Abstract
Presynaptic Ca2+ stores have been suggested to regulate Ca2+ dynamics within the nerve terminals at certain types of the synapse. However, little is known about their mode of activation, molecular identity, and detailed subcellular localization. Here, we show that the ryanodine-sensitive stores exist in axons and amplify presynaptic Ca2+ accumulation at the hippocampal mossy fiber synapses, which display robust presynaptic forms of plasticity. Caffeine, a potent drug inducing Ca2+ release from ryanodine-sensitive stores, causes elevation of presynaptic Ca2+ levels and enhancement of transmitter release from the mossy fiber terminals. The blockers of ryanodine receptors, TMB-8 or ryanodine, reduce presynaptic Ca2+ transients elicited by repetitive stimuli of mossy fibers but do not affect those evoked by single shocks, suggesting that ryanodine receptors amplify presynaptic Ca2+ dynamics in an activity dependent manner. Furthermore, we generated the specific antibody against the type 2 ryanodine receptor (RyR2; originally referred to as the cardiac type) and examined the cellular and subcellular localization using immunohistochemistry. RyR2 is highly expressed in the stratum lucidum of the CA3 region and mostly colocalizes with axonal marker NF160 but not with terminal marker VGLUT1. Immunoelectron microscopy revealed that RyR2 is distributed around smooth ER within the mossy fibers but is almost excluded from their terminal portions. These results suggest that axonal localization of RyR2 at sites distant from the active zones enables use dependent Ca2+ release from intracellular stores within the mossy fibers and thereby facilitates robust presynaptic forms of plasticity at the mossy fiber-CA3 synapse.
Keywords: calcium store, hippocampus, axon, plasticity
Calcium ions play central roles in use dependent modification of the synaptic efficacy. A buildup of Ca2+ within the presynaptic terminals during repetitive action potentials causes use dependent enhancement of transmitter release (1–3). In addition to this common mechanism of presynaptic plasticity, some types of the synapse adopt additional mechanisms to amplify presynaptic Ca2+ dynamics in an activity dependent manner. Ca2+-induced Ca2+ release (CICR) from intracellular stores is one such likely candidate and growing evidence suggests that ryanodine receptors mediating CICR regulate presynaptic Ca2+ dynamics at some, but not all, types of the synapse (4–12; for review, see ref. 13).
The roles of presynaptic Ca2+ stores in the hippocampus were studied extensively (14–17), but still remain largely unknown. Recent studies suggested functional significance of presynaptic ryanodine-sensitive stores at the mossy fiber-CA3 synapse. The presynaptic Ca2+ transients elicited by high frequency stimulus were suppressed by ryanodine (18) while those evoked by paired-pulse stimuli were not significantly affected (19). It should be noted that application of nicotine elicited synchronized release of a large amount of glutamate sufficient for firing of postsynaptic CA3 neurons (20). Since this synchronous release is blocked by ryanodine, Ca2+ sparks from ryanodine-sensitive stores might be involved in concerted release of multiple quanta, as demonstrated for inhibitory synapses onto the cerebellar Purkinje cell (7, 21).
In this study, we examined the exact experimental conditions to induce Ca2+ release from presynaptic Ca2+ stores through ryanodine receptors at hippocampal mossy fiber synapses. Our findings support the notion that the CICR mechanism amplifies accumulation of Ca2+ within the terminals in a use dependent manner and therefore is critically involved in presynaptic forms of plasticity at this synapse. Furthermore, we produced a specific antibody against RyR2 because of its high transcription levels in dentate gyrus granule cells (22, 23) and found preferential localization of RyR2 in non-terminal portions of mossy fibers. These results suggest that the axonal localization of RyR2 distant from active zones underlie use dependent activation of Ca2+ release from the presynaptic Ca2+ stores.
Results
Presynaptic Effects of Caffeine at the Mossy Fiber-CA3 Synapse.
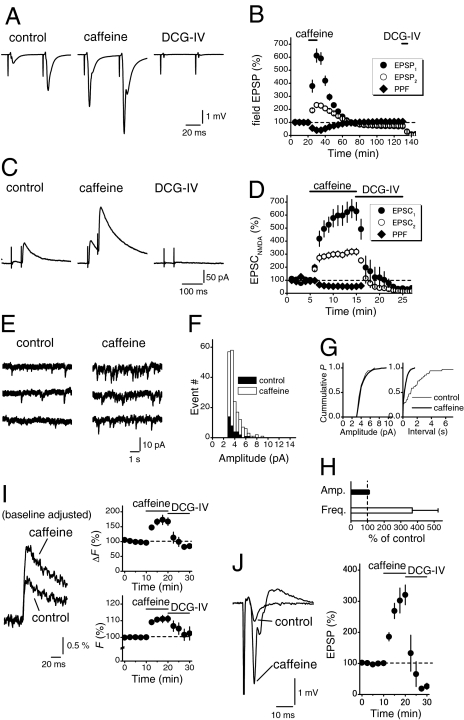
To explore the physiological significance of the presynaptic Ca2+ stores at the hippocampal mossy fiber synapses, we first addressed whether activation of ryanodine receptors could modulate transmitter release from the mossy fiber terminals. Application of 10 mM caffeine, a drug inducing Ca2+ release through ryanodine receptor channels, caused robust enhancement of the field excitatory postsynaptic potentials (EPSPs) (to 613 ± 51% of control, n = 9), while paired-pulse facilitation (PPF) at 50-ms intervals were reduced (to 40 ± 5% of control, Fig. 1 A and B). It should be noted that caffeine reproducibly induced long-term depression of the field EPSPs after prolonged washout (to 69 ± 4% after 100-min washout). DCG-IV at 1 μM was applied at the end of experiments to confirm that the mossy fibers were selectively stimulated (24).
Fig. 1.
Caffeine-induced enhancement of transmitter release and presynaptic Ca2+ levels at the mossy fiber-CA3 synapse. (A and B) Effects of 10 mM caffeine on the field EPSPs evoked by paired-stimuli (50-ms interval). DCG-IV (1 μM) was applied at the end of experiments to confirm that the mossy fibers were selectively stimulated. The time course of relative amplitudes of the first (closed circles) and the second (open circles) field EPSPs and relative values of paired-pulse facilitation (PPF; diamond) are shown in B. (C and D) Effects of 10 mM caffeine on monosynaptic mossy fiber transmission monitored as the NMDA-receptor component of EPSCs (EPSCNMDA) in the presence of the non-NMDA receptor blocker CNQX and at a positive holding potential (+40 mV). The time course of relative amplitudes of the first (closed circles) and second (open circles) EPSC and the PPF (50-ms interval; diamonds) are shown in D. The values of the first and the second responses are normalized to those before caffeine application in B and D. (E–H) Caffeine-induced increase in frequency of the kainate receptor-mediated miniature EPSCs (mEPSCKA) recorded in the selective AMPA receptor blocker GYKI 53655 (30 μM). Amplitude histograms of the mEPSCKA (F) or cumulative probability plots (G) of amplitudes (left) and interevent intervals (right) recorded before (filled bars or thin line) and after the caffeine application (open bars or thick line). Pooled data for relative changes in mean amplitude (filled bar) and mean frequency (open bar) are shown in H. (I and J) Effects of caffeine on presynaptic Ca2+ signals (I) and simultaneously-recorded EPSPs (J) at mossy fiber synapse. The graphs in I represent the time course of the Ca2+ transient (ΔF, upper) and baseline fluorescence (F, lower). F values were significantly increased with caffeine, suggesting the elevation of Ca2+ levels by the release from presynaptic Ca2+ stores.
The caffeine-induced enhancement of field EPSPs may reflect facilitation of transmission at the mossy fiber-CA3 synapse or at the associational synapse recruited disynaptically after caffeine treatment. To determine the relative contribution, monosynaptic responses at the mossy fiber-CA3 synapse were measured as NMDA receptor-mediated components of the excitatory postsynaptic currents (EPSCNMDA) recorded at a positive membrane potential and in the presence of 10 μM CNQX to block polysynaptic excitation (25). Caffeine at 10 mM increased the amplitude of EPSCNMDA to the almost same degree (to 629 ± 68% of control, n = 9; Fig. 1 C and D), suggesting that the transmission at the mossy fiber synapse was selectively enhanced by application of caffeine. The caffeine-induced enhancement of EPSCNMDA was accompanied by a marked reduction of paired-pulse facilitation (to 52 ± 7% of control, n = 9; Fig. 1D). These data suggest that caffeine enhanced the probability of transmitter release from the mossy fiber terminals.
We also examined the effect of caffeine on quantal release solely from the mossy fiber terminals. For this purpose, we monitored kainate receptor-mediated slow miniature EPSCs (mEPSCKA) recorded in the presence of GYKI 53655, a selective AMPA receptor blocker. Among excitatory synapses onto CA3 neurons, only mossy fiber synapses express postsynaptic kainate receptors (26, 27). Therefore, the slow mEPSCKA recorded in the presence of GYKI 53655 reflects quantal release solely from the mossy fiber terminals (28). Application of 10 mM caffeine significantly increased the frequency (to 369 ± 155% of control, n = 5), but not the amplitude (to 109 ± 2%), of the mEPSCKA recorded in the presence of GYKI 53655 (Fig. 1 E–H), suggesting that caffeine enhanced transmitter release from the mossy fiber terminals.
Furthermore we optically monitored presynaptic Ca2+ dynamics by selective loading of the mossy fibers with the fluorescent Ca2+ indicator in hippocampal slices (29). Background fluorescence (F) was significantly increased by caffeine (to 111 ± 2% of control; Fig. 1I), while simultaneously-recorded field EPSPs were enhanced (to 320 ± 32%, Fig. 1J). Since this increase in F by caffeine was suppressed by prior application of ryanodine [to 103 ± 2%, supporting information (SI) Fig. S1], the result suggested that caffeine caused elevation of Ca2+ levels by the release from presynaptic Ca2+ stores. Caffeine-induced decrease in PPF, increase in frequency of mEPSCs, and elevation of presynaptic Ca2+ levels are all consistent with enhanced transmitter release by Ca2+ release from ryanodine-sensitive stores.
Use-Dependent Effects of Ryanodine Receptor Blockers on Presynaptic Ca2+ Transients.
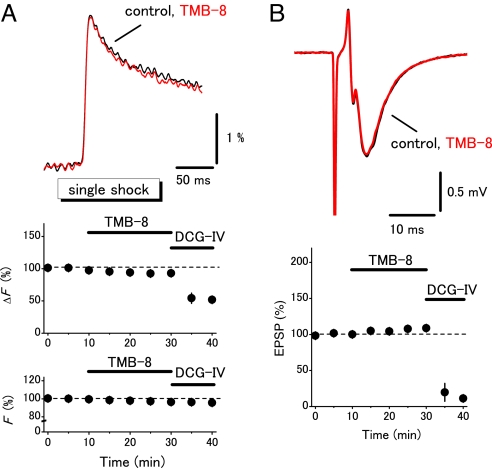
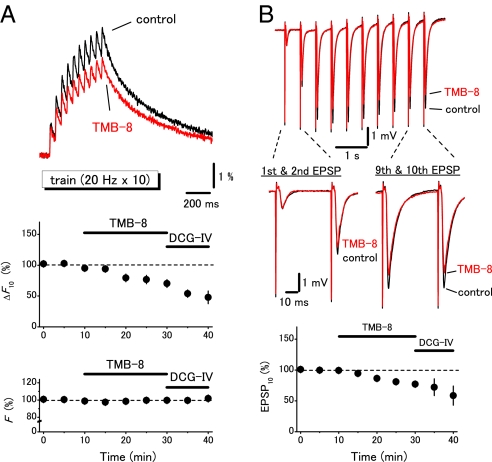
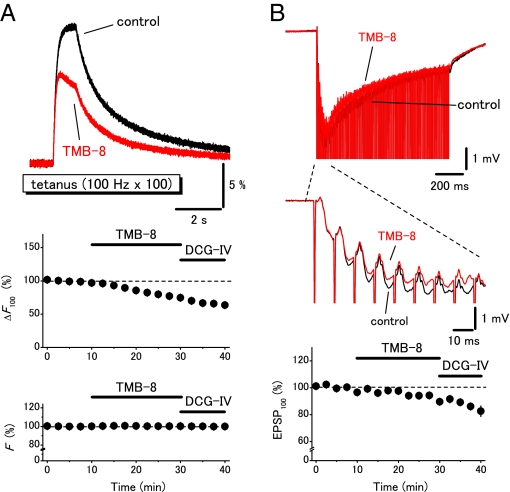
To explore the physiological functions of presynaptic ryanodine receptors, the effects of TMB-8, a blocker of ryanodine receptor, on the presynaptic Ca2+ transients were examined by changing the stimulus protocols systematically. TMB-8 at 10 μM did not affect the field EPSPs and the presynaptic Ca2+ transients elicited by single shocks (to 108 ± 4% and 93 ± 3%, respectively, n = 5; Fig. 2 A and B). DCG-IV nearly abolished the field EPSPs, suggesting that the mossy fibers were selectively stimulated (24). On the other hand, the presynaptic Ca2+ transients elicited by the train (20 Hz for 10 times; Fig. 3A) or by the tetanus (100 Hz for 100 times; Fig. 4A) were significantly suppressed (to 74 ± 5%, n = 6, or to 70 ± 4%, n = 8, respectively). It should be noted that the first response in the train stimuli (Fig. 3A) and the initial phase during the tetanic stimuli (Fig. 4A) were little affected while the later phases were suppressed by TMB-8. These results suggested that the CICR mechanism amplified the presynaptic Ca2+ signaling in a use dependent manner. It should be noted that this notion was also supported by the observation of simultaneously-recorded field EPSPs. Application of TMB-8 partially suppressed the 10th field EPSPs elicited by the train stimuli (to 82 ± 4%, n = 6) or 100th field EPSPs elicited by the tetanus (to 89 ± 2%, n = 8) without affecting the first responses (Fig. 3B and Fig. 4B). These results suggested that the CICR mechanism did modify the Ca2+ transients in the vicinity of active zone and modulate transmitter release from the mossy fiber terminals. We also examined the effects of ryanodine to further confirm that the effects of TMB-8 were mediated by suppression of ryanodine receptors. Ryanodine at 100 μM suppressed the presynaptic Ca2+ transients elicited by the train (to 82 ± 3%, n = 8, Fig. S2) or by the tetanus (to 87 ± 2%, n = 5, data not shown).
Fig. 2.
Minimal effects of a ryanodine receptor blocker TMB-8 on presynaptic Ca2+ transients evoked by single shocks. (A) Effects of TMB-8 (10 μM) on presynaptic Ca2+ transients evoked by single shocks to mossy fibers. Graphs represent the time course of amplitudes of Ca2+ transients (ΔF) and F. (B) Effects of TMB-8 on the field EPSPs recorded simultaneously.
Fig. 3.
Use dependent amplification of the presynaptic Ca2+ transients by ryanodine receptors. (A) The ryanodine receptor blocker TMB-8 (10 μM) partly reduced the presynaptic Ca2+ transients elicited by a stimulus train (20 Hz 10 times). Graphs represent the time course of amplitudes of Ca2+ transients to the 10th stimuli (ΔF10) and F. (B) Effects of TMB-8 on the field EPSPs recorded simultaneously. Graph represents the time course of the 10th EPSP (EPSP10). Note that the first responses during the train were almost unaffected while the latter responses were selectively suppressed by TMB-8 in both Ca2+ and EPSP recordings.
Fig. 4.
Effects of a ryanodine receptor blocker TMB-8 on the presynaptic Ca2+ transients elicited by the tetanic stimulation. (A) Presynaptic Ca2+ transients evoked by the tetanus (100 Hz 100 times) were suppressed by 10 μM TMB-8 while the initial phases were almost unaffected. Graphs represent the time course of amplitudes of Ca2+ transients to the 100th stimuli (ΔF100) and F. (B) Effects of TMB-8 on the field EPSPs recorded simultaneously. Graph represents the time course of the 100th EPSP (EPSP100).
Previous studies suggested that stimulation of α-bungarotoxin-sensitive presynaptic nicotinic receptors leads to CICR through ryanodine receptors at this synapse (20, 30). This raised the possibility that the observed use dependent effects of TMB-8 or ryanodine were mediated by activation of α7 nicotinic acetylcholine receptors (nAChRs) by costimulation of cholinergic inputs originated from the septo-hippocampal system (31). Therefore, we tested whether α7 nAChR-selective antagonist methyllycacontine (MLA) modifies the presynaptic calcium transients elicited by the train stimulus. Application of 10 nM MLA alone had little effect on the train-induced presynaptic Ca2+ transients (to 97 ± 2%, n = 7; Fig. S3). More importantly, TMB-8 suppressed the train-induced Ca2+ transients to a similar degree in both the absence (to 74 ± 5%, n = 6; Fig. 3A) and presence (to 77 ± 3%, n = 7; Fig. S3) of MLA (P = 0.33). These results suggested that the use dependent amplification of presynaptic Ca2+ transients was independent of modulation by α7 nAChR.
Localization of RyR2 in Nonterminal Axons of Mossy Fibers.
The results of electrophysiological examination all suggest the presence of functional presynaptic ryanodine receptors at the mossy fiber-CA3 synapse. Next, we examined molecular components and subcellular localization of the presynaptic ryanodine receptors by immunohistochemical approaches. To date, little information is available for subcellular localization of individual subtypes of the ryanodine receptor, partly because of the lack of subtype-specific antibodies. Here, we produced a specific antibody to RyR2 (the cardiac type), which is a highly transcribed in dentate gyrus granule cells (22, 23). For antigen, we selected the 139-aa sequence of RyR2, because the sequence shows little similarity to that of RyR1 (the skeletal muscle type) and is missing in RyR3 (the brain type) (Fig. S4A). In immunoblot, an affinity-purified rabbit RyR2 antibody recognized a single protein band (>500 kDa) in protein extracts prepared from the brain and heart but not those from the skeletal muscle (Fig. S4B). This is also true in immunohistochemistry (Fig. S4 C and E). In the brain, various brain regions were stained (Fig. S4F) in patterns similar to those of the distribution of RyR2 mRNA (Fig. S4H). After preabsorption with the antigen, all immunoblot and immunohistochemical signals disappeared (Fig. S4 B, D, and G). All of these results indicate the specificity of the RyR2 antibody.
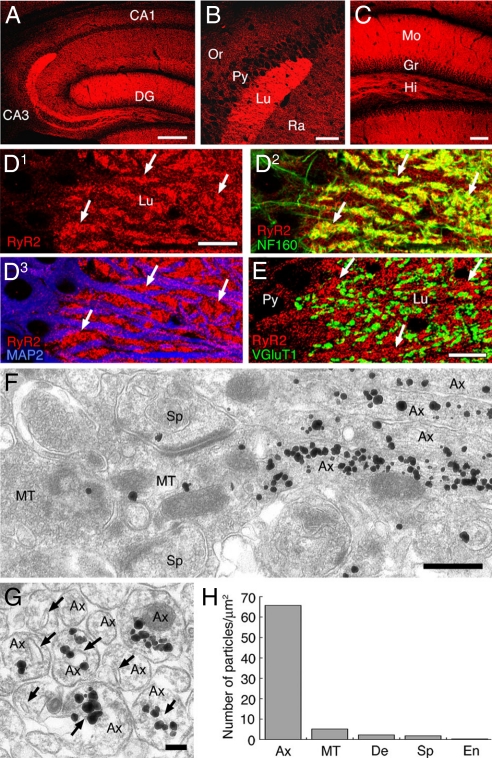
Using the specific antibody, we examined distribution of RyR2 in the adult mouse hippocampus by immunohistochemistry. RyR2 was distributed widely with the highest level in the molecular layer of the dentate gyrus, and also in fibrous bundles running through the hilus of the dentate gyrus and the stratum lucidum of the CA3 region (Fig. 5 A–C), mossy fiber-recipient regions. In the stratum lucidum, the labeling of RyR2 was intense in neurofilament-positive neuropil, while it was low to moderate on microtubule-associated protein-2 (MAP2)-positive dendritic shafts and perikarya of pyramidal cells (Fig. 5D), suggesting high contents of RyR2 in mossy fibers. When terminals of mossy fibers were visualized by immunofluorescence for vesicular glutamate transporter 1 (VGluT1), higher levels for RyR2 were detected outside VGluT1-positive terminals (Fig. 5E). These results suggest that RyR2 is distributed almost selectively in non-terminal axons.
Fig. 5.
Preferential localization of the type 2 ryanodine receptor (RyR2) in non-terminal axons of hippocampal mossy fibers. (A–C) Immunofluorescence for the RyR2 in the hippocampus. (D) Triple immunofluorescence for the RyR2 (red), neurofilament-160 (NF160, green), and microtubule-associated protein-2 (MAP2, blue) in the stratum lucidum of the CA3 region. (E) Double immunofluorescence for the RyR2 (red) and type 1 vesicular glutamate transporter (VGluT1, green). Arrows in D and E indicate the intense RyR2 signal in putative axon bundles. (F and G) Silver-enhanced immunogold labeling for the RyR2. Arrows indicate the smooth ER in non-terminal axons. (H) The density of RyR2 immunogold labeling. Ax, axon; MT, mossy fiber terminal; De, dendrite; Sp. spine; En, endothelial cell; Scale bars: A, 200 μm; B and C, 50 μm; D and E, 10 μm; F, 500 nm; G, 100 nm.
This was further tested by the silver-enhanced immunogold technique. Many metal particles were observed in mossy fibers (Fig. 5 F and G). When sectioned longitudinally, heavy deposits were detected in non-terminal portions of mossy fibers whereas there were relatively few metal particles in their huge terminals, forming asymmetrical synapses with multiple spines (Fig. 5F). When sectioned transversely, most particles were accumulated inside axon profiles; they were often distributed around flattened or tubular sacs of the smooth endoplasmic reticulum (sER), and a few appeared in narrow cytoplasmic space between the axolemma and sER (Fig. 5G). This distribution was assessed quantitatively by counting the density of metal particles (Fig. 5H). The number of metal particles per 1 μm2 was highest in non-terminal axons of mossy fibers (65.7). On the other hand, the densities in axon terminals (5.1), dendritic shafts (2.2), and spines (1.8) were >10 times lower than those in non-terminal axons but were higher than those in capillary endothelial cells (0.3). Inside non-terminal axons, most particles were separate from the plasma membrane and judged as intracellular labeling (96.5%), and the rest were found to attach to the plasma membrane (3.5%). Therefore, RyR2 is highly enriched inside the non-terminal portion of mossy fibers and mostly distributed around the smooth ER.
Predominant Postsynaptic Localization of RyR1 in the Hippocampus.
The above results strongly suggested that axonal RyR2 mediated activity dependent presynaptic CICR at the mossy fiber synapse. However, we could not exclude the possibility that the other types of ryanodine receptors were involved, since the granule cells of the dentate gyrus expressed the mRNA for all subtypes (i.e., RyR1–3). To address this possibility, we analyzed cellular distribution of RyR1 using the specific antibody (32). In contrast with the intense labeling of RyR2 within mossy fiber axons, RyR1 immunoreactivity in the hippocampus was generally weaker than in the cerebellum and predominantly expressed in postsynaptic neurons (Fig. S5). These results suggested that RyR1 was less likely to contribute to presynaptic CICR at the hippocampal mossy fiber synapse.
Discussion
In the present study, we examined the functional roles and detailed localization of presynaptic Ca2+ stores at the hippocampal mossy fiber synapse. Using fluorescence recordings of presynaptic Ca2+ transients in mice hippocampal slices, we demonstrate here that the CICR mechanism through ryanodine receptors amplifies accumulation of presynaptic Ca2+ in an activity dependent manner. Immunofluorescence staining reveals that the RyR2 is expressed specifically and intensely within axons of the mossy fibers rather than their terminals. Our findings suggest that axonal CICR mechanism amplifies accumulation of presynaptic Ca2+ in a use dependent manner, and is critically involved in presynaptic forms of plasticity unique to this synapse.
Activity Dependent Amplification of the Presynaptic Ca2+ Signaling by CICR.
To date, several reports suggested the presence and the significance of presynaptic ryanodine receptors at the hippocampal mossy fiber synapse. Pressure application of nicotine to mossy fiber terminals elicited concerted release of multiple quanta, which were large enough to generate action potentials in postsynaptic CA3 neurons (20). Since these responses were blocked by ryanodine, the authors suggested that concerted release of multiple quanta was evoked by nicotine possibly through local Ca2+ release from presynaptic ryanodine-sensitive stores. It was also reported that ryanodine suppressed the induction of presynaptic forms of long-term potentiation (LTP) unique to this synapse (33). Taking together, it was strongly suggested that functional ryanodine-sensitive Ca2+ stores existed in presynaptic sites of the mossy fiber-CA3 synapse. It should be noted that long-term depression (LTD) was induced after prolonged washout of caffeine (Fig. 1B). This caffeine-induced LTD was possibly induced by the increase in presynaptic Ca2+ concentration, since LTD at this synapse was demonstrated to be of presynaptic origin and dependent upon presynaptic Ca2+ (34).
However, previous results of direct presynaptic Ca2+ measurement were controversial. The presynaptic Ca2+ transient elicited by paired-pulse stimulus (50-ms interval) was not significantly affected by application of ryanodine (19). On the other hand, the presynaptic Ca2+ transients elicited by high-frequency stimulation were substantially suppressed by ryanodine (18). Because of low time resolution of the Ca2+ signals in the latter study, kinetic properties of the ryanodine-sensitive components are difficult to interpret. To clarify the reason for the discrepancy, we adopted different stimulus protocols (single shock, train, tetanus) and examined the use-dependency of activation systematically. Our results have unequivocally clarified the reason for the discrepancy and demonstrated that prolonged repetitive stimuli are necessary for the Ca2+ release from presynaptic Ca2+ stores. These findings also give confidence to a previous electrophysiological study showing that the presynaptic Ca2+ store is critically involved in facilitation of presynaptic LTP unique to this synapse (33).
The effects of caffeine on presynaptic Ca2+ signals at mossy fiber synapse were different from those observed in parallel fiber synapses in the cerebellum (19). Enhancement of the presynaptic Ca2+ transients was larger in this study, possibly due to the different sensitivity among the synapses. In addition, they also reported that increase in baseline [Ca]i induced by 40 mM caffeine was little affected by 10 μM ryanodine, whereas the effect of 10 mM caffeine was significantly suppressed by 100 μM ryanodine in this study (Fig. S1). The different concentration of drugs as well as difference in synapses may be the reason for the discrepancy.
It should be noted that α7 nAChR-selective antagonist MLA affected neither the train-induced presynaptic Ca2+ transients nor their suppression by TMB-8. These results were rather unexpected, since several previous studies demonstrated presynaptic effects of nicotine at the hippocampal mossy fiber synapse (20, 30; but see ref. 35). Almost no effect of MLA itself on the train-induced presynaptic Ca2+ transients, therefore, suggested that cholinergic fibers, possibly originating from the septo-hippocampal projection, were not strongly stimulated in our experimental conditions. Alternatively, presynaptic α7 nAChRs are less effectively activated by the train stimulus. In any case, we conclude that the use dependent effect of ryanodine receptor blockers observed in this study was independent of cholinergic modulation.
The ryanodine receptor blocker TMB-8 also suppressed the field EPSPs during tetanus, although the magnitude of suppression was smaller. Two possibilities may explain this finding. First, facilitation of transmitter release during tetanus may reach a nearly saturated level and reduction of presynaptic Ca2+ may no longer decrease transmitter release as much, as expected from the substantial tetanus-induced field EPSPs remaining in the presence of DCG-IV (Fig. 4B). Second, increase in volume-averaged Ca2+ during the repetitive stimulus, which we measured in this study, may not be linearly related to local Ca2+ at the active zone. Distal localization of the axonal ryanodine receptors from the active zone, as discussed below, supported this possibility.
Axonal Localization of the RyR2 at the Mossy Fiber-CA3 Synapse.
Thus far, little information is available for detailed subcellular localization of each subtype of ryanodine receptors. Here, we generated a specific antibody against the RyR2 and explored its cellular and subcellular distribution in detail. Rather unexpected findings of immunohistochemical studies are the intense labeling of the RyR2 at non-terminal portions of the mossy fibers. Immunoelectron microscopy clearly revealed that RyR2 was highly enriched inside the axons and mostly distributed around the smooth ER. Axonal enrichment of the RyR2, particularly in the non-terminal portion, seems to be well suited for activity dependent recruitment of the Ca2+ release, as demonstrated in this study. Since the RyR2 is localized at the relatively distal site from the active zone, but not in the vicinity of the active zone, low-frequency stimulus is hardly able to activate the CICR mechanism, while repetitive stimuli at high frequency, which should be likely to increase the Ca2+ concentration in the axon considerably, readily recruit Ca2+ release from the axonal store. Such strategic localization of presynaptic ryanodine receptors helps to support unique presynaptic forms of plasticity at this synapse. It should be mentioned that immunohistochemical examination revealed that RyR1 was predominantly localized in postsynaptic neurons and therefore is hardly involved in the presynaptic CICR at the mossy fiber synapse.
Functional Implications.
A prominent feature of synaptic transmission at the hippocampal mossy fiber (MF)-CA3 synapse involves robust presynaptic forms of plasticity with remarkably wide dynamic range. LTP at the mossy fiber-CA3 synapse does not require activation of postsynaptic NMDA receptors (36), but definitively needs Ca2+ accumulation within the presynaptic terminals (25). Therefore, activity dependent modification of the presynaptic Ca2+ dynamics is critically important for the induction process of mossy fiber LTP. R-type Ca2+ channels were demonstrated to amplify presynaptic Ca2+ dynamics in a use dependent manner and thereby facilitate LTP induction (37, 38). Highly strategic localization of key molecules for Ca2+ handling on the presynaptic side (i.e., R-type Ca2+ channels and RyR2) is beneficial for supporting presynaptic LTP as well as robust short-term plasticity characteristics of this synapse. In this respect, it should be noted that single granule cells in the dentate gyrus reliably generate action potentials in postsynaptic CA3 neurons, only when trains of action potentials were elicited in granule cells (39). Short-term plasticity at the mossy fiber synapses is important for regulation of information transfer in the hippocampal neuronal networks, and might be effectively regulated by the presynaptic RyR2 within the mossy fiber axons.
In summary, we have found that the CICR mechanism amplifies the presynaptic Ca2+ dynamics at the mossy fiber-CA3 synapse in an activity dependent manner. In addition, we generated a subtype-specific antibody against RyR2, and unequivocally clarified rather unexpected axonal localization of RyR2 at the synapses in which robust presynaptic forms of plasticity take place. It would be interesting to examine how the presynaptic CICR modulates the information transfer in the hippocampal networks in vivo.
Materials and Methods
Presynaptic Ca2+ Measurement and Electrophysiology.
All experiments were performed according to the guidelines for the care and use of laboratory animals of Kobe University and Hokkaido University School of Medicine. Simultaneous recordings of field EPSPs and presynaptic Ca2+ transients in hippocampal slices were carried out as described before (29). Briefly, transverse hippocampal slices were prepared from C57BL/6J mice (14–21 days old). Slices were continuously superfused with a solution composed of the following (in mM): 127 NaCl, 1.5 KCl, 1.2 KH2PO4, 26 NaHCO3, 10 glucose, 4 CaCl2, and 4 MgCl2, saturated with 95% O2 and 5% CO2. Mossy fibers were stimulated at the granule cell layer of the dentate gyrus, and the evoked field EPSPs were recorded in the stratum lucidum of the CA3 region. A membrane-permeable Ca2+ indicator, rhod 2-AM, was loaded into the mossy fiber terminals by injecting it into the axon bundle in the stratum lucidum of the CA3 region. The intensity of fluorescence from the recording site (≈100 μm diameter) was measured with a photodiode 2–3 h after the injection of the dye. The field EPSPs, Ca2+ transients (ΔF), and F were monitored every 5 min (except for the Fig. 1 I and J and Fig. 4 experiments in which a stimulus was given every 2.5 min). In some experiments, whole-cell recordings were made from visually-identified CA3 pyramidal cells. Patch pipettes were filled with an internal solution containing the following (in mM): 150 Cs gluconate, 0.2 EGTA, 8 NaCl, 2 MgATP, 5 QX-314 (lidocaine N-ethyl bromide quaternary salt), and 10 Hepes (pH 7.2). For paired-pulse experiments shown in Fig. 1C, monosynaptic mossy fiber responses were measured at positive potentials (+40 mV) as NMDA receptor-mediated synaptic currents (EPSCNMDA) in the presence of 10 μM CNQX that suppresses non-NMDA receptors and therefore blocks polysynaptic components. Slow mEPSCs mediated by kainate receptors (mEPSCKA), which reflected quantal release exclusively at mossy fiber synapses, were recorded (Fig. 1 E) in the presence of the AMPA receptor-selective blocker GYKI 53655 (30 μM), 1 μM tetrodotoxin, 100 μM picrotoxin, and 25 μM D-APV. The mEPSCKA were analyzed off-line using MiniAnalysis Program (Synaptosoft). The Kolmogorov-Smirnov test was used to assess the effects on the amplitude and interevent interval.
Immunohistochemistry.
Under deep pentobarbital anesthesia, mice were perfused transcardially with either 4% paraformaldehyde in the 0.1 M sodium phosphate buffer (PB, pH 7.2) or 4% paraformaldehyde/0.1% glutaraldehyde in the PB. Microslicer (50 μm) sections of the brain and cryostat sections (50 μm) of the heart and skeletal muscles were used. All immunohistochemical incubations were done at room temperature. For immunofluorescence, microslicer sections were incubated with 10% normal donkey serum for 20 min, a mixture of primary antibodies overnight (1 μg/ml), and a mixture of Alexa 488-, Cy3-, and Cy5-labeled species-specific secondary antibodies for 2 h at a dilution of 1:200 (Molecular probes; Jackson ImmunoResearch). PBS (pH 7.4) containing 0.1% Tween 20 was used as diluent of antibodies and a washing buffer. Images were taken with a light microscope (AX-70, Olympus Optical) equipped with a digital camera (DP70, Olympus Optical ) or with a confocal laser scanning microscope (FV1000, Olympus Optical ).
For immunoelectron microscopy, microslicer sections immunoreacted for the RyR2 were subjected to silver-enhanced immunogold using 1.4-nm gold particles conjugated with an anti-rabbit IgG (Nanogold; Nanoprobes Inc.) and a silver enhancement kit (HQ silver; Nanoprobes Inc.). Sections were further treated with 1% osmium tetroxide and uranyl acetate and embedded in Epon 812. Ultrathin sections (70 nm in thickness) were prepared with an ultramicrotome (Leica), and photographs were taken with an H7100 electron microscope (Hitachi). For additional Materials and Methods, please see SI Text.
Supplementary Material
Acknowledgments.
This work was supported by Grants-in-Aid for Science Research from the Ministry of Education, Science, Sports, Culture and Technology of Japan (M.W., T.M., and H.K.), and by the grants from Ichiro Kanehara Foundation and from NOVARTIS Foundation (to H.K.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0802175105/DCSupplemental.
References
- 1.Katz B, Miledi R. The role of calcium in neuromuscular facilitation. J Physiol (Lond) 1967;195:481–492. doi: 10.1113/jphysiol.1968.sp008469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kamiya H, Zucker RS. Residual Ca2+ and short-term synaptic plasticity. Nature. 1994;371:603–606. doi: 10.1038/371603a0. [DOI] [PubMed] [Google Scholar]
- 3.Zucker RS, Regehr WG. Short-term synaptic plasticity. Ann Rev Physiol. 2002;64:355–405. doi: 10.1146/annurev.physiol.64.092501.114547. [DOI] [PubMed] [Google Scholar]
- 4.Onodera K. Effect of caffeine on the neuromuscular junction of the frog, and its relation to external calcium concentration. Jap J Physiol. 1973;23:587–597. doi: 10.2170/jjphysiol.23.587. [DOI] [PubMed] [Google Scholar]
- 5.Peng YY. Ryanodine-sensitive component of calcium transients evoked by nerve firing at presynaptic nerve terminals. J Neurosci. 1996;16:6703–6712. doi: 10.1523/JNEUROSCI.16-21-06703.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Narita K, et al. Functional coupling of Ca2+ channels to ryanodine receptors at presynaptic terminals - Amplification of exocytosis and plasticity. J Gen Physiol. 2000;115:519–532. doi: 10.1085/jgp.115.4.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Llano I, et al. Presynaptic calcium stores underlie large-amplitude miniature IPSCs and spontaneous calcium transients. Nat Neurosci. 2000;3:1256–1265. doi: 10.1038/81781. [DOI] [PubMed] [Google Scholar]
- 8.Galante M, Marty A. Presynaptic ryanodine-sensitive calcium stores contribute to evoked neurotransmitter release at the basket cell-Purkinje cell synapse. J Neurosci. 2003;23:11229–11234. doi: 10.1523/JNEUROSCI.23-35-11229.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lelli A, et al. Presynaptic calcium stores modulate afferent release in vestibular hair cells. J Neurosci. 2003;23:6894–6903. doi: 10.1523/JNEUROSCI.23-17-06894.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lim R, Oleskevich S, Few AP, Leao RN, Walmsley B. Glycinergic mIPSCs in mouse and rat brainstem auditory nuclei: Modulation by ruthenium red and the role of calcium stores. J Physiol (Lond) 2003;546:691–699. doi: 10.1113/jphysiol.2002.035071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Collin T, Marty A, Llano I. Presynaptic calcium stores and synaptic transmission. Curr Opin Neurobiol. 2005;15:275–281. doi: 10.1016/j.conb.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 12.Liu Q, et al. Presynaptic ryanodine receptors are required for normal quantal size at the Caenorhabditis elegans neuromuscular junction. J Neurosci. 2005;25:6745–6754. doi: 10.1523/JNEUROSCI.1730-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bouchard R, Pattarini R, Geiger JD. Presence and functional significance of presynaptic ryanodine receptors. Prog Neurobiol. 2003;69:391–418. doi: 10.1016/s0301-0082(03)00053-4. [DOI] [PubMed] [Google Scholar]
- 14.Greene RW, Haas HL, Hermann A. Effects of caffeine on hippocampal pyramidal cells-in vitro. Brit J Pharmacol. 1985;85:163–169. doi: 10.1111/j.1476-5381.1985.tb08843.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Emptage NJ, Reid CA, Fine A. Calcium stores in hippocampal synaptic boutons mediate short-term plasticity, store-operated Ca2+ entry, and spontaneous transmitter release. Neuron. 2001;29:197–208. doi: 10.1016/s0896-6273(01)00190-8. [DOI] [PubMed] [Google Scholar]
- 16.Unni VK, Zakharenko SS, Zablow L, DeCostanzo AJ, Siegelbaum SA. Calcium release from presynaptic ryanodine-sensitive stores is required for long-term depression at hippocampal CA3-CA3 pyramidal neuron synapses. J Neurosci. 2004;24:9612–9622. doi: 10.1523/JNEUROSCI.5583-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Padua RA, et al. Autoradiographic analysis of [3H] ryanodine binding sites in rat brain: Regional distribution and the effects of lesions on sites in the hippocampus. J Chem Neuroanat. 1992;5:63–73. doi: 10.1016/0891-0618(92)90034-n. [DOI] [PubMed] [Google Scholar]
- 18.Liang Y, Yuan LL, Johnston D, Gray R. Calcium signaling at single mossy fiber presynaptic terminals in the rat hippocampus. J Neurophysiol. 2002;87:1132–1137. doi: 10.1152/jn.00661.2001. [DOI] [PubMed] [Google Scholar]
- 19.Carter AG, Vogt KE, Foster KA, Regehr WG. Assessing the role of calcium-induced calcium release in short-term presynaptic plasticity at excitatory central synapses. J Neurosci. 2002;22:21–28. doi: 10.1523/JNEUROSCI.22-01-00021.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sharma G, Vijayaraghavan S. Modulation of presynaptic store calcium induces release of glutamate and postsynaptic firing. Neuron. 2003;38:929–939. doi: 10.1016/s0896-6273(03)00322-2. [DOI] [PubMed] [Google Scholar]
- 21.Conti R, Tan YP, Llano I. Action potential-evoked and ryanodine-sensitive spontaneous Ca2+ transients at the presynaptic terminal of a developing CNS inhibitory synapse. J Neurosci. 2004;24:6946–6957. doi: 10.1523/JNEUROSCI.1397-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Giannini G, Conti A, Mammarella S, Scrobogna M, Sorrentino V. The ryanodine receptor calcium-channel genes are widely and differentially expressed in murine brain and peripheral-tissues. J Cell Biol. 1995;128:893–904. doi: 10.1083/jcb.128.5.893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mori F, Fukaya M, Abe H, Wakabayashi K, Watanabe M. Developmental changes in expression of the three ryanodine receptor mRNAs in the mouse brain. Neurosci Lett. 2000;285:57–60. doi: 10.1016/s0304-3940(00)01046-6. [DOI] [PubMed] [Google Scholar]
- 24.Kamiya H, Shinozaki H, Yamamoto C. Activation of metabotropic glutamate receptor type 2/3 suppresses transmission at rat hippocampal mossy fibre synapses. J Physiol (Lond) 1996;493:447–455. doi: 10.1113/jphysiol.1996.sp021395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nicoll RA, Schmitz D. Synaptic plasticity at hippocampal mossy fibre synapses. Nat Rev Neurosci. 2005;6:863–876. doi: 10.1038/nrn1786. [DOI] [PubMed] [Google Scholar]
- 26.Vignes M, Collingridge GL. The synaptic activation of kainate receptors. Nature. 1997;388:179–182. doi: 10.1038/40639. [DOI] [PubMed] [Google Scholar]
- 27.Castillo PE, Malenka RC, Nicoll RA. Kainate receptors mediate a slow postsynaptic current in hippocampal CA3 neurons. Nature. 1997;388:182–186. doi: 10.1038/40645. [DOI] [PubMed] [Google Scholar]
- 28.Cossart R, et al. Quantal release of glutamate generates pure kainate and mixed AMPA/kainate EPSCs in hippocampal neurons. Neuron. 2002;35:147–159. doi: 10.1016/s0896-6273(02)00753-5. [DOI] [PubMed] [Google Scholar]
- 29.Kamiya H, Ozawa S. Dual mechanism for presynaptic modulation by axonal metabotropic glutamate receptor at the mouse mossy fibre-CA3 synapse. J Physiol (Lond) 1999;518:497–506. doi: 10.1111/j.1469-7793.1999.0497p.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gray R, Rajan AS, Radcliffe KA, Yakehiro M, Dani JA. Hippocampal synaptic transmission enhanced by low concentrations of nicotine. Nature. 1996;383:713–716. doi: 10.1038/383713a0. [DOI] [PubMed] [Google Scholar]
- 31.Kása P. The cholinergic systems in brain and spinal cord. Prog Neurobiol. 1986;26:211–272. doi: 10.1016/0301-0082(86)90016-x. [DOI] [PubMed] [Google Scholar]
- 32.Kakizawa S, et al. Junctophilin-mediated channel crosstalk essential for cerebellar synaptic plasticity. EMBO J. 2007;26:1924–1933. doi: 10.1038/sj.emboj.7601639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lauri SE, et al. A role for Ca2+ stores in kainate receptor-dependent synaptic facilitation and LTP at mossy fiber synapses in the hippocampus. Neuron. 2003;39:327–341. doi: 10.1016/s0896-6273(03)00369-6. [DOI] [PubMed] [Google Scholar]
- 34.Kobayashi K, Manabe T, Takahashi T. Calcium-dependent mechanisms involved in presynaptic long-term depression at the hippocampal mossy fibre-CA3 synapse. Eur J Neurosci. 1999;11:1633–1638. doi: 10.1046/j.1460-9568.1999.00578.x. [DOI] [PubMed] [Google Scholar]
- 35.Vogt KE, Regehr WG. Cholinergic modulation of excitatory synaptic transmission in the CA3 area of the hippocampus. J Neurosci. 2001;21:75–83. doi: 10.1523/JNEUROSCI.21-01-00075.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zalutsky RA, Nicoll RA. Comparison of two forms of long-term potentiation in single hippocampal neurons. Science. 1990;248:1619–1624. doi: 10.1126/science.2114039. [DOI] [PubMed] [Google Scholar]
- 37.Dietrich D, et al. Functional specialization of presynaptic Cav2.3 Ca2+ channels. Neuron. 2003;39:483–496. doi: 10.1016/s0896-6273(03)00430-6. [DOI] [PubMed] [Google Scholar]
- 38.Breustedt J, Vogt KE, Miller RJ, Nicoll RA, Schmitz D. α1E-Containing Ca2+ channels are involved in synaptic plasticity. Proc Natl Acad Sci USA. 2003;100:12450–12455. doi: 10.1073/pnas.2035117100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Henze DA, Wittner L, Buzsaki G. Single granule cells reliably discharge targets in the hippocampal CA3 network in vivo. Nat Neurosci. 2002;5:790–795. doi: 10.1038/nn887. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.