Abstract
A decrease in the surface area per unit volume is a well known constraint setting limits to the size of organisms at both the cellular and whole-organismal levels. Similar constraints may apply to social groups as they grow in size. The communal three-dimensional webs that social spiders build function ecologically as single units that intercept prey through their surface and should thus be subject to this constraint. Accordingly, we show that web prey capture area per spider, and thus number of insects captured per capita, decreases with colony size in a neotropical social spider. Prey biomass intake per capita, however, peaks at intermediate colony sizes because the spiders forage cooperatively and larger colonies capture increasingly large insects. A peaked prey biomass intake function would explain not only why these spiders live in groups and cooperate but also why they disperse only at large colony sizes, thus addressing both sociality and colony size range in this social spider. These findings may also explain the conspicuous absence of social spiders from higher latitudes and higher elevations, areas that we have previously shown to harbor considerably fewer insects of the largest size classes than the lowland tropical rainforests where social spiders thrive. Our findings thus illustrate the relevance of scaling laws to the size and functioning of levels of organization above the individual.
Keywords: Anelosimus, cooperation, group foraging, sociality, allometry
Transitions between levels of organization, to the extent that they bring about an increase in organismal size, should constitute both an opportunity and a challenge. Accessing open ecological niches above the size range of previously existing organisms is clearly a benefit that might accompany the origin of a higher level of organization (1). Increasing size, however, also brings a variety of challenges. A major one is a decline in the surface area to volume ratio. Such a decline is expected because tridimensional objects of a constant shape grow in volume to the third power of their linear dimensions, whereas surface area increases to the square power. A declining surface to volume ratio constitutes a challenge for growing organisms because they require energy and resources as a function of their mass (volume) but must acquire them through their surface. Simple multicellular organisms, such as Volvox, have met this challenge through the use of flagellar structures that improve nutrient flow (2), whereas more complex organisms have developed space-filling fractal distribution networks (3, 4) and structures such as lungs and intestines that maximize surface area for the exchange of gases, food, and waste (1). Ultimately, however, surface area to volume ratio relationships and other scaling laws are expected to set a limit to organismal size.
Similar opportunities and challenges are likely to be encountered in the transition from individuals to social groups. As with multicellularity, sociality is also thought to allow the colonization of ecological niches not accessible to solitary individuals (5). Naked mole rats, for instance, are capable of inhabiting the extremely arid deserts of southern Africa by cooperatively searching for new food patches after heavy and unpredictable rains have softened the soil enough for digging (6), and emperor penguins are able to withstand the frigid winters of Antarctica by huddling together to maintain warmth (7, 8). Among cooperative foragers, tree-killing bark beetles and social carnivores are capable of obtaining resources—live trees or large animals, respectively—that solitary individuals are unable to access (9–12). To the extent that social groups are dependent on space, however, they should also be subject to the physical laws of scaling (13, 14).
Social spiders are notable among cooperative foragers for their ability to capture prey that are many times larger than the spiders themselves (15–17), thus gaining access to a rich supply of insects not available to most solitary spiders (15, 18). The nests and prey capture webs of these spiders tend to be internally filled, irregular three-dimensional structures that intercept prey moving through the environment (18) (Fig. 1A). In the species subject of this study—Anelosimus eximius Keyserling 1894 (Araneae: Theridiidae) (19, 20)—spiders contribute a roughly constant volume per capita to the prey interception portion of their webs (21). It is the surface area of this webbing exposed to the environment, however, that should determine the frequency at which prey items enter the webs. For a more or less constant shape, we therefore expect that web surface area per spider, and thus the number of incoming prey items per capita, will decrease to the −1/3 power of colony size. A decreasing surface area to volume ratio should intensify competition for resources as colony size increases, raising the question of how and to what extent spiders in this and other social species are able to overcome this scaling challenge to produce colonies with dozens to thousands of individuals.
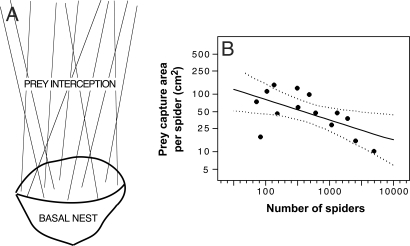
Fig. 1.
Diagram of a social Anelosimus nest and the relationship between the prey capture area per spider and colony size. (A) Diagram of an Anelosimus eximius nest showing the basal basket-shaped section used primarily for habitation and the superior webbing used primarily to intercept and capture prey. The relationship between colony size and prey capture web area per spider (log10 area per spider = 2.6201645 − 0.3546797 × log10 number of spiders) shown in B is based on the superior webbing. Data are log transformed to satisfy normal distribution requirements of linear regression. Dotted lines indicate 95% confidence intervals.
Here, we demonstrate the major role that cooperation plays in solving the problem of a declining surface area to volume ratio in this social spider. Anelosimus eximius is notable among cooperative spiders—also known as nonterritorial, permanent social, or simply social—for building the largest webs and colonies among species of this social system (18). Cooperative social spiders build and maintain communal webs in which members of a colony cooperate in the capture of prey, feeding, and brood care. Colony members are totipotent and mate with each other to produce new generations of spiders that continue to occupy and expand the natal nest. Colonies grow through this process of internal recruitment until, in species such as A. eximius, a single colony's population may on occasion reach into the tens of thousands (18). Here, we show that cooperative foraging in A. eximius allows the capture of increasingly large insects as colony size increases and that this effect is sufficient to overcome the decline in the number of insects caught per capita that results from the scaling of prey capture area per spider with increasing colony size. As a result, prey biomass intake per capita is maximized in colonies of intermediate size, thus explaining both sociality and colony size range in this social spider. This is an intriguing solution to a universal scaling problem, made possible because the “organism” in this case is a collective of units capable of cooperation.
Results
Matching the predicted relationships among colony size, prey capture area, and incoming prey items per capita, A. eximius's prey interception webs decreased in surface area per spider as colony size increased (Fig. 1B) and, although the overall number of prey items captured increased with colony size, prey items per capita decreased (Fig. 2A). The empirically estimated slope of the web surface area per capita (−0.35 ± 0.14 SE; r2 = 0.35; t = −2.56, P = 0.025, x and y axes log transformed) is not significantly different from the −1/3 slope expected (on log-transformed variables) or from the slope obtained for the relationship between prey items captured per capita and colony size (slope = −0.45 ± 0.13 SE; r2 = 0.42; t = −3.53, P = 0.003) (Fig. 2A).
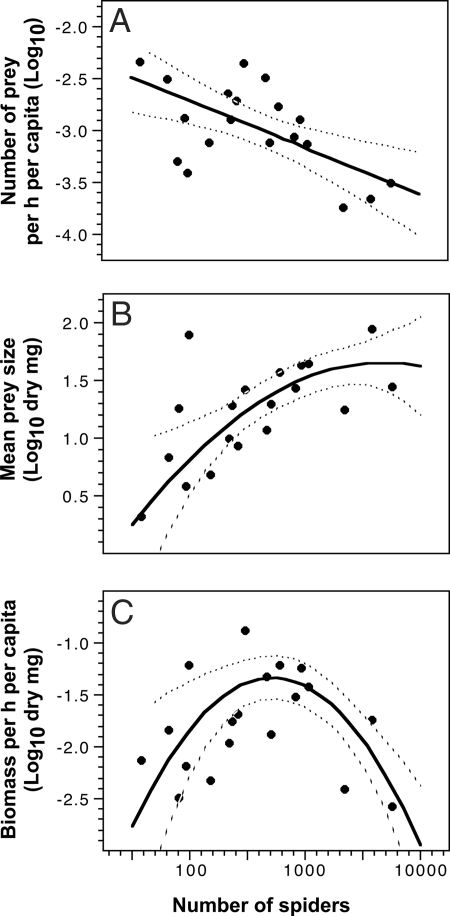
Fig. 2.
The relationships between colony size and the number of insects captured per hour per spider (A), the size of insects captured (dry mg) (B), and the prey mass (dry mg) captured per hour per spider (C). Dotted lines indicate 95% confidence intervals for the fits. Data are log transformed to satisfy normal distribution requirements of regression. Data points shown are each based on between 24 and 57 h of observation and one to several dozen prey capture events (median 27, range 1–62 prey capture events).
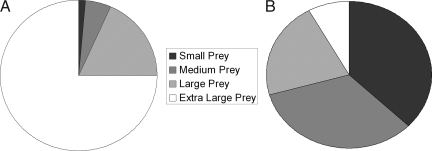
The average size of the prey captured (dry mg) by A. eximius colonies, however, increased 20-fold as colony size increased from <100 to ≈10,000 spiders (Fig. 2B), and large prey items (body length ≥2 cm), although making up only 8% of the diet by number, contributed nearly 75% of all nutritional mass across colonies (Fig. 3). We used both a quadratic polynomial and a linear regression to assess the relationship between colony size and prey size. The quadratic polynomial model (adj-r2 = 0.51; ANOVA, F2,16 = 10.27, P = 0.001; t-ratio, linear term: 4.37, P = 0.0005; quadratic term: −1.75, P = 0.10) was a slightly better fit than the linear one (adj-r2 = 0.45; ANOVA, F1,17 = 15.62, P = 0.001) but not significantly so (F1,15 = 2.85, P > 0.05, generalized likelihood ratio test) (linear fit not shown). By combining the number of prey items captured and their estimated masses to calculate the average per capita prey mass intake by colonies (see Materials and Methods), we show that the relationship between per capita prey intake and colony size has a significant quadratic component, with a peak at intermediate colony sizes of ≈500 adult and subadult spiders (adj-r2 = 0.56; ANOVA, F2,16 = 12.58, P = 0.0005; t-ratio, linear term: −2.69, P = 0.016; quadratic term: −4.05, P = 0.0009) (Fig. 2C).
Fig. 3.
The total amount of prey captured across all observation periods and colonies, divided into four size categories: small prey < 0.5 cm; 0.5 cm ≤ medium prey < 1.0 cm; 1.0 cm ≤ large prey < 2.0 cm; and 2.0 cm ≤ extra large prey. (A) Estimated summed dry mass of prey items: 0.22 g of small, 0.87 g of medium, 3.07 g of large, 12.44 g of extra large, and 16.60 g of total prey. (B) Number of prey items: 192 small, 173 medium, 110 large, 42 extra large, and 517 total.
Discussion
We show that cooperative foraging in a neotropical social spider leads to the capture of increasingly large insects as colony size increases, an effect that compensates for the decline in the number of prey captured per capita that results from the scaling of web surface area per spider with increasing colony size. These two effects combined result in a per capita food intake function that is maximal at intermediate colony sizes. Although the ability to capture large prey has long been recognized as a benefit of sociality in spiders (15–18, 22–24), the explicit relationship between colony size and per capita food intake had not been previously demonstrated for any social spider. That per capita prey biomass intake peaks at intermediate colony sizes, as well as the observation that fewer (on a per capita basis) but larger insects are captured as colony size increases, helps explain several biological phenomena associated with spider sociality. These phenomena include the conspicuous absence of social spiders from higher latitudes (18, 25) and higher elevations (26), as well as observed colony size distributions and dispersal and fitness patterns. For the genus Anelosimus, in particular, we have found that the higher latitude or higher elevation areas in the Americas, where only species forming small single-family groups occur (i.e., subsocial species) (26), have notably fewer insects belonging to large insect size classes than areas in the lowland rainforest where social species such as A. eximius occur (17, 27). Spiders in environments without a sufficient supply of large insects would not be able to make up for the decrease in prey items per capita with increasing colony size and may therefore disperse at smaller colony sizes (e.g., 24, 28–32). The shape and magnitude of the per capita prey intake function would thus, on the one hand, be a reflection of the range of insect sizes available in the environment and, on the other, mediate individual dispersal decisions. The latter suggestion and our estimated per capita prey intake function (Fig. 2C) are consistent with reports that A. eximius spiders will not disperse until a colony exceeds 1,000 individuals (18). That this threshold is twice the optimal foraging colony size estimated here, as well as the existence of considerably larger colonies, suggests that most spiders may not disperse until declining per capita resources come close to matching conditions in small newly established colonies. The broad range of colony sizes observed may also reflect an intrinsic inability of colonies to fine-tune their size associated with rapid colony growth (through internal recruitment) and discrete generations (5, 18, 33). Finally, a peaked per capita prey intake function would also explain why fitness peaks at intermediate colony sizes in this (33) and perhaps other social spiders (34). Increased food resources in colonies of intermediate size may promote juvenile survivorship, a fitness component shown to be positively correlated with colony size (33, 34). The capture in larger colonies of fewer, albeit larger, prey items per capita, on the other hand, may lead to inequitable sharing of prey typical of some social spiders (35–37). The latter phenomenon may explain the observed monotonic decrease with increasing colony size in the proportion of A. eximius females that reproduce (33). Such inequitable sharing of prey would counter the otherwise expected reduction in individual prey consumption variance that should result from the law of large numbers as colony size increases (38). Estimating individual prey consumption variance, however, is beyond the scope of this study because our per capita resource intake estimates were obtained from whole colony rather than from individual measures.
The extent to which access to large prey by groups interacts with other components of prey capture success and could thus be responsible for social evolution has remained unclear, both conceptually and empirically (39–41). Using game theory models, Packer and Ruttan (39) suggested that cooperative hunting is more likely to lead to gregariousness when groups hunt multiple prey that are small enough to be monopolized by the hunter and thus kept from cheaters. Our findings, as well as the observation that spider sociality is concentrated in areas where large insects are abundant (17, 27), are at odds with this prediction. The discrepancy probably arises because Packer and Ruttan's models considered prey size as a fixed parameter—either large or small—rather than a function of the size of the social group or of the number of individuals involved in a hunt, as our study has demonstrated for social spiders. Thus, it is not only prey capture efficiency that increases with colony size, but also the size of the insects captured. So, even if efficiency for a given prey size reached 100%, prey size and biomass captured would constitute moving targets potentially only limited by the range of prey sizes available in the environment. Furthermore, the prey capture success of single hunters, also a fixed parameter in the Packer and Ruttan models, is probably also a moving target because prey caught by larger colonies are increasingly beyond the reach of solitary hunters. In fact, insects at least four times as long and many times more massive than an A. eximius spider (where adult females average 4.6 mm in length; ref. 20) contributed a large majority of all prey mass caught by the observed colonies (Fig. 3). Studies on a solitary theridiid spider have shown that the capture efficiency of solitary individuals is close to zero when offered prey items greater than three times their size (16). According to the Packer and Ruttan models, it is under conditions in which the hunting success of groups far exceeds that of solitary individuals that cooperation in the capture of single large prey items may evolve (39). Above and beyond an increase in prey capture efficiency, therefore, cooperative hunting may allow access to a range of resources, and thus an ecological niche, unavailable to solitary hunters.
Although a declining surface area to volume ratio may represent a challenge for resource intake, it may on the other hand enhance the defensive nature of three-dimensional webs (42). Cooperative social spiders appear to in fact shield their offspring from predation by placing them in the innermost areas of their three-dimensional nests (unpublished observation). More elongated web shapes that would enhance foraging efficiency by maximizing surface relative to volume should conflict with this protective function. Accordingly, although there was a trend among the nests we studied to be slightly more elongated as their size increased (data not shown), such elongation was clearly not sufficient to counter the decline in surface area per unit volume that we observed as the colonies got larger. Individuals in other types of social groups may also take advantage of group-level surface to volume ratio relationships to gain protection from the elements or from predators and parasites for themselves or their offspring [e.g., huddling penguins (refs. 7 and 8) or antipredator tactics of schooling fish or of ungulate or other types of “selfish” herds (refs. 43–48)]. Foraging and predation tradeoffs may also be important to the colonial orb-weaving spiders. These spiders aggregate individual orb webs but, unlike the cooperative spiders, do not cooperate in prey capture. Nonetheless, larger colonies have increased capture efficiency through the “ricochet effect” whereby prey items that rebound off another web are more likely to be caught (49). Tradeoffs manifest themselves in terms of position within the web complex, because spiders in the interior are less likely to be attacked by predatory wasps but are also less likely to capture prey (50). Thus, scaling laws of the web or web complex may both limit prey intake and decrease predation risk as size increases, creating opposing selective pressures on web or complex size.
The ubiquity of scaling laws is hardly surprising as units in the biological hierarchy aggregated through time to form increasingly higher levels of organization. At each of the levels, selection may act on the individual units to manipulate group allometry to their advantage, although not necessarily to that of their neighbors' (i.e., the selfish herd) (43). Exactly how organisms should manipulate group allometry, however, may depend on scaling laws with complex and sometimes opposing fitness effects. Here, we have shown an example of how both the constraint imposed by scaling a declining surface area to volume ratio of communally-built webs of increasing size and the solution—foraging on increasingly large prey—are engendered through cooperation, suggesting that social behavior and the associated scaling laws may interact in complex and intriguing ways.
Materials and Methods
A. eximius webs consist of two main portions: a basket-shaped basal portion that surrounds a piece of vegetation and is used primarily for habitation and a superior web of primarily vertical and oblique lines, and no included vegetation, that is used to intercept and capture prey (Fig. 1A) (18, 51). What is relevant for our studies, and what we measured to estimate prey capture surface area per spider (Fig. 1B), is the superior web. For this purpose we used colonies seen at the colonies at the Jatun Sacha Biological Reserve (01°4′13.2″S, 77°36′41.4″W; 450-m elevation) in January 2002 (n = 6), June 2002 (n = 3), and May to June 2003 (n = 5). To estimate the surface area of the prey interception web we measured its circumference just above the basal basked-shaped nest and at the top where the webbing attached to vegetation. The distance between these two points was the web's height. We estimated the surface area by approximating the web's shape to a cylinder, a cone, or, for complex web formations, a series of geometric shapes whose surface areas we then combined.
We examined colonies for prey capture (prey size and number and biomass of prey caught per capita and unit time) at the Jatun Sacha Biological Reserve from May to June 2003 (n = 6) and at the Cuyabeno Nature Reserve (0°2′S, 76°20′W, 200- to 300-m elevation) from July to August 2004 (n = 6 forest interior and 7 river-edge colonies). We used primarily colonies where adults and late instar juveniles and subadults predominated to maintain homogeneity of life-cycle stages across colonies. We checked colonies for prey that spiders were capturing or consuming every 0.5–1.5 h for 4–5 h from 1 p.m. to 6 p.m. and 7 p.m. to 12 a.m. in 2003 and from 7 a.m. to 6 p.m. in 2004 (night observations were conducted under a red light). We did not remove prey items between observations in order not to damage the webs or disturb the spiders. Prey items were individually identifiable based on their taxonomic category, size, and location in the web. Double accounting was easily avoided given the relatively small number of insects being processed by a colony at any one time (range 0–8). Censuses were repeated over 6 days to obtain a single estimate per colony of the number of insects caught per capita and unit time (total number of insects caught divided by the number of spiders present in a colony and the number of hours of observation) and their size [dry insect mass estimated from the insect's length based on taxon-specific equations derived by Sage (52)]. These estimates were combined to obtain an estimate of total prey biomass caught per hour and per capita by each colony. We analyzed these data as a function of colony size (number of adult and subadult females), with individual colony estimates weighted in the analyses by either the number of hours of observation (for number of prey caught) or the number of insects (for prey size and biomass per capita) entering in that colony's estimate (range 24–57 h and 1–62 insects, median 27 insects, per colony). Some colonies were quite large, making direct population counts infeasible. For these we inferred colony size from previously derived relationships between number of spiders (log-transformed number of adults plus subadults) and nest size (log-transformed cross-sectional area of the nest at the widest part of the basket, as seen in an aerial view) (see ref. 51 for equations and diagram). We used adult and subadult females as a proxy for overall colony size because only individuals of these instars participate in prey capture, and only adult, subadult, and older instar juveniles participate in web maintenance and repair. So, it is only individuals of the older size classes that will have an effect on the size and condition of the web and on the success of prey capture and the size of the insects caught. The relationships between prey size, per capita prey capture, and colony size were examined through linear and polynomial regressions; competing models were tested by using a generalized likelihood ratio test (53).
Acknowledgments.
We thank J. Cochrane and J. Purcell for help in data collection; P. Durán-Ballen, J. Luna, the Jatun Sacha Foundation, and Don Melitón and Doña Zulema Llori for logistic support; and N. Brown, J. Guevara, J. Pepper, J. Purcell, and three anonymous reviewers for comments on the manuscript. We thank the Pontificia Universidad Católica del Ecuador for sponsoring our research in Ecuador and the Ministerio del Ambiente del Ecuador for research permits. This work was supported by grants from the U.S. National Science Foundation and the Canadian National Sciences and Engineering Research Council (to L.A.) and completed while L.A. was a visitor at the Institute for Advanced Study, Berlin. E.C.Y. was supported by the BRAVO! program at the University of Arizona, and K.P. was supported by the Center for Insect Science and the Department of Ecology and Evolutionary Biology at the University of Arizona.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
References
- 1.Bonner JT. Why Size Matters: From Bacteria to Blue Whales. Princeton: Princeton Univ Press; 2006. [Google Scholar]
- 2.Solari CA, Ganguly S, Kessler JO, Michod RE, Goldstein RE. Multicellularity and the functional interdependence of motility and molecular transport. Proc Natl Acad Sci USA. 2006;103:1353–1358. doi: 10.1073/pnas.0503810103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.West GB, Brown JH, Enquist BJ. A general model for the origin of allometric scaling laws in biology. Science. 1997;276:122–126. doi: 10.1126/science.276.5309.122. [DOI] [PubMed] [Google Scholar]
- 4.West GB, Brown JH, Enquist BJ. The fourth dimension of life: Fractal geometry and allometric scaling of organisms. Science. 1999;284:1677–1679. doi: 10.1126/science.284.5420.1677. [DOI] [PubMed] [Google Scholar]
- 5.Avilés L. Cooperation and non-linear dynamics: An ecological perspective on the evolution of sociality. Evol Ecol Res. 1999;1:459–477. [Google Scholar]
- 6.Jarvis JUM, Oriain MJ, Bennett NC, Sherman PW. Mammalian eusociality – a family affair. Trends Ecol Evol. 1994;9:47–51. doi: 10.1016/0169-5347(94)90267-4. [DOI] [PubMed] [Google Scholar]
- 7.Ancel A, Visser H, Handrich Y, Masman D, Le Maho Y. Energy saving in huddling penguins. Nature. 1997;385:304–305. [Google Scholar]
- 8.Gilbert C, Robertson G, Le Maho Y, Naito Y, Ancel A. Huddling behavior in emperor penguins: Dynamics of huddling. Physiol Behav. 2006;88:479–488. doi: 10.1016/j.physbeh.2006.04.024. [DOI] [PubMed] [Google Scholar]
- 9.Caraco T, Wolf LL. Ecological determinants of group sizes of foraging lions. Am Nat. 1975;109:343–352. [Google Scholar]
- 10.Nudds TD. Convergence of group size strategies by mammalian social carnivores. Am Nat. 1978;112:957–960. [Google Scholar]
- 11.Raffa KF, Berryman AA. Interacting selective pressures in conifer-bark beetle systems – a basis for reciprocal adaptations. Am Nat. 1987;129:234–262. [Google Scholar]
- 12.Baird RW, Dill LM. Ecological and social determinants of group size in transient killer whales. Behav Ecol. 1995;7:408–416. [Google Scholar]
- 13.Jun J, Pepper JW, Savage VM, Gillooly JF, Brown JH. Allometric scaling of ant foraging trail networks. Evol Ecol Res. 2003;5:297–303. [Google Scholar]
- 14.Hamilton MJ, Milne B, Walker RS, Brown JH. Nonlinear scaling of space use in human hunter-gatherers. Proc Natl Acad Sci USA. 2007;104:4765–4769. doi: 10.1073/pnas.0611197104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nentwig W. Social spiders catch larger prey: A study of Anelosimus eximius (Araneae: Theridiidae) Behav Ecol Sociobiol. 1985;17:79–85. [Google Scholar]
- 16.Rypstra AL. Prey capture and feeding efficiency of solitary and social spiders: A comparison. Acta Zool Fennica. 1990;190:339–343. [Google Scholar]
- 17.Powers KS, Avilés L. The role of prey size and abundance in the geographical distribution of spider sociality. J Anim Ecol. 2007;76:995–1003. doi: 10.1111/j.1365-2656.2007.01267.x. [DOI] [PubMed] [Google Scholar]
- 18.Avilés L. Causes and consequences of cooperation and permanent sociality in spiders. In: Choe JC, Crespi BJ, editors. Social Behavior in Insects and Arachnids. New York: Cambridge Univ Press; 1997. pp. 476–498. [Google Scholar]
- 19.Levi HW. The American spiders of the genus Anelosimus (Araneae: Theridiidae) Trans Am Microscop Soc. 1963;82:30–48. [Google Scholar]
- 20.Agnarsson I. A revision of the New World eximius lineage of Anelosimus (Araneae, Theridiidae) and a phylogenetic analysis using worldwide exemplars. Zool J Linn Soc. 2006;146:453–493. [Google Scholar]
- 21.Powers KS. Tucson: University of Arizona; 2004. Prey abundance and the evolution of sociality in the social spider genus Anelosimus. PhD thesis. [Google Scholar]
- 22.Buskirk R. Sociality in the Arachnida. In: Hermann HR, editor. Social Insects. Vol 2. New York: Academic; 1981. pp. 282–367. [Google Scholar]
- 23.Ward PI. Prey availability increases less quickly than nest size in the social spider Stegodyphus mimosarum. Behaviour. 1986;97:213–225. [Google Scholar]
- 24.Jones TC, Parker PG. Delayed juvenile dispersal benefits both mother and offspring in the cooperative spider Anelosimus studiosus (Araneae: Theridiidae) Behav Ecol. 2002;13:142–148. [Google Scholar]
- 25.Riechert SE, Roeloffs R, Echternacht AC. The ecology of the cooperative spider Agelena consociata in equatorial Africa (Araneaea, Agelenidae) J Arachnol. 1986;14:174–191. [Google Scholar]
- 26.Avilés L, et al. Altitudinal pattern of sociality in the spider genus Anelosimus and the biology of a new mid-elevation social species in Ecuador. Am Nat. 2007;170:783–792. doi: 10.1086/521965. [DOI] [PubMed] [Google Scholar]
- 27.Guevara J, Avilés L. Multiple sampling techniques confirm differences in insect size between low and high elevations that may influence levels of spider sociality. Ecology. 2007;88:2015–2033. doi: 10.1890/06-0995.1. [DOI] [PubMed] [Google Scholar]
- 28.Krafft B, Horel A, Julita JM. Influence of food supply on the duration of the gregarious phase of a maternal-social spider, Coelotes terrestris (Araneae, Agelenidae) J Arachnol. 1986;14:219–226. [Google Scholar]
- 29.Ruttan LM. Experimental manipulations of dispersal in the subsocial spider, Theridion pictum. Behav Ecol Sociobiol. 1990;27:169–173. [Google Scholar]
- 30.Avilés L, Gelsey G. Natal dispersal and demography of a subsocial Anelosimus species and its implications for the evolution of sociality in spiders. Can J Zool. 1998;76:2137–2147. [Google Scholar]
- 31.Kim KW. Dispersal behaviour in a subsocial spider: Group conflict and the effect of food availability. Behav Ecol Sociobiol. 2000;48:182–185. [Google Scholar]
- 32.Powers KS, Avilés L. Natal dispersal patterns of a subsocial spider Anelosimus cf. jucundus (Theridiidae) Ethology. 2003;109:725–737. [Google Scholar]
- 33.Avilés L, Tufiño P. Colony size and individual fitness in the social spider Anelosimus eximius. Am Nat. 1998;152:403–418. doi: 10.1086/286178. [DOI] [PubMed] [Google Scholar]
- 34.Bilde T, et al. Survival benefits select for group living in a social spider despite reproductive costs. J Evol Biol. 2007;20:2412–2426. doi: 10.1111/j.1420-9101.2007.01407.x. [DOI] [PubMed] [Google Scholar]
- 35.Rypstra AL. Prey size, social competition and the development of reproductive division of labor in social spider groups. Am Nat. 1993;142:868–880. [Google Scholar]
- 36.Amire N, Whitehouse MEA, Lubin Y. Food consumption rates and competition in a communally feeding social spider, Stegodyphus dumicola (Eresidae) J Arachnol. 2000;28:195–200. [Google Scholar]
- 37.Gonzaga M, Vasconcellos-Neto J. Influence of collective feeding on weight gain and size variability of. Anelosimus jabaquara Levi 1959 (Araneae, Theridiidae) Behaviour. 2002;139:1431–1442. [Google Scholar]
- 38.Wenzel J, Pickering J. Cooperative foraging, productivity, and the central limit theorem. Proc Natl Acad Sci USA. 1991;88:36–38. doi: 10.1073/pnas.88.1.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Packer C, Ruttan L. The evolution of cooperative hunting. Am Nat. 1988;132:159–198. [Google Scholar]
- 40.Packer C, Scheel D, Pusey AE. Why lions form groups: Food is not enough. Am Nat. 1990;136:1–19. [Google Scholar]
- 41.Creel S. Communal hunting and pack size in African wild dogs, Lycaon-pictus. Anim Behav. 1997;54:1319–1324. doi: 10.1006/anbe.1997.0481. [DOI] [PubMed] [Google Scholar]
- 42.Blackledge TA, Coddington JA, Gillespie RG. Are three-dimensional spider webs defensive adaptations? Ecol Lett. 2003;6:13–18. [Google Scholar]
- 43.Hamilton WD. Geometry for selfish herd. J Theor Biol. 1971;31:295–311. doi: 10.1016/0022-5193(71)90189-5. [DOI] [PubMed] [Google Scholar]
- 44.Skogland T. Comparative social organization in reindeer in relation to food, predator avoidance, and mates. Adv Ethol. 1989;29:1–71. [Google Scholar]
- 45.Nøttestad L, Axelsen BE. Herring schooling manoeuvres in response to killer whale attacks. Can J Zool. 1999;77:1540–1546. [Google Scholar]
- 46.Smith DW, et al. Wolf–bison interactions in Yellowstone National Park. J Mammal. 2000;81:1128–1135. [Google Scholar]
- 47.McLain DK, Pratt AE, Kirschstein K. Predator-driven fragmentation of fiddler crab droves into selfish miniherds of biased composition. J Exp Mar Biol Ecol. 2005;315:1–15. [Google Scholar]
- 48.Fauchald P, et al. Escaping parasitism in the selfish herd: Age, size and density-dependent warble fly infestation in reindeer. Oikos. 2007;116:491–499. [Google Scholar]
- 49.Uetz GW, Hieber CS. Colonial web-building spiders: Balancing the costs and benefits of group-living. In: Choe JC, Crespi BJ, editors. Social Behavior in Insects and Arachnids. New York: Cambridge Univ Press; 1997. pp. 458–475. [Google Scholar]
- 50.Rayor SL, Uetz G. Trade-offs in foraging success and predation risk with spatial position in colonial spiders. Behav Ecol Sociobiol. 1990;27:77–85. [Google Scholar]
- 51.Purcell J, Avilés L. Smaller colonies and more solitary living mark higher elevation populations of a social spider. J Anim Ecol. 2007;76:590–603. doi: 10.1111/j.1365-2656.2007.01228.x. [DOI] [PubMed] [Google Scholar]
- 52.Sage RD. Wet and dry-weight estimates of insects and spiders based on length. Am Midl Nat. 1982;108:407–411. [Google Scholar]
- 53.Borowiak DS. Model Discrimination for Non-linear Regression Models. New York: Dekker; 1989. [Google Scholar]