Abstract
In the developing cerebellum, switching of the subunit composition of NMDA receptors occurs in granule cells from NR2B-containing receptors to NR2C-containing ones. We investigated the mechanisms underlying switching of NR2B and NR2C subunit composition in primary cultures of mouse granule cells at the physiological KCl concentration (5 mM). Granule cells extensively extended their neuritic processes 48 h after having been cultured in serum-free medium containing 5 mM KCl. Consistent with this morphological change, NR2B mRNA and NR2C mRNA were down- and up-regulated, respectively, in the granule cells. This dual regulation of the two mRNAs was abrogated by blocking excitation of granule cells with TTX. This neuronal activity–dependent regulation of NR2B and NR2C mRNAs was abolished by the addition of selective antagonists of AMPA receptors and NMDA receptors. Furthermore, the dual regulation of NR2B and NR2C mRNAs in TTX-treated cells was restored by the addition of NMDA in the presence of the AMPA receptor antagonist, but not by that of AMPA in the presence of the NMDA receptor antagonist. Importantly, the NMDA receptor activation drove the NR2B/NR2C switching of NMDA receptors in the cell-surface membrane of granule cells. This investigation demonstrates that stimulation of NMDA receptors in conjunction with the AMPA receptor–mediated excitation of granule cells plays a key role in functional subunit switching of NMDA receptors in maturing granule cells at the physiological KCl concentration.
Keywords: cerebellar development, gene regulation, glutamate receptor, synaptic maturation
Switching of subunit composition of neurotransmitter receptors represents a hallmark of development and maturation of functional synapse formation during the early postnatal period (1, 2). NMDA receptors are glutamate-gated ion channels composed of an obligatory NR1 subunit and distinct combinations of NR2 subunits, known as NR2A-2D (3). Cerebellar granule cells express NR1, NR2A, and NR2B mRNAs after division in the external granule cell layer (4, 5). After their migration into the internal granule cell layer, these cells down-regulate their NR2B mRNA and markedly up-regulate their NR2C mRNA during maturation of synapses between mossy fibers and granule cells (4–6). This switching of subunit composition changes the properties of NMDA receptors and contributes to synaptic transmission at the mossy fiber–granule cell in the mature cerebellar network (6, 7).
The regulatory mechanism underlying switching of NMDA receptor subunit composition still largely remains to be clarified. Several lines of evidence have indicated that neural activity differently regulates NMDA receptor subunit expression in developing granule cells (8–11). This mechanism has been extensively investigated in cultures of dissociated rat granule cells. Primary cultures of rat granule cells require a high concentration of KCl (25 mM) because of their poor survival under the low-KCl (5 mM) condition (8, 9, 11). The high KCl concentration enhances granule cell survival, differentiation, and NMDA receptor responsiveness and has been proposed to mimic the activity of glutamatergic mossy fiber input to granule cells (8). However, the physiological concentration of extracellular K+ is ≈5 mM, and mossy fibers are not chronically active in vivo (12). Furthermore, the characterization of the properties of granule cells cultured at high KCl concentration has indicated that they are immature in terms of gene expression pattern, electrophysiological properties, and intracellular signaling mechanisms (13–16). In contrast to rat granule cells, the long-term viability of mouse granule cells is maintained at 5 mM KCl (14, 17). In this investigation, we addressed activity-dependent switching mechanisms of the NR2B and NR2C subunit composition in cultured mouse granule cells at 5 mM KCl. Here we report that stimulation of NMDA receptors plays a key role in both up-regulation of NR2C and down-regulation of NR2B in cultured cells at the physiological concentration of KCl.
Results
Regulation of NMDA Receptor Subunit Expression by Neuronal Activity.
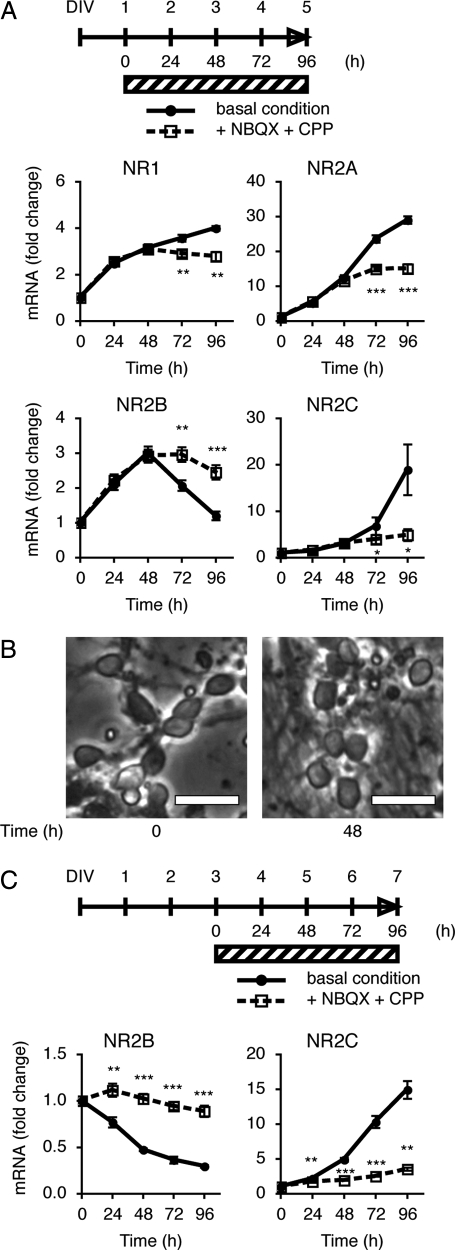
Subunit switching from NR2B to NR2C in the cerebellum occurs between postnatal days 8 and 21 (4, 5), when mossy fibers form mature synapses with granule cells (18). Primary cultures of cerebellar granule cells were prepared from Institute of Cancer Research (ICR) mouse pups at postnatal day 8. Granule cells were cultured in medium containing serum and 5 mM KCl for 24 h, and then for up to 96 h in serum-free medium containing 5 mM KCl. We first addressed how expression of the mRNAs for four different NMDA receptor subunits changed during continuing cell culture for 96 h in the serum-free medium containing 5 mM KCl (Fig. 1). The levels of these mRNAs were quantified by PCR analysis. NR1, NR2A, and NR2B mRNAs exhibited an increase for the first 48 h, but their expression patterns were different during the following 48 h. NR2A mRNA continuously increased up to at least 96 h, but NR1 mRNA gradually reached a plateau level during the 96-h culture period. In contrast, NR2B mRNA decreased from 48 h to 96 h. Conversely, NR2C mRNA failed to increase for the first 48 h and then became markedly elevated during the following 48 h.
Fig. 1.
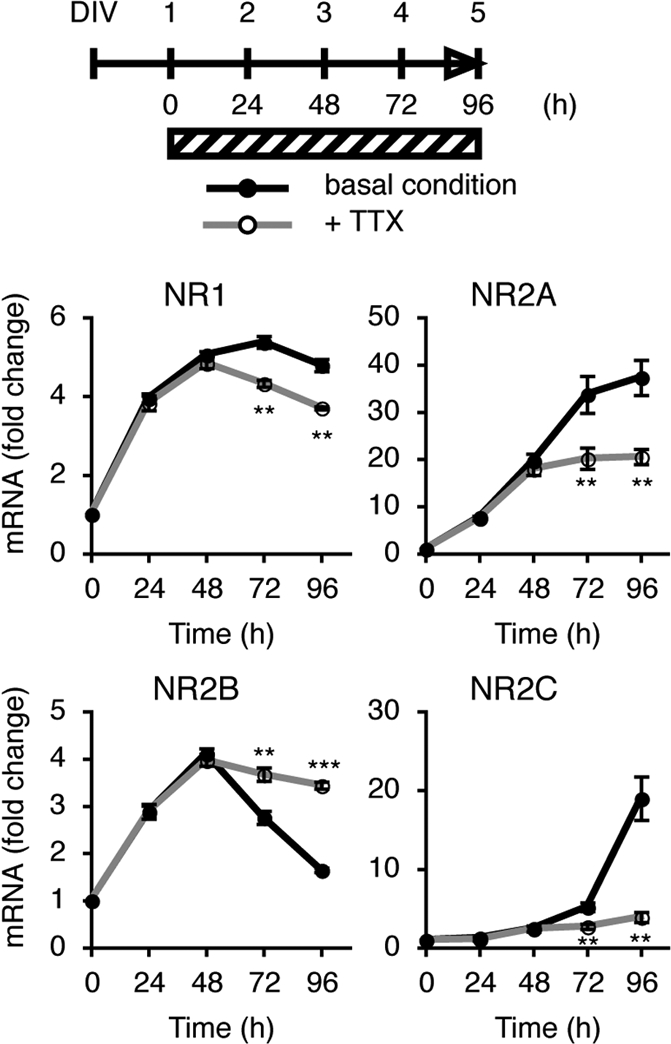
Inhibitory effects of TTX on mRNA expression of the NMDA receptor subunits. Granule cells were cultured in the serum-containing medium for 24 h. Culture was continued in the serum-free medium for 96 h after the addition or omission of 5 μM TTX, as indicated by the shaded bar. The time course of mRNA levels in the presence and absence of TTX was examined by PCR analysis (n = 4). Data are expressed as the mean ± SEM. ***P < 0.001, **P < 0.01, TTX-treated vs. untreated. DIV, days in vitro.
Next, we examined how blockade of granule cell excitation would influence the expression patterns of these four NR mRNAs by adding TTX to the granule cell cultures (Fig. 1). TTX had no effect on the expression patterns of any of the mRNAs during the first 48-h culture period but then later abolished the increase or decrease in expression characteristic of each NR mRNA.
Regulation of NMDA Receptor Subunit Expression by Glutamatergic Transmission.
Granule cells are excited by the neurotransmitter glutamate via its interaction with both AMPA and NMDA receptors. Because the aforementioned findings strongly suggested that excitation of granule cells via a neurotransmitter distinctly up- or down-regulated the four NR mRNAs during the culture period from 48 h to 96 h, we examined whether the inhibition by selective antagonists of AMPA receptors and NMDA receptors would block the changes in expression of the NR mRNAs in cultured granule cells (Fig. 2A). The AMPA receptor antagonist 2,3-dioxo-6-nitro-1,2,3,4-tetrahydrobenzo[f] quinoxaline-7-sulfonamide (NBQX) (19) and the NMDA receptor antagonist 3-(2-carboxypiperazin-4-yl)propyl-1-phosphonic acid (CPP) (20) were added together at the beginning of cell culture period in the serum-free medium, and the mRNA levels were quantified by PCR analysis. These antagonists had no effect on the expression pattern of any of the NR mRNAs during the first 48-h period. Thereafter, however, the antagonists abrogated the characteristic expression patterns of all four NR mRNAs.
Fig. 2.
Inhibition of changes in mRNA expression of the NMDA receptor subunits by NBQX and CPP. (A) Granule cells were cultured in the serum-containing medium for 24 h. The cultures were continued in the serum-free medium for 96 h after the addition or omission of 100 μM NBQX and 100 μM CPP, as indicated by the shaded bar. The time course of mRNA levels in the presence and absence of antagonists was examined by PCR analysis (n = 5). (B) Cell morphology of granule cells cultured in the serum-free medium at time points 0 and 48 h. Bar, 20 μm. (C) Cultures were treated as described in A, except that the antagonists were added after a 48-h culture period in the serum-free medium (n = 5). Data are expressed as the mean ± SEM. *P < 0.05, **P < 0.01, ***P < 0.001, antagonist-treated vs. untreated.
Granule cells changed their morphology between the first 48-h period of culture and the subsequent 48-h one (Fig. 2B). They showed variously shaped somas with poorly developed neurites during the first 48 h of culture and prominently extended neurites from rounded somas during the following 48 h. This morphological change was in good agreement with the distinct effects of the glutamate antagonists on the characteristic expression of the four NR mRNAs during the latter 48-h culture period. The extended neurites of granule cells were expected to release glutamate, and the resulting glutamate most likely excited neighboring granule cells in culture and differently regulated the expression of their NR mRNAs. To substantiate this notion, we added the glutamate receptor antagonists 48 h after having cultured the cells in the serum-free medium, and then examined their effects on switching of NR2B and NR2C mRNAs up to 96 h after the addition of the antagonists (Fig. 2C). The results of this experiment explicitly indicated that the receptor antagonists blocked both up-regulation of NR2C mRNA and down-regulation of NR2B at the maturation stage of the cultured granule cells. Thus stimulation of glutamate receptors plays a pivotal role in switching of the NMDA receptor subunit composition in maturing granule cells.
Regulation of NR2B and NR2C mRNA Expression by NMDA Receptors.
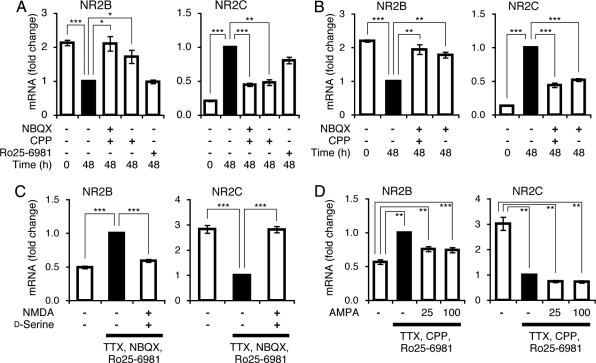
Next we examined which class of glutamate receptors was involved in the switching of the NMDA receptor subunits in the granule cells. NBQX or CPP alone or both were added 48 h after the cells had been cultured in the serum-free medium, and then their effects on changes in NR2B mRNA and NR2C mRNA levels were analyzed 48 h after the addition of these antagonists (Fig. 3 A and B). The addition of CPP and NBQX both together and alone blocked the changes in the levels of NR2B and NR2C mRNAs during the incubation of these antagonists for 48 h. The channel activity of NMDA receptors is known to be blocked by Mg2+ in a voltage-dependent manner (21). The culture medium contained Mg2+ (0.8 mM) to maintain the survival of the granule cells. The inhibition of AMPA receptors by NBQX could thus prevent excitation of granule cells and would inhibit relief of the voltage-dependent Mg2+ block of NMDA receptors to respond to endogenously released glutamate (21).
Fig. 3.
Regulation of NR2B and NR2C mRNA levels by NMDA receptor activation. Granule cells were cultured in the serum-containing medium for 24 h. They were then cultured in the serum-free medium for 48 h (time point, 0). (A,B) Cultures were continued for 48 h (time point, 48 h) after the addition or omission of the indicated antagonists. mRNA levels were quantified at both time points (n = 4). (C,D) Cultures were treated as described in A and B, except that after a 48-h culture period in the serum-free medium (time point, 0), culture was continued in the presence and absence of 5 μM TTX, 0.5 μM Ro25–6981, and either 100 μM NBQX (C) or 100 μM CPP (D) for 48 h (time point, 48 h). Either 100 μM NMDA together with 100 μM d-serine or the indicated concentrations (in micromolars) of AMPA were added or omitted at time point 0. mRNA levels were quantified at time point 48 h (n = 7 for C, n = 4 for D). mRNA levels at time point 48 h in the absence of antagonists (A,B) or in the presence of the indicated antagonists without agonists (C,D) were taken as 1 (black bars) and statistically analyzed. Data are expressed as the mean ± SEM. *P < 0.05, **P < 0.01, ***P < 0.001.
To examine the receptor specificity more directly, we investigated the stimulatory effect of either NMDA receptors or AMPA receptors on the mRNA levels in the presence of NBQX or CPP, respectively (Fig. 3 C and D). To maximally stimulate NMDA receptors, we added d-serine, an NMDA receptor coactivator, together with NMDA. In addition, we added TTX to avoid stimulation by endogenous glutamate, which results from either spontaneous excitation of granule cells or ligand-mediated recurrent stimulation by neighboring granule cells. Under this condition, we found that NMDA significantly caused cell death 48 h after its addition. To circumvent this problem, we added the NR2B-specific blocker Ro25–6981, which has been reported to protect against NMDA-mediated cell death in many neuronal cells (22, 23). The addition of Ro25–6981 (0.5 μM) protected against the NMDA-induced cell death of cultured granule cells up to 108 h. Ro25–6981 alone, however, had no effect on either NR2B or NR2C mRNA regulation during the 48-h cell culture period (Fig. 3A). The basal culture medium used in the subsequent experiments thus contained 5 μM TTX and 0.5 μM Ro25–6981 in serum-free medium containing 5 mM KCl.
Under these culture conditions, the addition of NMDA and d-serine significantly down-regulated NR2B mRNA and conversely up-regulated NR2C mRNA (Fig. 3C). Because Ro25–6981 was present in the culture medium, this finding indicates that the NR1/NR2A NMDA receptors are capable of regulating the expression of both mRNAs in culture granule cells. In contrast, neither NR2B mRNA down-regulation nor NR2C mRNA up-regulation was restored by the addition of AMPA to the CPP-treated granule cells during the 48-h culture period (Fig. 3D). Taking into consideration the inhibitory effect of the AMPA receptor antagonist on regulation of both mRNAs, these results demonstrate that the AMPA receptor–mediated excitation of granule cells is necessary for activation of NMDA receptors, but that the NMDA receptor activation plays a key role in both down-regulation of NR2B mRNA and up-regulation of NR2C mRNA in the granule cells.
Regulatory Expression of NR2B and NR2C in the Cell-Surface Membrane.
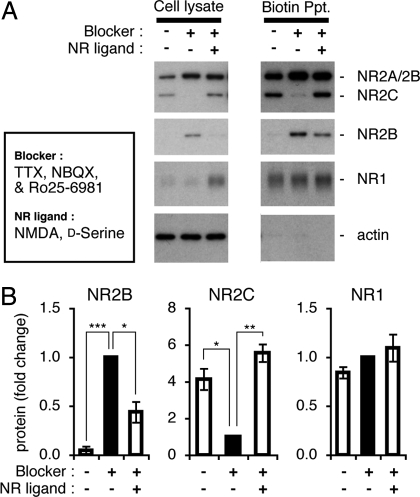
We next examined whether the effects of NMDA receptor activation on NR mRNAs would be reflected in the expression of the NR subunit proteins (Fig. 4). Granule cells were cultured in the presence and absence of NMDA and d-serine in the serum-free medium containing TTX, NBQX, and Ro25–6981 for 108 h. Supernatants of cell lysates were then subjected to Western blot analysis (Fig. 4A). The addition of NMDA and d-serine markedly decreased and increased the levels of NR2B and NR2C proteins, respectively. NR1 was also up-regulated by the addition of NMDA and d-serine.
Fig. 4.
Expression of NMDA receptor subunits on cell-surface membranes. Granule cells were cultured in the serum-containing medium for 24 h and then in the serum-free medium for 48 h. Cultures were continued in the presence and absence of 5 μM TTX, 100 μM NBQX, and 0.5 μM Ro25–6981 for 108 h. At the same time point, 100 μM NMDA and 100 μM d-serine were added when the aforementioned antagonists were added. Cell-surface membrane proteins were biotinylated and precipitated (Ppt) by using avidin beads. The amounts of NMDA receptor subunits were quantified by Western blot analysis of supernatants of cell lysates and biotinylated membrane proteins. (A) Representative data of Western blot analysis are indicated. Anti-NR2 reacting with NR2A, NR2B, and NR2C subunits, anti-NR2B, anti-NR1, and anti-actin were used as primary antibodies, respectively, in Western blots from the upper panel to the lower panel. (B) Relative amounts of NMDA receptor subunits in cell membranes are shown by receptor levels (black bars) in the absence of NMDA and d-serine as 1 (n = 4) and used for statistical comparison of other values. Data are expressed as the mean ± SEM. *P < 0.05, **P < 0.01, ***P < 0.001.
We then examined whether the NMDA receptor activation was driving the subunit switching of NMDA receptors in the cell-surface membranes of the granule cells (Fig. 4 A and B). Granule cells in culture were biotinylated with membrane-impermeable biotin ester. The solubilized membrane proteins were then precipitated with avidin beads and analyzed by Western blotting. The cytoplasmic actin was not biotinylated, confirming that only membrane proteins facing the exterior were biotinylated (Fig. 4A). The NR1, NR2B, and NR2C subunits were all biotinylated at cell-surface membranes. Importantly, the amounts of biotinylated cell-surface NR2B and NR2C decreased and markedly increased, respectively, in granule cells treated with NMDA and d-serine compared with their levels in agonist-untreated cells (Fig. 4 A and B). These increases and decreases were in contrast to the lack of any appreciable change in the cell-surface NR1 in the NMDA-treated cells. These results indicate that the NMDA receptor activation is a key event to exchange subunits for the assembly of the functional NMDA receptors in cell-surface membranes.
Downstream Signaling of NR2C Up-Regulation.
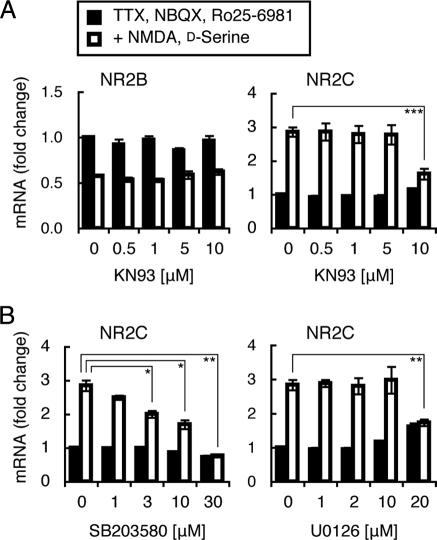
A number of studies have indicated that activation of NMDA receptors increases Ca2+ influx, which in turn regulates gene expression in neuronal cells through the activation of some Ca2+-dependent protein kinases and protein phosphatases (24–26). We therefore addressed whether any of these calcium signaling mechanisms were involved in the regulation of NR2B and NR2C mRNAs (Fig. 5). Cultured granule cells were treated with various kinase/phosphatase inhibitors together with NMDA and d-serine for 48 h and then subjected to PCR analysis. A high concentration of the calmodulin-dependent protein kinase (CaMK) inhibitor KN93 (27) abrogated the NMDA-mediated up-regulation of NR2C mRNA, but this inhibitor had no effect on the down-regulation of NR2B mRNA (Fig. 5A). Treatments with the protein kinase C inhibitor bisindolylmaleimide I (1 μM) or the calcineurin phosphatase inhibitor cyclosporin A (0.5 μM) failed to block NMDA-mediated regulation of either NR2B or NR2C mRNA (data not shown). Other signaling inhibitors including the tyrosine kinase inhibitor K252a (50 nM), the PI3K inhibitor LY294002 (10 μM), and the PLC inhibitor U73122 (2 μM) also had no effect on either mRNA (data not shown). These inhibitors were previously confirmed to effectively inhibit target protein kinases/phosphatases in cultured granule cells at the concentrations used (16). A member of the MAP kinase family is activated via the CaMK-mediated signaling cascades (24, 28). The P38 inhibitor SB203580 (29) inhibited the NMDA-mediated up-regulation of NR2C mRNA in a dose-dependent manner, whereas the MEK1/2/5 inhibitor U0126 (30) was effective in inhibiting it at only a high concentration (Fig. 5B). In contrast, these inhibitors had no appreciable effect on the NMDA-mediated down-regulation of NR2B mRNA (data not shown). These results indicate that NMDA receptors play a crucial role in the regulation of both NR2B and NR2C mRNAs but that its downstream signaling is different between these two mRNAs.
Fig. 5.
Involvement of the CaMK and MAP kinase cascades in NR2C mRNA up-regulation. Granule cells were cultured in the serum-containing medium for 24 h and then in the serum-free medium for 48 h. Cultures were continued for 48 h in the serum-free medium containing 5 μM TTX, 100 μM NBQX, and 0.5 μM Ro25–6981 with or without 100 μM NMDA and 100 μM d-serine. KN93 (n = 5), SB203580 (n = 4), and U0126 (n = 4) at the indicated concentrations were added 2 h before the addition or omission of NMDA and d-serine. mRNA levels were quantified at time point 48 h. Data are expressed as the mean ± SEM. *P < 0.05, **P < 0.01, ***P < 0.001, compared with mRNA levels in the absence of the indicated inhibitors.
Discussion
Primary cultures of the early postnatal cerebellum are highly enriched in granule cells that exhibit many properties characteristic of developing granule cells in vivo (8, 9). A number of studies of developing rat granule cells in primary culture have been conducted to explore the mechanism underlying proliferation and differentiation of immature granule cells and maturation of mossy fiber–granule cell synapses (8–11). Long-term exposure of granule cells in primary culture to 25 mM KCl increases Ca2+ influx and enhances granule cell survival, differentiation, and NMDA receptor responsiveness (2, 8). The treatment of cultured granule cells with high KCl concentration has thus been proposed to mimic the activity of mossy fiber input to granule cells (8). In culture, the resting membrane potentials of granule cells at 25 mM KCl and 5 mM KCl were reported to be approximately −35 mV and −50 mV, respectively (14). Importantly, the resting membrane potential of immature granule cells (≈−25 mV) becomes gradually more negative (≈−55 mV) during granule cell maturation in vivo (31). In addition, long-term exposure of cultured granule cells to high KCl abolishes the generation of the action potential and attenuates excitatory and inhibitory synaptic transmission (14). Furthermore, our recent study using organotypic cerebellar cultures showed that high KCl permits the development and migration of immature granule cells but blocks the synaptic maturation of granule cells (M.O., H.A., and S.N., unpublished observation). The KCl-induced depolarization could thus mimic the developmental processes of immature granule cells rather than the maturation processes of mossy fiber–granule cell synapse formation (2).
In this investigation, we examined the mechanism of subunit switching of NR2B and NR2C subunits of NMDA receptors in mouse granule cells at the physiological KCl concentration. The results indicated that both up-regulation of NR2C mRNA and down-regulation of NR2B mRNA were blocked by the addition of TTX. We interpret these findings to mean that ambient endogenous glutamate excites granule cells and in turn mediates the regulatory expression of both mRNAs in cultured granule cells. The blockade of NR2B down-regulation by TTX is consistent with the study using organotypic cerebellar cultures, although this study argued against the involvement of granule cell excitation in NR2C up-regulation (10). In cultures of rat granule cells, a large number of granule cells die at 5 mM KCl, but it has also been reported that a small portion of granule cells survive at 5 mM KCl and exhibit an increase and a decrease in their levels of NR2C and NR2B mRNAs, respectively (11). The present investigation combining the use of agonists and antagonists has provided compelling evidence that activation of NMDA receptors selectively and significantly regulates both NR2B and NR2C mRNA expression in granule cells at the physiological KCl concentration. Importantly, the present study has indicated that the NMDA receptor activation results in switching of functional NMDA receptors in the cell-surface membranes of granule cells. Although further study awaits analysis of the downstream signals of NMDA receptor activation for regulation of NR2B and NR2C mRNAs, the present study has explicitly demonstrated that NMDA receptors play a pivotal role in subunit switching of NR2B and NR2C during the maturation of granule cells.
A switch of NMDA receptor subunits during granule cell maturation alters the open time, burst lengths, and Mg2+ sensitivity of NMDA receptor channels (6). Gene targeting studies indicated that expression of NR2A and NR2C is essential for glutamatergic transmission in mature mossy fiber–granule synapses and participates in the coordination of complex motor movements (7). Long-term NR2B expression during development by incorporating the NR2B gene into the NR2C locus also showed a profound deteriorating effect on the cerebellar architecture and impaired motor coordination (32). The subunit switch of NMDA receptors thus significantly contributes to glutamatergic transmission in the mossy fiber–granule cell synapses. Primary cultures of mouse granule cells at the physiological KCl concentration thus provide a useful system to explore further the mechanisms underlying NMDA receptor subunit switching in maturing granule cells.
Materials and Methods
Culture of Cerebellar Granule Cells.
All animal handling procedures were performed according to the guidelines of Kyoto University Faculty of Medicine and Osaka Bioscience Institute. Cerebella were prepared from 8-day-old ICR mice (Japan SLC). Cell dissociation, plating, and culturing of granule cells were performed as described previously (15, 16, 33). Granule cells were plated on poly-d-lysine–coated dishes (Becton Dickinson) at a density of 2.5–3.0 × 105 cells/cm2 in the serum-containing medium supplemented with 5 mM KCl and incubated for 24 h. The culture medium was then switched to the serum-free medium containing 5 mM KCl (15, 16, 33). Staining with anti–β-tubulin type III antibody showed that the cultures contained ≈90% neuronal cells (33). Cell viability was measured with a LIVE/DEAD Viability/Cytotoxicity kit (Molecular Probes). NBQX, (R)-CPP, TTX, Ro25–6981, NMDA, d-serine, (S)-AMPA, U0126, and SB203580 were all purchased from Tocris. KN93 was from Calbiochem.
Quantitative PCR Analysis.
Total RNA was isolated from cultured granule cells and subjected to reverse transcription, followed by quantitative PCR analysis as described previously (16). A Transcriptor First Strand cDNA Synthesis Kit (Roche Diagnostics) was used for reverse transcription. Quantitative PCR was performed with the use of LightCycler 480 SYBR green I Master on LightCycler 480 System (Roche Diagnostics) with β-actin mRNA as an internal control for normalization of mRNA levels. According to GenBank databases of the National Center for Biotechnology Information, the primer sequences used for PCR were as follows: residues 2602–2621 and 2778–2758 of NM_008169 for NR1, 1199–1218 and 1348–1329 of NM_008170 for NR2A, 1728–1747 and 1918–1899 of NM_008171 for NR2B, 1793–1812 and 1943–1924 of NM_010350 for NR2C, and 1771–1790 and 1890–1870 of NM_007393 for β-actin.
Western Blot and Cell-Surface Biotinylation Analysis.
Western blot analysis and cell-surface biotinylation were carried out as described previously (16) except that a 4–12% gradient NuPAGE Novex Bis-Tris Gel (Invitrogen) was used for electrophoresis. Antibodies used were rabbit anti-NR1 (1:2,000; Chemicon), anti-NR2B (1:1,000; Chemicon), anti-NR2 (1:2,000; Affinity BioReagents), and anti-actin (1:5,000; Sigma). The band intensity of immunoblots was quantified with a GS-800 Calibrated Densitometer (Bio-Rad).
Statistical Analysis.
Data were statistically analyzed by using the two-tailed t test.
Acknowledgments.
This work was supported by Research Grant KAKENHI 17002016 (S.N.) from the Ministry of Education, Culture, Sports, Science, and Technology of Japan and by a grant from Takeda Science Foundation.
Footnotes
The authors declare no conflict of interest.
References
- 1.Sanes JR, Lichtman JW. Induction, assembly, maturation and maintenance of a postsynaptic apparatus. Nat Rev Neurosci. 2001;2:791–805. doi: 10.1038/35097557. [DOI] [PubMed] [Google Scholar]
- 2.Nakanishi S, Okazawa M. Membrane potential-regulated Ca2+ signalling in development and maturation of mammalian cerebellar granule cells. J Physiol. 2006;575:389–395. doi: 10.1113/jphysiol.2006.113340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nakanishi S. Molecular diversity of glutamate receptors and implications for brain function. Science. 1992;258:597–603. doi: 10.1126/science.1329206. [DOI] [PubMed] [Google Scholar]
- 4.Akazawa C, Shigemoto R, Bessho Y, Nakanishi S, Mizuno N. Differential expression of five N-methyl-d-aspartate receptor subunit mRNAs in the cerebellum of developing and adult rats. J Comp Neurol. 1994;347:150–160. doi: 10.1002/cne.903470112. [DOI] [PubMed] [Google Scholar]
- 5.Watanabe M, Mishina M, Inoue Y. Distinct spatiotemporal expressions of five NMDA receptor channel subunit mRNAs in the cerebellum. J Comp Neurol. 1994;343:513–519. doi: 10.1002/cne.903430402. [DOI] [PubMed] [Google Scholar]
- 6.Farrant M, Feldmeyer D, Takahashi T, Cull-Candy SG. NMDA-receptor channel diversity in the developing cerebellum. Nature. 1994;368:335–339. doi: 10.1038/368335a0. [DOI] [PubMed] [Google Scholar]
- 7.Kadotani H, et al. Motor discoordination results from combined gene disruption of the NMDA receptor NR2A and NR2C subunits, but not from single disruption of the NR2A or NR2C subunit. J Neurosci. 1996;16:7859–7867. doi: 10.1523/JNEUROSCI.16-24-07859.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gallo V, Kingsbury A, Balázs R, Jørgensen OS. The role of depolarization in the survival and differentiation of cerebellar granule cells in culture. J Neurosci. 1987;7:2203–2213. doi: 10.1523/JNEUROSCI.07-07-02203.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bessho Y, Nawa H, Nakanishi S. Selective up-regulation of an NMDA receptor subunit mRNA in cultured cerebellar granule cells by K+-induced depolarization and NMDA treatment. Neuron. 1994;12:87–95. doi: 10.1016/0896-6273(94)90154-6. [DOI] [PubMed] [Google Scholar]
- 10.Audinat E, Lambolez B, Rossier J, Crépel F. Activity-dependent regulation of N-methyl-d-aspartate receptor subunit expression in rat cerebellar granule cells. Eur J Neurosci. 1994;6:1792–1800. doi: 10.1111/j.1460-9568.1994.tb00572.x. [DOI] [PubMed] [Google Scholar]
- 11.Vallano ML, Lambolez B, Audinat E, Rossier J. Neuronal activity differentially regulates NMDA receptor subunit expression in cerebellar granule cells. J Neurosci. 1996;16:631–639. doi: 10.1523/JNEUROSCI.16-02-00631.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chadderton P, Margrie TW, Häusser M. Integration of quanta in cerebellar granule cells during sensory processing. Nature. 2004;428:856–860. doi: 10.1038/nature02442. [DOI] [PubMed] [Google Scholar]
- 13.Condorelli DF, et al. Growth conditions differentially regulate the expression of α-amino-3-hydroxy-5-methylisoxazole-4-propionate (AMPA) receptor subunits in cultured neurons. J Neurochem. 1993;61:2133–2139. doi: 10.1111/j.1471-4159.1993.tb07451.x. [DOI] [PubMed] [Google Scholar]
- 14.Mellor JR, Merlo D, Jones A, Wisden W, Randall AD. Mouse cerebellar granule cell differentiation: electrical activity regulates the GABAA receptor α6 subunit gene. J Neurosci. 1998;18:2822–2833. doi: 10.1523/JNEUROSCI.18-08-02822.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sato M, Suzuki K, Yamazaki H, Nakanishi S. A pivotal role of calcineurin signaling in development and maturation of postnatal cerebellar granule cells. Proc Natl Acad Sci USA. 2005;102:5874–5879. doi: 10.1073/pnas.0501972102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Suzuki K, Sato M, Morishima Y, Nakanishi S. Neuronal depolarization controls brain-derived neurotrophic factor-induced upregulation of NR2C NMDA receptor via calcineurin signaling. J Neurosci. 2005;25:9535–9543. doi: 10.1523/JNEUROSCI.2191-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mogensen HS, Hack N, Balázs R, Jørgensen OS. The survival of cultured mouse cerebellar granule cells is not dependent on elevated potassium-ion concentration. Int J Dev Neurosci. 1994;12:451–460. doi: 10.1016/0736-5748(94)90029-9. [DOI] [PubMed] [Google Scholar]
- 18.Mason CA, Gregory E. Postnatal maturation of cerebellar mossy and climbing fibers: transient expression of dual features on single axons. J Neurosci. 1984;4:1715–1735. doi: 10.1523/JNEUROSCI.04-07-01715.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sheardown MJ, Nielsen EØ, Hansen AJ, Jacobsen P, Honoré T. 2,3-Dihydroxy-6-nitro-7-sulfamoyl-benzo(F)quinoxaline: a neuroprotectant for cerebral ischemia. Science. 1990;247:571–574. doi: 10.1126/science.2154034. [DOI] [PubMed] [Google Scholar]
- 20.Davies J, et al. CPP, a new potent and selective NMDA antagonist. Depression of central neuron responses, affinity for [3H]d-AP5 binding sites on brain membranes and anticonvulsant activity. Brain Res. 1986;382:169–173. doi: 10.1016/0006-8993(86)90127-7. [DOI] [PubMed] [Google Scholar]
- 21.Nowak L, Bregestovski P, Ascher P, Herbet A, Prochiantz A. Magnesium gates glutamate-activated channels in mouse central neurones. Nature. 1984;307:462–465. doi: 10.1038/307462a0. [DOI] [PubMed] [Google Scholar]
- 22.von Engelhardt J, et al. Excitotoxicity in vitro by NR2A- and NR2B-containing NMDA receptors. Neuropharmacology. 2007;53:10–17. doi: 10.1016/j.neuropharm.2007.04.015. [DOI] [PubMed] [Google Scholar]
- 23.Zhou M, Baudry M. Developmental changes in NMDA neurotoxicity reflect developmental changes in subunit composition of NMDA receptors. J Neurosci. 2006;26:2956–2963. doi: 10.1523/JNEUROSCI.4299-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kennedy MB, Beale HC, Carlisle HJ, Washburn LR. Integration of biochemical signalling in spines. Nat Rev Neurosci. 2005;6:423–434. doi: 10.1038/nrn1685. [DOI] [PubMed] [Google Scholar]
- 25.Chakravarthy B, Morley P, Whitfield J. Ca2+-calmodulin and protein kinase Cs: a hypothetical synthesis of their conflicting convergences on shared substrate domains. Trends Neurosci. 1999;22:12–16. doi: 10.1016/s0166-2236(98)01288-0. [DOI] [PubMed] [Google Scholar]
- 26.Xia Z, Storm DR. The role of calmodulin as a signal integrator for synaptic plasticity. Nat Rev Neurosci. 2005;6:267–276. doi: 10.1038/nrn1647. [DOI] [PubMed] [Google Scholar]
- 27.Sumi M, et al. The newly synthesized selective Ca2+/calmodulin dependent protein kinase II inhibitor KN-93 reduces dopamine contents in PC12h cells. Biochem Biophys Res Commun. 1991;181:968–975. doi: 10.1016/0006-291x(91)92031-e. [DOI] [PubMed] [Google Scholar]
- 28.Pláteník J, Kuramoto N, Yoneda Y. Molecular mechanisms associated with long-term consolidation of the NMDA signals. Life Sci. 2000;67:335–364. doi: 10.1016/s0024-3205(00)00632-9. [DOI] [PubMed] [Google Scholar]
- 29.Cuenda A, et al. SB 203580 is a specific inhibitor of a MAP kinase homologue which is stimulated by cellular stresses and interleukin-1. FEBS Lett. 1995;364:229–233. doi: 10.1016/0014-5793(95)00357-f. [DOI] [PubMed] [Google Scholar]
- 30.Favata MF, et al. Identification of a novel inhibitor of mitogen-activated protein kinase kinase. J Biol Chem. 1998;273:18623–18632. doi: 10.1074/jbc.273.29.18623. [DOI] [PubMed] [Google Scholar]
- 31.Rossi P, De Filippi G, Armano S, Taglietti V, D'Angelo E. The weaver mutation causes a loss of inward rectifier current regulation in premigratory granule cells of the mouse cerebellum. J Neurosci. 1998;18:3537–3547. doi: 10.1523/JNEUROSCI.18-10-03537.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schlett K, et al. Long-term NR2B expression in the cerebellum alters granule cell development and leads to NR2A down-regulation and motor deficits. Mol Cell Neurosci. 2004;27:215–226. doi: 10.1016/j.mcn.2004.05.008. [DOI] [PubMed] [Google Scholar]
- 33.Sato M, Suzuki K, Nakanishi S. NMDA receptor stimulation and brain-derived neurotrophic factor upregulate homer 1a mRNA via the mitogen-activated protein kinase cascade in cultured cerebellar granule cells. J Neurosci. 2001;21:3797–3805. doi: 10.1523/JNEUROSCI.21-11-03797.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]