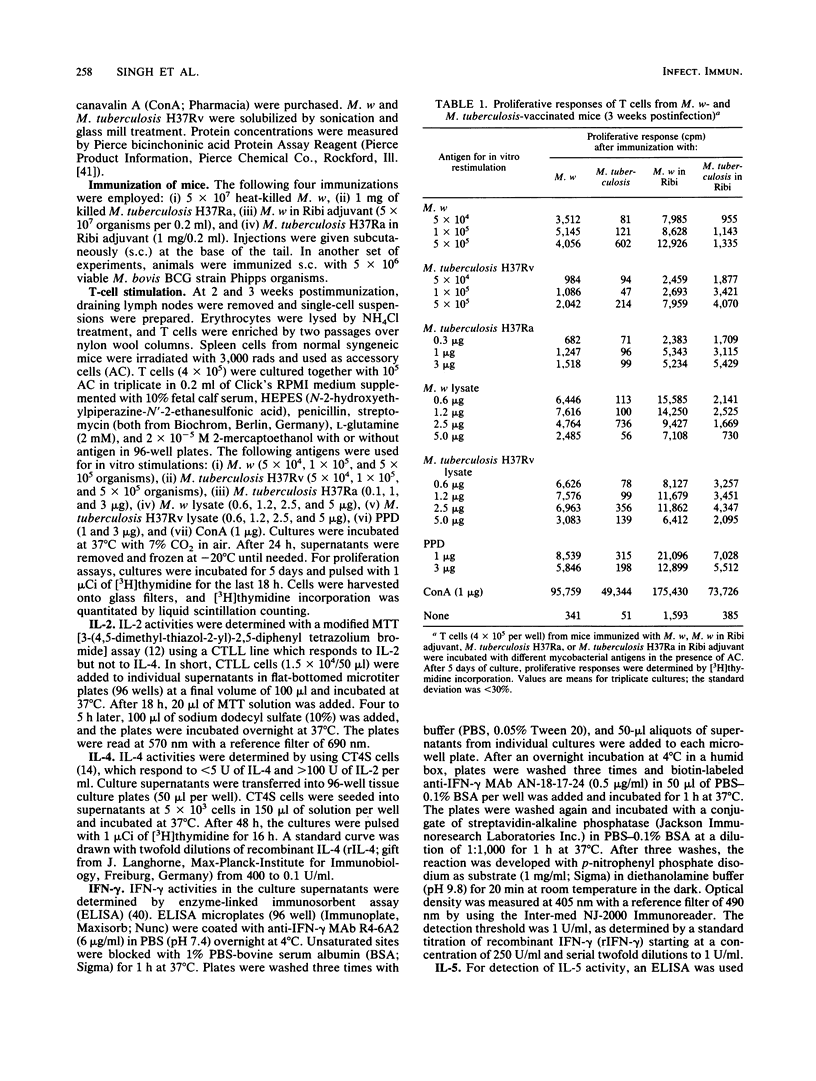
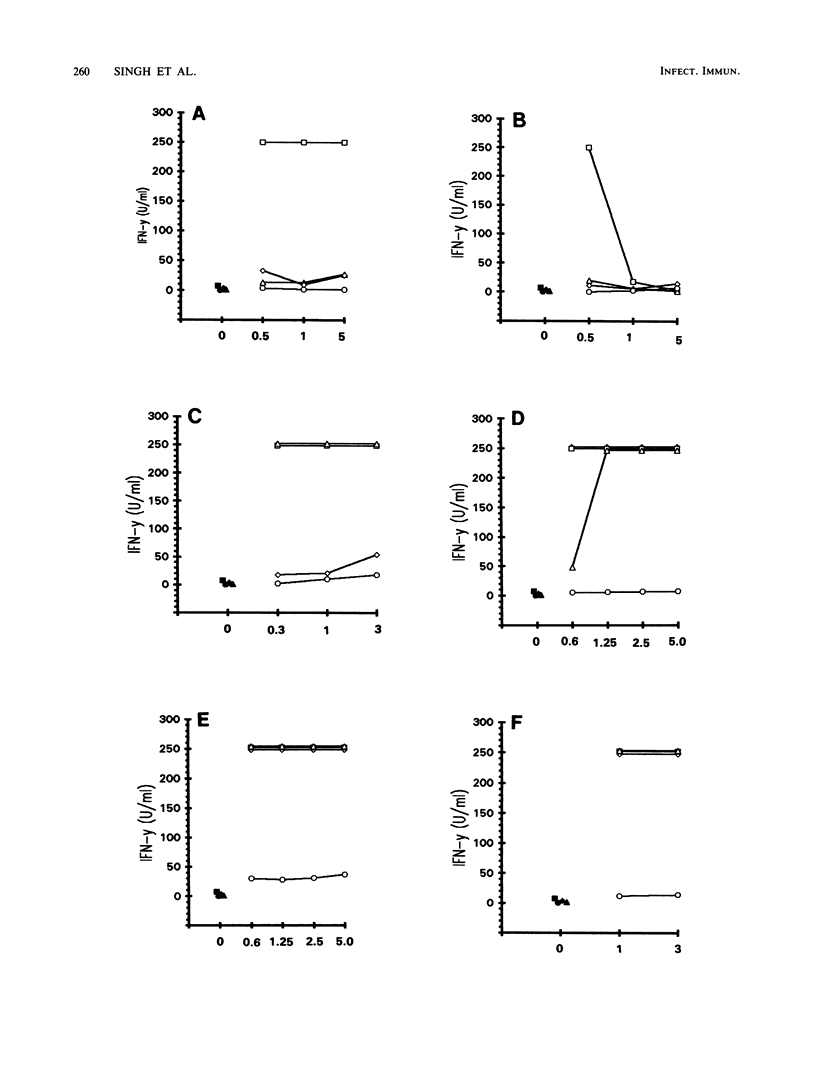
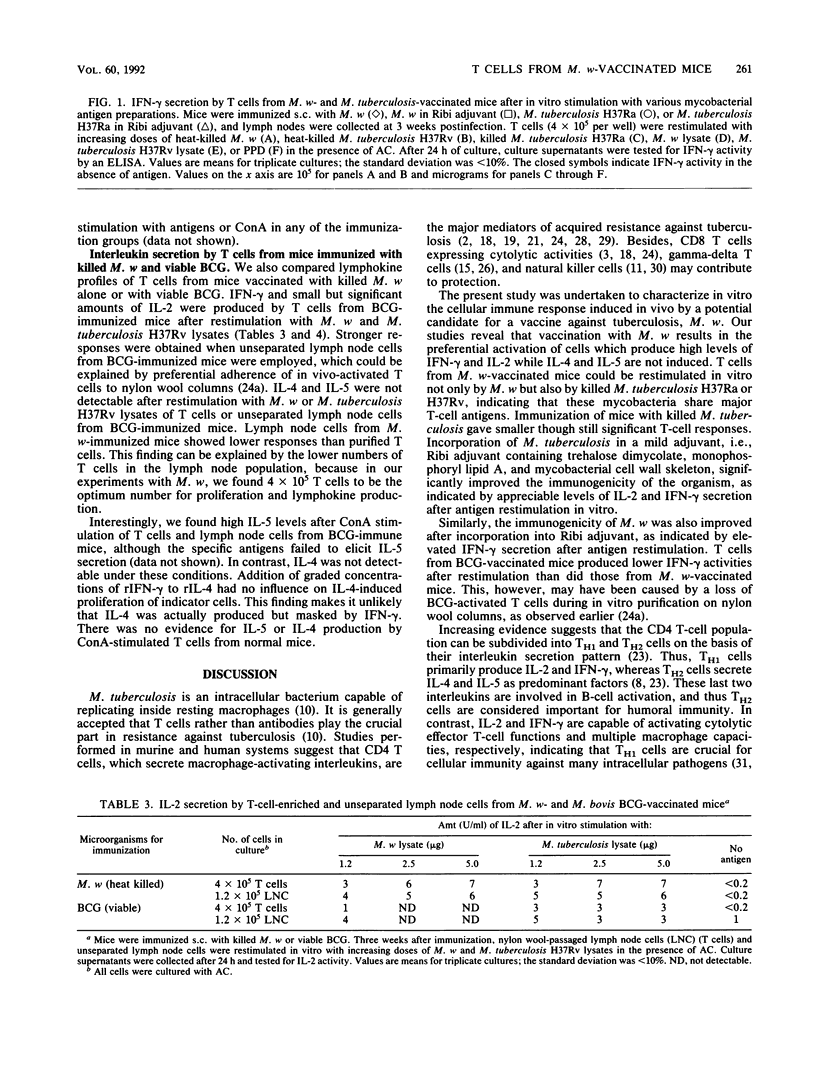
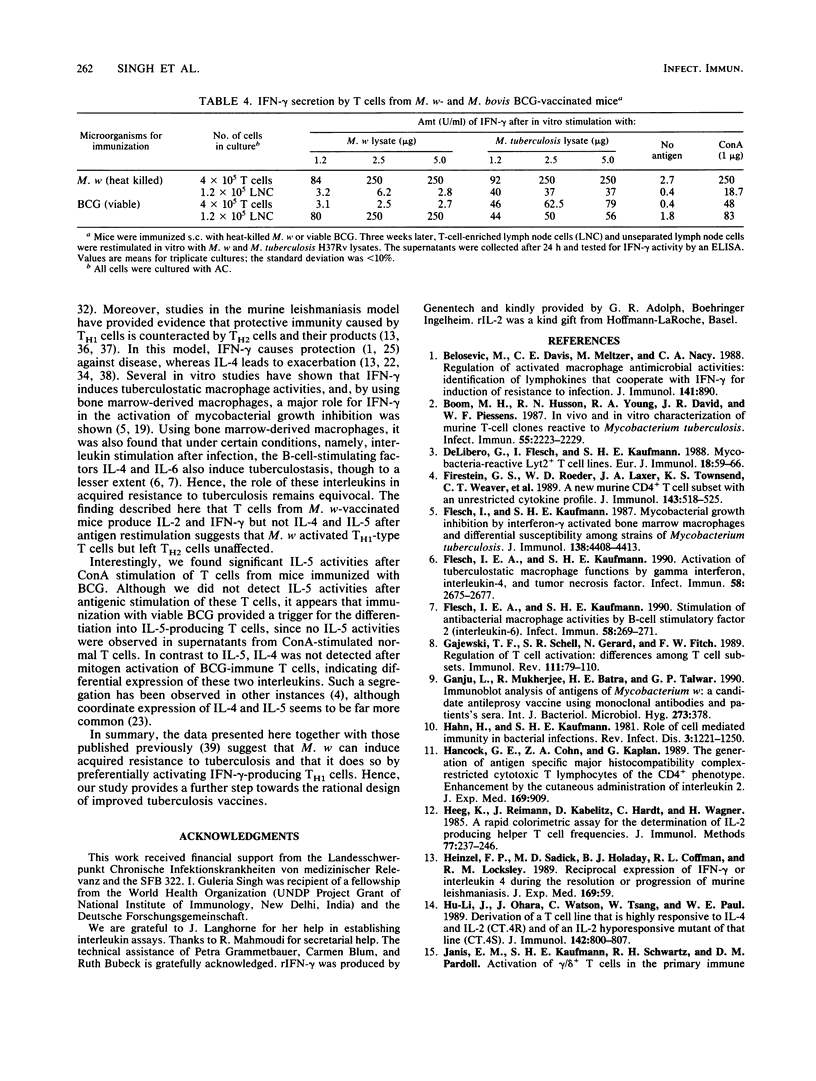
Abstract
Tuberculosis caused by the intracellular bacterial pathogen Mycobacterium tuberculosis still represents a major health problem, and its effective control would best be accomplished by active vaccination. Although vaccination with M. bovis BCG has proven highly effective in certain parts of the world, in several developing countries it has been found to confer only marginal protection. Hence, novel vaccination strategies are warranted. Mycobacterium w is a saprophytic cultivable mycobacterium which shares several antigens with M. tuberculosis. In the murine system, vaccination with killed M. w was found to protect against subsequent tuberculosis. In order to characterize the responsible immune mechanisms more precisely, mice were vaccinated with killed M. w and T cells restimulated in vitro with mycobacterial antigens. These T cells produced interleukin 2 and gamma interferon but no detectable interleukin 4 and interleukin 5. Killed M. w induced significantly stronger T-cell responses than killed M. tuberculosis, and both vaccination regimes were markedly improved by administration in a mild adjuvant, i.e., the Ribi adjuvant containing trehalose dimycolate, monophosphoryl lipid A, and mycobacterial cell wall skeleton. Our data suggest that M. w-induced immunity against M. tuberculosis rests primarily on TH1 cells, which are thought to be of major relevance for acquired antituberculosis resistance. Our study therefore provides a further step toward the identification of a novel tuberculosis vaccine.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Belosevic M., Davis C. E., Meltzer M. S., Nacy C. A. Regulation of activated macrophage antimicrobial activities. Identification of lymphokines that cooperate with IFN-gamma for induction of resistance to infection. J Immunol. 1988 Aug 1;141(3):890–896. [PubMed] [Google Scholar]
- Boom W. H., Husson R. N., Young R. A., David J. R., Piessens W. F. In vivo and in vitro characterization of murine T-cell clones reactive to Mycobacterium tuberculosis. Infect Immun. 1987 Sep;55(9):2223–2229. doi: 10.1128/iai.55.9.2223-2229.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dam H. G., Toman K., Hitze K. L., Guld J. Present knowledge of immunization against tuberculosis. Bull World Health Organ. 1976;54(3):255–269. [PMC free article] [PubMed] [Google Scholar]
- De Libero G., Flesch I., Kaufmann S. H. Mycobacteria-reactive Lyt-2+ T cell lines. Eur J Immunol. 1988 Jan;18(1):59–66. doi: 10.1002/eji.1830180110. [DOI] [PubMed] [Google Scholar]
- Firestein G. S., Roeder W. D., Laxer J. A., Townsend K. S., Weaver C. T., Hom J. T., Linton J., Torbett B. E., Glasebrook A. L. A new murine CD4+ T cell subset with an unrestricted cytokine profile. J Immunol. 1989 Jul 15;143(2):518–525. [PubMed] [Google Scholar]
- Flesch I. E., Kaufmann S. H. Activation of tuberculostatic macrophage functions by gamma interferon, interleukin-4, and tumor necrosis factor. Infect Immun. 1990 Aug;58(8):2675–2677. doi: 10.1128/iai.58.8.2675-2677.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flesch I. E., Kaufmann S. H. Stimulation of antibacterial macrophage activities by B-cell stimulatory factor 2 (interleukin-6). Infect Immun. 1990 Jan;58(1):269–271. doi: 10.1128/iai.58.1.269-271.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flesch I., Kaufmann S. H. Mycobacterial growth inhibition by interferon-gamma-activated bone marrow macrophages and differential susceptibility among strains of Mycobacterium tuberculosis. J Immunol. 1987 Jun 15;138(12):4408–4413. [PubMed] [Google Scholar]
- Gajewski T. F., Schell S. R., Nau G., Fitch F. W. Regulation of T-cell activation: differences among T-cell subsets. Immunol Rev. 1989 Oct;111:79–110. doi: 10.1111/j.1600-065x.1989.tb00543.x. [DOI] [PubMed] [Google Scholar]
- Ganju L., Mukherjee R., Batra H. V., Talwar G. P. Immuno-blot analysis of antigens of Mycobacterium w: a candidate anti-leprosy vaccine using monoclonal antibodies and patient sera. Zentralbl Bakteriol. 1990 Aug;273(3):378–385. doi: 10.1016/s0934-8840(11)80441-x. [DOI] [PubMed] [Google Scholar]
- Hahn H., Kaufmann S. H. The role of cell-mediated immunity in bacterial infections. Rev Infect Dis. 1981 Nov-Dec;3(6):1221–1250. doi: 10.1093/clinids/3.6.1221. [DOI] [PubMed] [Google Scholar]
- Hancock G. E., Cohn Z. A., Kaplan G. The generation of antigen-specific, major histocompatibility complex-restricted cytotoxic T lymphocytes of the CD4+ phenotype. Enhancement by the cutaneous administration of interleukin 2. J Exp Med. 1989 Mar 1;169(3):909–919. doi: 10.1084/jem.169.3.909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heeg K., Reimann J., Kabelitz D., Hardt C., Wagner H. A rapid colorimetric assay for the determination of IL-2-producing helper T cell frequencies. J Immunol Methods. 1985 Mar 18;77(2):237–246. doi: 10.1016/0022-1759(85)90036-5. [DOI] [PubMed] [Google Scholar]
- Heinzel F. P., Sadick M. D., Holaday B. J., Coffman R. L., Locksley R. M. Reciprocal expression of interferon gamma or interleukin 4 during the resolution or progression of murine leishmaniasis. Evidence for expansion of distinct helper T cell subsets. J Exp Med. 1989 Jan 1;169(1):59–72. doi: 10.1084/jem.169.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu-Li J., Ohara J., Watson C., Tsang W., Paul W. E. Derivation of a T cell line that is highly responsive to IL-4 and IL-2 (CT.4R) and of an IL-2 hyporesponsive mutant of that line (CT.4S). J Immunol. 1989 Feb 1;142(3):800–807. [PubMed] [Google Scholar]
- Karasuyama H., Melchers F. Establishment of mouse cell lines which constitutively secrete large quantities of interleukin 2, 3, 4 or 5, using modified cDNA expression vectors. Eur J Immunol. 1988 Jan;18(1):97–104. doi: 10.1002/eji.1830180115. [DOI] [PubMed] [Google Scholar]
- Kaufmann S. H., Chiplunkar S., Flesch I., De Libero G. Possible role of helper and cytolytic T cells in mycobacterial infections. Lepr Rev. 1986 Dec;57 (Suppl 2):101–111. doi: 10.5935/0305-7518.19860060. [DOI] [PubMed] [Google Scholar]
- Kaufmann S. H., Flesch I. Function and antigen recognition pattern of L3T4+ T-cell clones from Mycobacterium tuberculosis-immune mice. Infect Immun. 1986 Nov;54(2):291–296. doi: 10.1128/iai.54.2.291-296.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufmann S. H., Hahn H. Biological functions of t cell lines with specificity for the intracellular bacterium Listeria monocytogenes in vitro and in vivo. J Exp Med. 1982 Jun 1;155(6):1754–1765. doi: 10.1084/jem.155.6.1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufmann S. H., Väth U., Thole J. E., Van Embden J. D., Emmrich F. Enumeration of T cells reactive with Mycobacterium tuberculosis organisms and specific for the recombinant mycobacterial 64-kDa protein. Eur J Immunol. 1987 Mar;17(3):351–357. doi: 10.1002/eji.1830170308. [DOI] [PubMed] [Google Scholar]
- Kaufmann S. Towards new leprosy and tuberculosis vaccines. Microbiol Sci. 1987 Nov;4(11):324–328. [PubMed] [Google Scholar]
- Liew F. Y. Functional heterogeneity of CD4+ T cells in leishmaniasis. Immunol Today. 1989 Feb;10(2):40–45. doi: 10.1016/0167-5699(89)90302-2. [DOI] [PubMed] [Google Scholar]
- Mosmann T. R., Cherwinski H., Bond M. W., Giedlin M. A., Coffman R. L. Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J Immunol. 1986 Apr 1;136(7):2348–2357. [PubMed] [Google Scholar]
- Munk M. E., Gatrill A. J., Kaufmann S. H. Target cell lysis and IL-2 secretion by gamma/delta T lymphocytes after activation with bacteria. J Immunol. 1990 Oct 15;145(8):2434–2439. [PubMed] [Google Scholar]
- Mustafa A. S. Identification of T-cell-activating recombinant antigens shared among three candidate antileprosy vaccines, killed M. leprae, M. bovis BCG, and mycobacterium w. Int J Lepr Other Mycobact Dis. 1988 Jun;56(2):265–273. [PubMed] [Google Scholar]
- Müller I., Cobbold S. P., Waldmann H., Kaufmann S. H. Impaired resistance to Mycobacterium tuberculosis infection after selective in vivo depletion of L3T4+ and Lyt-2+ T cells. Infect Immun. 1987 Sep;55(9):2037–2041. doi: 10.1128/iai.55.9.2037-2041.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller I., Pedrazzini T., Farrell J. P., Louis J. T-cell responses and immunity to experimental infection with leishmania major. Annu Rev Immunol. 1989;7:561–578. doi: 10.1146/annurev.iy.07.040189.003021. [DOI] [PubMed] [Google Scholar]
- Orme I. M., Collins F. M. Adoptive protection of the Mycobacterium tuberculosis-infected lung. Dissociation between cells that passively transfer protective immunity and those that transfer delayed-type hypersensitivity to tuberculin. Cell Immunol. 1984 Mar;84(1):113–120. doi: 10.1016/0008-8749(84)90082-0. [DOI] [PubMed] [Google Scholar]
- Orme I. M. The kinetics of emergence and loss of mediator T lymphocytes acquired in response to infection with Mycobacterium tuberculosis. J Immunol. 1987 Jan 1;138(1):293–298. [PubMed] [Google Scholar]
- Ottenhoff T. H., Ab B. K., Van Embden J. D., Thole J. E., Kiessling R. The recombinant 65-kD heat shock protein of Mycobacterium bovis Bacillus Calmette-Guerin/M. tuberculosis is a target molecule for CD4+ cytotoxic T lymphocytes that lyse human monocytes. J Exp Med. 1988 Nov 1;168(5):1947–1952. doi: 10.1084/jem.168.5.1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pace J. L., Russell S. W., LeBlanc P. A., Murasko D. M. Comparative effects of various classes of mouse interferons on macrophage activation for tumor cell killing. J Immunol. 1985 Feb;134(2):977–981. [PubMed] [Google Scholar]
- Pace J. L., Russell S. W., Torres B. A., Johnson H. M., Gray P. W. Recombinant mouse gamma interferon induces the priming step in macrophage activation for tumor cell killing. J Immunol. 1983 May;130(5):2011–2013. [PubMed] [Google Scholar]
- Prat M., Gribaudo G., Comoglio P. M., Cavallo G., Landolfo S. Monoclonal antibodies against murine gamma interferon. Proc Natl Acad Sci U S A. 1984 Jul;81(14):4515–4519. doi: 10.1073/pnas.81.14.4515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadick M. D., Heinzel F. P., Holaday B. J., Pu R. T., Dawkins R. S., Locksley R. M. Cure of murine leishmaniasis with anti-interleukin 4 monoclonal antibody. Evidence for a T cell-dependent, interferon gamma-independent mechanism. J Exp Med. 1990 Jan 1;171(1):115–127. doi: 10.1084/jem.171.1.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott P., Caspar P., Sher A. Protection against Leishmania major in BALB/c mice by adoptive transfer of a T cell clone recognizing a low molecular weight antigen released by promastigotes. J Immunol. 1990 Feb 1;144(3):1075–1079. [PubMed] [Google Scholar]
- Scott P., Natovitz P., Coffman R. L., Pearce E., Sher A. Immunoregulation of cutaneous leishmaniasis. T cell lines that transfer protective immunity or exacerbation belong to different T helper subsets and respond to distinct parasite antigens. J Exp Med. 1988 Nov 1;168(5):1675–1684. doi: 10.1084/jem.168.5.1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott P., Pearce E., Cheever A. W., Coffman R. L., Sher A. Role of cytokines and CD4+ T-cell subsets in the regulation of parasite immunity and disease. Immunol Rev. 1989 Dec;112:161–182. doi: 10.1111/j.1600-065x.1989.tb00557.x. [DOI] [PubMed] [Google Scholar]
- Singh I. G., Mukherjee R., Talwar G. P. Resistance to intravenous inoculation of Mycobacterium tuberculosis H37Rv in mice of different inbred strains following immunization with a leprosy vaccine based on Mycobacterium w. Vaccine. 1991 Jan;9(1):10–14. doi: 10.1016/0264-410x(91)90309-t. [DOI] [PubMed] [Google Scholar]
- Slade S. J., Langhorne J. Production of interferon-gamma during infection of mice with Plasmodium chabaudi chabaudi. Immunobiology. 1989 Oct;179(4-5):353–365. doi: 10.1016/S0171-2985(89)80041-5. [DOI] [PubMed] [Google Scholar]
- Smith P. K., Krohn R. I., Hermanson G. T., Mallia A. K., Gartner F. H., Provenzano M. D., Fujimoto E. K., Goeke N. M., Olson B. J., Klenk D. C. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985 Oct;150(1):76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- Spitalny G. L., Havell E. A. Monoclonal antibody to murine gamma interferon inhibits lymphokine-induced antiviral and macrophage tumoricidal activities. J Exp Med. 1984 May 1;159(5):1560–1565. doi: 10.1084/jem.159.5.1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland I. Future policy for BCG vaccination in Britain. Postgrad Med J. 1971 Nov;47(553):756–758. doi: 10.1136/pgmj.47.553.756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talwar G. P. Concluding comments on development of a vaccine against leprosy. Lepr India. 1978 Oct;50(4):597–598. [PubMed] [Google Scholar]
- Talwar G. P., Zaheer S. A., Mukherjee R., Walia R., Misra R. S., Sharma A. K., Kar H. K., Mukherjee A., Parida S. K., Suresh N. R. Immunotherapeutic effects of a vaccine based on a saprophytic cultivable mycobacterium, Mycobacterium w in multibacillary leprosy patients. Vaccine. 1990 Apr;8(2):121–129. doi: 10.1016/0264-410x(90)90134-8. [DOI] [PubMed] [Google Scholar]
- Talwar G. P., Zaheer S. A., Suresh N. R., Parida S. K., Mukherjee R., Singh I. G., Sharma A. K., Kar H. K., Misra R. S., Mukherjee A. Immunotherapeutic trials with a candidate anti-leprosy vaccine based on Mycobacterium w. Trop Med Parasitol. 1990 Sep;41(3):369–370. [PubMed] [Google Scholar]



