Abstract
Cancer-associated carbohydrate antigens are often found on the surface of cancer cells. Understanding their roles in cancer progression will lead to the development of new therapeutics and high-sensitivity diagnostics for cancers. Globo H is a member of this family, which is highly expressed on breast cancer cells. Here, we report the development of a glycan microarray of Globo H and its analogs for measurement of the dissociation constants on surface (KD,surf) with three different monoclonal antibodies (VK-9, Mbr1, and anti-SSEA-3), to deduce their binding specificity. The glycan microarray was also used to detect the amount of antibodies present in the plasma of breast cancer patients and normal blood donors. It was shown that the amount of antibodies against Globo H from breast cancer patients were significantly higher than normal blood donors, providing a new tool for possible breast cancer diagnosis. Compared with the traditional ELISA method, this array method required only atto-mole amounts of materials and is more effective and more sensitive (5 orders of magnitude). The glycan microarray thus provides a new platform for use to monitor the immune response to carbohydrate epitopes after vaccine therapy or during the course of cancer progression.
Keywords: array, diagnosis
Globo H is a hexasaccharide, which is a member of a family of antigenic carbohydrates that are highly expressed on a various types of cancers, especially cancers of breast, prostate and lung (1–4). It is expressed on the cancer cell surface as a glycolipid and possibly as a glycoprotein (5, 6). Furthermore, it has been established that the serum of breast cancer patients contains high levels of antibodies against the Globo H epitope, and this epitope is also targeted by the monoclonal antibodies Mbr1 (5, 7–8) and VK9 (9) in immunohistochemistry studies. Although certain normal tissues also react with Mbr1, including normal breast, pancreas, small bowel, and prostate tissue (2, 6, 10), the antigen in these tissues is predominantly localized at the secretary borders where access to the immune system is restricted. These two findings provide the rationale for evaluating human antibody against Globo H as a diagnosis for breast cancer.
Glycan microarrayis a very powerful tool for glycobiological study (11–36). It may effectively mimic the presentation of glycans on the cell membrane to exhibit multivalent interactions with receptors with high affinity and specificity (37). In addition, only a very small amount of material is required for arraying. Thus, the microarray may provide a more appropriate and more sensitive system for studying protein–carbohydrate interactions than the traditional solution-phase ELISA analysis. Recently, our group has used Globo H and its truncated analog microarrays to profile the binding specificity of its monoclonal and polyclonal antibodies in a qualitative manner (38). In that preliminary study, we found that the polyclonal antibodies from a breast cancer patient can bind both Globo H and its truncated analog without fucose (Gb5), whereas Mbr1 is specific for Globo H (38). The binding specificity studies of monoclonal antibodies and human polyclonal antibodies associated with Globo H have prompted us to further investigate the following questions: (i) Are Globo H and Gb5 differentially expressed in disease stages? (ii) Can the glycan array be used for the quantitative and high-sensitivity detection of anti-Globo H antibody present in the serum as an indication of cancer? (iii) Can the array be used to measure the activity of fucosyltransferase in breast cancer cells at different stages, to screen inhibitors of the enzyme and to monitor the immune response to the Globo H-based vaccine?
Results and Discussion
Specificities of Antibodies.
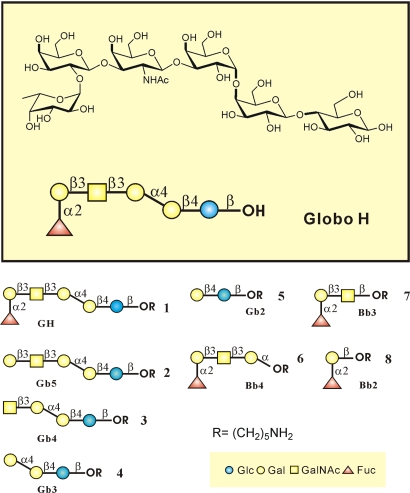
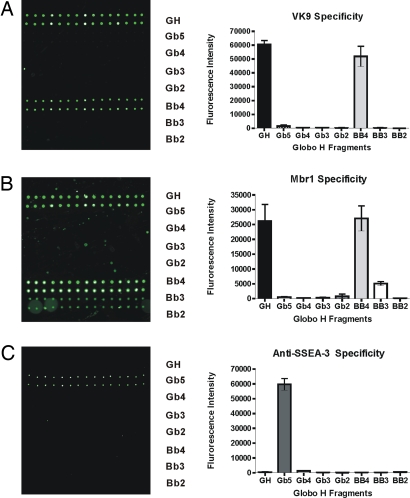
The Globo H and its analogs (Fig. 1) were prepared according to the one-pot programmable protocol (38) and were covalently attached onto NHS-coated glass slides based on the standard microarray robotic printing technology, as reported in refs. 38 and 39. In addition, two more glycans (lactose and Gb3) were included to give a complete array of Globo H and truncated structures. These glycans were printed on the glass slide by taking an aliquot from a stock solution of sugar at a fixed concentration (80 μM). The array was designed in a 16-row slide format for development of high-throughput screening. We printed two rows for every glycan (their structure are shown in Fig. 1), and the array was used for binding experiments with Mbr1 (a mouse IgM anti-Globo H monoclonal antibody), VK9 (a mouse IgG anti-Globo H monoclonal antibody), and anti-mouse/human SSEA-3 (stage-specific embryonic antigen-3) monoclonal antibody. Briefly, these primary antibodies were incubated in a well on the slide, followed by incubation with a fluorophore-tagged secondary antibody against its primary antibody. After washing, scanning the slide for fluorescence yielded images as shown in Fig. 2, which reflect the extent of binding of the antibody to printed oligosaccharides. VK9 and Mbr1 recognize Globo H and Bb4 specifically (Fig. 2 A and B), indicating that both IgG and IgM of anti-Globo H antibodies (5, 7–9) recognize the outer tetrasaccharide of Globo H, and the presence of the fucose moiety is required for binding, consistent with previous solution phase assays (9). The other antibody, anti-mouse/human SSEA-3 (stage-specific embryonic antigen-3) recognizes Gb5 (SSEA-3 antigen) without any cross-reactivity to other Globo H analogs.
Fig. 1.
Chemical structure of Globo H and abbreviations of Globo H analogs.
Fig. 2.
Binding of monoclonal antibodies to Globo H and its analogues. (A) Slide image obtained from fluorescence scan after antibody incubation assay with VK-9. The grid contain sugars 1–8 printed at an 80 μM concentration. (B and C) Slide images obtained by assay with MBr1 (B) and anti-SSEA-3 monoclonal antibody (C).
Determination of Surface Dissociation Constants (KD,surf).
Both protein and carbohydrate microarrays have been used to investigate the kinetics and thermodynamics of the interactions between biomolecules in solution phase and solid phase (40–42). To determine the dissociation constants of Globo H and truncated analogs on surface interacting with antibodies in a multivalent manner, we adopted the direct measurement method reported by our group in ref. 42, using different concentrations of antibodies and printed sugars. The Langmuir isotherm was then used for analyzing the binding curves to generate the dissociation constants on surface (KD,surf). At the equilibrium conditions during incubation, the mean fluorescence of the replicate spots (Fobs) can be described by
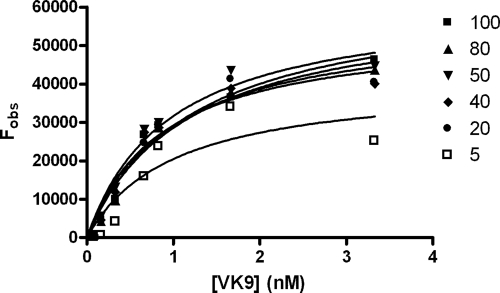
where Fmax is the maximum fluorescence intensity, a measure of the amount of active carbohydrate on the surface, [P] is the total antibody concentration, and KD,surf is the equilibrium dissociation constant for surface carbohydrate and the antibody. The anti-Globo H antibody VK9 was diluted to different concentrations from 3.3 to 0.08 nM and incubated in each array with different concentrations of printed sugars ranging from 100 μM to 0.01 μM (100, 80, 50, 40, 20, 10, 5, 1, 0.1, and 0.01 μM). This was followed by incubation with the Cy3-labeled goat anti-mouse IgG secondary antibody. After washing, the slide was scanned to measure the fluorescence intensities to generate the saturated curve for dissociation constant (KD,surf) determination. We found that the binding curve reached saturation at 5 μM printing concentration. To determine the dissociation constant on surface, we plotted VK9 concentrations against the fluorescence intensity at different concentrations of printed sugar. At the printing concentrations from 100 μM to 5 μM, the KD,surf values determined were narrowly distributed from the individual curves (Fig. 3 and Table 1). However, if the printing concentration was <5 μM, the fluorescence intensity of each spot was too weak to be detected with a significant signal-to-noise (S/N) ratio (data not shown). In addition, the intensities of those spots at lower printing concentrations did not converge to the binding curves generated by the Langmuir isotherm. This result illustrates that at the printing concentrations <5 μM, the sugar density on surface is too low to exhibit a multivalent interaction.
Fig. 3.
Binding curves for Globo H printed at different concentrations (100, 80, 50, 40, 20, 10, and 5 μM) are shown. The curves were obtained by using Cy3-labeled goat anti mouse IgG secondary antibodies.
Table 1.
Surface dissociation constants (KD,surf) of antibody VK9 and Globo H on microarray
| Printing concentration Globo H, μM | Fmax | KD,surf, nM |
|---|---|---|
| 100 | 64,018 | 1.198 |
| 80 | 61,517 | 1.152 |
| 50 | 61,988 | 0.9587 |
| 40 | 54,623 | 0.8694 |
| 20 | 57,157 | 0.9561 |
| 10 | 46,473 | 0.8951 |
| 5 | 41,029 | 1.004 |
Shown are KD,surf values (nM) and the corresponding fluorescence intinsities (Fmax) under different printing concentrations.
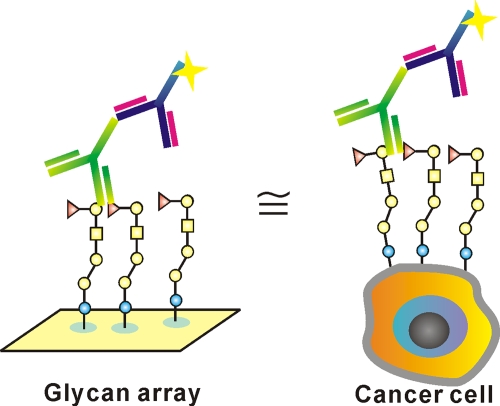
Using the same method, the KD,surf values were measured for the truncated Globo H analogs interacting with antibodies [see the supporting information (SI), Fig. 2, and Table 2]. The relative binding specificity of both VK9 and Mbr1 for the sugar epitopes was Globo H > tetrasacchride Bb4. In addition, we observed that the relative binding affinity of antibodies for Globo H was Mbr1> VK9 but that for Bb4 was in an opposite order. This study indicates that the glycan microarray is a good mimic of cell surface expressing multiple glycans and is thus useful for the study of multivalent interaction (Fig. 4).
Table 2.
KD,surf (nM) values of different antibodies and different Globo H analogs
| Antibodies |
KD, surf (nM) ± SD(nM) |
||
|---|---|---|---|
| Globo H | Tetrasaccharide, 6 | gal-globoside, 2 | |
| VK9 | 1 ± 0.116 | 2.192 ± 0.567 | — |
| Mbr1 | 0.56 ± 0.129 | 4.287 ± 0.59 | — |
| anti- SSEA-3 | — | — | 15.723 ± 3.896 |
Fig. 4.
Glycan array is a mimic of cell surface expressing glycans for study of multivalent interaction.
Detection of Serum Antibody Against Globo H.
The binding intensities in a range of Globo H and tetrasaccharide Bb4 concentration (5–100 μM) for the two antibodies (VK-9 and Mbr1), as measured by the Cy3-labeled secondary antibody were relatively high and similar, whereas the binding to other truncated sequences is very weak. This result provides a rationale for development of a glycan microarray to detect the presence of anti-Globo H antibody in the serum of patients. Because we only loaded 0.7 nL to each spot, the minimum amount of sugar required for detection was 10−15 mol per spot if a 50 μM solution is used (Table 2 and Fig. 3). For the purpose of detection only, the concentration can further be reduced to 10−18 mol per spot, as shown in the study in ref. 42. This is indeed a highly sensitive method and the sensitivity could be further improved with the use of more effective fluorescent probes.
Glycan Microarray of Globo H and Its Truncated Analogs as a Tool for Breast Cancer Diagnosis.
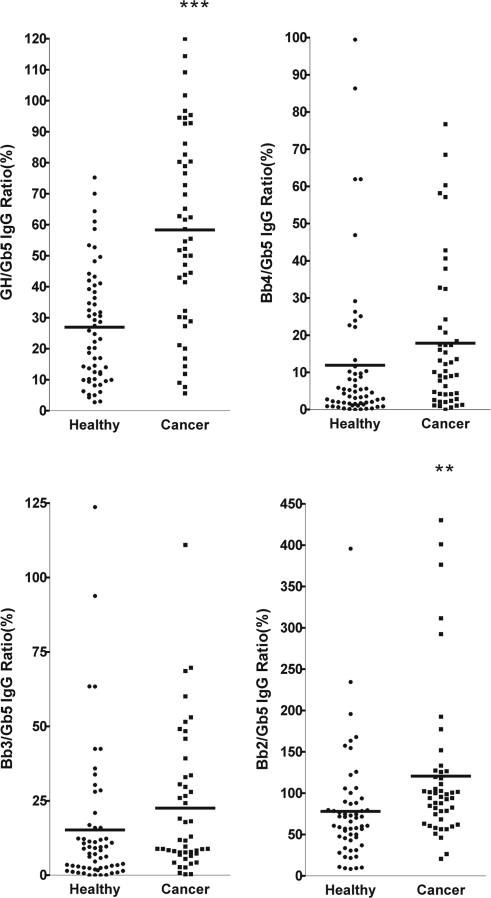
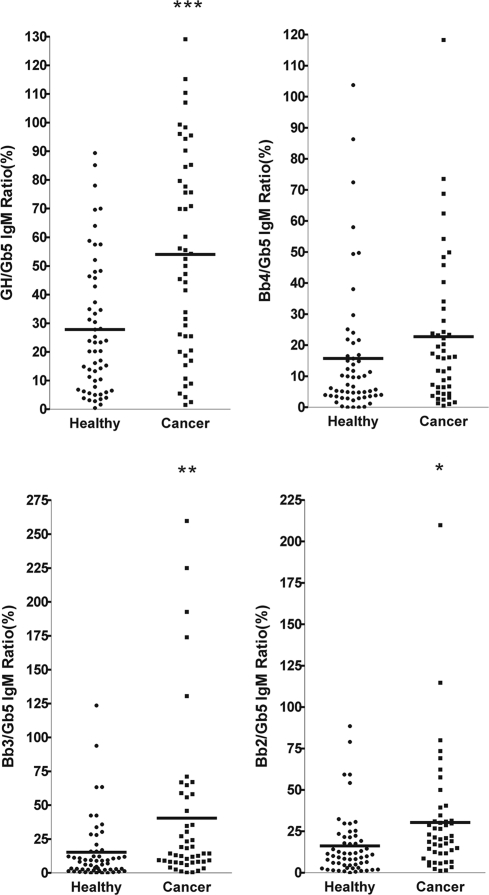
Breast cancer is the most common malignancy in many parts of the world for women, with an increase in mortality of ≈33% in the past 10 years (43). Development of a rapid and reliable method for use to diagnose breast cancer at an early stage should lead to the reduction of mortality rate. To take advantage of our highly sensitive Globo H microarray (38), we examined the plasma samples of 58 breast cancer patients and 47 healthy blood donors for antibodies that bound to Globo H and its truncated fragments on glycan microarrays. Microarrays of Globo H and its truncated fragments were treated with the plasma of patients or healthy donors, and the bound antibodies, after washing, were detected with Cy3-labeled goat anti-human IgM or IgG secondary antibody. As shown in Figs. 5 and 6, the presence of antibodies that bound to Gb5 was high in patients and normal donors, but the number of antibodies bound to Globo H was less in normal donors when compared with breast cancer patients. The levels of antibodies bound to other glycans were much lower than Globo H or Gb5 in patients or healthy donors. The fluorescence data reflecting the antibody reactivity to each glycan was normalized to Gb5 for each sample, and the relative fluorescence intensities for IgG and IgM antibodies from patients and normal subjects are presented in Fig. 5 and Fig. 6, respectively. It was evident that both the levels of IgG and IgM against Globo H were significantly higher in breast cancer patients than in normal individuals (P < 0.0001) (Table 3 and 4). However, the normalized antibody levels for Bb4 displayed no significant differences between patients and normal donors and those for Bb3 and Bb2 showed a trend of higher levels in cancer patients with borderline significance (P = 0.1106 and 0.0063 for IgG; P = 0.0043 and 0.0105 for IgM, respectively) (Tables 3 and 4). It is noted that the background intensities of Globo H binding from normal subject could be the result of nonselective binding.
Fig. 5.
Ratios of IgG levels against Globo H analogs in sera from breast cancer patients and normal blood donors. The relative fluorescence ratios were obtained from fluorescence intensity of Globo H or Globo H analogs divided by fluorescence intensity of Gb5. The mean of Globo H/Gb5 IgG ratios was significantly higher in sera of breast cancer patients.
Fig. 6.
Ratios of IgM levels against Globo H analogs in sera from breast cancer patients and normal blood donors. The relative fluorescence ratios were obtained from fluorescence intensity of Globo H or Globo H analogs/fluorescence intensity of Gb5. The mean of Globo H/Gb5 IgM ratios was significantly higher in sera of breast cancer patients.
Table 3.
IgG ratios of Globo H analogs to Gb5 from breast cancer patients (n = 58) and healthy individuals (n = 47)
| IgG ratio, % | GH/Gb5 |
Bb4/Gb5 |
Bb3/Gb5 |
Bb2/Gb5 |
||||
|---|---|---|---|---|---|---|---|---|
| Healthy | Cancer | Healthy | Cancer | Healthy | Cancer | Healthy | Cancer | |
| Mean | 26.92 | 58.31 | 11.91 | 17.84 | 15.22 | 22.54 | 78.02 | 120.8 |
| SD | 18.49 | 31.14 | 20.54 | 19.60 | 23.12 | 23.22 | 63.63 | 92.26 |
| P | P < 0.0001*** | 0.1360 (ns) | 0.1106 (ns) | 0.0063** | ||||
***, P < 0.001, extremely significant; **, P = 0.001–0.01, very significant; ns, P < 0.05, not significant.
Table 4.
IgM ratios of Globo H analogs to Gb5 from breast cancer patients (n = 57) and healthy individuals (n = 47)
| IgM ratio, % | GH/Gb5 |
Bb4/Gb5 |
Bb3/Gb5 |
Bb2/Gb5 |
||||
|---|---|---|---|---|---|---|---|---|
| Healthy | Cancer | Healthy | Cancer | Healthy | Cancer | Healthy | Cancer | |
| Mean | 27.82 | 53.98 | 15.73 | 22.74 | 15.36 | 40.51 | 16.19 | 30.36 |
| SD | 23.42 | 35.41 | 21.60 | 23.98 | 23.30 | 59.76 | 18.83 | 35.44 |
| P | P < 0.0001*** | 0.1259* | 0.0043** | 0.0105* | ||||
***, P < 0.001, extremely significant; **, P = 0.001–0.01, very significant; *, P = 0.01–0.05, significant.
Monitoring the Immune Response to Globo H Vaccine by Using Globo H Microarray.
Traditionally, the immune response to carbohydrate based vaccine was evaluated by ELISA method. Recently, Bovin and coworkers (44) compared carbohydrate microarray and ELISA methods for the assessment of carbohydrate–protein interaction. They used biotinylated glycoconjugates attached to streptavidin coated on gold as glycan microarray and 96-well plates coated with sugar-polyacrylamide (Sug-PAA) for the ELISA assy. In their comparative assays, they found that the sensitivity of the array method was lower than the ELISA method. Therefore, we decided to compare our glycan microarray system with routine ELISA again.
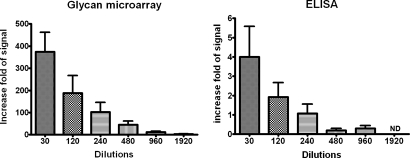
Mice were immunized with KLH conjugated Globo H once weekly for 3 weeks, and sera were collected 10 days after third immunization. Anti-Globo H antibodies were tested at serial dilutions from 30- to 1,920-fold, on the Globo H microarray chip (3.5 × 10−14 mol per spot) or on Globo H coated ELISA plate (1.28 × 10−10 mol per well). A significant rise in anti-Globo H specific IgG antibody was observed in Globo H-KLH treated mice compared with the preimmune sera. As shown in Fig. 7 and Table 5, using glycan microarray, the intensity of positive signal (postimmune–preimmune) for anti-Globo H was 374.7 ± 87.8-fold over the background signal at 1:30 serum dilution and 3.2 ± 1.0-fold at 1:1,920 dilution. By ELISA method, the signal was only 4.0 ± 1.6-fold at 1: 30 dilution and dropped to near background level at 1:240 dilution (Fig. 7). Additionally, given that the working concentration of Globo H in array is 4 orders of magnitude lower than ELISA, the overall sensitivity of Globo H microarray is 5 orders of magnitude higher than the routine ELISA. As a result, minimal amounts of serum samples, reagents and Globo H antigens were necessary for glyan microarray analysis compared with ELISA. Based on these findings, we believe that the glycan microarray method may be developed as a sensitive rapid device for screening of breast cancer and other applications.
Fig. 7.
The comparison of glycan microarray and ELISA for mornitoring anti-Globo H response. Sera from immunized mice were serially diluted and analyzed for anti-Globo H specific IgG antibody on glycan microarray and ELISA plates. The fold level of signal over background was calculated as (mean of fluorescence of postserum − preserum)/background intensity in glycan micorarray or (OD value of postserum − preserum)/background in ELISA. The antibody levels were detectable up to 1:1,920 dilution method (3.2 ± 1.0-fold of background signal), but only 1:240 dilution in ELISA (1.08 ± 0.48-fold of background OD). Values shown were mean ± SD of four mice.
Table 5.
The comparison of sensitivity of glycan microarray and ELISA
| Dilution | Increase fold of signal |
|
|---|---|---|
| Glycan microarray | ELISA | |
| 30 | 374.7 ± 87.83 | 4.01 ± 1.58 |
| 120 | 188.4 ± 78.93 | 1.92 ± 0.75 |
| 240 | 102.2 ± 44.21 | 1.08 ± 0.48 |
| 480 | 44.86 ± 17.05 | 0.20 ± 0.10 |
| 960 | 12.13 ± 4.08 | 0.30 ± 0.14 |
| 1,920 | 3.203 ± 1.048 | ND |
Glycan microarray showed much higher sensitivity than ELISA. The increase fold of signal defined as (signal intensity of post-immune − pre-immune)/background intensity. Values are mean ± SEM. ND, not detectable.
Conclusion
In this study, we have shown that the glycan microarray can be used for quantitative analysis of the breast cancer-associated carbohydrate Globo H interacting with monoclonal antibodies Mbr1 and VK-9 and the anti-Globo H polyclonal antibodies present in the serum of breast cancer patients with the sensitivity down to the atto-mol level. Our results showed a very significant difference in anti-Globo H levels between patient and normal plasma, suggesting that the Globo H microarray may be developed as a new method for breast cancer diagnosis. In other research (45), we found the level of Globo H on the breast cancer stem cells is relatively low as stained with the monoclonal antibody and increases when the disease progresses. We also demonstrated the superiority of our glycan microarray over conventional ELISA for evaluation of immune responses to carbohydrates, which thereby minimizing the amount of serum and carbohydrate required for the assay. Furthermore, this highly sensitive glycan microarray may offer a new platform for the analysis of fucosyltransferase activity and identification of fucosyltransferase inhibitors (21) as possible therapeutics.
Materials and Methods
General.
NHS-coated glass slides were purchased from Nexterion H slide (SCHOTT North America). Primary antibodies used were mouse anti-Globo H monoclonal antibodies Mbr1 (IgM; Alexis Biochemicals), VK-9 [IgG; a gift from Philip Livingston (Memorial Sloan-Kettering Cancer Center, New York)], and A488 anti-mouse/human SSEA-3 (stage-specific embryonic antigen-3) (eBioscience). The secondary antibodies used were Cy3-conjuagted Goat anti-mouse IgG, goat anti-mouse IgM, goat anti-human IgG, and goat anti-human IgM (Jackson ImmunoResearch). Human plasma samples from healthy individuals and caner patients were obtained from the Genomics Research Center; Academia Sinica; and the Tri-Service General Hospital, Taipei, Taiwan, respectively. Samples were fully encoded to protect patient confidentiality and were used under a protocol approved by the Institutional Review Board of Human Subjects Research Ethics Committee of Academia Sinica, Taipei, Taiwan. Globo H and its truncated analogs were synthesized as in ref. 38.
Glycan Microarray Fabrication.
Microarrays were printed (BioDot; Cartesian Technologies) by robotic pin (SMP3; TeleChem International) deposition of ≈0.7 nL of various concentrations of amine-containing glycans in printing buffer [300 mM phosphate buffer (pH 8.5), containing 0.005% Tween-20] from a 96-well microtiter plate onto NHS-coated glass slides. The slides for Globo H analogs were spotted with solutions of Globo H analogs 1–8 with concentrations 50 μM every two rows for one glycan from bottom to top with 15 replicates horizontally placed in each subarray, and each slide was designed for 16 grids for further incubation experiments. Printed slides were allowed to react in an atmosphere of 80% humidity for an hour followed by desiccation overnight. These slides were designed for 16 grids in one slides, and stored at room temperature in a desiccator until use. Before the binding assay, these slides were blocked with ethanolamine [50 mM ethanolamine in 50 mM borate buffer (pH 9.2)] and then washed with water and PBS buffer (pH 7.4) twice.
Antibody Binding Assay.
Mbr1, a mouse IgM anti-Globo H monoclonal antibody, VK-9, a mouse IgG anti-Globo H monoclonal antibody, or A488 anti-mouse/human SSEA-3 were prepared in 0.05% Tween 20/PBS buffer (pH 7.4) and added to cover the grid with application of a coverlip. After incubation in a humidifying chamber with shaking for 1 h, the slides were washed three times each with 0.05% Tween 20/PBS buffer (pH 7.4), PBS buffer (pH 7.4), and water. Next, Cy3-conjugated goat anti-mouse IgM (for MBr1) or IgG (for VK-9) antibody was added to the slide and incubated in a humidifying chamber with shaking under cover lip for 1 h. The slide was washed three times each with 0.05% Tween20/PBS buffer (pH 7.4), PBS buffer (pH 7.4), and H2O and dried. The slide was scanned at 532 nm (for Cy3-conjugated secondary antibody) and 488 nm (for A488 anti-SSEA-3 antigen antibody) with a microarray fluorescence chip reader (ArrayWorx microarray reader).
Microarray Analysis of Cancer Patient Plasma.
The plasma samples from breast cancer patients and healthy individuals were diluted 1:20 with 0.05% Tween 20/3% BSA/PBS buffer (pH 7.4), and applied to the grids on the Globo H analogs microarrays and then incubated in a humidifying chamber with shaking for 1 h. Then the slides were washed three times each with 0.05% Tween 20/PBS buffer (pH 7.4), PBS buffer (pH 7.4), and water. Next, Cy3-conjugated goat anti-human IgM or IgG antibody was added to the slide as described above and incubated in a humidifying chamber incubation with shaking under a coverlid for 1 h. The slide was washed three times each with 0.05% Tween20/PBS buffer (pH 7.4), PBS buffer (pH7.4), and H2O and dried. The slide was scanned at 532 nm (for Cy3-conjugated secondary antibody) with a microarray fluorescence chip reader (ArrayWorx microarray reader).
Immunization.
Groups of three mice (6-week-old female C57BL/6 mice; BioLASCO) were immunized s.c. with Globo H-KLH (Optimer) once weekly for 3 weeks. Each vaccination contained 0.6 μg of Globo H with 2 μg of α-galactosylceramide as an adjuvant. Control mice were injected with PBS. The mouse sera were obtained before 1st immunization and 10 days after the third immunization. The serological responses were analyzed by glycan microarray with the same method as described for human plasma except that Cy3-conjugated goat anti-mouse IgG antibody was used as a secondary antibody. 0.2 μg of Globo H–ceramide in 100 μl of carbonate bicarbonate buffer (pH 10) were coated in 96-well plate (NUNC) at 4°C for overnight, washed with PBS, and blocked with 3% BSA for 30 min at room temperature. Serial dilutions of mice sera were added into each well and incubated for 1 h at room temperature, followed by washing with Dulbecco's phosphate buffered saline (DPBST) and 0.05% Tween 20. Goat anti-mouse IgG-AP (1:200, Southern Biotech.) was added and incubated for 45 min at room temperature. The plates were washed with PBST five times and then incubated with an alkaline phosphates substrate, p-nitrophenyl phosphate (Sigma), for 8 min at 37°C. After incubation, the reaction was stopped by adding 3 M NaOH, and the plates were read at 405 nm on the ELISA reader (SpectraMax; Molecular Devices).
Data Analysis.
The software GenePix Pro (Axon Instruments) was used for the fluorescence analysis of the extracted data. The local background was subtracted from the signal at each antibody spot. The spots with obvious defects, no detectable signal, or a net fluorescence of <100 were removed from the analysis. The “medians of ratios” from replicate spots were averaged in the same array.
To determine the KD,surf value, the equilibrium binding data were analyzed by fitting the data to the appropriate equation (1), assuming that ligands bound to one or two independent sites, using the commercial nonlinear regression program GradPad PRISM (GraphPad). To obtain the relative binding intensities of Globo H analogs in human plasma, we used the binding intensity to Bg5 as 100%, and normalized the relative binding intensities of Globo H analogs in each plasma sample. For example, the ratio of GH/Gb5 in sera was calculated by dividing mean of Globo H replicates by mean of Gb5 replicates. Finally, the statistical analysis of IgG or IgM levels in breast cancer patients and normal individuals was performed with an unpaired t test, using the program GraphPad Prism (GraphPad).
Supplementary Material
Acknowledgments.
We thank Ms. Pei-Lan Hsu, a registered nurse in the management of clinical samples. This work was supported by Academia Sinica.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0804923105/DCSupplemental.
References
- 1.Kannagi R, et al. New globo series glycospingolipids in human tetraocarcinoma reactive with the monoclonal-antibody directed to a developmentally regulated antigen, stage-specific embryonic antigen-3. J Biol Chem. 1983;258:8934–8942. [PubMed] [Google Scholar]
- 2.Zhang SL, et al. Selection of tumor antigens as targets for immune attack using immunohistochemistry. 1. Focus on gangliosides. Int J Cancer. 1997;73:42–49. doi: 10.1002/(sici)1097-0215(19970926)73:1<42::aid-ijc8>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 3.Hakomori S, Zhang YM. Glycosphingolipid antigens and cancer therapy. Chem Biol. 1997;4:97–104. doi: 10.1016/s1074-5521(97)90253-2. [DOI] [PubMed] [Google Scholar]
- 4.Dube DH, Bertozzi CR. Glycans in cancer and inflammation. Potential for therapeutics and diagnostics. Nat Rev Drug Discov. 2005;4:477–488. doi: 10.1038/nrd1751. [DOI] [PubMed] [Google Scholar]
- 5.Menard S, Tagliabue E, Canevari S, Fossati G, Colnaghi MI. Generation of monoclonal-antibodies reacting with normal and cancer-cells of human-breast. Cancer Res. 1983;43:1295–1300. [PubMed] [Google Scholar]
- 6.Livingston PO. Augmenting the immunogenicity of carbohydrate tumor antigens. Cancer Biol. 1995;6:357–366. doi: 10.1016/1044-579x(95)90005-5. [DOI] [PubMed] [Google Scholar]
- 7.Canevari S, Fossati G, Balsari A, Sonnino S, Colnaghi MI. Immunochemical analysis of the determinant recognized by a monoclonal-antibody (Mbr1) which specifically binds to human mammary epithelial-cells. Cancer Res. 1983;43:1301–1305. [PubMed] [Google Scholar]
- 8.Bremer EG, et al. Characterization of a glycosphinolipid antigen defined by the monoclonal-antibody Mbr1 expressed in normal and neoplastic epithelial-cells of human mammary-gland. J Biol Chem. 1984;259:14773–14777. [PubMed] [Google Scholar]
- 9.Kudryashov V, et al. Characterization of a mouse monoclonal IgG3 antibody to the tumor-associated globo H structure produced by immunization with a synthetic glycoconjugate. Glycoconjugate J. 1998;15:243–249. doi: 10.1023/a:1006992911709. [DOI] [PubMed] [Google Scholar]
- 10.Zhang S, et al. Expression of potential target antigens for immunotherapy on primary and metastatic prostate cancers. Clin Cancer Res. 1998;4:295–302. [PubMed] [Google Scholar]
- 11.Park S, Shin I. Fabrication of carbohydrate chips for studying protein–carbohydrate interactions. Angew Chem Int Ed. 2002;41:3180–3182. doi: 10.1002/1521-3773(20020902)41:17<3180::AID-ANIE3180>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 12.Houseman BT, Mrksich M. Carbohydrate arrays for the evaluation of protein binding and enzymatic modification. Chem Biol. 2002;9:443–454. doi: 10.1016/s1074-5521(02)00124-2. [DOI] [PubMed] [Google Scholar]
- 13.Fazio F, Bryan MC, Blixt O, Paulson JC, Wong CH. Synthesis of sugar arrays in microtiter plate. J Am Chem Soc. 2002;124:14397–14402. doi: 10.1021/ja020887u. [DOI] [PubMed] [Google Scholar]
- 14.Bryan MC, et al. Saccharide display on microtiter plates. Chem Biol. 2002;9:713–720. doi: 10.1016/s1074-5521(02)00155-2. [DOI] [PubMed] [Google Scholar]
- 15.Fukui S, Feizi T, Galustian C, Lawson AM, Chai W. Oligosaccharide microarrays for high-throughput detection and specificity assignments of carbohydrate–protein interactions. Nat Biotechnol. 2002;20:1011–1017. doi: 10.1038/nbt735. [DOI] [PubMed] [Google Scholar]
- 16.Wang DN, Liu SY, Trummer BJ, Deng C, Wang AL. Carbohydrate microarrays for the recognition of cross-reactive molecular markers of microbes and host cells. Nat Biotechnol. 2002;20:275–281. doi: 10.1038/nbt0302-275. [DOI] [PubMed] [Google Scholar]
- 17.Mellet CO, Garcia Fernandez JM. Carbohydrate microarrays. Chembiochem. 2002;3:819–822. doi: 10.1002/1439-7633(20020902)3:9<819::AID-CBIC819>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 18.Feizi T, Fazio F, Chai W, Wong CH. Carbohydrate microarrays-a new set of technologies at the frontiers of glycomics. Curr Opin Struct Biol. 2003;13:637–645. doi: 10.1016/j.sbi.2003.09.002. [DOI] [PubMed] [Google Scholar]
- 19.Disney MD, Magnet S, Blanchard JS, Seeberger PH. Aminoglycoside microarrays to study antibiotic resistance. Angew Chem Int Ed. 2004;43:1591–1594. doi: 10.1002/anie.200353236. [DOI] [PubMed] [Google Scholar]
- 20.Ratner DM, et al. Probing protein–carbohydrate interactions with microarrays of synthetic oligosaccharides. ChemBioChem. 2004;5:379–382. doi: 10.1002/cbic.200300804. [DOI] [PubMed] [Google Scholar]
- 21.Bryan MC, et al. Covalent display of oligosaccharide arrays in microtiter plates. J Am Chem Soc. 2004;126:8640–8641. doi: 10.1021/ja048433f. [DOI] [PubMed] [Google Scholar]
- 22.Disney MD, Seeberger PH. Aminoglycoside microarrays to explore interactions of antibiotics with RNAs and proteins. Chem Eur J. 2004;10:3308–3314. doi: 10.1002/chem.200306017. [DOI] [PubMed] [Google Scholar]
- 23.Adams EW, et al. Oligosaccharide and glycoprotein Microarrays as tools in HIV glycobiology: Glycan-dependent gp120/protein interactions. Chem Biol. 2004;11:875–881. doi: 10.1016/j.chembiol.2004.04.010. [DOI] [PubMed] [Google Scholar]
- 24.Shin I, Park S, Lee MR. Carbohydrate microarrays: An advanced technology for functional studies of glycans. Chem Eur J. 2005;11:2894–2901. doi: 10.1002/chem.200401030. [DOI] [PubMed] [Google Scholar]
- 25.Bochner BS, et al. Glycan array screening reveals a candidate ligand for Siglect-8. J Biol Chem. 2005;280:4307–4312. doi: 10.1074/jbc.M412378200. [DOI] [PubMed] [Google Scholar]
- 26.Calarese DA, et al. Dissection of the carbohydrate specificity of the broadly neutralizing anti-HIV-1 antibody 2G12. Proc Natl Acad Sci USA. 2005;102:13372–13377. doi: 10.1073/pnas.0505763102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Manimala JC, Roach TA, Li Z, Gildersleeve JC. High-Throughput carbohydrate microarray analysis of 24 lectins. Angew Chem Int Ed. 2006;45:3507–3610. doi: 10.1002/anie.200600591. [DOI] [PubMed] [Google Scholar]
- 28.Dotan N, Altstock RT, Schwarz M, Dukler A. Anti-glycan antibodies as biomarkers for diagnosis and prognosis. Lupus. 2006;15:442–450. doi: 10.1191/0961203306lu2331oa. [DOI] [PubMed] [Google Scholar]
- 29.Lee JC, Wu CY, Apon JV, Siuzdak G, Wong CH. Reactivity-based one-pot synthesis of the tumor-associated antigen N3 minor octasaccharide for the development of a photo cleavable DIOS-MS sugar array. Angew Chem Int Ed. 2006;45:2753–2757. doi: 10.1002/anie.200504067. [DOI] [PubMed] [Google Scholar]
- 30.Lawrie CH, et al. Cancer-associated carbohydrate identification in Hodgkin's lymphotna by carbohydrate array profiling. Int J Cancer. 2006;118:3161–3166. doi: 10.1002/ijc.21762. [DOI] [PubMed] [Google Scholar]
- 31.Paulson JC, Blixt O, Collins BE. Nat Chem Biol. 2006;2:238–248. doi: 10.1038/nchembio785. [DOI] [PubMed] [Google Scholar]
- 32.Stevens J, et al. Structure and receptor specificity of the hemagglutinin from an H5N1 influenza virus. Science. 2006;312:404–410. doi: 10.1126/science.1124513. [DOI] [PubMed] [Google Scholar]
- 33.Stevens J, Blixt O, Paulson JC, Wilson IA. Glycan microarray technologies: Tools to survey host specificity of influenza viruses. Nat Rev Microbiol. 2006;4:857–864. doi: 10.1038/nrmicro1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stevens J, et al. Glycan microarray analysis of the hemagglutinins from modern and pandemic influenza viruses reveals different receptor specificities. J Mol Biol. 2006;355:1143–1155. doi: 10.1016/j.jmb.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 35.Manimala JC, Roach TA, Li ZT, Gildersleeve JC. High-throughput carbohydrate microarray profiling of 27 antibodies demonstrates widespread specificity problems. Glycobiology. 2007;17:17C–23C. doi: 10.1093/glycob/cwm047. [DOI] [PubMed] [Google Scholar]
- 36.Ratner DM, Seeberger PH. Carbohydrate microarrays as tools in HIV glycobiology. Curr Pharm Des. 2007;13:173–183. doi: 10.2174/138161207779313650. [DOI] [PubMed] [Google Scholar]
- 37.Mammen M, Choi SK, Whitesides GM. Polyvalent interactions in biological systems: Implications for design and use of multivalent ligands and inhibitors. Angew Chem Int Ed. 1998;37:2755–2794. doi: 10.1002/(SICI)1521-3773(19981102)37:20<2754::AID-ANIE2754>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 38.Hung CY, et al. Carbohydrate microarray for profiling the antibodies interacting with Globo H tumor antigen. Proc Natl Acad Sci USA. 2006;103:15–20. doi: 10.1073/pnas.0509693102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Blixt O, et al. Printed covalent glycan array for ligand profiling of diverse glycan binding proteins. Proc Natl Acad Sci USA. 2004;101:17033–17038. doi: 10.1073/pnas.0407902101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jones RB, Gordus A, Krall JA, MacBeath G. A quantitative protein interaction network for the ErbB receptors using protein microarrays. Nature. 2006;439:168–174. doi: 10.1038/nature04177. [DOI] [PubMed] [Google Scholar]
- 41.Gordus A, MacBeath G. Circumventing the problems caused by protein diversity in microarrays: Implications for protein interaction networks. J Am Chem Soc (2006) 2006;28:13668–13669. doi: 10.1021/ja065381g. [DOI] [PubMed] [Google Scholar]
- 42.Liang PH, Wang SK, Wong CH. Quantitative analysis of carbohydrate–protein interactions using glycan microarrays: Determination of surface and solution dissociation constants. J Am Chem Soc. 2007;129:11177–11184. doi: 10.1021/ja072931h. [DOI] [PubMed] [Google Scholar]
- 43.Boyle P. Global burden of cancer. Lancet. 1997;349:S23–S26. doi: 10.1016/s0140-6736(97)90017-9. [DOI] [PubMed] [Google Scholar]
- 44.Galanina OE, Mecklenburg M, Nifantiev NE, Pazynina GV, Bovin NV. GlycoChip: Multiarray for the study of carbohydrate-binding proteins. Lab Chip. 2003;3:260–265. doi: 10.1039/b305963d. [DOI] [PubMed] [Google Scholar]
- 45.Chang WW, et al. Expression of Globo H and SSEA3 in breast cancer stem cells and the involvement of fucosyl transferase 1 & 2 in Globo H synthesis. Proc Natl Acad Sci USA. 2008;105:11667–11672. doi: 10.1073/pnas.0804979105. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.