoding PAX5-C20orf112S. We used a PAX5 antibody detecting the C-terminal region of the protein, which could detect wild-type PAX5, but not PAX5-C20orf112S, as the C-terminal end of PAX5 was replaced by C20orf112S in this fusion protein. Although wild-type specific PAX5 antibody precipitated the promoter region of BLK after transfection of the empty vector, the amount of DNA of the BLK promoter region bound to wild-type PAX5 was reduced after transfection of the PAX5-C20orf112S gene (Fig. 3 E and F).
To examine the effect of PAX5-fusion proteins on B cell development in murine hematopoietic cells, we infected murine hematopoietic cells from the bone marrow with retroviral vectors encoding either PAX5-C20orf112S or PAX5-C20orf112L. GFP-positive infected bone marrow cells were sorted by FACS and plated in media containing cytokines that are know to stimulate B cell differentiation (Fig. 3F). Murine hematopoietic cells infected with the empty vector showed abundant colonies (Fig. 3G Left), and 79% of the cells were B220 positive B cells (Fig. 3G Upper Right). In contrast, murine hematopoietic cells infected with either PAX5-C20orf112S or PAX5-C20orf112L formed very few colonies (Fig. 3G Left). Most of these colonies were GFP-negative (data not shown), suggesting that these PAX5-fusion proteins impaired B cell development from murine hematopoietic cells.
Discussion
In this study, we describe a paradigm for discovering fusion genes in malignancy by taking advantage of samples with unbalanced translocations and using high density SNP-chip analysis. This technique allows us to identify genes involved in translocations even if chromosomal analysis is not available, especially in solid tumors.
Steps to identify novel fusion genes using SNP chip analysis include (i) identify either a deletion or duplication that occurs within two genes; (ii) determine whether transcription of both genes is in the same direction; (iii) take advantage of ancillary tests such as standard chromosomal analysis or spectral karyotyping (14), which can grossly show that two chromosomes are fused; and (iv) design primers of candidate genes and perform RT-PCR to clone fusion genes. Rapid amplication of cDNA ends (RACE) (15) or long-distance PCR (12) also help the cloning of genes involved in translocations. In our SNP-chip data, a number of regions of segmental deletions or duplications were detected (9). Although some of them are simple deletions or duplications at the original sites of the chromosomes, the others are deletions that occurred during chromosomal translocations or when duplicated fragments were inserted into chromosomal sites other than the original region (data not shown). Therefore, data of chromosomal analysis help to define translocations, leading to identification of candidate genes in novel fusion genes.
Recently, Tomlins et al. found the fusion genes TMPRSS2/ERG and TMPRSS2/ETV1 in prostate cancers by using expression microarray data (16). They focused on the genes ERG and ETV1, which are highly expressed in this cancer and examined levels of individual exons of these two genes (16). They found differences in expression of 5′ and 3′ regions of the genes, suggesting that these genes are fused to each other (16). In these fusion genes, the 5′ regions were replaced by the TMPRSS2 gene, resulting in the differences in the expression of the 5′ and 3′ region of the ERG/ETV1 genes (16). They also used SNP-chip analysis to identify these fusion genes and found a deletion of a genomic region between TMPRSS2 (21q22.3) and ERG (21q22.2), leading to fusion of these two genes (17). These new technologies, based on oligonucleotide microarrays and bioinformatics, will help to identify fusion genes in cancers.
Our study found that the PAX5 gene was frequently fused to one of a variety of partner genes. PAX5 is a key transcription factor in the development of B cells (18, 19). We found that these PAX5 fusion proteins suppressed the function of wild-type PAX5 in a dominant-negative fashion and suppressed expression of downstream target genes of wild-type PAX5 in leukemic cells.
We found that when PAX5 was joined to one of its fusion partner genes, its C-terminal end was replaced by one of the partner genes. Elimination of the C-terminal end of PAX5 may play an important role in generation of a dominant negative form of mutated PAX5. In in vitro assays, PAX5-fusion proteins showed a similar affinity as wild-type PAX5 for the PAX5 recognition sequences. Although expression of several downstream targets of wild-type PAX5 was repressed by expression of PAX5-fusion proteins, others were not affected. Binding of transcription factors to DNA can be modulated by cofactors and/or neighboring transcription factors (20). Compared to PAX5, PAX5-fusion proteins may bind more strongly to some target genes and more weakly to others, depending on the contextual environment of the target genes.
Further, our data showed that PAX5-fusion protein inhibited B cell development of hematopoietic cells in a colony formation assay. This result may suggest that PAX5 fusion protein blocked differentiation of hematopoietic cells into mature B cells. PAX5-deficient mice have impairment of B cell differentiation (18). These data suggest that PAX5-fusion proteins may contribute to leukomegenesis by blocking B cell differentiation. It has been suggested that two distinct genetic abnormalities contribute to leukemogenesis in acute myelogenous leukemia (AML); one is mutations promoting cellular proliferation, for example FLT3 or RAS mutations, and the other is mutations blocking differentiation, for example PML-RARA or RUNX1-ETO (21, 22). PAX5-fusion proteins may cooperate with unidentified mutations promoting cellular proliferation in the ALL cells.
Recently, Mullighan et al. have analyzed pediatric ALL samples by high density SNP-chips and found frequent abnormalities of PAX5 gene (23). Their data also showed that PAX5 fusion products suppressed transcriptional activity of PAX5 in a dominant-negative fashion (23). In addition, other researcher have reported PAX5 fusion genes, including PAX5 fused to ETV6 (12p13) (23, 24), FOXP1 (3p14) (23), ZNF521 (18q11) (23), ELN (7q11.23) (25), and PML (15q24) (26). We have found PAX5 fused to either ETV6, FOXP1, C20orf112 (20q11), or AUTS2 (7q11.22).
In our study, the function of PAX5 was attenuated by the dominant-negative forms of the fusion products in B cell lineage ALL, suggesting that PAX5 behaves as a tumor suppressor in early B cells, and that impairment of its function can be associated with the development of ALL. In contrast, translocation of the PAX5 gene to the enhancer region of the Ig heavy chain gene [t(9;14)(p13.2;q32)] or point mutations of the 5′ regulatory region of the PAX5 gene leads to its overexpression, which is associated with B cell lineage lymphomas (27–29). Also, experimental overexpression of wild-type PAX5 can transform lymphocytes (30, 31). Therefore, an aberrant PAX5 may behave in a dominant-negative fashion at the pre-B stage of B cell development, resulting in ALL; its forced expression in a more mature B cell can lead to lymphoma. Our study showed that PAX5-fusion proteins blocked differentiation of B cells but did not transform them. B cells at different stages of differentiation may need alteration of distinct sets of pathways to transform. Why PAX5 can act as a tumor suppressor in ALL and as an oncoprotein in lymphoma is unclear. Further studies are needed to clarify the mechanism of this paradoxical phenomenon in carcinogenesis.
In summary, we identified multiple fusion genes in ALL by SNP-chip analysis, leading to the exploration of a B cell differentiation block as a contributing factor to the development of ALL. This methodology should help researchers to identify oncogenic fusion genes and explore the mechanism of tumorigenesis in other types of cancers as well.
Materials and Methods
Samples and DNA/RNA Preparation.
SNP-chip was performed on 399 pediatric ALL patients consecutively enrolled in the ALL-BFM 2000 trial of the Berlin-Frankfurt-Münster (BFM) study at diagnosis and during remission (350 cases were B cell lineage ALL and 49 cases were T cell lineage ALL) (9). Detailed results of the SNP-chip analysis are published separately (9). The ALL-BFM 2000 study was approved by the local ethics committee. DNA and RNA were extracted from the ALL samples and cell lines by using standard techniques (32). Nalm 6, a human pre-B ALL cell line, was generously provided by Dr. G. Crook (Los Angeles Children's Hospital, Los Angeles, CA) and maintained in RPMI medium 1640 with 10% FBS.
SNP-Chip Analysis.
SNP-chip of GeneChip Human mapping 50k array XbaI 240 and/or 250k Nsp were used for this study (Affymetrix Japan). Preparation of samples was reported previously (4, 5). The data were analyzed by CNAG program as previously described (4, 5). All 399 ALL samples and their matched control samples were analyzed by using 50K-SNP chip; selected cases with genomic abnormalities were also analyzed by using 250K SNP-chip.
RT-PCR.
RT-PCR was performed by using ThermoScript RT-PCR Systems (Invitrogen) according to the manufacturer's protocol. The primers used for detection of PAX5 fusion transcripts are listed in Table S2. Expression of PAX5 downstream target genes in Nalm 6 cells after transfection was examined by semiquantitative RT-PCR. The gene names and their primer sequences are listed in Table S3.
Reporter Gene Constructs and Expression Vectors.
The PAX5 reporter gene construct with the luciferase gene and PAX5 binding region of the CD19 promoter, as well as the human PAX5 cDNA constructs, were kindly provided by Dr. M. Busslinger (Research Institute of Molecular Pathology, Vienna, Austria). PAX5-fusion constructs were generated by using PCR. All coding regions were ligated into the pcDNA3.1 vectors (Stratagene). Wild-type PAX5 cDNA was ligated into pMSCV vector (Clontech), and EGFP cDNA was ligated under the control of pGK promoter as a marker (pMSCV-GFP-wtPAX5). PAX5-C20orf112S and PAX5-C20orf112L cDNA sequences were also ligated into pMSCV-GFP vectors.
Transfection and Reporter Gene Assay.
For reporter gene assays, pMSCV-GFP-wtPAX5 and pcDNA vectors encoding PAX5-fusion genes were cotransfected with the PAX5 reporter construct and pRL (Renilla luciferase) vector into 293T cells by using the Effecten transfection kit (Qiagen). Firefly and Renilla luciferase activities were measured with the Dual-Luciferase Reporter Assay System (Promega). Transfection into Nalm6 human pre-B cell ALL cell line was performed with Amaxa nucleofector. GFP-positive cells were sorted by using the MoFlo cell sorter (Dako). Detailed information about the procedure is described in SI Text.
Retrovirus Transduction into Murine Hematopietic Cells.
Retrovirus containing pMSCV-GFP (empty), pMSCV-GFP-PAX5-C20orf112S, and pMSCV-GFP-PAX5-C20orf112L was generated. The retrovirus was transfected into murine bone marrow cells as previously reported (33). After the transfection, GFP-positive cells were sorted and plated into methylcellulose cultures (M3231; Stem Cell Technologies) as previously described (33). Surface antigens (CD43, B220, c-kit, and CD11b) of these GFP-positive cells were examined by using FACScan (Becton–Dickinson). Detailed information of the procedure is described in the SI Text.
Subcellular Fractionation of Proteins and EMSA.
Forty-eight hours after transfection of vectors into 293T cells, the cells were subjected to subcellular fractionation with the CelLytic NuCLEAR Extraction Kit (Sigma-Aldrich). Detailed information of the procedure is described in the SI Text.
Purified nuclear proteins from the cells were also subjected to EMSA as previously reported (34). Detailed information of the procedure is described in the SI Text.
Chromatin Immunoprecipitation (ChIP) Assay.
ChIP assay was performed with the Magna ChIP A kit from Millipore according to the manufacturer's protocol. pMSCV-GFP or pMSCV-GFP-C20orf112S were transfected into Nalm 6 cells as described above, and GFP-positive cells were sorted by MoFlo (Dako). Precipitated DNA was recovered and subjected to PCR to amplify the BLK promoter region and the 3′ end of the BLK gene (internal control). The primer sequences used for ChIP assay are listed in Table S4. Detailed information of the procedure is described in the SI Text.
Supplementary Material
Acknowledgments.
We thank Dr. M. Busslinger (Research Institute of Molecular Pathology, Vienna, Austria) for generously providing the CD19 promoter reporter construct and the human PAX5 cDNA. This study was supported by the Parker Hughes Fund and by grants from the National Institutes of Health. N.K. is supported by a fellowship from The Tower Cancer Research Foundation. H.P.K. holds the Mark Goodson Chair in Oncology Research at Cedars-Sinai and is a member of the Jonsson Cancer Center and the Molecular Biology Institute of the University of California, Los Angeles. This work was also supported by a grant-in-aid from the Department of Health, Welfare and Labor; by the Ministry of Education, Culture, Sports, Science and Technology (Japan); by European Union Grant FOOD-CT-2005-016320; by grants from the Deutsche Krebshilfe to Cambridge Research Biochemicals; and by the Fritz-Thyssen Foundation (C.S.). The ALL-BFM 2000 trial is supported by Grant 50-2698-Schr 1 of the Deutsche Krebshilfe.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. J.W.G. is a guest editor invited by the Editorial Board.
Data deposition: The sequences reported in this paper have been deposited in the GenBank database (accession nos. EU784145, PAX5-FOXP1; EU784146, PAX5-AUTS2; EU784147, PAX5-C20orf112 short isoform; and EU784148, PAX5-C20orf112 long isoform).
This article contains supporting information online at www.pnas.org/cgi/content/full/0711039105/DCSupplemental.
References
- 1.Armstrong SA, Look AT. Molecular genetics of acute lymphoblastic leukemia. J Clin Oncol. 2005;23:6306–6315. doi: 10.1200/JCO.2005.05.047. [DOI] [PubMed] [Google Scholar]
- 2.Pui CH, Evans WE. Treatment of acute lymphoblastic leukemia. N Engl J Med. 2006;354:166–178. doi: 10.1056/NEJMra052603. [DOI] [PubMed] [Google Scholar]
- 3.Pui CH, Relling MV, Downing JR. Acute lymphoblastic leukemia. N Engl J Med. 2004;350:1535–1548. doi: 10.1056/NEJMra023001. [DOI] [PubMed] [Google Scholar]
- 4.Nannya Y, et al. A robust algorithm for copy number detection using high-density oligonucleotide single nucleotide polymorphism genotyping arrays. Cancer Res. 2005;65:6071–6079. doi: 10.1158/0008-5472.CAN-05-0465. [DOI] [PubMed] [Google Scholar]
- 5.Yamamoto G, et al. Highly sensitive method for genomewide detection of allelic composition in nonpaired, primary tumor specimens by use of affymetrix single-nucleotide-polymorphism genotyping microarrays. Am J Hum Genet. 2007;81:114–126. doi: 10.1086/518809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lindblad-Toh K, et al. Loss-of-heterozygosity analysis of small-cell lung carcinomas using single-nucleotide polymorphism arrays. Nat Biotechnol. 2000;18:1001–1005. doi: 10.1038/79269. [DOI] [PubMed] [Google Scholar]
- 7.Raghavan M, et al. Genome-wide single nucleotide polymorphism analysis reveals frequent partial uniparental disomy due to somatic recombination in acute myeloid leukemias. Cancer Res. 2005;65:375–378. [PubMed] [Google Scholar]
- 8.Lehmann S, et al. Molecular allelokaryotyping of early-stage, untreated chronic lymphocytic leukemia. Cancer. 2008;112:1296–1305. doi: 10.1002/cncr.23270. [DOI] [PubMed] [Google Scholar]
- 9.Kawamata N, et al. Molecular allelokaryotyping of pediatric acute lymphoblastic leukemias by high resolution single nucleotide polymorphism oligonucleotide genomic microarray. Blood. 2008;111:776–784. doi: 10.1182/blood-2007-05-088310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lin YH, Shin EJ, Campbell MJ, Niederhuber JE. Transcription of the blk gene in human B lymphocytes is controlled by two promoters. J Biol Chem. 1995;270:25968–25975. doi: 10.1074/jbc.270.43.25968. [DOI] [PubMed] [Google Scholar]
- 11.Schebesta A, et al. Transcription factor Pax5 activates the chromatin of key genes involved in B cell signaling, adhesion, migration, and immune function. Immunity. 2007;27:49–63. doi: 10.1016/j.immuni.2007.05.019. [DOI] [PubMed] [Google Scholar]
- 12.Delogu A, et al. Gene repression by Pax5 in B cells is essential for blood cell homeostasis and is reversed in plasma cells. Immunity. 2006;24:269–281. doi: 10.1016/j.immuni.2006.01.012. [DOI] [PubMed] [Google Scholar]
- 13.Schröck E, Padilla-Nash H. Spectral karyotyping and multicolor fluorescence in situ hybridization reveal new tumor-specific chromosomal aberrations. Semin Hematol. 2000;37:334–347. doi: 10.1016/s0037-1963(00)90014-3. [DOI] [PubMed] [Google Scholar]
- 14.Frohman MA, Dush MK, Martin GR. Rapid production of full-length cDNAs from rare transcripts by amplification using a single gene-specific oligonucleotide primer. Proc Natl Acad Sci USA. 1988;85:8998–9002. doi: 10.1073/pnas.85.23.8998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cheng S, Fockler C, Barnes WM, Higuchi R. Effective amplification of long targets from cloned inserts and human genomic DNA. Proc Natl Acad Sci USA. 1994;91:5695–5699. doi: 10.1073/pnas.91.12.5695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tomlins SA, et al. Recurrent fusion of TMPRSS2 and ETS transcription factor genes in prostate cancer. Science. 2005;310:644–648. doi: 10.1126/science.1117679. [DOI] [PubMed] [Google Scholar]
- 17.Perner S, et al. TMPRSS2:ERG fusion-associated deletions provide insight into the heterogeneity of prostate cancer. Cancer Res. 2006;66:8337–8341. doi: 10.1158/0008-5472.CAN-06-1482. [DOI] [PubMed] [Google Scholar]
- 18.Urbanek P, Wang ZQ, Fetka I, Wagner EF, Busslinger M. Complete block of early B cell differentiation and altered patterning of the posterior midbrain in mice lacking Pax5/BSAP. Cell. 1994;79:901–912. doi: 10.1016/0092-8674(94)90079-5. [DOI] [PubMed] [Google Scholar]
- 19.Xie H, Ye M, Feng R, Graf T. Stepwise reprogramming of B cells into macrophages. Cell. 2004;117:663–676. doi: 10.1016/s0092-8674(04)00419-2. [DOI] [PubMed] [Google Scholar]
- 20.Kumaran RI, Thakar R, Spector DL. Chromatin dynamics and gene positioning. Cell. 2008;132:929–934. doi: 10.1016/j.cell.2008.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gilliland DG, Griffin JD. The roles of FLT3 in hematopoiesis and leukemia. Blood. 2002;100:1532–1542. doi: 10.1182/blood-2002-02-0492. [DOI] [PubMed] [Google Scholar]
- 22.Tenen DG. Disruption of differentiation in human cancer: AML shows the way. Nat Rev Cancer. 2003;3:89–101. doi: 10.1038/nrc989. [DOI] [PubMed] [Google Scholar]
- 23.Mullighan CG, et al. Genome-wide analysis of genetic alterations in acute lymphoblastic leukaemia. Nature. 2007;446:758–764. doi: 10.1038/nature05690. [DOI] [PubMed] [Google Scholar]
- 24.Cazzaniga G, et al. The paired box domain gene PAX5 is fused to ETV6/TEL in an acute lymphoblastic leukemia case. Cancer Res. 2001;61:4666–4670. [PubMed] [Google Scholar]
- 25.Bousquet M, et al. A novel PAX5-ELN fusion protein identified in B cell acute lymphoblastic leukemia acts as a dominant negative on wild-type PAX5. Blood. 2007;109:3417–3423. doi: 10.1182/blood-2006-05-025221. [DOI] [PubMed] [Google Scholar]
- 26.Nebral K, et al. Identification of PML as novel PAX5 fusion partner in childhood acute lymphoblastic leukaemia. Br J Haematol. 2007;139:269–274. doi: 10.1111/j.1365-2141.2007.06731.x. [DOI] [PubMed] [Google Scholar]
- 27.Busslinger M, Klix N, Pfeffer P, Graninger PG, Kozmik Z. Deregulation of PAX-5 by translocation of the Emu enhancer of the IgH locus adjacent to two alternative PAX-5 promoters in a diffuse large-cell lymphoma. Proc Natl Acad Sci USA. 1996;93:6129–6134. doi: 10.1073/pnas.93.12.6129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Iida S, et al. The t(9;14)(p13;q32) chromosomal translocation associated with lymphoplasmacytoid lymphoma involves the PAX-5 gene. Blood. 1996;88:4110–4117. [PubMed] [Google Scholar]
- 29.Pasqualucci L, et al. Hypermutation of multiple proto-oncogenes in B-cell diffuse large-cell lymphomas. Nature. 2007;412:341–346. doi: 10.1038/35085588. [DOI] [PubMed] [Google Scholar]
- 30.Anderson K, et al. Ectopic expression of PAX5 promotes self renewal of bi-phenotypic myeloid progenitors co-expressing myeloid and B-cell lineage associated genes. Blood. 2007;109:3697–3705. doi: 10.1182/blood-2006-05-026021. [DOI] [PubMed] [Google Scholar]
- 31.Souabni A, Jochum W, Busslinger M. Oncogenic role of Pax5 in the T-lymphoid lineage upon ectopic expression from the immunoglobulin heavy-chain locus. Blood. 2007;109:281–289. doi: 10.1182/blood-2006-03-009670. [DOI] [PubMed] [Google Scholar]
- 32.Sambrook J, Russell DW. Molecular Cloning: A Laboratory Manual. 3rd Ed. Cold Spring Harbor, NY: Cold Spring Harbor Press; 2001. [Google Scholar]
- 33.Schwieger M, et al. AML1-ETO inhibits maturation of multiple lymphohematopoietic lineages and induces myeloblast trnsformation in syngergy with ICSBP deficiency. J Exp Med. 2002;196:1227–1240. doi: 10.1084/jem.20020824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sato H, Wang D, Kudo A. Dissociation of Pax-5 from KI and KII sites during kappa-chain gene rearrangement correlates with its association with the underphosphorylated form of retinoblastoma. J Immunol. 2001;166:6704–6710. doi: 10.4049/jimmunol.166.11.6704. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.