Abstract
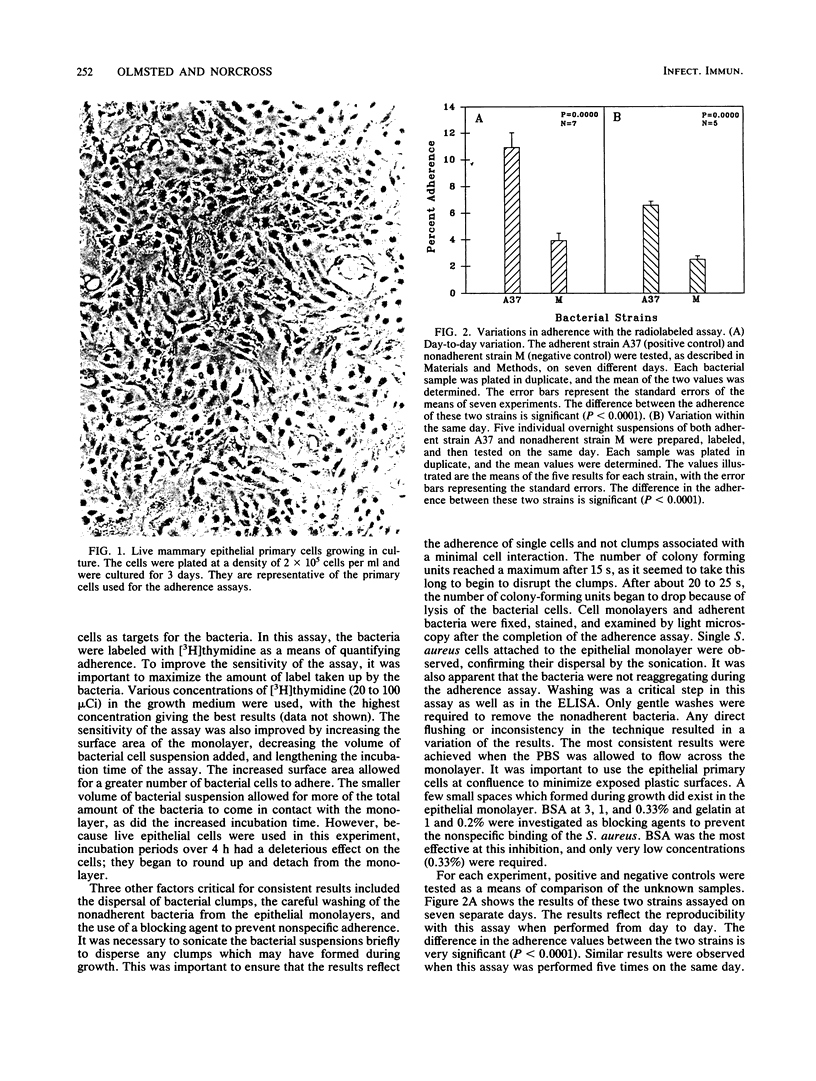
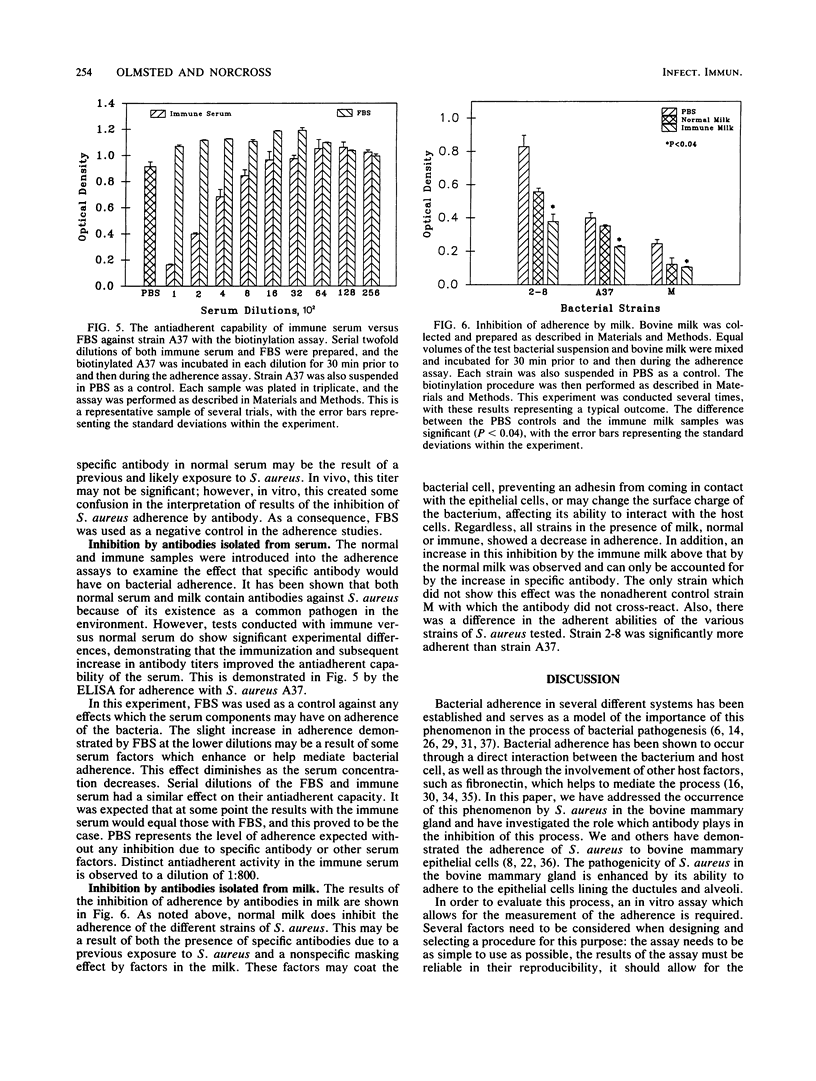
Staphylococcus aureus is a major pathogen in the bovine mammary gland. The ability of S. aureus to adhere to epithelial cells in the ductules and alveoli of the bovine mammary gland is believed to add greatly to its virulence and may be necessary for colonization. Two in vitro methods were developed for the purposes of quantifying adherence and of determining the effect which specific antibody may have in inhibiting the adherence of this organism. Both methods utilize bovine mammary epithelial primary cells as targets for labeled bacteria. In one assay, the bacteria are labeled with [methyl-3H]thymidine and incubated on the primary epithelial monolayers. The second assay involves labeling the bacteria with biotin. An enzyme-linked immunosorbent assay is then performed with streptavidin conjugated to horseradish peroxidase. Both methods have proven to be reliable and allow for the testing of many criteria in one assay. Cows were immunized with a whole-cell vaccine, and immune serum and milk were collected. The bacteria were then incubated in the presence of serum or milk as a test for antiadherent capability. By using the methods described, distinct antiadherent activity in both serum and milk was demonstrated.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Annuar B. O., Wilcox G. E. Adherence of Moraxella bovis to cell cultures of bovine origin. Res Vet Sci. 1985 Sep;39(2):241–246. [PubMed] [Google Scholar]
- Cole G. W., Silverberg N. L. The adherence of Staphylococcus aureus to human corneocytes. Arch Dermatol. 1986 Feb;122(2):166–169. [PubMed] [Google Scholar]
- Collier R. J., Bauman D. E., Hays R. L. Lactogenesis in explant cultures of mammary tissue from pregnant cows. Endocrinology. 1977 Apr;100(4):1192–1200. doi: 10.1210/endo-100-4-1192. [DOI] [PubMed] [Google Scholar]
- De Graaf F. K., Mooi F. R. The fimbrial adhesins of Escherichia coli. Adv Microb Physiol. 1986;28:65–143. doi: 10.1016/s0065-2911(08)60237-4. [DOI] [PubMed] [Google Scholar]
- Dunkle L. M., Blair L. L., Fortune K. P. Transformation of a plasmid encoding an adhesin of Staphylococcus aureus into a nonadherent staphylococcal strain. J Infect Dis. 1986 Apr;153(4):670–675. doi: 10.1093/infdis/153.4.670. [DOI] [PubMed] [Google Scholar]
- Edén C. S., Hanson L. A., Jodal U., Lindberg U., Akerlund A. S. Variable adherence to normal human urinary-tract epithelial cells of Escherichia coli strains associated with various forms of urinary-tract infection. Lancet. 1976 Sep 4;1(7984):490–492. [PubMed] [Google Scholar]
- Ellen R. P., Gibbons R. J. M protein-associated adherence of Streptococcus pyogenes to epithelial surfaces: prerequisite for virulence. Infect Immun. 1972 May;5(5):826–830. doi: 10.1128/iai.5.5.826-830.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frost A. J. Selective adhesion of microorganisms to the ductular epithelium of the bovine mammary gland. Infect Immun. 1975 Nov;12(5):1154–1156. doi: 10.1128/iai.12.5.1154-1156.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frost A. J., Wanasinghe D. D., Woolcock J. B. Some factors affecting selective adherence of microorganisms in the bovine mammary gland. Infect Immun. 1977 Jan;15(1):245–253. doi: 10.1128/iai.15.1.245-253.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman G. T., Akers R. M., Friderici K. H., Tucker H. A. Hormonal regulation of alpha-lactalbumin secretion from bovine mammary tissue cultured in vitro. Endocrinology. 1983 Apr;112(4):1324–1330. doi: 10.1210/endo-112-4-1324. [DOI] [PubMed] [Google Scholar]
- Greenberg D. P., Ward J. I., Bayer A. S. Influence of Staphylococcus aureus antibody on experimental endocarditis in rabbits. Infect Immun. 1987 Dec;55(12):3030–3034. doi: 10.1128/iai.55.12.3030-3034.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper M., Turvey A., Bramley A. J. Adhesion of fimbriate Escherichia coli to bovine mammary-gland epithelial cells in vitro. J Med Microbiol. 1978 May;11(2):117–123. doi: 10.1099/00222615-11-2-117. [DOI] [PubMed] [Google Scholar]
- Jones G. W., Abrams G. D., Freter R. Adhesive properties of Vibrio cholerae: adhesion to isolated rabbit brush border membranes and hemagglutinating activity. Infect Immun. 1976 Jul;14(1):232–239. doi: 10.1128/iai.14.1.232-239.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuusela P. Fibronectin binds to Staphylococcus aureus. Nature. 1978 Dec 14;276(5689):718–720. doi: 10.1038/276718a0. [DOI] [PubMed] [Google Scholar]
- Martin D., Mathieu L. G., Lecomte J., deRepentigny J. Adherence of gram-positive and gram-negative bacterial strains to human lung fibroblasts in vitro. Exp Biol. 1986;45(4):323–334. [PubMed] [Google Scholar]
- McGrath C. M., Soule H. D. Renewal inhibition of human mammary cell growth in vitro: cortisol and the recruitment of cells to terminal differentiation. J Cell Physiol. 1983 Sep;116(3):385–396. doi: 10.1002/jcp.1041160317. [DOI] [PubMed] [Google Scholar]
- Norcross N. L., Opdebeeck J. P. Encapsulation of Staphylococcus aureus isolated from bovine milk. Vet Microbiol. 1983 Aug;8(4):397–404. doi: 10.1016/0378-1135(83)90052-4. [DOI] [PubMed] [Google Scholar]
- Ofek I., Courtney H. S., Schifferli D. M., Beachey E. H. Enzyme-linked immunosorbent assay for adherence of bacteria to animal cells. J Clin Microbiol. 1986 Oct;24(4):512–516. doi: 10.1128/jcm.24.4.512-516.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opdebeeck J. P., Frost A. J., O'Boyle D. Adhesion of Staphylococcus aureus and Escherichia coli to bovine udder epithelial cells. Vet Microbiol. 1988 Jan;16(1):77–86. doi: 10.1016/0378-1135(88)90128-9. [DOI] [PubMed] [Google Scholar]
- Opdebeeck J. P., Norcross N. L. Frequency and immunologic cross-reactivity of encapsulated Staphylococcus aureus in bovine milk in New York. Am J Vet Res. 1983 Jun;44(6):986–988. [PubMed] [Google Scholar]
- Porras O., Svanborg-Edén C., Lagergård T., Hanson L. A. Method for testing adherence of Haemophilus influenzae to human buccal epithelial cells. Eur J Clin Microbiol. 1985 Jun;4(3):310–315. doi: 10.1007/BF02013659. [DOI] [PubMed] [Google Scholar]
- Reid G., Brooks H. J. A fluorescent antibody staining technique to detect bacterial adherence to urinary tract epithelial cells. Stain Technol. 1985 Jul;60(4):211–217. doi: 10.3109/10520298509113915. [DOI] [PubMed] [Google Scholar]
- Sanford B. A., Davison V. E., Ramsay M. A. Staphylococcus aureus adherence to influenza A virus-infected and control cell cultures: evidence for multiple adhesins. Proc Soc Exp Biol Med. 1986 Jan;181(1):104–111. doi: 10.3181/00379727-181-42230. [DOI] [PubMed] [Google Scholar]
- Sanford B. A., Ramsay M. A. Detection of staphylococcal membrane receptors on virus-infected cells by direct adhesin overlay. Infect Immun. 1986 Jun;52(3):671–675. doi: 10.1128/iai.52.3.671-675.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stampfer M., Hallowes R. C., Hackett A. J. Growth of normal human mammary cells in culture. In Vitro. 1980 May;16(5):415–425. doi: 10.1007/BF02618365. [DOI] [PubMed] [Google Scholar]
- Switalski L. M., Rydén C., Rubin K., Ljungh A., Hök M., Wadström T. Binding of fibronectin to Staphylococcus strains. Infect Immun. 1983 Nov;42(2):628–633. doi: 10.1128/iai.42.2.628-633.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torian B. E., Connelly R. J., Stephens R. S., Stibbs H. H. Specific and common antigens of Trichomonas vaginalis detected by monoclonal antibodies. Infect Immun. 1984 Jan;43(1):270–275. doi: 10.1128/iai.43.1.270-275.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tramont E. C. Adhesion of Neisseria gonorrhoeae and disease. Ciba Found Symp. 1981;80:188–201. doi: 10.1002/9780470720639.ch12. [DOI] [PubMed] [Google Scholar]
- Tramont E. C. Specificity of inhibition of epithelial cell adhesion of Neisseria gonorrhoeae. Infect Immun. 1976 Aug;14(2):593–595. doi: 10.1128/iai.14.2.593-595.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuomanen E. Piracy of adhesins: attachment of superinfecting pathogens to respiratory cilia by secreted adhesins of Bordetella pertussis. Infect Immun. 1986 Dec;54(3):905–908. doi: 10.1128/iai.54.3.905-908.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaudaux P., Suzuki R., Waldvogel F. A., Morgenthaler J. J., Nydegger U. E. Foreign body infection: role of fibronectin as a ligand for the adherence of Staphylococcus aureus. J Infect Dis. 1984 Oct;150(4):546–553. doi: 10.1093/infdis/150.4.546. [DOI] [PubMed] [Google Scholar]
- Vercellotti G. M., Lussenhop D., Peterson P. K., Furcht L. T., McCarthy J. B., Jacob H. S., Moldow C. F. Bacterial adherence to fibronectin and endothelial cells: a possible mechanism for bacterial tissue tropism. J Lab Clin Med. 1984 Jan;103(1):34–43. [PubMed] [Google Scholar]
- Wanasinghe D. D. In vitro adherence of Staphylococcus aureus to bovine mammary gland epithelial cells. Acta Vet Scand. 1981;22(1):99–108. doi: 10.1186/BF03547211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson M. R., Hohmann A. W. Immunity to Escherichia coli in pigs: adhesion of enteropathogenic Escherichia coli to isolated intestinal epithelial cells. Infect Immun. 1974 Oct;10(4):776–782. doi: 10.1128/iai.10.4.776-782.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J., Elias J. J., Petrakis N. L., Wellings S. R., Nandi S. Effects of hormones and growth factors on human mammary epithelial cells in collagen gel culture. Cancer Res. 1981 Mar;41(3):1021–1027. [PubMed] [Google Scholar]
- Yang J., Guzman R., Richards J., Jentoft V., DeVault M. R., Wellings S. R., Nandi S. Primary culture of human mammary epithelial cells embedded in collagen gels. J Natl Cancer Inst. 1980 Aug;65(2):337–343. [PubMed] [Google Scholar]
- Yang N. S., Kube D., Park C., Furmanski P. Growth of human mammary epithelial cells on collagen gel surfaces. Cancer Res. 1981 Oct;41(10):4093–4100. [PubMed] [Google Scholar]



