Abstract
Diacylglycerol (DAG) kinases (DGKs) are a family of enzymes that convert DAG to phosphatidic acid (PA), the physiologic functions of which have been poorly defined. We report here that DGK α and ζ synergistically promote T cell maturation in the thymus. Absence of both DGKα and ζ (DGKα−/−ζ−/−) results in a severe decrease in the number of CD4+CD8− and CD4−CD8+ single-positive thymocytes correlating with increased DAG-mediated signaling. Positive selection, but not negative selection, is impaired in DGKα−/−ζ−/− mice. The developmental blockage in DGKα−/−ζ−/− mice can be partially overcome by treatment with PA. Furthermore, decreased DGK activity also promotes thymic lymphomagenesis accompanying elevated Ras and Erk1/2 activation. Our data demonstrate a synergistic and critical role of DGK isoforms in T cell development and tumor suppression, and indicate that DGKs not only terminate DAG signaling but also initiate PA signaling in thymocytes to promote positive selection.
Keywords: phosphatidic acid, signaling, tumorigenesis
Diacylglycerol (DAG) kinases (DGKs) are a family of enzymes that catalyze phosphorylation of DAG, converting it to phosphatidic acid (PA). Ten DGK isoforms have been identified in mammals and are divided into 5 subtypes based on unique structural features (1, 2). Within a single tissue, multiple DGK isoforms can be expressed. A notable feature of the DGK-mediated reaction is that both the substrate, DAG, and the product, PA, can be important second messengers. DAG can associate with Ras guanyl nucleotide-releasing proteins (RasGRPs), protein kinase Cs (PKCs), protein kinase Ds, chimaerins, and Munc-13s through their cysteine-rich (C1) domains (3). PA has been reported to bind to the SH-2 domain containing tyrosine phosphatase-1 (SHP-1), mammalian target of Rapamycin (mTOR), cAMP-specific phosphodiesterase 4 (PDE4), protein phosphatase-1 (PP-1), son of sevenless (Sos), and type I phosphatidylinositol 4-phosphate 5-kinase (PI5K) (4–8). Through the regulation of the activities and subcellular localizations of these signaling molecules, DAG and PA play critical roles in signaling from many cell surface receptors (3, 4). It has been hypothesized that DGKs act as crucial regulators of receptor signaling and cellular function by modulating DAG and PA concentrations. However, the physiologic importance of most DGKs and the role of DGK-derived PA in receptor signaling have been poorly defined.
T cell maturation in the thymus occurs through CD4−CD8− double-negative (DN) to CD4+CD8+ double-positive (DP) and finally to the CD4+CD8− or CD4−CD8+ single-positive (SP) stage (9). Engagement of TCR expressed on DP thymocytes with self-peptide presented by MHCs on thymic stromal cells and bone marrow-derived dendritic cells induces intracellular signaling that can lead to either maturation (positive selection) or cell death (negative selection). In general, TCRs with high affinity to self-antigens elicit strong signals directing negative selection, whereas TCRs with low affinity to self-antigens induce weak signals, allowing positive selection to occur (9–11). Thymic selection results in the generation of a repertoire of T cells that recognize foreign antigens and, at the same time, are self-tolerant. Abnormal TCR signaling in thymocytes can skew thymic selection and cause autoimmune diseases.
Following TCR engagement, phospholipase Cγ1 (PLCγ1) produces inositol trisphosphate (IP3) and DAG. IP3 and DAG induce activation of the Ca2+-calcineurin and RasGRP1-Ras-Erk1/2 cascades. Ras and Erk1/2 have been implicated in both positive selection and negative selection (12–17). Recent evidence indicates that DGKα and ζ, isoforms known to be expressed in T cells negatively control DAG and the Ras-Erk1/2 signaling pathway after TCR engagement (18–22). Deficiency of either DGKα or ζ causes enhanced T cell activation and resistance to anergy induction (19, 20). We report here that loss of both DGKα and ζ (DGKα−/−ζ−/−, DKO) in mice selectively inhibits positive selection that can be rescued by PA. Furthermore, decreased DGK activity promotes thymic lymphomagenesis in the HY-TCR transgenic model, which correlates with elevated Ras and Erk1/2 activation. These observations demonstrate that two structurally distinct DGK isoforms perform synergistic roles in T cell maturation through the action of their product PA, and that DGK activity is involved in tumor suppression.
Results
Deficiency of both DGKα and ζ Inhibits T Cell Maturation.
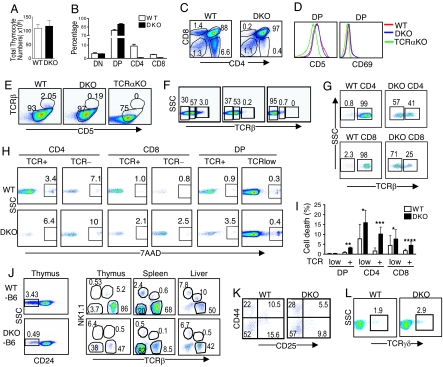
Deficiency of either DGKα or ζ promotes peripheral T cell activation without obviously altering T cell numbers (19, 20). In DGKα−/−ζ−/− mice, we observed severe decreases of SP thymocytes, indicating a synergistic role of these 2 isoforms in promoting DP to SP maturation. The total thymic cellularity in DGKα−/−ζ−/− mice was similar to WT mice, although the ratio of DGKα−/−ζ−/− DP thymocytes increased in compensation (Fig. 1 A–C). TCRα deficiency causes T cell developmental blockade at the DP stage, and positive selection is not initiated in TCRα−/− DP thymocytes due to lack of a functional TCR (23). In TCRα−/− DP thymocytes, cell surface CD69, CD5, and TCRβ were low or hardly detectable. Compared with TCRα−/− mice and WT mice, CD69 and CD5 up-regulation and up-regulation of TCRβ or CD3 on cell surface from negative to low levels, events occurring during positive selection, were not inhibited in DGKα−/−ζ−/− DP thymocytes (Fig. 1 D–F). However, the TCRβhigh population in DGKα−/−ζ−/− DP thymocytes was decreased 90% (Fig. 1 E and F). Furthermore, the majority of DGKα−/−ζ−/− CD4 and CD8 SP thymocytes expressed low levels of TCR, whereas 98% or more WT controls expressed high levels of TCR (Fig. 1G). Increased cell death was detected in DGKα−/−ζ−/− CD4 SP, CD8 SP, and TCRhigh DP thymocytes but not in DGKα−/−ζ−/− TCRlow DP (Fig. 1 H and I). These results indicate that increased death of thymocytes during and after positive selection may cause the decrease of SP thymocytes in the DGKα−/−ζ−/− thymus.
Fig. 1.
Loss of both DGKα and ζ inhibits αβ T-cell development at the DP stage. Thymocytes from DGKα−/−ζ−/− mice and WT mice were stained with different fluorescently labeled antibodies and analyzed by FACS. (A) Total thymocyte numbers (n = 10). (B) Percentage of thymocyte subsets (n = 3). (C) Representative FACS plots for CD4 and CD8 staining of total thymocytes. (D) CD69 and CD5 staining of WT, DGKα−/−ζ−/−, and TCRα−/− DP thymocytes. (E) CD5 and TCRβ staining of WT, DGKα−/−ζ−/−, and TCRα−/− DP thymocytes. (F) TCRβ staining of WT, DGKα−/−ζ−/−, and TCRα−/− DP thymocytes. (G) TCRβ staining of WT and DGKα−/−ζ−/− SP thymocytes. (H and I). Increased cell death of DGKα−/−ζ−/− thymocytes. Thymocytes were stained for CD4, CD8, TCRβ, and 7-AAD. Representative FACS plots of 7-AAD staining (H) in indicated populations are shown. Bar graph (I) shows 7-AAD positive cells in indicated populations (n = 3, *, P > 0.05; **, P = 0.002; ***, P = 0.015; ****, P = 0.03). (J) Deficiency of NKT cells in DGKα−/−ζ−/− mice. Representative TCRβ and NK1.1 staining of gated CD24− populations from C57BL/6 and DGKα−/−ζ−/− mice backcrossed to C57BL/6 background for 7 to 8 generations is shown. (K) CD25 and CD44 staining of CD4−CD8−CD3− thymocytes. (L) TCRγδ staining of CD4−CD8− DN thymocytes.
NKT cells, expressing both TCR and NK1.1 but low levels of CD24, are generated at the DP stage (24). NKT cells were much more dramatically decreased in DGKα−/−ζ−/− thymi, spleen, and livers compared with total αβ T cells (Fig. 1J). In contrast, DN III (CD25+CD44−) to DN IV (CD25−CD44−) maturation was not blocked in DGKα−/−ζ−/− mice (Fig. 1K). CD4−CD8−TCRγδ+ thymocytes were slightly increased in DGKα−/−ζ−/− mice (Fig. 1L). Thus, DGKα and ζ deficiency preferentially inhibits DP to SP maturation and NKT cell development but does not inhibit DN to DP maturation or γδ T cell development.
T-Cell Intrinsic Mechanisms Cause Developmental Defects in DGKα−/−ζ−/− Mice.
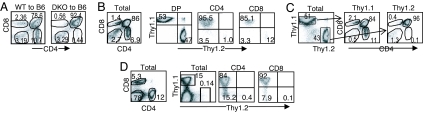
Both DGKα and ζ are expressed in multiple cell lineages and tissues inside and outside the hematopoietic system (18, 22, 25). To determine whether the developmental defect in DGKα−/−ζ−/− mice is T cell autonomous, WT and DGKα−/−ζ−/− bone marrow (BM) cells were injected into lethally irradiated C57BL/6 mice to generate chimeric mice. An obvious blockade of T cell maturation at the DP stage was observed in recipients reconstituted with DGKα−/−ζ−/− BM but not in WT BM recipients (Fig. 2A). Furthermore, in sublethally irradiated Rag-1−/− mice reconstituted with equal portions of WT (Thy1.1) and DGKα−/−ζ−/− (Thy1.2) BM, very few DGKα−/−ζ−/− SP T cells developed. In contrast, DGKα−/−ζ−/− DP thymocyte numbers were similar to WT control (Fig. 2 B and C). Very few DGKα−/−ζ−/− T cells were presented in the peripheral lymphoid organs in the recipient mice (Fig. 2D). Thus, the presence of WT BM-derived elements including WT thymocytes cannot correct the developmental defects of DGKα−/−ζ−/− thymocytes.
Fig. 2.
Developmental defect caused by DGKαζ deficiency is T cell autonomous. (A) Lethally irradiated C57BL/6 mice were reconstituted with BM cells from WT and DGKα−/−ζ−/− mice. Thymocytes from chimeric mice were stained for CD4 and CD8 and analyzed by flow cytometry 6–8 weeks after reconstitution. (B–D) Analysis of thymocytes and splenocytes from Rag-1−/− mice reconstituted with Thy1.1 WT and Thy1.2 DGKα−/−ζ−/− BM mixed at equal portions. Recipients were analyzed 8 weeks after reconstitution. (B) CD4 and CD8 staining in total thymocytes and Thy1.1 and Thy1.2 staining in gated DP, CD4, and CD8 thymocytes. (C) Thy1.1 and Thy1.2 staining in total thymocytes and CD4 and CD8 staining in gated Thy1.1+Thy1.2− and Thy1.1−Thy1.2+ populations. (D) FACS plots of splenocytes. All data shown are representative of at least four experiments.
Impairment of Positive Selection but Not Negative Selection in DGKα−/−ζ−/− Mice.
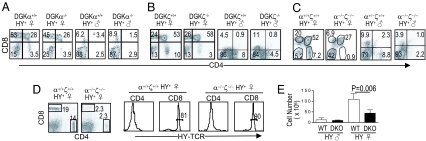
The HY-TCR is MHC class I-restricted and directs thymocytes to the CD8 lineage in female mice but induces negative selection in male mice because of the presence of the cognate male antigen (26). In female HY-mice, absence of either DGKα or ζ did not obviously affect positive selection of HY thymocytes to CD8 lineage, although the number of CD4+CD8− SP thymocytes decreased in HY-DGKζ−/− female mice (Fig. 3 A and B). However, in female DGKα−/−ζ−/−-HY mice, total thymic cellularity and the numbers of DP, CD8 SP, and CD4 SP thymocytes were decreased (Fig. 3 C and E). The ratio of DN thymocytes in these mice was relatively increased but the absolute numbers were similar to WT HY mice. In the peripheral lymphoid organs, very few T cells were detected in DGKα−/−ζ−/−-HY mice (Fig. 3D). In male mice, very few DP or SP thymocytes were presented and the total cellularity was low in WT-HY, DGKα−/−-HY, DGKζ−/−-HY, and DGKα−/−ζ−/−-HY mice (Fig. 3 A–C, and E). Thus, at least in the HY-TCR transgenic model, deficiency of both DGKα and ζ causes impairment of positive selection but does not inhibit negative selection.
Fig. 3.
Effects of DGKα and/or ζ deficiency on positive selection and negative selection in the HY-TCR transgenic model. (A–C) Representative FACS plots of thymocytes of at least four mice of each genotype of 6–8 weeks of age are shown. (D) FACS plots of splenocytes of indicated genotypes. (E) Bar graph shows total thymic cellularity of indicated genotypes (n = 4, mean ± SD).
Elevated DAG and Activation of Ras and Erk1/2 in DGKα−/−ζ−/− DP Thymocytes.
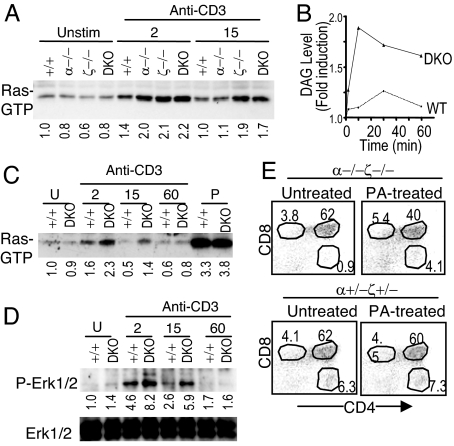
To investigate how DGKα and ζ deficiency may affect TCR signaling in thymocytes, we first assessed TCR-induced Ras activation, which is downstream of DAG through its effector molecules RasGRP1 and PKCθ (13, 27), in total thymocytes from WT, DGKα−/−, DGKζ−/−, or DGKα−/−ζ−/− mice. Ras activation was elevated in DGKα−/−, DGKζ−/−, and DGKα−/−ζ−/− thymocytes after TCR stimulation for 2 min. Fifteen min after TCR stimulation, increased Ras activation was only observed in DGKζ−/− and DGKα−/−ζ−/− thymocytes but not in DGKα−/− thymocytes, suggesting a stronger role of DGKζ in regulating Ras activation than DGKα (Fig. 4A).
Fig. 4.
Enhanced DAG signaling in DGKα and/or ζ deficient thymocytes after TCR stimulation. (A) TCR-induced Ras activation in total thymocytes deficient of DGK α, ζ, or both α and ζ. Numbers below the blot represent relative signal intensity. Data shown are representative of three experiments. (B) Measurement of DAG in DP thymocytes. Fold induction of DAG in WT and DGKα−/−ζ−/− DP thymocytes both before and after TCR stimulation is shown. Data are representative of two experiments. (C and D) Enhanced TCR-induced Ras and Erk1/2 activation in DGKα−/−ζ−/− DP thymocytes. WT and DGKα−/−ζ−/− DP thymocytes were stimulated with an anti-CD3ε antibody or PMA, and Ras activation (C) was measured as described in A. Erk1/2 phosphorylation (D) was determined by immunoblotting cell lysates with an anti-phospho-Erk1/2 antibody. The blot was stripped and probed with an anti-Erk1/2 antibody for loading control. U, unstimulated; P, PMA stimulated. (E) Promoting DGKα−/−ζ−/− SP thymocyte generation by PA. Embryonic day 15 thymi of indicated genotypes were left untreated or treated with 100 μM PA for 7 days during FTOC followed by FACS analysis.
Because thymic selection occurs at the DP stage, we further examined how deficiency of both DGKα and ζ may affect TCR signaling in purified DP thymocytes. TCR stimulation induced slight elevation of total DAG concentrations in WT DP thymocytes (Fig. 4B). In DGKα−/−ζ−/− DP thymocytes, a more robust elevation of total DAG concentrations was observed, indicating that deficiency of DGK activity leads to decreased conversion of DAG to PA.
TCR-induced Ras activation was enhanced in DGKα−/−ζ−/− DP thymocytes as compared with WT DP thymocytes (Fig. 4C). Stimulation with PMA, a functional analogue of DAG resistant to DGK activity, caused similar Ras activation in both WT and DGKα−/−ζ−/− DP thymocytes. Erk1/2 phosphorylation, which correlates with their activation and is downstream of the Ras pathway, was also enhanced in DGKα−/−ζ−/− DP thymocytes (Fig. 4D). These observations indicate that DGK activity inhibits the TCR-induced Ras-Erk1/2 pathway downstream of DAG in DP thymocytes.
Exogenous PA Promotes DGKα−/−ζ−/− T-Cell Maturation.
In addition to terminating DAG, DGKs produce PA. We investigated a potential role of DGK-derived PA in TCR signaling and T cell maturation. Fetal thymic organ culture (FTOC) in the presence or absence of exogenous PA was used. In the absence of exogenous PA, DGKα−/−ζ−/− thymi showed decreased SP thymocytes as compared with DGKα+/−ζ−/− thymi or DGKα+/−ζ+/− thymi (Fig. 4E and data not shown). Addition of PA during FTOC resulted in an increase of SP thymocytes in DGKα−/−ζ−/− thymi without obvious effects on control thymi. Thus, exogenous PA can partially promote the positive selection of DGKα−/−ζ−/− thymocytes, suggesting that, in addition to terminating DAG-mediated signaling, DGKα and ζ may produce PA, which in turn promotes positive selection.
Decreased DGKα and ζ Activities Promote Thymic Lymphomagenesis.
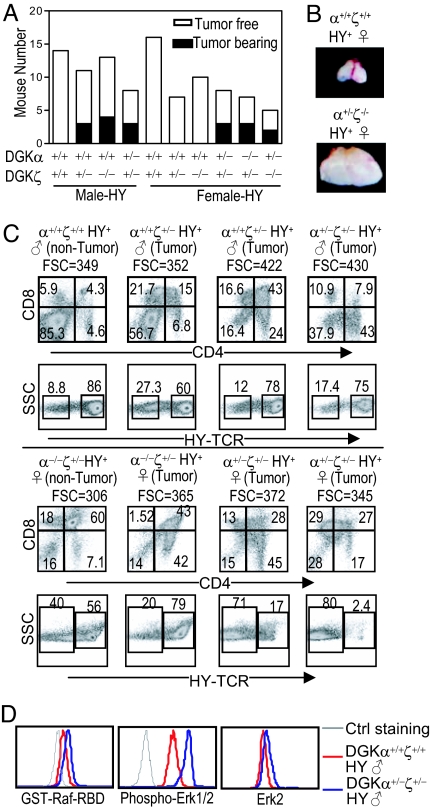
Approximately 2–3% HY-TCR transgenic mice develop thymic lymphoma at 5 months of age, with increased tumor occurrence at older ages (28). At 3 to 5 months of age, some DGKα+/+ζ+/−-HY or DGKα+/+ζ−/−-HY male but not female mice developed thymic lymphomas. Higher percentages of both female and male DGKα+/−ζ+/−-HY mice developed thymic lymphoma (named as DGK-HY lymphoma), suggesting a synergistic role of DGKα and ζ in suppressing lymphomagenesis (Fig. 5 A and B). DGK-HY lymphomas appeared heterogeneous among different mice, as CD4lowCD8+, CD4+CD8−, or CD4lowCD8low. Most of them expressed the HY-TCR (Fig. 5C). Some of them did not express the canonic HY-TCR but did express the β chain of the HY-TCR (Vβ8). Ras and Erk1/2 activation was increased in DGK-HY lymphomas (Fig. 5D). No thymic lymphomas have been observed in DGKα−/−, DGKζ−/−, DGKα+/−ζ+/−, DGKα+/−ζ−/−, DGKα−/−ζ+/−, or DGKα−/−ζ−/− mice without a TCR transgene. However, DGKα−/−ζ−/− mice have shortened life spans because of autoimmunity (Wan et al., manuscript submitted). We cannot exclude the possibility that thymic lymphoma might have developed in these mice if they had normal lifespans. Together, these observations indicate that DGKα and ζ inhibit the oncogenic potential caused by the HY-TCR transgene.
Fig. 5.
Decreased DGK activity promotes thymic lymphomagenesis in mice carrying the HY-TCR transgene. (A) Incidence of thymic lymphomas in 3–5-month-old mice with decreased DGKα and/or ζ activity. (B) Pictures of a pair of littermate of female WT-HY and DGKα+/−ζ+/−-HY thymi. (C) FACS plots of representative male and female HY mice of indicated genotypes. FSC indicates increased cell sizes of DGK-HY lymphomas. (D) Elevated Ras and Erk1/2 activation in DGK-HY lymphomas. Thymocytes from DGKα+/+ζ+/+-HY male mice and DGKα+/−ζ+/−-HY male mice were stained for CD4 and CD8 followed by intracellular staining for Ras-GTP, phosphor-Erk1/2, and Erk2.
Discussion
This study demonstrated that DGKα and ζ synergistically promote positive selection, which also provides evidence that 2 DGK isoforms perform synergistic function in vivo. In DGKα−/−ζ−/− DP thymocytes, positive selection events such as CD69, CD5, and TCRβ up-regulation, are not inhibited. The developmental defect in DGKα−/−ζ−/− DP thymocytes appears to occur when they reach the TCRhigh stage and may be caused by increased death of TCRhigh DP and SP thymocytes. These observations suggest that DGK activity might be dispensable for the initiation of positive selection but critical for successful completion of positive selection.
Our data suggest that DGKα and ζ function both as signal terminator and initiator by converting DAG to PA in thymocytes. In DGKα−/−ζ−/− DP thymocytes, DAG concentration and activation of Ras and Erk1/2 are increased after TCR engagement. Ras and Erk1/2 are known to be important for positive selection but may also contribute to negative selection (12–15, 29–31). At present, the role of increased DAG concentration and enhanced Ras and Erk1/2 activation in the developmental defect of DGKα−/−ζ−/− thymocytes is not clear. Transient but robust Erk1/2 activation after strong engagement of TCR in DP thymocytes induces negative selection. By contrast, weaker but prolonged Erk1/2 activation after low-affinity engagement of TCR in DP thymocytes promotes positive selection (14, 16, 17). The Ras and Erk1/2 activation in DGKα−/−ζ−/− DP thymocytes is enhanced which could convert positive selection into negative selection. Furthermore, activation of Ras and Erk1/2 in the cytoplasm membrane but not in the intracellular compartments is correlated with negative selection whereas activation of these 2 molecules in both cytoplasm membrane and intracellular compartments is correlated with positive selection (17). Deficiency of DGKs could cause abnormal accumulation of DAG and activation of Ras and Erk1/2 in abnormal subcellular compartments, leading to impairment of positive selection. Because overexpression of RasGRP1 does not cause developmental blockage of thymocytes (32), enhanced DAG-RasGRP1-Ras-Erk1/2 pathway alone in DGKα−/−ζ−/− mice might not be sufficient to cause the developmental defects seen in these mice. It remains to be determined whether other DAG-mediated signaling mechanism(s) may contribute to the failure of positive selection.
Although much evidence has implicated phospholipase D-derived PA as the major source of PA in receptor signaling (4), accumulating data reveal that DGK-derived PA is also important for receptor signaling. DGKζ-derived PA can promote mTOR signaling in HEK293 cells, enhance PI5K activity, and increase TLR-induced IL-12 production in macrophages (8, 33, 34). We show here that PA can promote DGKα−/−ζ−/− DP thymocyte maturation to the SP stage in FTOC, indicating a critical role of PA for positive selection. PA has been found to associate with and to promote the activities of numerous effector molecules such as mTOR, SHP-1, Sos, PI5K, PDE4, and PP-1 (4–8). PA may promote positive selection by regulating one or several of its effector molecules. A particular interesting candidate is mTOR, which promotes protein translation, glucose uptake, cell survival, and proliferation, and inhibits autophagy (35). Inhibition of mTOR by rapamycin causes death of thymocytes (36). A potential decrease of mTOR activity in the absence of DGK activity could cause death of positive selecting thymocytes. In addition to specific PA-protein interactions, PA may also affect positive selection indirectly, such as by serving as an intermediate for the production of other messengers.
We observed accelerated thymic lymphomagenesis in HY-TCR transgenic mice when DGKα and/or ζ activities were decreased. In some human cancer patients, DGKα expression is correlated with good prognosis (37), and the tumor suppressor p53 promotes DGKα expression (38, 39). Thus, DGK activity may function as tumor suppressor both in mice and in humans.
Sustained DAG signaling may associate with tumorigenesis, because phorbol esters, functional analogues of DAG that have long-half lives and are strong activators of RasGRPs and PKCs, are carcinogenic. In DGKHY-thymic lymphoma, there is an obvious increase of Ras and Erk1/2 activation, suggesting that DGK activity may suppress lymphomagenesis by terminating DAG and preventing overactivation of RasGRP1 and Ras. Overexpression of RasGRP1 induces transformation in cell line models and thymic lymphomas in mice (32, 40). RasGRP1 expression is elevated in many human acute T lymphocytic leukemias that originate predominantly from transformed thymocytes (41). Constitutive elevation of Ras activity has been detected in many human solid tumors and a subset of T lymphocytic leukemia (42). The causes of increased Ras activation in some of these tumors have not been revealed. It would be interesting to determine whether loss-of-function mutations in DGK loci may exist and contribute to elevated Ras activation and tumorigenesis in humans. Of note, the tumorigenesis caused by DGKα and ζ deficiency occurs only in mice carrying the HY-TCR transgene, whereas lymphomagenesis caused by RasGRP1 overexpression is independent on a TCR (32). It is possible that other DAG-metabolizing mechanisms might also be involved in limiting DAG-mediated signaling. In addition to inhibiting RasGRP1 and Ras activation, DGK activity may also influence tumorigenesis through other mechanisms. For example, the retinoblastoma protein (pRB) binds to DGKζ and activates DGKζ to promote cell cycle arrest (43, 44). In addition, DGK-derived PA could induce signaling events that suppress tumorigenesis. However, this possibility is less likely because of the mitogenic function of PA demonstrated in cell line models (6).
In summary, our studies demonstrate a synergistic role of 2 DGK isoforms with distinct structural features in T cell development and in tumor suppression. We propose that DGK activity may serve two functions in developing T cells, by terminating DAG signaling and by initiating PA signaling to ensure proper T cell development and to suppress tumorigenesis.
Experimental Procedures
Mice.
C56BL/6 mice, TCRα−/−, Rag-1−/−, and the HY-TCR transgenic mice were obtained from the Jackson Laboratory. DGKα and ζ deficient mice were reported (19, 20). All mice were used in accordance with protocols approved by the Institutional Animal Care and Use Committee at Duke University.
FACS Analysis.
Thymocytes were incubated with fluorescently labeled antibodies in 5% FBS-PBS at 4°C for 30 min. Stained cells were collected in a FACScan or FACSCalibur with Cellquest software and analyzed with FlowJo software. Fluorescently labeled anti-CD3 (145–2C11), CD4 (GK1.5), CD8 (53–6.7), CD25 (7D4), TCRβ (H57–597), CD5 (53–7.3), CD69 (H1.2F3), CD24 (M1/69), NK1.1 (PK136), and CD44 (G44–26) were purchased from BD Biosciences. The phycoerithrin (PE)-conjugated anti-HY TCR antibody (T3.70) was from eBiosciences.
Isolation of DP Thymocytes.
Thymocytes (1 × 108) from WT and DGKα−/−ζ−/− mice were stained with a biotin-labeled anti-Db antibody (2 μl) in 400 μl 0.5% BSA- and 0.2 mM EDTA-PBS (BEP) at 4°C for 20 min. After washing two times, cells were resuspended in 500 μL BEP and then stained with 2 μl streptavidin-conjugated PE at 4° for 20 min. Cells were washed and mixed with 20 μl anti-PE microbeads (Miltenyi Biotec GmbH). After incubation at 4°C for 15 min, DP thymocytes were isolated by using the LD depletion column (Miltenyi Biotec GmbH). The purities of DP thymocytes were usually >95%.
Western Blot.
A 5- to 10-million quantity of total or CD4+CD8+ DP thymocytes was rested in 0.5 ml PBS at 37°C for 20 min. Cells were then left unstimulated, stimulated with an anti-CD3 antibody (500A2, 5 μg/ml) for different times, or stimulated with PMA (50 ng/ml) for 5 min. Cells were lysed in 1% Nonidet P-40 lysis buffer (1% Nonidet P-40, 150 mM NaCl, 50 mM Tris, pH 7.4) with protease and phosphatase inhibitors. Proteins in lysates were separated by SDS/PAGE and transferred onto nitrocellularose membrane. The blots were probed with anti-phospho-Erk1/2 antibody and reprobed with anti-Erk1/2 for loading control. Western blot was quantified by using the ImageJ software (National Institutes of Health).
Measurement of Ras-GTP.
Thymocytes were stimulated as described above except lysed in Mg2+ lysis/wash buffer (25 mM Hepes, 150 mM NaCl, 1% (vol/vol) Nonidet P-40, 0.25% (wt/vol) Na deoxycholate, 10 mM MgCl2, 1 mM EDTA, 10% (vol/vol) glycerol, pH 7.5) with freshly added PMSF (Sigma) and protease inhibitor mixture. GTP-bound Ras was pulled down by GST-Raf-RBD agarose beads and detected by Western blot with an anti-Pan Ras antibody, as described in ref. 18.
Detection of Erk1/2 Phosphorylation and GTP-Ras by Intracellular Staining.
Thymocytes were stained with PE-Cy5 conjugated anti-CD4 and PE-conjugated CD8 antibodies at 4°C. Cells were pelleted, fixed, permeabilized, and stained with an anti-phospho-Erk1/2 antibody (Cell Signaling Technology), Erk2 antibody (Santa Cruz Biotechnology), or GST-Raf-RBD fusion protein. These cells were further stained with an Alexa Fluor 488 conjugated anti-mouse, anti-rabbit Ig, or anti-GST antibody (Invitrogen), respectively, and followed by FACS analysis.
Measurement of DAG.
A 5- to 10-million quantity of DP thymocytes was left unstimulated or stimulated with an anti-CD3ε antibody (500A2, 5 μg/ml) for the indicated lengths of time. Cells were pelleted and total lipids were collected according to the method of Bligh and Dyer (45). DAG was measured with an in vitro DAG kinase assay. DAG levels were always normalized to lipid phosphates, which was measured according to the method of Rouser et al. (46).
Fetal Thymic Organ Culture (FTOC).
Embryonic Day 15 thymi from DGKα+/−ζ−/− male x DGKα−/−ζ+/− female mating were cultured on Millipore filters in a 48-well plate with 1 ml RPMI-1640 medium supplemented with 10% FBS and penicillin/streptomycin/glutamine. One thymic lobe was treated with 100 μM PA and the other lobe was left untreated. After 7 days' incubation at 37°C with 5% CO2, single cell suspensions were stained for cell surface molecules followed by FACS analysis. Genotypes of fetuses were determined by PCR.
Statistical Analysis.
Experimental data are expressed as the mean ± SD. The statistical significance of differences between means was determined by using the unpaired two-tailed Student's t test.
Acknowledgments.
We thank Dr. Erik Peterson for many helpful discussions; Houde Zhou for technique support; Cheryl Bock and David Snyder for generating DGKα knockout mice; and Aileen Liu for editing the manuscript. This work was supported by a career development grant from the American Heart Association (X.-P.Z.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
References
- 1.Sakane F, Imai S, Kai M, Yasuda S, Kanoh H. Diacylglycerol kinases: Why so many of them? Biochim Biophys Acta. 2007;1771:793–806. doi: 10.1016/j.bbalip.2007.04.006. [DOI] [PubMed] [Google Scholar]
- 2.Merida I, Avila-Flores A, Merino E. Diacylglycerol kinases: At the hub of cell signalling. Biochem J. 2008;409:1–18. doi: 10.1042/BJ20071040. [DOI] [PubMed] [Google Scholar]
- 3.Kazanietz MG. Novel “nonkinase” phorbol ester receptors: The C1 domain connection. Mol Pharmacol. 2002;61:759–767. doi: 10.1124/mol.61.4.759. [DOI] [PubMed] [Google Scholar]
- 4.Wang X, Devaiah SP, Zhang W, Welti R. Signaling functions of phosphatidic acid. Prog Lipid Res. 2006;45:250–278. doi: 10.1016/j.plipres.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 5.Frank C, Keilhack H, Opitz F, Zschornig O, Bohmer FD. Binding of phosphatidic acid to the protein-tyrosine phosphatase SHP-1 as a basis for activity modulation. Biochemistry. 1999;38:11993–12002. doi: 10.1021/bi982586w. [DOI] [PubMed] [Google Scholar]
- 6.Fang Y, Vilella-Bach M, Bachmann R, Flanigan A, Chen J. Phosphatidic acid-mediated mitogenic activation of mTOR signaling. Science. 2001;294:1942–1945. doi: 10.1126/science.1066015. [DOI] [PubMed] [Google Scholar]
- 7.Zhao C, Du G, Skowronek K, Frohman MA, Bar-Sagi D. Phospholipase D2-generated phosphatidic acid couples EGFR stimulation to Ras activation by Sos. Nat Cell Biol. 2007;9:706–712. doi: 10.1038/ncb1594. [DOI] [PubMed] [Google Scholar]
- 8.Luo B, Prescott SM, Topham MK. Diacylglycerol kinase ζ regulates phosphatidylinositol 4-phosphate 5-kinase Iα by a novel mechanism. Cell Signal. 2004;16:891–897. doi: 10.1016/j.cellsig.2004.01.010. [DOI] [PubMed] [Google Scholar]
- 9.Starr TK, Jameson SC, Hogquist KA. Positive and negative selection of T cells. Annu Rev Immunol. 2003;21:139–176. doi: 10.1146/annurev.immunol.21.120601.141107. [DOI] [PubMed] [Google Scholar]
- 10.Hogquist KA, et al. T cell receptor antagonist peptides induce positive selection. Cell. 1994;76:17–27. doi: 10.1016/0092-8674(94)90169-4. [DOI] [PubMed] [Google Scholar]
- 11.Ashton-Rickardt PG, et al. Evidence for a differential avidity model of T cell selection in the thymus. Cell. 1994;76:651–663. doi: 10.1016/0092-8674(94)90505-3. [DOI] [PubMed] [Google Scholar]
- 12.Alberola-Ila J, Forbush KA, Seger R, Krebs EG, Perlmutter RM. Selective requirement for MAP kinase activation in thymocyte differentiation. Nature. 1995;373:620–623. doi: 10.1038/373620a0. [DOI] [PubMed] [Google Scholar]
- 13.Dower NA, et al. RasGRP is essential for mouse thymocyte differentiation and TCR signaling. Nat Immunol. 2000;1:317–321. doi: 10.1038/79766. [DOI] [PubMed] [Google Scholar]
- 14.Werlen G, Hausmann B, Palmer E. A motif in the αβ T-cell receptor controls positive selection by modulating ERK activity. Nature. 2000;406:422–426. doi: 10.1038/35019094. [DOI] [PubMed] [Google Scholar]
- 15.Fischer AM, Katayama CD, Pages G, Pouyssegur J, Hedrick SM. The role of erk1 and erk2 in multiple stages of T cell development. Immunity. 2005;23:431–443. doi: 10.1016/j.immuni.2005.08.013. [DOI] [PubMed] [Google Scholar]
- 16.Mariathasan S, et al. Duration and strength of extracellular signal-regulated kinase signals are altered during positive versus negative thymocyte selection. J Immunol. 2001;167:4966–4973. doi: 10.4049/jimmunol.167.9.4966. [DOI] [PubMed] [Google Scholar]
- 17.Daniels MA, et al. Thymic selection threshold defined by compartmentalization of Ras/MAPK signalling. Nature. 2006;444:724–729. doi: 10.1038/nature05269. [DOI] [PubMed] [Google Scholar]
- 18.Zhong XP, et al. Regulation of T cell receptor-induced activation of the Ras-ERK pathway by diacylglycerol kinase ζ. J Biol Chem. 2002;277:31089–31098. doi: 10.1074/jbc.M203818200. [DOI] [PubMed] [Google Scholar]
- 19.Zhong XP, et al. Enhanced T cell responses due to diacylglycerol kinase ζ deficiency. Nat Immunol. 2003;4:882–890. doi: 10.1038/ni958. [DOI] [PubMed] [Google Scholar]
- 20.Olenchock BA, et al. Disruption of diacylglycerol metabolism impairs the induction of T cell anergy. Nat Immunol. 2006;7:1174–1181. doi: 10.1038/ni1400. [DOI] [PubMed] [Google Scholar]
- 21.Zha Y, et al. T cell anergy is reversed by active Ras and is regulated by diacylglycerol kinase α. Nat Immunol. 2006;7:1166–1173. doi: 10.1038/ni1394. [DOI] [PubMed] [Google Scholar]
- 22.Sanjuan MA, et al. T cell activation in vivo targets diacylglycerol kinase α to the membrane: A novel mechanism for Ras attenuation. J Immunol. 2003;170:2877–2883. doi: 10.4049/jimmunol.170.6.2877. [DOI] [PubMed] [Google Scholar]
- 23.Mombaerts P, et al. Mutations in T-cell antigen receptor genes alpha and beta block thymocyte development at different stages. Nature. 1992;360:225–231. doi: 10.1038/360225a0. [DOI] [PubMed] [Google Scholar]
- 24.Gapin L, Matsuda JL, Surh CD, Kronenberg M. NKT cells derive from double-positive thymocytes that are positively selected by CD1d. Nat Immunol. 2001;2:971–978. doi: 10.1038/ni710. [DOI] [PubMed] [Google Scholar]
- 25.Olenchock BA, et al. Impaired degranulation but enhanced cytokine production after FcεRI stimulation of diacylglycerol kinase ζ-deficient mast cells. J Exp Med. 2006;203:1471–1480. doi: 10.1084/jem.20052424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.von Boehmer H. Developmental biology of T cells in T cell-receptor transgenic mice. Annu Rev Immunol. 1990;8:531–556. doi: 10.1146/annurev.iy.08.040190.002531. [DOI] [PubMed] [Google Scholar]
- 27.Roose JP, Mollenauer M, Gupta VA, Stone J, Weiss A. A diacylglycerol-protein kinase C-RasGRP1 pathway directs Ras activation upon antigen receptor stimulation of T cells. Mol Cell Biol. 2005;25:4426–4441. doi: 10.1128/MCB.25.11.4426-4441.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Strzadala L, Miazek A, Matuszyk J, Kisielow P. Role of thymic selection in the development of thymic lymphomas in TCR transgenic mice. Int Immunol. 1997;9:127–138. doi: 10.1093/intimm/9.1.127. [DOI] [PubMed] [Google Scholar]
- 29.Swan KA, et al. Involvement of p21ras distinguishes positive and negative selection in thymocytes. EMBO J. 1995;14:276–285. doi: 10.1002/j.1460-2075.1995.tb07001.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Delgado P, Fernandez E, Dave V, Kappes D, Alarcon B. CD3δ couples T-cell receptor signaling to ERK activation and thymocyte positive selection. Nature. 2000;406:426–430. doi: 10.1038/35019102. [DOI] [PubMed] [Google Scholar]
- 31.Pages G, et al. Defective thymocyte maturation in p44 MAP kinase (Erk 1) knockout mice. Science. 1999;286:1374–1377. doi: 10.1126/science.286.5443.1374. [DOI] [PubMed] [Google Scholar]
- 32.Klinger MB, Guilbault B, Goulding RE, Kay RJ. Deregulated expression of RasGRP1 initiates thymic lymphomagenesis independently of T-cell receptors. Oncogene. 2005;24:2695–2704. doi: 10.1038/sj.onc.1208334. [DOI] [PubMed] [Google Scholar]
- 33.Avila-Flores A, Santos T, Rincon E, Merida I. Modulation of the mammalian target of rapamycin pathway by diacylglycerol kinase-produced phosphatidic acid. J Biol Chem. 2005;280:10091–10099. doi: 10.1074/jbc.M412296200. [DOI] [PubMed] [Google Scholar]
- 34.Liu CH, et al. Diacylglycerol kinase ζ regulates microbial recognition and host resistance to Toxoplasma gondii. J Exp Med. 2007;204:781–792. doi: 10.1084/jem.20061856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sarbassov DD, Ali SM, Sabatini DM. Growing roles for the mTOR pathway. Curr Opin Cell Biol. 2005;17:596–603. doi: 10.1016/j.ceb.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 36.Tian L, Lu L, Yuan Z, Lamb JR, Tam PK. Acceleration of apoptosis in CD4+CD8+ thymocytes by rapamycin accompanied by increased CD4+CD25+ T cells in the periphery. Transplant. 2004;77:183–189. doi: 10.1097/01.TP.0000101005.44661.3E. [DOI] [PubMed] [Google Scholar]
- 37.Berrar D, Sturgeon B, Bradbury I, Downes CS, Dubitzky W. Survival trees for analyzing clinical outcome in lung adenocarcinomas based on gene expression profiles: Identification of neogenin and diacylglycerol kinase α expression as critical factors. J Comput Biol. 2005;12:534–544. doi: 10.1089/cmb.2005.12.534. [DOI] [PubMed] [Google Scholar]
- 38.Kannan K, et al. DNA microarrays identification of primary and secondary target genes regulated by p53. Oncogene. 2001;20:2225–2234. doi: 10.1038/sj.onc.1204319. [DOI] [PubMed] [Google Scholar]
- 39.Hammond EM, et al. Genome-wide analysis of p53 under hypoxic conditions. Mol Cell Biol. 2006;26:3492–3504. doi: 10.1128/MCB.26.9.3492-3504.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ebinu JO, et al. RasGRP, a Ras guanyl nucleotide-releasing protein with calcium- and diacylglycerol-binding motifs. Science. 1998;280:1082–1086. doi: 10.1126/science.280.5366.1082. [DOI] [PubMed] [Google Scholar]
- 41.Yeoh EJ, et al. Classification, subtype discovery, and prediction of outcome in pediatric acute lymphoblastic leukemia by gene expression profiling. Cancer Cell. 2002;1:133–143. doi: 10.1016/s1535-6108(02)00032-6. [DOI] [PubMed] [Google Scholar]
- 42.Malumbres M, Barbacid M. RAS oncogenes: The first 30 years. Nat Rev Cancer. 2003;3:459–465. doi: 10.1038/nrc1097. [DOI] [PubMed] [Google Scholar]
- 43.Evangelisti C, et al. Nuclear diacylglycerol kinase-zeta is a negative regulator of cell cycle progression in C2C12 mouse myoblasts. FASEB J. 2007;21:3297–3307. doi: 10.1096/fj.07-8336com. [DOI] [PubMed] [Google Scholar]
- 44.Los AP, et al. The retinoblastoma family proteins bind to and activate diacylglycerol kinase ζ. J Biol Chem. 2006;281:858–866. doi: 10.1074/jbc.M502693200. [DOI] [PubMed] [Google Scholar]
- 45.Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959;37:911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- 46.Rouser G, Fkeischer S, Yamamoto A. Two dimensional then layer chromatographic separation of polar lipids and determination of phospholipids by phosphorus analysis of spots. Lipids. 1970;5:494–496. doi: 10.1007/BF02531316. [DOI] [PubMed] [Google Scholar]