Abstract
Thymic stromal lymphopoietin (TSLP) is a cytokine expressed by epithelial cells, including keratinocytes, and is important in allergic inflammation. Allergic skin inflammation elicited by epicutaneous immunization of mice with ovalbumin (OVA), a potential model of atopic dermatitis, was severely impaired in TSLPR−/− mice, as evidenced by decreased infiltration of eosinophils and decreased local expression of T helper 2 (Th2) cytokines. However, secretion of Th2 cytokines by splenocytes from epicutaneous sensitized TSLPR−/− mice in response to OVA was normal. Skin dendritic cells from TSLPR−/− mice were normal in their ability to migrate to draining lymph nodes, express activation markers, and induce proliferation and Th2 cytokine production by naïve T cells. CD4+ T cells from TSLPR−/− mice expressed the skin homing receptor E-selectin ligand normally, and homed to the skin normally, but failed to transfer allergic skin inflammation to WT recipients. TSLP enhanced Th2 cytokine secretion in vitro by targeting TSLPR on antigen specific T cells. Intradermal injection of anti-TSLP blocked the development of allergic skin inflammation after cutaneous antigen challenge of OVA immunized WT mice. These findings suggest that TSLP is essential for antigen driven Th2 cytokine secretion by skin infiltrating effector T cells and could be a therapeutic target in allergic skin inflammation.
Atopic dermatitis (AD) is an inflammatory skin disorder characterized by allergen-driven T helper 2 (Th2) cell polarization, skin infiltration with CD4+ T cells and eosinophils, and local expression of the Th2 cytokines IL-4 and IL-13, whereas chronic AD lesions have a mixed Th1 and Th2 pattern (1). Recent evidence has suggested an important role of skin epithelium, composed mainly of keratinocytes, in the pathogenesis of AD (2). In response to danger signals (e.g., physical injury, microbial products, or allergens), keratinocytes secrete a variety of proinflammatory mediators, which regulate innate and adaptive immune reactions (3).
Thymic stromal lymphopoietin (TSLP) is a cytokine expressed by keratinocytes and other epithelial cells (4). TSLP exerts its biological activities by binding to a heterodimeric receptor consisting of the IL-7 receptor α-chain (IL-7Rα) and the TSLP receptor chain (TSLPR), which is closely related to the common receptor-γ-like chain (5, 6). TSLPR is expressed on a variety of cell types, including T cells, B cells, dendritic cells (DCs), and monocytes (5, 6).
Studies in humans have suggested that TSLP polarizes DCs to induce the differentiation of naïve T cells into Th2 cells; this is mediated in part by induction of OX40L expression on DCs (7, 8). It was initially reported that TSLP had no effect on mouse DCs (4), but it was later reported that it causes a moderate increase in the expression level of costimulatory molecules on mouse DCs and reduced IFN-γ production by CD4+ T cells (9). TSLP promotes the proliferation of human and mouse T cells to T cell receptor ligation, and directly drives mouse Th2 cell differentiation in the absence of DCs in vitro (10, 11). TSLP also up-regulates Th2 cytokine production by mast cells (12).
TSLP expression by epithelial cells is up-regulated by proinflammatory and Th2 cytokines (13, 14). TSLP is highly expressed by keratinocytes in AD skin lesions (4), and in bronchial epithelial cells in asthma (4, 14). Overexpression of murine TSLP in keratinocytes or lung epithelial cells causes spontaneous dermatitis and airway inflammation, respectively (15, 16). Skin-specific expression of TSLP also caused skin inflammation in T cell-deficient RAG2−/− mice, with infiltration by mast cells and eosinophils, suggesting that it can act in a T cell-independent way directly on these myeloid cells, which express TSLPR (15). TSLPR−/− mice exhibit a severely attenuated lung inflammation with less infiltration of inflammatory cells in response to inhaled antigen (9, 16). These results suggest that the TSLP–TSLPR pathway is intimately involved in the development of allergic inflammation. However, the mechanisms by which TSLP contributes to allergic diseases are not well understood.
In the current study, we have investigated the role of the TSLP–TSLPR pathway in a mouse model of allergic skin inflammation elicited by repeated epicutaneous (EC) sensitization with ovalbumin (OVA) to tape-stripped skin (17). In this model, tape stripping may mimic the mechanical injury inflicted by scratching, a hallmark of AD. Our results indicate that TSLPR plays no detectable role in the elicitation of a Th2 response to EC sensitization. In contrast, TSLP plays an important role in the effector phase of Th2-dominated allergic skin inflammation by enhancing local Th2 cytokine production by skin-infiltrating antigen-specific CD4+ T cells.
Results
Allergic Skin Inflammation Is Impaired in TSLPR−/− Mice.
As reported (17), EC sensitization of BALB/c mice with OVA resulted in epidermal thickening and dermal infiltration with CD4+ cells and eosinophils and significant up-regulation of mRNA expression of the Th2 cytokines IL-4 and IL-13, but not of IFN-γ mRNA. Dermal infiltration with eosinophils was significantly less in OVA-sensitized skin of TSLPR−/− mice (Fig. 1 A and B). In contrast, there was no significant difference in CD4+ T cells infiltration (Fig. 1B). There was significantly decreased expression of IL-4 and IL-13 mRNA in OVA-sensitized skin of TSLPR−/− mice (Fig. 1C), with no increase in IFN-γ mRNA expression (data not shown). These observations were confirmed in two additional experiments that used four to seven mice per group (data not shown). Cells recruited to inflamed tissues later mobilize to draining lymph nodes (DLNs) (18). Antigen-stimulated cells from skin DLNs of TSLPR−/− mice secreted significantly less IL-4 and IL-13 than those from WT controls, but normal amounts of IFN-γ (Fig. 1D). Intracellular staining revealed weak expression of IL-4 by CD4+ cells in DLNs of WT mice and TSLPR−/− mice. IFN-γ expression was comparable between the two groups [supporting information (SI) Fig. S1].
Fig. 1.
Decreased allergic skin inflammation in EC-sensitized TSLPR−/− mice. (A) H&E staining of sections from saline- and OVA-sensitized skin at ×200 magnification. Further magnification of the red bordered box shows the presence of multiple eosinophils (arrows). (B) Skin infiltration of eosinophils and CD4+ T cells. (C) Quantitative PCR analysis of mRNA levels of IL-4 and IL-13 in sensitized skin expressed as fold induction compared with saline-sensitized skin sites. (D) IL-4, IL-13, and IFN-γ secretion by cells from skin DLNs after stimulation for 4 days with OVA. Columns and error bars represent the mean ± SEM (n = 5–7 per group). *, P < 0.05, **, P < 0.01, ***, P < 0.0001. N.D., not detectable.
Splenocytes from EC-Sensitized Mice Proliferate and Secrete Cytokines Normally in Response to OVA Stimulation.
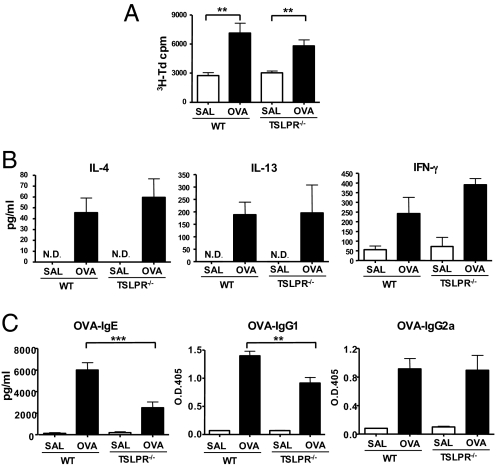
We tested whether impaired allergic skin inflammation in TSLPR−/− mice was a result of impaired ability to mount a systemic Th2 response to EC sensitization. Splenocytes from EC-sensitized TSLPR−/− mice proliferated normally in response to OVA stimulation (Fig. 2A), and secreted comparable amounts of IL-4, IL-13, and IFN-γ as splenocytes from WT controls (Fig. 2B). This observation was confirmed in two additional experiments that used four to seven mice per group (data not shown). However, TSLPR−/− mice exhibited a significantly lower IgG1 and IgE, but not IgG2a, antibody response to OVA than WT controls (Fig. 2C).
Fig. 2.
Systemic immune response in EC-sensitized TSLPR−/− mice. Proliferation (A) and secretion of IL-4, IL-13, and IFN-γ (B) by splenocytes from OVA EC-sensitized WT and TSLPR−/− mice after in vitro OVA stimulation. (C) Serum levels of OVA-specific IgE, IgG1, and IgG2a in EC-sensitized WT and TSLPR−/− mice. Columns and error bars represent the mean ± SEM (n = 6 per group), and similar results were obtained in two other independent experiments with 4–6 mice per group. *, P < 0.05, **, P < 0.01, ***, P < 0.0001.
Skin DCs Migrate Normally to Lymph Nodes of TSLPR−/− Mice and Induce Normal Expression of Cytokines in Naïve T Cells.
Skin DCs capture antigen and up-regulate a number of surface antigens and traffic to DLNs, where they prime naïve T cells to become effector cells that can home to the skin (19, 20). TSLP expression in AD is associated with Langerhans cell migration and activation in situ, and TSLP has been shown to promote the ability of DCs to polarize naïve T cells into Th2 cells partly by up-regulating OX40L on these cells (7, 8, 21). To examine the migration and function of skin-derived DCs in TSLPR−/− mice, we applied FITC to the skin and isolated CD11c+FITC+ from DLNs 24 h later. The percentage and number of CD11c+FITC+ DCs in DLNs were comparable in TSLPR−/− mice and WT controls (Fig. 3 A and B). The mean fluorescence intensities of FITC on these cells were also comparable (913 ± 181 for TSLPR−/− mice vs. 842 ± 100 for WT controls, n = 3). Furthermore, up-regulation of surface expression of MHC class II, CD40, CD86, CD80, and OX40L on CD11c+FITC+ DCs was comparable in TSLPR−/− mice and WT controls (Fig. 3C). We also examined the migration and expression of surface markers of CD11c+FITC+ DCs under conditions in which the cells are exposed to exogenous TSLP injected intradermally 1 hour before FITC painting. Intradermal injection of exogenous TSLP had no effect on the migration of skin DCs or on the expression of activation markers by skin DCs in DLNs (Fig. S2), in agreement with the recent results by Sokol et al. (22). More importantly, CD11c+FITC+ cells from DLNs of TSLPR−/− mice and WT controls were comparable in their capacity to induce proliferation and cytokine secretion by naïve DO11.10 CD4+ T cells after stimulation with OVA323–339 peptide (Fig. 3D). These results suggest that lack of TSLPR does not alter the capacity of mouse skin DCs that have captured antigen to migrate to DLNs and cause naïve T cells to proliferate and secrete cytokines.
Fig. 3.
Skin DCs migrate, express activation markers, and present antigen normally in TSLPR−/− mice. Representative FACS analysis of percentage (A) and numbers (B) of CD11c+FITC+ cells in DLNs from WT and TSLPR−/− mice 24 h after FITC application to shaved skin. (C) Representative FACS analysis of expression of MHC class II, CD40, CD86, CD80, and OX40L on CD11c+FITC+ DCs of WT and TSLPR−/− mice. (D) Proliferation and secretion of IL-4, IL-13, and IFN-γ by DO11.10 CD4+ T cells stimulated with OVA323–339 peptide in the presence of skin-derived DCs from WT or TSLPR−/− mice. Data are representative of three experiments.
TSLPR−/− T Cells Express Skin Homing Receptors Normally.
Impaired allergic skin inflammation in the face of normal proliferation and Th2 cytokine secretion by splenocytes of EC-sensitized TSLPR−/− mice may have been caused by defective expression of skin homing receptors by T cells from these mice. E-selectin ligand (E-lig) induced on naïve T cells by DCs from peripheral lymph nodes (PLNs), but not mesenteric lymph nodes, is expressed on CD4+CD44+ memory T cells and plays an important role in T cell homing to the skin by allowing the T cells to roll on endothelial cells that line high endothelial venules that express E-selectin (23, 24). The fraction of E-lig+ memory T cells in PLN of mice increases with age because of cumulative exposure to environmental antigens via scratched skin. We compared the percentage of CD4+CD44+E-lig+ cells in PLNs of young (age, 8 weeks) and older (age, 6 months) TSLPR−/− mice and WT controls. The age-dependent increase in the percentages of CD4+CD44+ and CD4+CD44+E-lig+ cells was comparable in PLNs of TSLPR−/− mice and WT controls (Fig. S3). These results suggest that lack of TSLP does not interfere with expression of the skin homing receptor E-lig on CD4+ T cells.
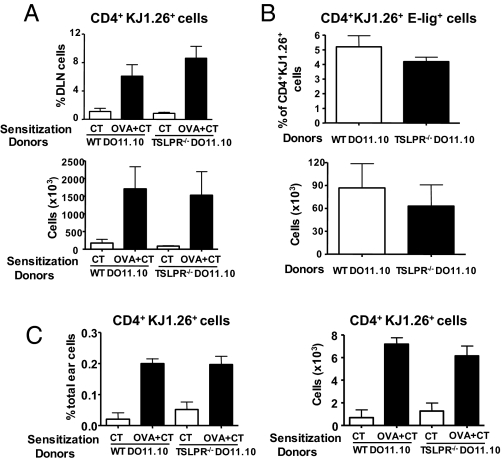
To directly verify that CD4+ TSLPR−/− T cells migrate normally to the skin, naïve DO11.10 CD4+ cells from TSLPR−/− mice or WT controls were administered i.v. to WT recipients, who were then challenged with OVA plus cholera toxin (CT) or CT alone on ear skin. Six days later, cells were isolated from DLNs and ear skin and analyzed for expression of the transgenic T cell receptor β chain by using mAb KJ1–26. The percentage and numbers of CD4+KJ1–26+ cells increased comparably in DLNs of WT recipients that received DO11.10 CD4+ cells from WT and TSLPR−/− background (Fig. 4A). Furthermore, the percentage of CD4+KJ1–26+ cells that expressed E-lig and the total number of CD4+KJ1–26+E-lig+ cells in DLNs of OVA-challenged ears were comparable in recipients of transgenic T cells from WT and TSLPR−/− donors (Fig. 4B). This finding is consistent with normal expansion of antigen-specific TSLPR−/− CD4+ T cells and normal expression of E-lig on these cells in response to cutaneous sensitization. The numbers of CD4+KJ1–26+E-lig+ cells in DLNs of control challenged ears were very few and not significantly different between recipients of WT and TSLPR−/− transgenic T cells (data not shown). Fig. 4C shows that OVA challenge resulted in a comparable increase in the percentage and number of KJ1–26+ CD4+cells in the ear of WT recipients that received DO11.10 CD4+ cells from WT and TSLPR−/− background. This result suggests that T cells from TSLPR−/− mice home normally to the skin after EC sensitization
Fig. 4.
TSLPR−/− T cells express skin homing receptors and traffic to skin normally. Percentages and numbers of donor-derived CD4+KJ1–26+ cells (A) and CD4+KJ1–26+E-lig+ cells (B) in the DLNs of WT recipient mice challenged with OVA or control. (C) Percentages and numbers of donor-derived KJ1–26+ CD4+ T cells in the total cells extracted from the challenged ears of WT recipient mice. Columns and error bars represent the mean ± SEM (n = 3 per group).
CD4+ Splenocytes from TSLPR−/− Mice Fail to Transfer Allergic Skin Inflammation.
Having demonstrated that OVA-specific CD4+ T cells from TSLPR−/− mice home normally to the skin in response to antigen challenge, we tested the hypothesis that these T cells may be impaired in their ability to secrete Th2 cytokines locally in the skin. Splenocytes from EC-sensitized mice were cultured with OVA for 5 days, then CD4+ cells were purified and adoptively transferred by i.v. injection into naïve recipients. Transferred T cells from OVA-sensitized WT and TSLPR−/− donors contained a comparable percentage of E-lig+ cells (2.72 ± 0.77% vs. 2.39 ± 0.55%, n = 4). Recipients were challenged the same day with OVA application to shaved and tape stripped dorsal skin. T cells from OVA-sensitized, but not saline-sensitized, WT mice transferred allergic skin inflammation to both WT and TSLPR−/− recipients. This was evidenced by comparable accumulation of CD4+ cells (Fig. 5A) and comparably increased expression of mRNA for IL-4 and IL-13 in OVA-challenged skin (Fig. 5B) of WT and TSLPR−/− recipients, but with no detectable increase in eosinophils and no change in IFN-γ mRNA expression (data not shown). This result demonstrates that the skin of TSLPR−/− mice supported local Th2 cytokine production by T cells in response to antigen challenge. Transfer of T cells from OVA-sensitized TSLPR−/− mice resulted in normal accumulation of CD4+ cells, but severely impaired expression of IL-4 and IL-13 mRNA in OVA-challenged skin of both WT and TSLPR−/− recipients (Fig. 5 A and B), with no increase in IFN-γ expression (data not shown). These results suggest that TSLPR signaling in T cells is important for their production of Th2 cytokines in the skin.
Fig. 5.
CD4+ T cells from TSLPR−/− mice fail to transfer allergic skin inflammation. Number of infiltrating CD4+ cells (A) and expression of mRNA for IL-4 and IL-13 (B) in OVA-challenged skin of WT or TSLPR−/− recipient mice that received CD4+ T cells from EC-sensitized WT or TSLPR−/− donor mice. Columns and error bars represent the mean ± SEM of two experiments (n = 3–5 recipients per group). *, P < 0.05; **, P < 0.01.
TSLP Amplifies Th2 Cytokine Secretion by Engaging TSLPR on T Cells.
We examined whether TSLPR signaling enhances the activation of OVA-specific T cells in vitro and whether it is important for their activation in OVA-challenged skin sites in vivo. Addition of TSLP significantly potentiated the ability of OVA-stimulated splenocytes from EC-sensitized WT mice to secrete the Th2 cytokines IL-4 and IL-13 (Fig. 6A). The effect of TSLP was specific, because addition of TSLP did not enhance IL-4 and IL-13 secretion by splenocytes of EC-sensitized TSLPR−/− mice. TSLP had no significant effect on the ability of splenocytes to proliferate or secrete IFN-γ.
Fig. 6.
TSLP amplifies Th2 cytokine secretion by engaging TSLPR on T cells. (A) Secretion of IL-4, IL-13, and IFN-γ and proliferation by splenocytes from EC-sensitized mice with OVA after in vitro OVA stimulation with or without TSLP. (B) Secretion of IL-4, IL-13, and IFN-γ and proliferation by DO11.10 CD4+ T cells stimulated with OVA323–339 peptide with or without TSLP in the presence of DCs from the spleens of WT or TSLPR−/− mice. Number of infiltrating eosinophils and CD4+ cells (C) and expression of mRNA for IL-4 and IL-13 (D) in OVA-challenged skin of i.p. immunized mice treated with neutralizing antibodies to TSLP or isotype control. Columns and error bars represent the mean ± SEM of 5 mice per group in A, C, and D, and mean ± SEM of four experiments in B. *, P < 0.05; **, P < 0.01; ***, P < 0.0001.
We next investigated whether the enhancing effect of TSLP on Th2 cytokine secretion targeted the DCs or the T cells. To this purpose, we examined the effect of TSLP on WT DO11.10 CD4+ T cells that were stimulated with OVA323–339 peptide in the presence of splenic DCs from WT or TSLPR−/− mice. TSLP enhanced IL-4 and IL-13 secretion by the T cells to a comparable extent in the presence of WT and TSLPR−/− DCs (Fig. 6B). Similar results were obtained with DCs isolated from PLN (data not shown). There was no significant effect of TSLP on proliferation or IFN-γ secretion by the transgenic CD4+ T cells.
To investigate the role of TSLP in Th2 cytokine secretion by skin infiltrating cells in vivo, we examined the effect of local TSLP blockade on the development of allergic skin inflammation at sites of EC antigen challenge in WT mice. We previously found that EC challenge of mice immunized (i.p.) with OVA/alum and of EC-sensitized mice yielded similar results (R.H., unpublished data). Because i.p. immunization involves a shorter time frame and less labor, mice were immunized i.p. with saline or OVA, then challenged with OVA application to tape stripped skin. OVA-sensitized mice received anti-TSLP mAb or control IgG2a mAb by intradermal injection at the challenge site immediately before each OVA application. EC challenge of OVA immunized mice treated with control IgG2a resulted in significant accumulation of eosinophils and CD4+ T cells and in significant increase in IL-4 and IL-13 mRNA expression compared with EC challenge of saline-immunized mice (Fig. 6C), with no change in IFN-γ mRNA expression (data not shown). Anti-TSLP treatment caused a significant reduction of allergic inflammation in OVA-immunized and challenged mice, as evidenced by significant decrease in infiltration by eosinophils and in Th2 cytokine expression. TSLP blockade had little effect on the accumulation of CD4+ cells. Taken together, these data suggest that TSLP plays an important role in antigen-driven activation of Th2 cells in the skin.
Discussion
We have identified a role for TSLP in allergic skin inflammation, namely driving Th2 cytokine production by skin-infiltrating T cells.
Allergic skin inflammation was markedly reduced in TSLPR−/− mice as evidenced by decreased dermal infiltration with eosinophils and decreased expression of the Th2 cytokines IL-4 and IL-13 in OVA-sensitized skin sites and in DLNs, which are enriched in T cells that have migrated through the skin (Fig. 1 A–C). It is unlikely that eosinophils and mast cells are responsible for the decreased Th2 cytokine expression because cytokine expression in EC-sensitized skin is normal in CCR3−/− mice that fail to mobilize eosinophils into tissues and in mast cell-deficient mice (25, 26). It is also unlikely that basophils play a major role in the cytokine secretion by DLNs, because all intracellular IL-4+ cells in DLNs of WT mice were CD4+ (data not shown). In marked contrast to the decreased eosinophil infiltration and Th2 cytokine expression, dermal infiltration with CD4+ T cells was not significantly affected in EC-sensitized skin of TSLPR−/− mice (Fig. 1B). In three independent experiments, splenocytes from EC-sensitized TSLPR−/− mice proliferated normally and secreted normal amounts of Th2 cytokines and IFN-γ in response to in vitro stimulation with OVA (Fig. 2 A and B). These findings indicate that in our in vivo model of EC sensitization, TSLP is not important for the elicitation of a systemic Th2 immune response to EC sensitization. This, together with the normal numbers of skin-infiltrating CD4+ T cells but decreased Th2 cytokine expression at sites of EC sensitization, suggest that TSLP may not be important for the recruitment and accumulation of T cells at antigen-sensitized sites, but may be essential for their local activation to express Th2 cytokines. It was previously reported that IL-4, but not IFN-γ, secretion by naïve TSLPR−/− T cells in vitro was reduced by approximately half (9). It is possible that repeated EC sensitization may have overcome the partial defect in Th2 cytokine secretion observed in vitro. The partially reduced IgG1 and IgE, but not IgG2a, antibody responses of TSLPR−/− mice to EC sensitization with OVA may be a result of the decreased Th2 cytokine expression in their skin and DLNs.
Impaired allergic skin inflammation in EC-sensitized TSLPR−/− mice was not a result of defective function of their skin DCs. DLNs of FITC painted skin of TSLPR−/− and WT mice contained comparable percentages and numbers of CD11c+FITC+ DCs (Fig. 3A), and these DCs had comparable mean fluorescence intensities. TSLP expression in the skin is up-regulated by the acetone/dibutyl phthalate used to dissolve the FITC (S.Z., unpublished observations). Thus, our results suggest that skin DCs that capture antigen migrate to regional lymph nodes independently of TSLP. Furthermore, CD11c+FITC+ DCs in DLNs of TSLPR−/− mice up-regulated normally all five activation antigens examined (Fig. 3B). These included MHC class II, CD40, CD80, CD86, and OX40L, which has been implicated in TSLP skewing of human DCs to support Th2 cell development (7). More importantly, CD11c+FITC+ DCs from DLNs of TSLPR−/− mice supported normally the production of Th2 cytokines by DO11.10 CD4+ cells stimulated with OVA peptide (Fig. 3C), consistent with the ability of TSLPR−/− mice to mount a normal systemic Th2 response to EC sensitization with OVA. Thus, TSLP–TSLPR interactions are not essential for the polarization of skin DCs to induce a Th2 response in vivo in our EC sensitization model. The normal ability of skin-derived DCs from TSLPR−/− mice to induce a Th2 response is in apparent contradiction with the ability of exogenous TSLP to promote the induction of aTh2 response by human DCs in vitro (7). This may reflect species differences, because the effect of TSLP on DC polarization in vitro is observed with human but not mouse DCs (4). It is also possible that, in our in vivo model, cytokines and other molecules released in response to mechanical skin injury inflicted by tape stripping are able to skew DCs to promote Th2 cell differentiation in the absence of a TSLPR signal.
A defect in skin homing of T cells was ruled out as the underlying cause for impaired allergic skin inflammation in EC-sensitized TSLPR−/− mice. This was based on several lines of evidence. First, the age-dependent increase in the percentage of CD4+CD44+ memory cells that express E-lig+ cells in PLN was comparable in TSLPR−/− mice and WT controls. Second, DO11.10 CD4+ T cells from TSLPR−/− and WT mice adoptively transferred to WT recipients that were then challenged with OVA on ear skin were comparable in their ability to expand in the DLNs and express E-lig (Fig. 4 A and B). Third, in the same experimental setting, comparable numbers of DO11.10 CD4+ cells from TSLPR−/− and WT donors accumulated in the antigen-challenged ears of the recipients (Fig. 4C). Normal homing of TSLPR−/− T cells to skin is consistent with the finding that the accumulation of skin-infiltrating CD4+ cells in OVA-sensitized sites was not significantly reduced in EC-sensitized skin of TSLPR−/− mice, although they failed to express Th2 cytokines and accumulate eosinophils in these sites (Fig. 1 B and C).
Despite normal capacity to home to skin, CD4+ T cells from splenocytes of EC-sensitized TSLPR−/− mice failed to transfer allergic skin inflammation to WT recipients. Consistent with our finding in EC-sensitized TSLPR−/− mice (Fig. 1B), there was normal accumulation of CD4+ cells in EC-challenged skin of WT recipients of TSLPR−/− cells (Fig. 5A). However, there was failure to up-regulate IL-4 and IL-13 mRNA expression at these sites (Fig. 5B). It was recently shown that intense proliferation of antigen-specific CD4+ T cells occurs locally in antigen-challenged skin sites and accounts for most of the accumulation of CD4+ T cells at these sites (27). In view of this observation, our findings suggest that TSLPR−/− T cells that home normally to skin are able to proliferate in response to antigen challenge, but are unable to secrete Th2 cytokines locally. Thus, receipt of a TSLPR signal by skin-infiltrating T cells is essential for their ability to secrete Th2 cytokines locally.
An important role of TSLPR signaling in T cells for Th2 cytokine production was established by the observation that TSLP selectively enhanced Th2 cytokine secretion in vitro by splenocytes from EC-sensitized WT mice and CD4+ cells from DO11.10 transgenic WT mice, with no significant effect on IFN-γ secretion or proliferation (Fig. 6 A and B). The Th2 enhancing effect of TSLP was exerted by targeting TSLPR on the T cells, because TSLP enhanced Th2 cytokine secretion by DO11.10 CD4+ cells to the same extent regardless of whether the DCs that were used to present OVA peptide were derived from WT or TSLPR−/− mice (Fig. 6B). TSLP enhancement of Th2 cytokine secretion by antigen-stimulated mouse CD4+ cells via a direct effect on T cells is consistent with our previous observations that TSLP enhances IL-4 secretion by mouse CD4+ T cells in response to T cell receptor ligation in the absence of APCs (11). We finally demonstrated that TSLP–TSLPR interactions are important for antigen-driven activation of Th2 cells in normal skin. Intradermal injection of anti-TSLP blocked the development of allergic skin inflammation after EC antigen challenge, as evidenced by significantly decreased infiltration by eosinophils and significantly decreased expression of IL-4 and IL-13 mRNA, with little effect on the accumulation of CD4+ cells (Fig. 6B). These observations suggest that TSLP–TSLPR interactions are important for activating local Th2 cytokine expression by skin-infiltrating T cells, in part by directly targeting T cells. We cannot rule out indirect effects on the T cells, e.g., via up-regulation the expression of OX40L on skin DCs that present antigen to infiltrating T cells at the site of challenge. In this regard, in vivo blockade of OX40 ligand inhibits TSLP driven atopic inflammation in lung and skin, including Th2 inflammatory cell infiltration and cytokine secretion (28).
We used an oxazolone-induced contact hypersensitivity (CHS) model to examine the effect of lack of TSLPR in another model of skin inflammation that involves local expression of both Th2 and Th1 cytokines. TSLPR−/− mice developed significantly less ear swelling 24 h after hapten challenge than WT mice (Fig. S4A), but had comparable ear swelling at 48 and 72 h. At all three time points, the total number of cells and the percentage of CD4+ and CD8+ cells recovered from the ears were comparable in WT and TSLPR−/− mice, but the percentages of neutrophils at 24 and 48 h and of macrophages at 24 h were significantly lower in TSLPR−/− mice than WT controls (Fig. S4B). TSLPR−/− mice up-regulated significantly less IL-4 and IL-13 mRNA expression at all three time points after hapten challenge compared with WT controls (Fig. S4C). There was less up-regulation of IFN-γ mRNA expression in TSLPR−/− mice, but the decrease was significant only at 72 h. These results confirm that lack of TSLPR impairs the expression of Th2 cytokines in a CHS model. Because CHS involves not only T cells, but also natural killer cells (29) and neutrophils, which can produce IFN-γ (30), as well as macrophages, which regulate IFN-γ production by T cells via IL-12 (31), decreased IFN-γ mRNA expression could be secondary to the decreased accumulation of macrophages and neutrophils. This is supported by our finding, in a CHS model using 2,4-dinitrofluorobenzene that elicits a Th1 restricted response, that ear swelling and IFN-γ expression in skin DLNs are actually higher in TSLPR−/− mice (S.Z., unpublished observations).
Our results establish a role for TSLP in the effector phase of allergic skin inflammation by demonstrating that TSLP, acting on T cells, is essential for local antigen-driven Th2 cytokine secretion by skin-infiltrating T cells. Our finding that local blockade of TSLP inhibits the development of allergic skin inflammation after skin challenge of immunized mice has important clinical implications, because it suggests that this therapeutic approach may be useful in patients with AD who are already sensitized to allergens.
Methods
Mice and Sensitization.
BALB/c mice (Charles River) and TSLPR−/− mice on BALB/c background were bred in our facility. DO11.10 /TSLPR−/− mice were generated by crossing TSLPR−/− mice with DO11.10 mice (Jackson Laboratory). EC sensitization of 8- to 10-week-old female mice was performed as described previously (17). For i.p. immunization, 50 μg of OVA or saline with alum were administered i.p. on days 0 and 7. Mice were challenged with OVA application to tape stripped skin on days 14 and 17, and 20 μg of anti-TSLP or control IgG2a mAb was intradermally injected 30 min before EC challenge.
Adoptive Transfer and T Cell Homing.
Splenocytes from EC sensitized WT or TSLPR−/− mice were cultured with OVA for 5 days, then CD4+ T cells were purified and injected i.v. into naïve recipients (3 × 106 CD4+ T cells per mouse), which were challenged the same day and 3 days later with 100 μg of OVA application to shaved and tape stripped dorsal skin, and then killed on day 7.
To examine homing, 4 × 106 naïve transgenic CD62L+CD4+ cells prepared from spleens of DO11.10 donors were adoptively transferred to WT recipients. One day later, OVA was applied to ear skin was as described (18), and 6 days later, cells were isolated from DLNs and ears as described (18).
Cell Preparation and Culture.
Single-cell suspensions from spleen and DLNs were cultured at 3 × 106/ml with the stimulation OVA (100 μg/ml) for 4 days. Skin- and spleen-derived CD11c+ DCs from WT or TSLPR−/− mice were prepared as described (32) and were cocultured in triplicate at a 1:10 ratio with WT DO11.10 CD4+ T cells stimulated with OVA323–339 peptide (4 μM) with or without TSLP (50 ng/ml) for 4 days. Wells were pulsed with 1 μCi [3H] thymidine for proliferation measurement and cells were harvested for staining for E-lig. Supernatants were collected for cytokine measurements.
Quantitative PCR Analysis of Cytokines.
Total RNA was extracted from homogenized dorsal or ear skin tissue, and quantitative real-time PCR was performed, and fold induction of target gene expression was determined as described (32).
Ig Ab Determinations.
Serum levels of OVA-specific IgE, IgG1, and IgG2a Abs were measured by ELISAs as described (17).
FITC-Induced in Vivo Migration and Maturation of Skin-Derived DCs.
FITC treatment was performed as described (33). Total cells in DLNs were enumerated and analyzed for FITC fluorescence and expression of surface markers using mAbs to CD11c, MHC class II, CD40, CD80, CD86, OX40L, KJ1–26, CD4, and CD44 (eBioscience). E-lig staining was performed as described (33).
Skin Histopathology and Immunohistochemistry.
Eosinophils in H&E-stained sections and CD4+ T cells in immunoperoxidase-stained sections were counted blinded in 10 high-power fields at a magnification of ×400 (17).
Statistics.
Statistical significance was determined by using Student's t test for unpaired data. A P value of <0.05 was considered to indicate statistical significance.
Supplementary Material
Acknowledgments.
We thank James Campbell and Hans Oettgen for suggestions and for reading the manuscript. This work was supported by U.S. Public Health Service Grant AR-047417 and Atopic Dermatitis Vaccinia Network contract N01AI40030.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0801532105/DCSupplemental.
References
- 1.van Reijsen FC, et al. Skin-derived aeroallergen-specific T-cell clones of Th2 phenotype in patients with atopic dermatitis. J Allergy Clin Immunol. 1992;90:184–193. doi: 10.1016/0091-6749(92)90070-i. [DOI] [PubMed] [Google Scholar]
- 2.Holgate ST. The epithelium takes centre stage in asthma and atopic dermatitis. Trends Immunol. 2007;28:248–251. doi: 10.1016/j.it.2007.04.007. [DOI] [PubMed] [Google Scholar]
- 3.Esche C, de Benedetto A, Beck LA. Keratinocytes in atopic dermatitis: Inflammatory signals. Curr Allergy Asthma Rep. 2004;4:276–284. doi: 10.1007/s11882-004-0071-8. [DOI] [PubMed] [Google Scholar]
- 4.Soumelis V, et al. Human epithelial cells trigger dendritic cell mediated allergic inflammation by producing TSLP. Nat Immunol. 2002;3:673–680. doi: 10.1038/ni805. [DOI] [PubMed] [Google Scholar]
- 5.Park LS, et al. Cloning of the murine thymic stromal lymphopoietin (TSLP) receptor: Formation of a functional heteromeric complex requires interleukin 7 receptor. J Exp Med. 2000;192:659–670. doi: 10.1084/jem.192.5.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pandey A, et al. Cloning of a receptor subunit required for signaling by thymic stromal lymphopoietin. Nat Immunol. 2000;1:59–64. doi: 10.1038/76923. [DOI] [PubMed] [Google Scholar]
- 7.Ito T, et al. TSLP-activated dendritic cells induce an inflammatory T helper type 2 cell response through OX40 ligand. J Exp Med. 2005;202:1213–1223. doi: 10.1084/jem.20051135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ebner S, et al. Thymic stromal lymphopoietin converts human epidermal Langerhans cells into antigen-presenting cells that induce proallergic T cells. J Allergy Clin Immunol. 2007;119:982–990. doi: 10.1016/j.jaci.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 9.Al-Shami A, Spolski R, Kelly J, Keane-Myers A, Leonard WJ. A role for TSLP in the development of inflammation in an asthma model. J Exp Med. 2005;202:829–839. doi: 10.1084/jem.20050199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Al-Shami A, et al. A role for thymic stromal lymphopoietin in CD4(+) T cell development. J Exp Med. 2004;200:159–168. doi: 10.1084/jem.20031975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Omori M, Ziegler S. Induction of IL-4 expression in CD4(+) T cells by thymic stromal lymphopoietin. J Immunol. 2007;178:1396–1404. doi: 10.4049/jimmunol.178.3.1396. [DOI] [PubMed] [Google Scholar]
- 12.Allakhverdi Z, et al. Thymic stromal lymphopoietin is released by human epithelial cells in response to microbes, trauma, or inflammation and potently activates mast cells. J Exp Med. 2007;204:253–258. doi: 10.1084/jem.20062211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bogiatzi SI, et al. Cutting edge: Proinflammatory and Th2 cytokines synergize to induce thymic stromal lymphopoietin production by human skin keratinocytes. J Immunol. 2007;178:3373–3377. doi: 10.4049/jimmunol.178.6.3373. [DOI] [PubMed] [Google Scholar]
- 14.Kato A, Favoreto S, Jr, Avila PC, Schleimer RP. TLR3- and Th2 cytokine-dependent production of thymic stromal lymphopoietin in human airway epithelial cells. J Immunol. 2007;179:1080–1087. doi: 10.4049/jimmunol.179.2.1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yoo J, et al. Spontaneous atopic dermatitis in mice expressing an inducible thymic stromal lymphopoietin transgene specifically in the skin. J Exp Med. 2005;202:541–549. doi: 10.1084/jem.20041503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhou B, et al. Thymic stromal lymphopoietin as a key initiator of allergic airway inflammation in mice. Nat Immunol. 2005;6:1047–1053. doi: 10.1038/ni1247. [DOI] [PubMed] [Google Scholar]
- 17.Spergel JM, et al. Epicutaneous sensitization with protein antigen induces localized allergic dermatitis and hyperresponsiveness to methacholine after single exposure to aerosolized antigen in mice. J Clin Invest. 1998;101:1614–1622. doi: 10.1172/JCI1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Campbell JJ, O'Connell DJ, Wurbel MA. Cutting edge: Chemokine receptor CCR4 is necessary for antigen-driven cutaneous accumulation of CD4 T cells under physiological conditions. J Immunol. 2007;178:3358–3362. doi: 10.4049/jimmunol.178.6.3358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Robert C, et al. Interaction of dendritic cells with skin endothelium: A new perspective on immunosurveillance. J Exp Med. 1999;189:627–636. doi: 10.1084/jem.189.4.627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kissenpfennig A, et al. Dynamics and function of Langerhans cells in vivo: Dermal dendritic cells colonize lymph node areas distinct from slower migrating Langerhans cells. Immunity. 2005;22:643–654. doi: 10.1016/j.immuni.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 21.Liu YJ. Thymic stromal lymphopoietin and OX40 ligand pathway in the initiation of dendritic cell-mediated allergic inflammation. J Allergy Clin Immunol. 2007;120:238–244. doi: 10.1016/j.jaci.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 22.Sokol CL, Barton GM, Farr AG, Medzhitov R. A mechanism for the initiation of allergen-induced T helper type 2 responses. Nat Immunol. 2008;9:310–318. doi: 10.1038/ni1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tietz W, et al. CD4+ T cells migrate into inflamed skin only if they express ligands for E- and P-selectin. J Immunol. 1998;161:963–970. [PubMed] [Google Scholar]
- 24.Campbell DJ, Butcher EC. Rapid acquisition of tissue-specific homing phenotypes by CD4(+) T cells activated in cutaneous or mucosal lymphoid tissues. J Exp Med. 2002;195:135–141. doi: 10.1084/jem.20011502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ma W, et al. CCR3 is essential for skin eosinophilia and airway hyperresponsiveness in a murine model of allergic skin inflammation. J Clin Invest. 2002;109:621–628. doi: 10.1172/JCI14097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Alenius H, et al. Mast cells regulate IFN-gamma expression in the skin and circulating IgE levels in allergen-induced skin inflammation. J Allergy Clin Immunol. 2002;109:106–113. doi: 10.1067/mai.2002.120553. [DOI] [PubMed] [Google Scholar]
- 27.Reinhardt RL, Bullard DC, Weaver CT, Jenkins MK. Preferential accumulation of antigen-specific effector CD4 T cells at an antigen injection site involves CD62E-dependent migration but not local proliferation. J Exp Med. 2003;197:751–762. doi: 10.1084/jem.20021690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Seshasayee D, et al. In vivo blockade of OX40 ligand inhibits thymic stromal lymphopoietin driven atopic inflammation. J Clin Invest. 2007;117:3868–3878. doi: 10.1172/JCI33559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.O'Leary JG, Goodarzi M, Drayton DL, von Andrian UH. T cell- and B cell-independent adaptive immunity mediated by natural killer cells. Nat Immunol. 2006;7:507–516. doi: 10.1038/ni1332. [DOI] [PubMed] [Google Scholar]
- 30.Ethuin F, et al. Human neutrophils produce interferon gamma upon stimulation by interleukin-12. Lab Invest. 2004;84:1363–1371. doi: 10.1038/labinvest.3700148. [DOI] [PubMed] [Google Scholar]
- 31.Kim E, Kim SH, Kim S, Kim TS. The novel cytokine p43 induces IL-12 production in macrophages via NF-kappaB activation, leading to enhanced IFN-gamma production in CD4+ T cells. J Immunol. 2006;176:256–264. doi: 10.4049/jimmunol.176.1.256. [DOI] [PubMed] [Google Scholar]
- 32.He R, Oyoshi MK, Jin H, Geha RS. Epicutaneous antigen exposure induces a Th17 response that drives airway inflammation after inhalation challenge. Proc Natl Acad Sci USA. 2007;104:15817–15822. doi: 10.1073/pnas.0706942104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu L, Fuhlbrigge RC, Karibian K, Tian T, Kupper TS. Dynamic programming of CD8+ T cell trafficking after live viral immunization. Immunity. 2006;25:511–520. doi: 10.1016/j.immuni.2006.06.019. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.