Abstract
Embryonic stem (ES) cells differentiate into neuroectodermal progenitors when cultured as floating aggregates in serum-free conditions. Here, we show that strict removal of exogenous patterning factors during early differentiation steps induces efficient generation of rostral hypothalamic-like progenitors (Rax+/Six3+/Vax1+) in mouse ES cell-derived neuroectodermal cells. The use of growth factor-free chemically defined medium is critical and even the presence of exogenous insulin, which is commonly used in cell culture, strongly inhibits the differentiation via the Akt-dependent pathway. The ES cell-derived Rax+ progenitors generate Otp+/Brn2+ neuronal precursors (characteristic of rostral–dorsal hypothalamic neurons) and subsequently magnocellular vasopressinergic neurons that efficiently release the hormone upon stimulation. Differentiation markers of rostral–ventral hypothalamic precursors and neurons are induced from ES cell-derived Rax+ progenitors by treatment with Shh. Thus, in the absence of exogenous growth factors in medium, the ES cell-derived neuroectodermal cells spontaneously differentiate into rostral (particularly rostral–dorsal) hypothalamic-like progenitors, which generate characteristic hypothalamic neuroendocrine neurons in a stepwise fashion, as observed in vivo. These findings indicate that, instead of the addition of inductive signals, minimization of exogenous patterning signaling plays a key role in rostral hypothalamic specification of neural progenitors derived from pluripotent cells.
Keywords: chemically defined, hypothalamus, patterning, vasopressin
Differentiation culture of mouse ES (mES) cells is a versatile in vitro tool for understanding molecular and cellular controls in early mammalian neurogenesis (1–5). We previously established a mES cell culture system with reduced exogenous signals, namely, serum-free culture of embryoid body-like aggregates (SFEB culture) (4). In this method, ES cells are dissociated (to minimize possible effects of culture substrate matrix), reaggregated (over one day), and cultured as floating aggregates in serum-free medium containing knockout serum replacement (KSR) (6), but with no major exogenous inductive factors, such as Fgf, BMP, Wnt, or Nodal. SFEB-cultured mES cells spontaneously differentiate into neural progenitors that acquire a rostral forebrain fate and efficiently generate telencephalic progenitors positive for Bf1 (FoxG1; (7); see Fig. 1A). Efficient forebrain differentiation is also seen in SFEB-cultured human ES cells (8).
Fig. 1.
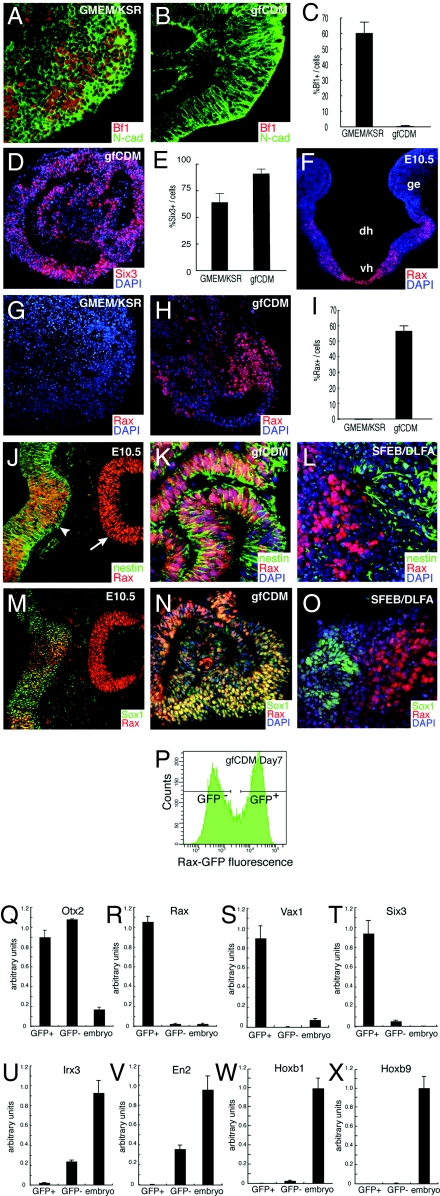
SFEBq culture in growth factor-free medium generates rostral hypothalamic progenitors from ES cells. (A, C, E, G, and I) SFEBq-cultured ES cells using KSR-containing medium, (B, C–E, H, I, K, and N) SFEBq-cultured ES cells using gfCDM. Immunostaining analysis of cryostat sections. (F) Coronal section of mouse rostral diencephalon at E10.5. (J and M) Expression in the rostral–dorsal hypothalamic neuroepithelium (arrowhead) and optic cup (arrow) at E10.5. (L and O) SFEB/DLFA-cultured ES cells for retinal differentiation. The sections were stained with antibodies against Bf1 (A and B), Six3 (D), Rax (G, H, J–O), N-cadherin (A and B), Nestin (J–L), Sox1 (M–O). DAPI for counter staining. (P–X) SFEBq-cultured Rax–EGFP cells are sorted by FACS (P) and analyzed by qPCR on day 7 for the expression of Otx2 (Q), Rax (R), Vax1 (S), Six3 (T), Irx3 (U), En2 (V), Hoxb1 (W), and Hoxb9 (X). Total RNA from E10.5 whole embryos was used as a control.
In contrast to telencephalic development, relatively little has been known about regulatory signals for early diencephalic development, including the initial specification of the mammalian hypothalamic anlage (9, 10). In the present study, using a similar SFEB culture approach, we wished to steer ES cell differentiation into hypothalamic tissues, which arises from the rostral forebrain region adjacent to the embryonic telencephalon in vivo. However, we noticed at the beginning of this study that hypothalamic markers were rarely induced in neural cells generated from mouse ES cells under the original SFEB conditions. We then paid attention to the fact that the serum-free medium used in the original SFEB culture still included some exogenous signals that might affect the differentiation pathway followed. In particular, KSR, widely used for the maintenance and differentiation of mouse and human ES cells (2, 4, 6, 8, 11), contains bioactive (growth) factors such as a high concentration of insulin (6.7 μg/ml in 10% KSR) and lipid-rich albumin partially purified from bovine serum (6) (PCT patent no. WO 98/30679).
In this report, we show that ES cell-derived neuroectodermal cells robustly differentiate into cells expressing regional markers of the embryonic rostral hypothalamus when cultured in a strictly chemically defined medium (CDM; growth factor-free chemically defined medium is gfCDM hereafter), free of KSR and other growth factors including insulin.
Results
Spontaneous Differentiation of mES Cell Aggregates into Rostral Hypothalamic Progenitor-Like Cells in Growth Factor-Free Suspension Culture.
Dissociated mES cells quickly reaggregated in a low cell-adhesion culture well (3,000 cells per well; U-bottomed 96-well plate) and were cultured as floating aggregates in gfCDM. In this quick reaggregation procedure (SFEBq, hereafter), the ES cells grew as healthy aggregates even in this CDM free of exogenous growth factors. They reproducibly underwent selective neural differentiation (Fig. 1B; N-cadherin (cadherin2)+ cells >90% of total cells) and developed well formed neuroepithelia. Interestingly, while SFEBq-cultured ES cells in KSR-containing medium efficiently differentiated into Bf1+ telencephalic cells (day 10; Fig. 1 A and C), ES cells cultured in gfCDM rarely expressed Bf1 (<1%; Fig. 1 B and C). The majority of the SFEBq/gfCDM-cultured neuroepithelial cells expressed Six3 (day 5; Fig. 1 D and E), indicating that their regional identity was within the rostral forebrain (12).
The Bf1− (nontelencephalic) portions of the rostral forebrain constitute the rostral diencephalon, which includes two major structures: the hypothalamus and the neural retina [see supporting information (SI) Fig. S1 A–C]. Rax is specifically expressed in the rostral hypothalamic neuroepithelia (anterior–dorsal and tuberal subregions, which constitute the neuroendocrine/homeostasis center) and retinal neuroepithelia (13) (Fig. 1F and Fig. S1C), but not in the caudal hypothalamus. Immunostaining showed that a large proportion of the SFEBq/gfCDM-cultured ES cells (55–70%; but not those cultured in KSR-containing medium) expressed Rax on day 7 (Fig. 1 G–I).
Rax+ tissues in the embryonic neural retina differ from those in the rostral hypothalamus in their coexpression of early neural markers (Fig. 1 J–O). The Rax+ embryonic neural retina expresses neither Nestin (Fig. 1J, arrow) nor Sox1 (Fig. 1M) (14) while the Rax+ hypothalamic neuroepithelium is clearly positive for both markers (Fig. 1J, arrowhead and Fig. 1M). SFEBq/gfCDM-induced Rax+ tissues strongly and uniformly coexpressed Nestin (Fig. 1K) and Sox1 (Fig. 1N). This finding is in contrast to our previous observation (14) that Rax+ retinal tissues generated from ES cells by the SFEB/DLFA method (SFEB culture combined with Dkk1, LeftyA, fetal calf serum, and activin treatment) were Nestin− and Sox1− (Fig. 1 L and O). Moreover, no substantial expression of the early retinal progenitor marker Chx10 [Vsx2; (15)] (Fig. S2A) was not detected in SFEBq/gfCDM-treated cells (Fig. S2B). These findings indicate that SFEBq/gfCDM cells have a marker expression profile typical for rostral hypothalamic progenitors.
We next produced ES cell lines with GFP cDNA knocked in at the Rax locus (Fig. S2 C and D for the targeting vector and E–G for GFP and Rax coexpression). Rax–GFP+ and Rax–GFP− cells were sorted by FACS on day 7 (Fig. 1P) and subjected to quantitative PCR analysis (qPCR) (Fig. 1 Q–X). Both Rax–GFP+ and Rax–GFP− cell populations expressed a high level of the fore-midbrain marker Otx2 (compare to the control E10.5 whole embryo) and not the caudal CNS markers Hoxa2, Hoxa3, Hoxb1, and Hoxb9 (Fig. 1 W and X and Fig. S2 H and I). However, the Rax–GFP+ and Rax–GFP− populations substantially differed in the expression of other regional marker genes. Rax–GFP+ cells (but not Rax–GFP− cells) expressed a high level of Rax (Fig. 1R), Vax1 (Fig. 1S; a rostral–ventral forebrain marker) (16) and Six3 (Fig. 1T; Six3 expression in vivo is initially found throughout the rostral forebrain, whereas it later becomes limited to parts of the rostral forebrain and largely overlaps with Rax expression in the hypothalamus; (17); in immunostaining of SFEBq aggregates, Six3 expression on day 7, unlike that on day 5, was limited to ≈60% of SFEBq/gfCDM cells and mostly colocalized with Rax expression; Fig. S2 J–L). In contrast, more caudal marker genes such as Irx3 (caudal diencephalon and brain tissues caudal to it) and En2 (typically midbrain) were expressed at a moderate level in Rax–GFP− cells but not substantially in Rax–GFP+ cells (Fig. 1 U and V).
Collectively, these findings indicate that Rax+ cells in SFEBq/gfCDM culture have a regional identity of the rostral forebrain, in particular, the rostral hypothalamus.
Insulin Inhibits Rostral Hypothalamic Differentiation via the Akt Pathway.
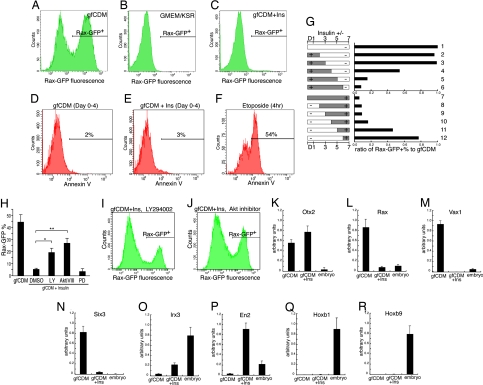
In contrast to a high Rax+ percentage of SFEBq/gfCDM-cultured ES cells (56%; Fig. 2A), few Rax–GFP+ cells (<3%) were present among ES cells cultured in medium containing KSR (Fig. 2B) or insulin, which KSR contains (Fig. 2C; also see Fig. S3A for dose-response analysis of insulin and IGF1 treatments). The presence or absence of insulin in culture medium (from day 0) did not substantially influence the percentage of Annexin V+ apoptotic cells (2–3% of total cells on day 4 in both cases; Fig. 2 D and E), suggesting that insulin impairs differentiation and does not act by actively eliminating a major population via selective apoptosis. The presence of insulin during the first 3 days had little inhibitory effect on the Rax–GFP+ percentage (Fig. 2G, upper graph, rows 2 and 3), whereas the Rax–GFP+ percentage decreased substantially when insulin was present in the CDM until day 4 or later (rows 4–6). Conversely, the addition of insulin to gfCDM on day 5 or earlier suppressed Rax–GFP expression (Fig. 2G, lower graph, rows 7–11), suggesting that the absence of high insulin signals during days 4 and 5 is a key condition for efficient Rax expression in SFEBq-cultured ES cells.
Fig. 2.
Insulin suppresses Rax expression on SFEBq culture. (A–C) FACS analysis of Rax–GFP knocked-in ES cells that were cultured by SFEBq for 7 days in gfCDM (A), GMEM/KSR (B), and gfCDM + 7 μg/ml insulin C. (D–F) Annexin V+ apoptotic cells were analyzed (day 4) with (E) or without (D) insulin. For positive control, cells were preincubated with 10 μM etoposide for 4 h (F). (G) Time-window analysis of insulin's effect on Rax–GFP+ percentages by FACS on day 7. Insulin (7 μg/ml) was removed (upper graph) or added (lower graph) as shown in the left bars. The ratio of the Rax–GFP+ percentages in the presence of insulin to that of SFEBq/gfCDM culture (row 1; referred to as 1.0) is shown in the graphs at the right. (H–J) Effects of inhibitors on SFEBq-cultured ES cells in gfCDM with or without insulin (7 μg/ml) were analyzed by FACS on day 7. LY294002 (H and I), Akt inhibitor VIII (H and J), PD98059 (10 μM), or DMSO (vehicle) was added on day 2. Statistical significance vs. the control (gfCDM + insulin with DMSO) was evaluated by the Dunnette test. *, P < 0.05; **, P < 0.01. (K–R) qPCR analysis of insulin's effect on day 7 was performed as in Fig. 1 P–X.
We next examined the effects of inhibitors of the insulin-downstream pathways on the differentiation of ES cells cultured in the insulin-added CDM. The treatment with the MAPK inhibitor PD48059 (0.5–10 μM; days 2–7) did not increase the Rax–GFP+ percentage in the differentiating ES cells on day 7 (Fig. 2H, PD and data not shown). In contrast, the treatment with either the PI3K inhibitor LY294002 (5 μM; LY) or the Akt inhibitor (inhibitor VIII; 2 μM; AktiVIII) significantly reversed the insulin-induced suppression of the Rax–GFP+ percentage, suggesting that the inhibitory effect of insulin signaling on rostral hypothalamic differentiation depends on the PI3K/Akt pathway (Fig. 2 H–J).
In qPCR analysis of regional marker genes (Fig. 2 K–R), insulin suppressed the expression of Rax, Vax1, and Six3 (Fig. 2 L–N, middle columns), while Otx2 expression was largely unaffected (Fig. 2K). The expression levels of Irx3 and En2 (but not Hoxa2, Hoxa3, Hoxb1, or Hoxb9) were increased by insulin (Fig. 2 O–R and Fig. S3 B and C). These findings show that insulin has suppressing effects on the expression of the rostralmost CNS markers and its treatment moderately induces caudal marker expression.
Differential Induction of Dorsal and Ventral Marker Expression in SFEBq/gfCDM-Induced Rax+ Hypothalamic Progenitors.
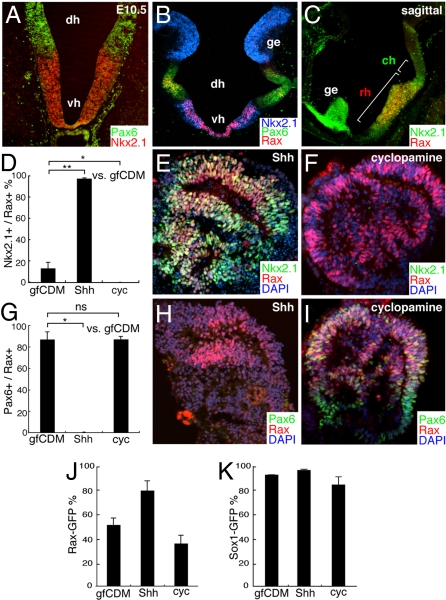
Shh is expressed specifically in the ventral subregion of the embryonic hypothalamus ((9, 10); Fig. S1E). Within the rostral hypothalamus, Rax expression is found in both the dorsal domain (Pax6+, Nkx2.1−; Fig. 3A and B) and the ventral domain (Pax6−, Nkx2.1+; Fig. 3 A–C). The Nkx2.1+ population (ventral) in ES cell-derived Rax+ cells was substantially increased by Shh treatment (days 4–7; Fig. 3 D and E; these cells were Bf1−/Pax6−, data not shown) and decreased by cyclopamine treatment (days 4–7; Fig. 3 D and F). The Nkx2.1+ population was also increased by treating with the Shh agonist purmorphamine [Fig. S4A; (18)]. Conversely, the Pax6+ population (dorsal) in Rax+ cells was decreased by Shh (Fig. 3 G–I). These findings indicate that Shh signaling promotes the specification of rostral–ventral hypothalamic progenitors at the cost of rostral–dorsal progenitors in vitro.
Fig. 3.
ES cell-derived Rax+ progenitors differentiate into early neuronal precursors of the dorsal and ventral hypothalamus. (A–C) Immunohistochemical analysis of the E10.5 mouse brain with Nkx2.1 (A–C), Pax6 (A and B) and Rax (B and C) antibodies. Coronal (A and B) and parasagittal (C). dh, vh, rh, and ch, dorsal, ventral, rostral, and caudal portions of the hypothalamus; ge, ganglionic eminence. The caudal hypothalamus (its ventral portion) is Nkx2.1+ and Rax−. (D–I) Subregional marker expression in SFEBq/gfCDM-cultured ES cells on day 7. Frozen sections of ES cell aggregates treated with 30 nM Shh (D, E, G, and H) or 10 μM cyclopamine (D, F, G, and I) were immunostained for Rax and Nkx2.1 (D and F) or Pax6 (G–I). (D and G) Statistical significance vs. the control (gfCDM) was evaluated by the Dunnette test. *, P < 0.05; **, P < 0.01; ns, not significant. (J and K) Effects of Shh (30 nM) and cyclopamine (10 μM) on the percentages of Rax–GFP+ (J) and Sox1–GFP+ (K) cells in SFEBq/gfCDM culture.
Interestingly, treatment with Shh from day 4 increased Rax–GFP+ percentage (81% of the total cells; Fig. 3J) while treatment with the hedgehog inhibitor cyclopamine (days 4–7) only slightly reduced it (Fig. 3J; the efficiency of general neural differentiation is shown in Fig. 3K). Given that Rax+ cells comprise higher percentages in the ventral domain than in the dorsal domain (Fig. 3B), it is possible that the Shh-induced increase in the Rax+ percentage (up to 81%; Fig. 3J) was caused, at least in part, by the generation of the high Rax+ ventral hypothalamic tissues.
Collectively, these observations indicate that mES cells cultured under exogenous growth factor-free suspension conditions preferentially generate neural progenitors with rostral hypothalamic characteristics, which can be secondarily patterned dorsally or ventrally.
FACS-Purified Rax+ Progenitors Derived from ES Cells Differentiate into Vasopressinergic Neurons.
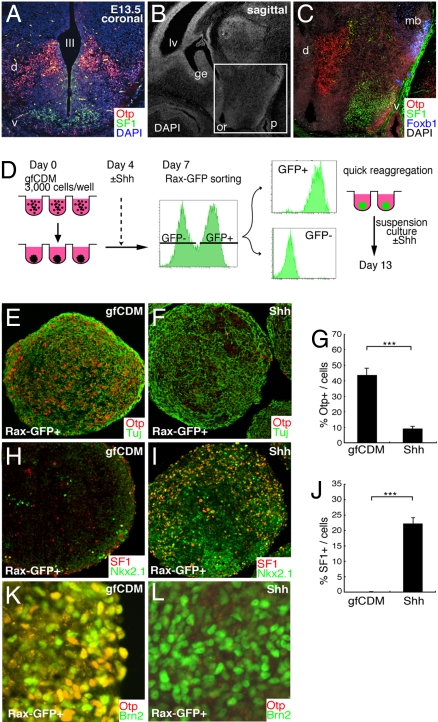
The rostral–dorsal domain of the hypothalamus contains a number of early neuronal precursors positive for Otp (red cells in Fig. 4A; coronal section at E13.5; also see Fig. S5 A–C) (19). The ventromedial nucleus (VMH; satiety center of appetite) is a typical nucleus present in the ventral part of the rostral hypothalamus (tuberal subdomain; Fig. S5D). SF1 (Nr5a1) is a bona-fide marker that specifically demarcates the postmitotic VMH precursors (Fig. 4 A–C, green) (20). Foxb1 serves as a specific marker for the mammillary body neurons (MB) (21) in the caudal hypothalamus (blue cells in Fig. 4C; parasagittal section; also see Fig. S1A).
Fig. 4.
ES cell-derived Rx+ progenitors differentiate into early precursor neurons of the dorsal and ventral hypothalamus. (A–C) E13.5 mouse hypothalamus. Coronal (A) and parasagittal (B and C) sections were stained with DAPI and antibodies against Otp (A and C; dorsal domain, d), SF1 (A and C; ventral domain, v), and Foxb1 (C; mb). III, third ventricle; lv, lateral ventricle; or, optic recess; and p, pituitary. (C) Immunostaining within the square (hypothalamic region) in panel B. (D) Schematic of reaggregation culture of FACS-sorted Rax–GFP+ progenitors. (E–J) The aggregates were cultured in DMEM/F12/KSR medium with (F, G, I, J, and L) or without (E, G, H, J, and K) 30 nM Shh (days 7–13). Frozen sections were immunostained for Otp (E–G, K, and L), SF1 (H, I, and J), Tuj (E and F), Nkx2.1 (H and I), and Brn2 (K and L). (G and J) Statistical significance was examined by Mann–Whitney's test. ***, P < 0.001.
Rax+ cells were isolated from SFEBq/gfCDM-cultured ES cells (with or without Shh treatment) by FACS on day 7, quickly reaggregated again in low cell-adhesion wells (5000 cells per well), and cultured until day 13 (Fig. 4D). When Shh was not added, numerous Otp+ neurons were found in the Rax–GFP+ cell aggregates (44% of total cells; Fig. 4 E and G) but not in aggregates of Rax− cells (<1%; data not shown). Shh treatment significantly reduced the number of Otp+ neurons in the Rax–GFP+ aggregates (Fig. 4 F and G), while the number of SF1+ neurons greatly increased (22% of cells in the Rax–GFP+ aggregates; Fig. 4 H–J). Consistent with the expression profile in the embryonic hypothalamus (19), a substantial percentage of Otp+ cells derived from untreated Rax–GFP+ cells coexpressed Brn2 (Pou3f2; 46%; Fig. 4K; Fig. 4L is the immunostaining of Shh-treated Rax–GFP+ aggregates). Regardless of Shh treatment, Rax–GFP+ cells differentiated into neither Foxb1+ MB neurons nor Crx+ photoreceptor progenitors (for both, n = 16 aggregates, >2000 cells each; Fig. S5 E and F and data not shown).
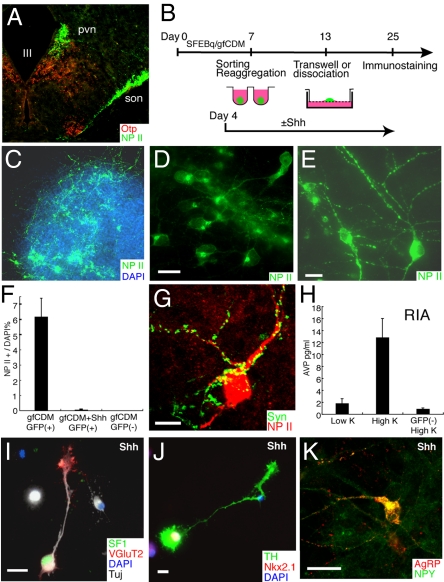
Arginine-vasopressin (AVP)-producing neurons [immunostained with the AVP precursor protein neurophysin II; (22)] in the paraventricular and supraoptic nuclei (PVN and SON; Fig. 5A) are derived from Otp+/Brn2+ precursors [the SON neurons are known to originate and migrate radially from the Otp+ subventricular zone; Fig. S5 B–D; (19, 23)]. To examine the differentiation of mature neurons, Rax–GFP+ progenitors were isolated by FACS and reaggregated on day 7, followed by suspension culture (without Shh treatement) for 6 days and Transwell culture on porous filters for an additional 12 days (Fig. 5B). Consistent with efficient Otp/Brn2 induction (Fig. 4 E and K), a number of AVP+ neurons were found in all of the aggregates (n = 212; Fig. 5 C–E), and represented 6.0% of the total population of cells on day 20 (n = 2000 cells; Fig. 5F). In contrast, few AVP+ neurons were produced in the Shh-treated aggregates, data not shown). Most of the AVP+ neurons had large round- or oval-shaped somata (20–30 μm in the long axis) and showed a massive accumulation of neurophysin II in the perinuclear (Golgi) area (Fig. 5D) and in the characteristic varicosities (Herring's bodies) of the neurites (Fig. 5E), as seen in the AVP+ magnocellular neurons in the rodent hypothalamus (23). AVP+ axonal swellings were also frequently in dissociation culture (from day 13; Fig. 5B, Fig. S5 G–J), while AVP+ dendrites received numerous presynaptic inputs (Fig. 5G and Fig. S5 K–M). A RIA (RIA; Fig. 5H) showed that a substantial amount of AVP [13 pg/ml from 10 cell aggregates incubated in 0.5 ml solution; 3–4 times higher concentration than the AVP level in peripheral blood; (24)] was released into the conditioned medium of Rax–GFP+ progenitor-derived aggregates, but not of Rax–GFP− control aggregates, upon high K+ stimulation for 10 min.
Fig. 5.
Generation of characteristic dorsal and ventral hypothalamic neurons. (A) Otp and neurophysin II (NP II) expression in the mouse hypothalamus at E15.5. At this stage, although Otp expression is found predominantly in the dorsal domain, a small number of neurons in ventrally located nuclei such as the lateroanterior hypothalamic nucleus and arcuate nucleus (data not shown) also express Otp. (B–K) Long-term neuronal culture following FACS sorting (day 7) of Rax–GFP+ progenitors from SFEBq/gfCDM-cultured ES cells. (B) Schematic of the culture procedure for rostral hypothalamic differentiation. (C–H) Immunostaining of a Transwell-cultured aggregate cultured without Shh. A number of NP II+ neurons were seen (C and F). High magnification views of NP II+ neurons showed predominantly large somas with a well developed NP II+ Golgi area (D) and characteristic varicosities in their neurites (E). (G) Immunostaining for NP II and synaptophysin in dissociation culture of Rax–GFP+ cells on day 35. (H) AVP-release analysis upon high K+ stimulation on day 25. AVP concentrations in conditioned media of Rax–GFP+ aggregates were measured by RIA. (I–K) Immunostaining of dissociation culture of Rax–GFP+ cells cultured with Shh (day 25). (I) SF1 (green), VGluT2 (located mainly in the growth cones; red), and TuJ1 (white). (J) Immunostaining for TH and Nkx2.1. (K) Immunostaining for AgRP and NPY. (Scale bars, 20 μm.)
In the presence of exogenous Shh (from day 4; Fig. 5B), the FACS-purified Rax–GFP+ progenitors efficiently differentiate into SF1+/VGLUT2 (Slc17a6)+ neurons (Fig. 4I and 5I and Fig. S5N; 13% of the total cells on day 25 of dissociation culture), a characteristic type of neurons that, within the embryonic brain, are specifically found in the ventral hypothalamus (VMH). In addition to these neurons, Shh-treated Rax–GFP+ progenitors (but not untreated ones) differentiate into other types of neurons found in the rostral–ventral hypothalamus, including TH+/Nkx2.1+ dopaminergic neurons [Fig. 5J and Fig. S5O; 14% of the Nkx2.1+ neurons, which made up 61% of the total cells; A12 type (25)] and AgRP+/NPY+ neurons [Fig. 5K; 1.0% of the total cells; typically found in the arcuate nucleus (26)].
Discussion
The rostral hypothalamus is a very complex structure (10, 27) containing a large diversity of neurons, whose developmental mechanisms are still poorly understood. SFEBq/gfCDM cultures could also be useful in the search for inductive signals for other intriguing hypothalamic neurons such as oxytocin neurons (some additional signals may be necessary as we have so far failed to observe strong oxytocin accumulation on days 25 and 29 under the present conditions; data not shown), orexin-expressing neurons, and suprachiasmatic nucleus neurons, which are involved in important physiological regulations such as feeding and circadian rhythm.
The present study has identified a key condition necessary for differentiation of rostral hypothalamic progenitors from ES cells, i.e., no exogenous patterning signals added at the naïve neuroectodermal stage. Whether endogenous extracellular signals [for instance (28)] have any active (rather than permissive) roles in the tissue-autonomous hypothalamic differentiation of ES cells remains to be understood.
An intriguing, yet challenging question then is whether the fate specification mechanism found in this study also works in the rostral hypothalamic specification within the embryonic neuroectoderm in vivo. The hypothalamic anlage is located adjacent to signaling tissues such as the anterior visceral endoderm and prechordal plate, which secrete anti-caudalizing factors such as Dkk1 and Cerberus-like (29, 30). In SFEBq/gfCDM culture, the addition of factors with caudalizing activity [Wnt3a, Nodal, Fgf8b, or RA; (31)] decreased the Rax–GFP+ percentage, while the treatment with the Wnt blocker Dkk1 further induces the level of rostral hypothalamic differentiation (Fig. S3 D, F, and G). These findings suggest that caudal positional information prevents the ES cell-derived neuroectodermal cells from acquiring a rostral hypothalamic identity, consistent with the rostralmost position of the rostral hypothalamus in the embryonic brain. In contrast to a previous report (32), we have so far failed to see a positive effect of BMP7 on hypothalamic differentiation in our system.
An unexpected finding regarding SFEBq culture conditions is that insulin, commonly used in various serum-free media, has a substantial inhibitory effect on hypothalamic marker expression via the PI3K/Akt pathway (Fig. 2 A–J and Fig. S4 B–E). A recent report has also indicated an inhibitory effect of Akt-mediated insulin signaling on ES cell differentiation into cardiac muscles (33). The qPCR analysis (Fig. 2 K–R) showed that insulin suppresses the rostralmost brain markers and induces (albeit moderately) the more caudal markers, suggesting that this weak caudalizing activity may be, in part, involved in this phenomenon. This idea is consistent with the report that insulin can activate β-catenin via the PI3K/Akt pathway as does the caudalizaing factor Wnt (34). The addition of insulin alone did not induce Bf1 expression in SFEBq/gfCDM culture (Fig. S3H) in contrast to the addition of KSR (Fig. S3I). The time-course analysis (Fig. 2G) showed that the presence or absence of exogenous insulin during days 4 and 5 had the strongest effects on the efficiency of hypothalamic differentiation. Our previous study has shown that differentiation of mES cells into Sox1+ neuroectodermal progenitors occurs primarily by day 4 [around day 3 in particular in SFEB culture (4); also in SFEBq culture], suggesting that insulin signals are likely affecting newly committed neuroectodermal cells rather than uncommitted pluripotent cells (illustrated in Fig. S6).
An intriguing question for future investigation is whether the differentiation of hypothalamic progenitors from human ES cells is regulated by similar mechanisms. One technical obstacle is the poor survival of human ES cell culture in SFEBq/gfCDM culture, unlike mouse ES cells. Although the addition of insulin to human ES cells [in the presence of ROCK inhibitor; (8)] improves their survival and growth (our unpublished observations), the dependence on and sensitivity to insulin need to be more carefully compared between human and mouse ES cells. In our preliminary analysis, the Akt inhibitor promoted the induction of Rax and Vax1 expression in human SFEBq aggregates cultured in gfCDM + insulin (Fig. S4 F and G), suggesting a certain common function of Akt.
Finally, the rostral hypothalamus is presumably assigned as the rostralmost region of the neural plate, although the exact assignment of the rostral–caudal axis in this area is still under some debate (27, 35, 36). One hypothesis that emerges from the present study and should be examined in the future is that the rostral–ventral forebrain (rostral hypothalamic anlage) represents the origin (or zero point) of the Cartesian coordinates for patterning of the naïve neuroectoderm. This possibility may be particularly intriguing from a phylogenic point of view, because the hypothalamus (particularly its rostral neuroendocrine portion) is a homeostasis center that is highly conserved during brain evolution, even across vertebrates and invertebrates (polychaetes), as has been shown in a recent study (37).
Materials and Methods
ES Cell Culture.
Mouse ES cells (EB5, TT2), Sox1-GFP ES cells (46C), and Rax–GFP cells (116–2, 116–18, 20–10, 20–14) were maintained as described in ref. 4. For SFEBq culture, ES cells were dissociated to single cells in 0.25% trypsin-EDTA and quickly reaggregated in differentiation medium (3000 cells per 150 μl per well) using 96-well low cell-adhesion plates (Lipidure Coat, NOF). Unless stated otherwise, the differentiation medium used during days 0–7 was growth factor-free CDM [modified from (38)], which contains Iscove's modified Dulbecco's medium/Ham's F-12 1:1, 1× chemically defined lipid concentrate, penicillin/streptomycin, monothioglycerol (450 μM) and purified BSA (>99% purified by crystallization; Sigma). The addition of human apo-transferrin (15 μg/ml final) to this medium caused no substantial changes in the differentiation of Rax+ hypothalamic progenitors. Generation of knockin ES cells is described in SI Methods. Primers used are listed in Table S1.
Neuronal Differentiation Culture.
Rax–GFP+ and Rax–GFP− cells were sorted on day 7 from SFEBq/gfCDM-cultured Rax–GFP ES cells, quickly reaggregated using low cell-adhesion 96-well culture plates (5000 cells per well for GFP+ cells and 2500 cells per well for GFP− cells), and cultured in DFK medium (DMEM/F12 supplemented with 7 g/liter glucose, 10% KSR, and penicillin/streptomycin). On day 10, half the medium was replaced with DFNB medium (DMEM/F12 supplemented with 7 g/liter glucose, N2 and B27) + 10 ng/ml CNTF. For long-term culture, these aggregates were subjected to filter culture using a Transwell culture insert (Corning) in DFNB + 10 ng/ml CNTF. A one-half volume of the medium was changed every other day. Technical details of dissociation culture are described in SI Methods.
Vasopressin Release Analysis.
Aggregates cultured on Transwell filters in DFNB medium + CNTF for 25 days were subjected to analysis. Ten aggregates per filter were incubated with 500 μl of artificial cerebrospinal fluid (aCSF; 124 mM NaCl, 3 mM KCl, 26 mM NaHCO3, 2 mM CaCl2, 1 mM MgSO4, 1.25 mM KH2PO4, and 10 mM d-glucose, pH 7.4) for 10 min at 37°C, followed by stimulation with high K+ aCSF (100 mM KCl) for an additional 10 min. Each incubated solution was individually frozen and its AVP content was analyzed by RIA using the double-antibody technique.
Supplementary Material
Acknowledgments.
We are grateful to E. De Robertis and D. Arendt for discussion on body plan evolution, to R. Ladher and C. Hanashima for invaluable comments, to A. Smith and K. Yamamura for the Sox1-GFP ES cell line and the Cre plasmid, to M. Ikeya for advice on gene targeting, and to D. Sipp and members of the Sasai lab for helpful discussion. This work was supported by grants-in-aid from the Ministry of Education, Culture, Sports, Science, and Technology, the Kobe Cluster Project, and the Leading Project (Y.S.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0803078105/DCSupplemental.
References
- 1.Mizuseki K, et al. Generation of neural crest-derived peripheral neurons and floor plate cells from mouse and primate embryonic stem cells. Proc Natl Acad Sci USA. 2003;100:5828–5833. doi: 10.1073/pnas.1037282100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tropepe V, et al. Direct neural fate specification from embryonic stem cells: A primitive mammalian neural stem cell stage acquired through a default mechanism. Neuron. 2001;30:65–78. doi: 10.1016/s0896-6273(01)00263-x. [DOI] [PubMed] [Google Scholar]
- 3.Wichterle H, Lieberam I, Porter JA, Jessell TM. Directed differentiation of embryonic stem cells into motor neurons. Cell. 2002;110:385–397. doi: 10.1016/s0092-8674(02)00835-8. [DOI] [PubMed] [Google Scholar]
- 4.Watanabe K, et al. Directed differentiation of telencephalic precursors from embryonic stem cells. Nat Neurosci. 2005;8:288–296. doi: 10.1038/nn1402. [DOI] [PubMed] [Google Scholar]
- 5.Ying QL, et al. Conversion of embryonic stem cells into neuroectodermal precursors in adherent monoculture. Nat Biotechnol. 2003;21:183–186. doi: 10.1038/nbt780. [DOI] [PubMed] [Google Scholar]
- 6.Goldsborough M, et al. Serum-free culture of murine embryonic stem (ES) cells. Focus. 1998;20:8–12. [Google Scholar]
- 7.Tao W, Lai E. Telencephalon-restricted expression of BF-1, a new member of the HNF-3/fork head gene family, in the developing rat brain. Neuron. 1992;8:957–966. doi: 10.1016/0896-6273(92)90210-5. [DOI] [PubMed] [Google Scholar]
- 8.Watanabe K, et al. A ROCK inhibitor permits survival of dissociated human embryonic stem cells. Nat Biotechnol. 2007;25:681–686. doi: 10.1038/nbt1310. [DOI] [PubMed] [Google Scholar]
- 9.Chiang C, et al. Cyclopia and defective axial patterning in mice lacking Sonic hedgehog gene function. Nature. 1996;383:407–413. doi: 10.1038/383407a0. [DOI] [PubMed] [Google Scholar]
- 10.Markakis EA. Development of the neuroendocrine hypothalamus. Front Neuroendocrinol. 2002;23:257–291. doi: 10.1016/s0091-3022(02)00003-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lebkowski JS, et al. Human embryonic stem cells: Culture, differentiation, and genetic modification for regenerative medicine applications. Cancer J. 2001;7(Suppl 2):S83–93. [PubMed] [Google Scholar]
- 12.Lagutin OV, et al. Six3 repression of Wnt signaling in the anterior neuroectoderm is essential for vertebrate forebrain development. Genes Dev. 2003;17:368–379. doi: 10.1101/gad.1059403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Furukawa T, Kozak CA, Cepko CL. rax, a novel paired-type homeobox gene, shows expression in the anterior neural fold and developing retina. Proc Natl Acad Sci USA. 1997;94:3088–3093. doi: 10.1073/pnas.94.7.3088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ikeda H, et al. Generation of Rx+/Pax6+ neural retinal precursors from embryonic stem cells. Proc Natl Acad Sci USA. 2005;102:11331–11336. doi: 10.1073/pnas.0500010102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Horsford DJ, et al. Chx10 repression of Mitf is required for the maintenance of mammalian neuroretinal identity. Development. 2005;132:177–187. doi: 10.1242/dev.01571. [DOI] [PubMed] [Google Scholar]
- 16.Hallonet M, Hollemann T, Pieler T, Gruss P. Vax1, a novel homeobox-containing gene, directs development of the basal forebrain and visual system. Genes Dev. 1999;13:3106–3114. doi: 10.1101/gad.13.23.3106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Oliver G, et al. Six3, a murine homologue of the sine oculis gene, demarcates the most anterior border of the developing neural plate and is expressed during eye development. Development. 1995;121:4045–4055. doi: 10.1242/dev.121.12.4045. [DOI] [PubMed] [Google Scholar]
- 18.Sinha S, Chen JK. Purmorphamine activates the Hedgehog pathway by targeting Smoothened. Nat Chem Biol. 2006;2:29–30. doi: 10.1038/nchembio753. [DOI] [PubMed] [Google Scholar]
- 19.Acampora D, et al. The role of Otx and Otp genes in brain development. Int J Dev Biol. 2000;44:669–677. [PubMed] [Google Scholar]
- 20.Dellovade TL, et al. Disruption of the gene encoding SF-1 alters the distribution of hypothalamic neuronal phenotypes. J Comp Neurol. 2000;423:579–589. doi: 10.1002/1096-9861(20000807)423:4<579::aid-cne4>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 21.Alvarez-Bolado G, et al. Winged helix transcription factor Foxb1 is essential for access of mammillothalamic axons to the thalamus. Development. 2000;127:1029–1038. doi: 10.1242/dev.127.5.1029. [DOI] [PubMed] [Google Scholar]
- 22.Caverson MM, Ciriello J, Calaresu FR, Krukoff TL. Distribution and morphology of vasopressin-, neurophysin II-, and oxytocin-immunoreactive cell bodies in the forebrain of the cat. J Comp Neurol. 1987;259:211–236. doi: 10.1002/cne.902590204. [DOI] [PubMed] [Google Scholar]
- 23.Armstrong ME. In: The Rat Nervous System. Paxinos G, editor. San Diego: Academic Press; 2004. pp. 369–383. [Google Scholar]
- 24.Cisowska-Maciejewska A, Ciosek J. Galanin influences vasopressin and oxytocin release from the hypothalamo-neurohypophysial system of salt loaded rats. J Physiol Pharmacol. 2005;56:673–688. [PubMed] [Google Scholar]
- 25.Bjorklund A, Moore RY, Nobin A, Stenevi U. The organization of tubero-hypophyseal and reticulo-infundibular catecholamine neuron systems in the rat brain. Brain Res. 1973;51:171–191. doi: 10.1016/0006-8993(73)90371-5. [DOI] [PubMed] [Google Scholar]
- 26.Tong Q, et al. Synaptic glutamate release by ventromedial hypothalamic neurons is part of the neurocircuitry that prevents hypoglycemia. Cell Metab. 2007;5:383–393. doi: 10.1016/j.cmet.2007.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Altman J, Bayer SA. The Development of the Rat Hypothalamus. Berlin: Springer-Verlag; 1986. pp. 10–26. [Google Scholar]
- 28.Maye P, et al. Hedgehog signaling is required for the differentiation of ES cells into neurectoderm. Dev Biol. 2004;265:276–290. doi: 10.1016/j.ydbio.2003.09.027. [DOI] [PubMed] [Google Scholar]
- 29.Belo JA, et al. Cerberus-like is a secreted BMP and nodal antagonist not essential for mouse development. Genesis. 2000;26:265–270. [PubMed] [Google Scholar]
- 30.Rallu M, Corbin JG, Fishell G. Parsing the prosencephalon. Nat Rev Neurosci. 2002;3:943–951. doi: 10.1038/nrn989. [DOI] [PubMed] [Google Scholar]
- 31.Nordstrom U, Jessell TM, Edlund T. Progressive induction of caudal neural character by graded Wnt signaling. Nat Neurosci. 2002;5:525–532. doi: 10.1038/nn0602-854. [DOI] [PubMed] [Google Scholar]
- 32.Ohyama K, Ellis P, Kimura S, Placzek M. Directed differentiation of neural cells to hypothalamic dopaminergic neurons. Development. 2005;132:5185–5197. doi: 10.1242/dev.02094. [DOI] [PubMed] [Google Scholar]
- 33.Freund C, et al. Insulin redirects differentiation from cardiogenic mesoderm and endoderm to neuroectoderm in differentiating human embryonic stem cells. Stem Cells. 2008;26:724–733. doi: 10.1634/stemcells.2007-0617. [DOI] [PubMed] [Google Scholar]
- 34.Desbois-Mouthon C, et al. Insulin and IGF-1 stimulate the beta-catenin pathway through two signalling cascades involving GSK-3β inhibition and Ras activation. Oncogene. 2001;20:252–259. doi: 10.1038/sj.onc.1204064. [DOI] [PubMed] [Google Scholar]
- 35.Puelles L, Rubenstein JL. Forebrain gene expression domains and the evolving prosomeric model. Trends Neurosci. 2003;26:469–476. doi: 10.1016/S0166-2236(03)00234-0. [DOI] [PubMed] [Google Scholar]
- 36.Rubenstein JL, Shimamura K, Martinez S, Puelles L. Regionalization of the prosencephalic neural plate. Annu Rev Neurosci. 1998;21:445–477. doi: 10.1146/annurev.neuro.21.1.445. [DOI] [PubMed] [Google Scholar]
- 37.Tessmar-Raible K, et al. Conserved sensory-neurosecretory cell types in annelid and fish forebrain: Insights into hypothalamus evolution. Cell. 2007;129:1389–1400. doi: 10.1016/j.cell.2007.04.041. [DOI] [PubMed] [Google Scholar]
- 38.Johansson BM, Wiles MV. Evidence for involvement of activin A and bone morphogenetic protein 4 in mammalian mesoderm and hematopoietic development. Mol Cell Biol. 1995;15:141–151. doi: 10.1128/mcb.15.1.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.