Abstract
Brain glucose sensing is critical for healthy energy balance, but how appropriate neurocircuits encode both small changes and large background values of glucose levels is unknown. Here, we report several features of hypothalamic orexin neurons, cells essential for normal wakefulness and feeding: (i) A distinct group of orexin neurons exhibits only transient inhibitory responses to sustained rises in sugar levels; (ii) this sensing strategy involves time-dependent recovery from inhibition via adaptive closure of leak-like K+ channels; (iii) combining transient and sustained glucosensing allows orexin cell firing to maintain sensitivity to small fluctuations in glucose levels while simultaneously encoding a large range of baseline glucose concentrations. These data provide insights into how vital behavioral orchestrators sense key features of the internal environment while sustaining a basic activity tone required for the stability of consciousness.
Keywords: brain, glucose, hypocretin, orexin, hypothalamus
To survive, living organisms need to vary their behavior according to internal energy levels. In mammals, this involves translating the hormone and nutrient content of the extracellular fluid into appropriate combinations of brain states such as hunger, arousal, and motivation (1). This translation critically relies on neurons producing the peptide neurotransmitters orexins/hypocretins (orexin neurons) (2, 3). Orexin neurons are located in the hypothalamus but innervate most of the brain, with major inputs to arousal and reward centers, where orexins are released and act on two specific G protein coupled receptors (4, 5). The firing of orexin neurons promotes wakefulness (6) and is so important for maintaining normal consciousness that loss of orexin cells causes severe narcolepsy/cataplexy (7, 8). Orexins are also a powerful stimulus for reward-seeking behavior, and destruction of orexin neurons prevents fasting from stimulating foraging (9–12). Besides promoting wakefulness and reward-seeking, orexin cells are involved in memory, stress, and cardiovascular control (reviewed in ref. 5).
Recent data show that orexin neurons are not only key effectors of vital behaviors but are also specialized sensors of the body's internal environment. In particular, they act as electrical detectors of glucose, a fundamental signal informing the brain of changes in body energy reserves (12–14). Small physiological rises in ambient [glucose] are sufficient to trigger large K+ currents in orexin cell bodies, causing hyperpolarization and suppression of action potential firing (15). Presumably, it is this combination of critical “sensor” and “effector” tasks that makes the orexin system such a prominent link between body energy status and behavior (12). However, this multitasking also poses an unsolved paradox. If orexin cells are shut down by even a small rise in glucose and loss of their activity causes narcolepsy, how is narcolepsy-free consciousness maintained after a meal or during diabetic hyperglycemia? One theoretical solution could be to delegate different functions to different sets of orexin neurons, whereby some cells measure energy status while others maintain cognitive arousal. Another theoretical solution, used by classical sensory systems such as the eye, is to encode changes in stimulus levels but not unchanging (baseline) stimulus levels, a phenomenon called adaptation (16). This would allow orexin cells to maintain a firing tone within a wide range of glucose levels.
Evaluating these theories is central to understanding the brain control of adaptive behavior and requires solving the following unknowns: (i) Are there functional subgroups of orexin cells and do they have different energy-sensing properties? (ii) Is the orexin system capable of adaptive glucose-sensing? To address these issues, here we detect living orexin neurons by transgenic GFP labeling, explore the temporal pattern of their glucose responses, and relate it to their intrinsic electrical properties and anatomical locations. We demonstrate that ≈70% of orexin cells exhibit “adaptive” responses to glucose, self-restoring their firing after inhibition by the sugar. This adaptation involves closure of leak-like K+ channels. Adapting and nonadapting orexin cells have different membrane properties and anatomical distributions. These results provide insights into how behaviorally critical brain circuits capture vital information about body energy resources without losing their basic activity tone.
Results
Transient and Sustained Glucose Sensors Among Orexin Neurons.
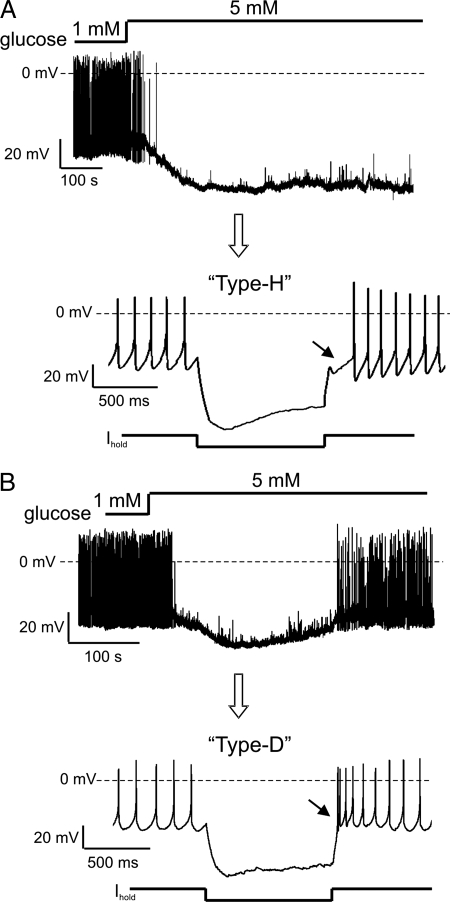
To study the time course of glucose responses, we monitored the membrane potential of individual orexin neurons in living brain slices at physiological temperatures (see Methods). Orexin neurons were precisely and unambiguously identified by transgenic labeling with eGFP, as in our previous studies (15, 17). Brain interstitial glucose levels are typically 10–30% of those in the blood and vary between ≈0.2 and 5 mM (14, 18). In 30% of orexin cells, switching from 1 to 5 mM bath glucose induced sustained membrane hyperpolarization and inhibition of firing, as previously reported (Fig. 1A, n = 18 of 60 cells). However, in the remaining 70% of orexin cells the time course of the membrane response did not follow that of the glucose stimulus. Instead, the membrane potential of these cells first fell but then, despite high glucose levels, spontaneously crept back to values close to the initial resting potential, causing the cells to resume firing (Fig. 1B, n = 42 of 60 cells). In the rest of the article, we call this type of transient response to glucose adaptive by analogy with adaptation in classical sensory systems (16).
Fig. 1.
Adaptive and nonadaptive glucose sensors among orexin neurons. (A) Nonadaptive membrane potential response to glucose (Upper) and corresponding whole-cell electrical fingerprint (Lower). Current-clamp protocol is shown below the trace. (B) Adaptive response to glucose (Upper) and corresponding whole-cell electrical fingerprint (Lower). Current-clamp protocol is shown below the trace.
To assess whether adaptive and nonadaptive orexin neurons have distinct intrinsic properties as well as distinct glucosensory abilities, we analyzed their membrane potential responses to hyperpolarizing current pulses, an established method of “electrical fingerprinting” of hypothalamic neurons (19, 20). For this analysis, cells were classified as adapting if they showed membrane repolarization within 15 min of peak glucose response, and as nonadapting if they did not display any repolarization during this time period. Nonadapting orexin cells exhibited a pronounced hyperpolarized rebound potential upon termination of the current pulse, which we called a “Type-H” response (H for hyperpolarized) (Fig. 1A, n = 15 cells). This electrical fingerprint was previously described for orexin neurons (21, 22). In contrast, adapting orexin cells exhibited a strikingly different type of rebound potential: a transient depolarization crowned with spikes; we called this new type of orexin cell fingerprint “Type-D” (Fig. 1B, n = 30 cells).
Membrane Properties and Anatomical Location of Adapting and Nonadapting Cells.
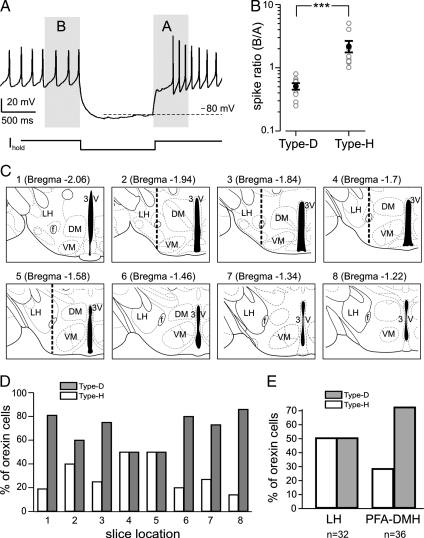
To quantify the electrophysiological differences between Type-H (nonadapting) and Type-D (adapting) orexin cells, we calculated “spike ratio,” the number of spikes in the 500 ms before the inhibitory current pulse divided by that in the 500 ms after the pulse (Fig. 2A). In this quantification, we created the same biophysical conditions for all cells by choosing current injections that hyperpolarized the cells to −80 mV (Fig. 2A) and using a K-gluconate intracellular solution (see Methods). In Type-H cells, the spike ratio was 2.15 ±0.42 (largest = 4, smallest = 1), but in Type-D cells, the spike ratio was significantly smaller, 0.51 ±0.06 (largest = 0.8, smallest = 0.25) (Fig. 2B, n = 10 for each cell type, P <0.005). In terms of spontaneous firing, Type-D cells tended to fire slightly faster than Type-H cells (4.13 ± 0.44 Hz and 3.75 ± 0.31 Hz respectively, n = 8 for each type), but this difference was not significant (P = 0.5), indicating that the differences in spike ratio are produced by currents that are more active during postinhibitory rebound than during baseline firing.
Fig. 2.
Electrical and anatomical properties of adaptive and nonadaptive cells. (A) Electrical fingerprint protocol showing time regions analyzed in B. (B) Spike ratios (see text) of Type-D (adaptive) and Type-H (nonadaptive) cells. Gray circles are raw data, black circles and bars are means ±SEM. ***, P <0.005. (C) Schematic drawings of coronal hypothalamic slices from which we recorded. Corresponding adult Bregma coordinates are given in brackets. In slices 3–5, the dotted vertical line shows the border between LH and PFA-DMH used to collect data in E. (D) Anterioposterior distribution of the two cell types. Proportions of Type-D and Type-H cells are given as percentages of total number of orexin neurons recorded in the series of slices shown in C (n = 128 cells). (E) Lateromedial distribution of the two cell types. Proportions of Type-D and Type-H cells are given as percentages of total number of orexin neurons recorded in LH and PFA-DMH regions (left and right sides respectively of the vertical dotted line in slices 3–5.
We next compared the anatomical locations of Type-H and Type-D cells by first performing electrical fingerprinting of orexin-GFP cells and then noting the anterioposterior locations of the coronal slice from which they were recorded. Within the anterioposterior field in which orexin-eGFP neurons were found (Fig. 2C), Type-D neurons were on average 3.5-fold more numerous than Type-H neurons everywhere except in the middle of the field (adult Bregma coordinate range approximately −1.55 to −1.75 mm), where the relative numbers appeared equal (Fig. 2D). Because recent in vivo studies implied different postbehavioral activation profiles for medially vs. laterally located orexin neurons (9, 23), we also compared the relative numbers of Type-D and Type-H orexin cells in the lateral hypothalamus (LH) and the perifornical/dorsomedial hypothalamus region (PFA-DMH). Because the postbehavioral staining studies focused on the middle portion of the anterioposterior orexin field, to facilitate comparisons with our data, we restricted our lateromedial mapping to these slices (slices 2–5 Fig. 2C). We found that Type-D (adapting) cells were more numerous in the PFA-DMH than in the LH, whereas Type-H (nonadapting cells) were more numerous in the LH than in the PFA-DMH (Fig. 2E). This difference between the percentage compositions of Type-D and Type-H cells in the LH and PFA-DMH (Fig. 2E) is unlikely to arise by chance (P <0.005, binomial test). This is intriguing because orexin neurons in the LH and PFA-DMH have been proposed to be differentially engaged in arousal vs. reward (see Discussion).
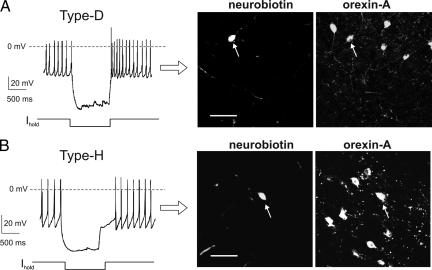
In orexin-eGFP transgenic mice (B6D2 strain, ref. 15), Type-D and Type-H electrical fingerprints persisted in different intracellular solutions: K-gluconate (Fig. 1), K-chloride (Fig. 3A, n = 10 for each cell type), and K-methylsulphate (Fig. 3B, n = 3 for each cell type). Type-D and Type-H fingerprints were also clearly apparent at 22°C as well as 35°C (n = 60 and 35, respectively; data not shown). We also tested whether the two types of cells are present in wild-type mice (C57BL6J strain), by performing electrical fingerprinting followed by neurobiotin filling and immunocytochemistry (Fig. 4 A and B). As in orexin-GFP mice, Type-D or Type-H fingerprints were exhibited by all orexin neurons in wild-type mice (Fig. 4 A and B, n = 60 cells, of which 17 were Type-Hs and 43 Type-Ds). This suggests that the cellular dichotomy in the orexin system is a robust phenomenon, independent of recording conditions and type of mouse.
Fig. 3.
Two orexin cell types persist in different experimental solutions. (A) Type-H and Type-D fingerprints recorded with a K-chloride intracellular solution in slices from orexin-eGFP mice. (B) Type-H and Type-D fingerprints recorded with a K-methylsulphate intracellular solution in slices from orexin-eGFP mice.
Fig. 4.
Two orexin cell types persist in different strains of mice. (A) (Left) Type-D fingerprint recorded in slice from wild-type (C57BL6J) mice. (Right) immunofluorescence imaging of the recorded cell, identified by neurobiotin staining; the cell contains orexin-A. Scale bar, 50 μm. (B) (Left) Type-H fingerprint recorded in slice from wild-type (C57BL6J) mice. (Right) immunofluorescence imaging of the recorded cell, identified by neurobiotin staining; the cell contains orexin-A. Scale bar, 50 μm.
Dynamics of Adaptive Glucose-Sensing.
To compare the dynamics of glucose responses in Type-D and Type-H orexin cells in more detail, we plotted the average values of their membrane potentials against time in high [glucose] (Fig. 5A). In Type-D neurons, the membrane potential depolarized back toward prestimulation levels with a half-recovery time of ≈200 s (Fig. 5A). This membrane potential adaptation was complete or nearly complete in some Type-D cells (n = 25) but appeared partial (50–70% recovery) in other Type-D cells (n = 17, Fig. 5B). This partial adaptation was seen at both high (5 mM) and low (2.5 mM) levels of the glucose stimulus. When we switched [glucose] back to low control levels, the partially adapted Type-D cells completely depolarized back to control levels (Fig. 5B), suggesting that partial adaptation is not an artifact caused by a hyperpolarizing drift during long-term recordings. Both partial and complete adaptation in Type-D cells was minimal or absent when the neurons were stimulated with glucose at 22°C instead of 35°C (cells monitored for up to 1 h, n = 10). This temperature dependence presumably explains why adaptation was not apparent when glucose-sensing in orexin cells was previously studied at room temperature (15, 22).
Fig. 5.
Dynamics of adaptive glucose-sensing. (A) Mean time course of glucose responses. Data are means ±SEM (n = 4). (B) Example of partial adaptation in a Type-D orexin cell. (C) Responses to sequential rises in [glucose] in a Type-D orexin cell.
In contrast, the inhibition of Type-H neurons by glucose was always persistent, with no membrane potential drift toward control levels at either 35°C (n = 15, see Fig. 5 A) or 22°C (cells monitored for up to 1 h, n = 30). Importantly, we verified that, despite long-term recordings, Type-H cells still had the ability to depolarize and fire, by reducing [glucose] back to control levels at the end of recording, which invariably led to reversal of glucose effects on the membrane potential (n = 10).
In terms of neural encoding of stimuli, a potential advantage of adaptive sensing is its ability to “filter out” baseline stimulus levels, thereby greatly expanding the range of stimulus intensities within which stimulus changes can be sensed (16). To test whether this occurs in the case of adaptive glucose-sensing by Type-D orexin neurons, we sequentially stimulated the cells with two [glucose] steps, first from 0.2 to 2.5 mM and then from 2.5 to 5 mM, thereby covering the full physiological range of brain [glucose] (18). Upon the switch from 0.2 to 2.5 mM, the cells hyperpolarized and stopped firing (Fig. 5C). Without adaptation, this would preclude their firing from encoding further elevations in [glucose]. However, the cells adapted to the continuing presence of 2.5 mM glucose, repolarizing and resuming their firing, which then allowed the firing to be reduced again by the switch to 5 mM glucose (Fig. 5C, n = 4). This “double-sensing” experiment demonstrates that adaptive sensing is necessary for orexin cell firing to encode fluctuations in glucose levels within the full physiological spectrum of its concentrations.
Mechanism of Adaptive Glucose-Sensing.
To investigate membrane mechanisms of glucose adaptation in Type-D orexin neurons, we monitored changes in membrane resistance and currents during adaptation. Theoretically, there are two fundamentally different mechanisms that could underlie the membrane repolarization during adaptation: (i) up-regulation of depolarizing membrane conductances or (ii) down-regulation of inhibitory membrane conductances. To discriminate between these possibilities, we first monitored membrane conductance during adaptation by examining the amplitude of membrane responses to periodic injections of fixed-magnitude hyperpolarizing current (see Methods). Membrane depolarization during adaptation was associated with a progressive reduction in the membrane conductance (Fig. 6A, n = 5), which persisted during synaptic isolation with tetrodotoxin (Fig. 6B, n = 3). These data imply that the adaptation is a postsynaptic phenomenon involving a net reduction in inhibitory membrane currents.
Fig. 6.
Mechanism of adaptive glucose-sensing in Type-D orexin cells. (A) Membrane resistance changes during adaptation in a Type-D orexin cell. (B) Response to glucose in the presence of 1 μM tetrodotoxin in a Type-D cell. (C) Membrane I-V relationships during different phases of the glucose response. (D) I-V relationship of the net current reduced during adaptation in Type-D cells. Data are means ±SEM (n = 4). Line shows a fit of GHK equation to the data (see Methods).
Glucose-induced hyperpolarization of orexin neurons involves activation of a postsynaptic K+ conductance exhibiting Goldman-Hodgkin-Katz (GHK) current–voltage rectification (15). To test whether the same or different inhibitory conductance becomes down-regulated during adaptation, we directly measured membrane currents during glucose responses and subsequent adaptation, using a K-chloride intracellular solution to minimize junction potential errors (24). As reported (15), glucose activated a current with an equilibrium potential of approximately −100 mV, which under our recording conditions (high [Cl−] in cytosol), can only be due to K+ channels (Fig. 6C). In Type-D orexin cells, adaptation involved a suppression of current with the same equilibrium potential (Fig. 6C, n = 4), suggesting that the current turned off during adaptation is the same as that initially turned on by glucose. To evaluate this further, we isolated the net current reduced during adaptation by subtracting whole-cell currents after 10–20 min of adaptation from those recorded from the same cell during its peak response to glucose (Fig. 6D). The net membrane current reduced during adaptation in Type-D cells had a reversal potential of −98 ±3 mV (Fig. 6D, n = 4), confirming its selectivity for K+ ions (EK ∼−100 mV). Furthermore, the current exhibited an outwardly rectifying current–voltage relation well described by the GHK equation (Fig. 6D). This implies that glucose adaptation involves closure of leak-like K+ channels. In contrast, in Type-H orexin cells, glucose triggered a persistent K+ that did not become smaller during the period of glucose application (n = 17).
Discussion
Here, we describe adaptive glucose-sensing, a mode of energy monitoring in hypothalamic networks orchestrating appetite, arousal, and reward. In particular, we identify three unexpected features of orexin/hypocretin networks. First, ≈70% of orexin neurons exhibit adapting firing responses to glucose (Fig. 1). This allows a large proportion of the orexin system to maintain electrical excitability irrespective of ambient glucose levels. Second, adapting and nonadapting orexin cells are also significantly different in their innate firing properties and anatomical distribution (Fig. 2). This reveals an intrinsic electrophysiological dichotomy among orexin neurons. Third, glucose adaptation is mediated by closure of leak-like K+ channels (Fig. 6 C and D) and acts as an automatic “sliding scale” that allows orexin cells to shift their firing sensitivity to match different ranges in glucose levels (Fig. 5C). This way of sensory encoding potentially suggests how the orexin system can track a wide range of sugar fluctuations without destabilizing consciousness.
Cellular and Sensory Dichotomy in the Orexin System.
The sensory dichotomy among orexin neurons corresponded to significant differences in their intrinsic membrane properties. Adapting orexin cells exhibited Type-D electrical fingerprints, characterized by transient postinhibitory depolarization and excitation (Figs. 1B and 2B). Nonadapting orexin cells exhibited Type-H fingerprints, showing transient postinhibitory hyperpolarization and inhibition (Fig. 1A and 2B). These differences in membrane potential response to depolarization indicate differences in the expression of depolarization-activated ion channels. For example, postinhibitory hyperpolarization of orexin cells is likely to be caused by A-type K+ channels (21), while postinhibitory excitation could theoretically be due to ICAN channels present in orexin cells (25). However, our voltage-clamp analysis (Fig. 6 C and D) argued against causal links between such channels and glucose adaptation. Instead, our data favor a simpler explanation, that selective down-regulation of nonvoltage-gated (leak) K+ channels, presumably the same channels as those activated by glucose (15), underlies glucose adaptation in Type-D cells (Fig. 6 C and D). What makes these channels progressively reduce their activity in Type-D but not Type-H orexin cells is a key question for future investigation. An answer probably lies in the currently unknown intracellular transduction pathways that couple extracellular glucose changes to the activity of background K+ channels (15).
Our anatomical mapping of adapting and nonadapting orexin neurons suggested that these cell types remain intermixed throughout the field where orexin cells are found (Fig. 2 D and E). However, they appear to be intermixed in different proportions. In particular, the percentage of Type-H cells is highest in the middle portion of the anterioposterior field in which orexin neurons are found (Fig. 2 D and E). Of note, in these “midfield” slices, the nonadapting Type-H cells are preferentially distributed in the LH region, whereas the adapting Type-D cells tend to be located more in the PFA-DMH (Fig. 2E). Based on postbehavioral activation mapping of orexin neurons in the same locations, it was recently suggested that LH orexin neurons are more involved in regulating reward, whereas PFA-DMH neurons are more involved in maintaining cognitive arousal (23). It is thus tempting to speculate that adaptive and nonadaptive cells are differentially involved in the diverse behaviors orchestrated by the orexin system. For example, whether adaptive and nonadaptive glucose sensors could respectively regulate arousal and reward merits further investigation.
Implications for Brain State Control.
Orexin neurons are vital for promoting wakefulness and regulating feeding behavior; elucidating the temporal profile of their activity is important not only for basic knowledge but also potentially for understanding the obesity epidemic in the developed world (26). We propose that adaptive glucose responses of orexin neurons, described here, represent a previously overlooked sensory strategy contributing to appropriate control of behavior by internal energy supplies. Our data indicate that the orexin system combines adapting and nonadapting sensory elements to capture diverse features of vital stimuli. Adaptive glucose-sensing may enable a large part of the orexin system to “filter out” baseline glucose levels while continuing to translate changes in glucose levels into changes in firing (Fig. 5C) and hence behavior (6). This may help to ensure that vital behaviors continue to be appropriately matched to trends in body energy levels irrespective of baseline glucose levels. Importantly, adaptive sensing enables much of the orexin system to maintain an active and excitable state, which may protect consciousness from becoming narcoleptically unstable after meals. In turn, nonadaptive sensing by a smaller population of orexin cells could encode the absolute levels of glucose, information that is presumably critical for long-term control of body weight and energy homeostasis. Integrating the firing output from transient and sustained glucosensors, for example on the level of net orexin release at projection targets, may thus allow the orexin system to signal small fluctuations in glucose levels while simultaneously encoding larger background levels of the sugar.
Methods
Preparation of Brain Slices from Transgenic and Wild-Type Mice.
Procedures involving animals were carried out in accordance with the Animals (Scientific Procedures) Act, 1986 (U.K.). We used transgenic orexin-eGFP mice in all experiments, except where indicated (Fig. 4 A and B). The transgenic mice (B6D2 strain) expressed enhanced GFP under the control of prepro-orexin promoter, as described in our previous study (15). This results in very specific targeting of eGFP only to orexin neurons (15). In experiments shown in Fig. 4 A and B, we used wild-type mice (C57BL6J strain). Mice were maintained on a 12-h light:dark cycle (lights on 08:00 a.m.) and had free access to food and water. Animals (14–20 days postnatal) were killed during the light phase, and coronal slices (250–300 μm thick) containing the lateral hypothalamus were prepared as described (17). Adult Bregma coordinates corresponding to different hypothalamic slices we studied (Fig. 2C) were taken from the stereotaxic mouse brain atlas of Franklin and Paxinos (27).
Electrophysiology.
Solutions.
Extracellular solution was ACSF gassed with 95% O2 and 5% CO2, and contained: 125 mM NaCl, 3 mM KCl, 2 mM MgCl2, 2 mM CaCl2, 1.2 mM NaH2PO4, 26 mM NaHCO3, glucose, as indicated. Three types of intracellular (pipette) solutions were used. K-chloride pipette solution (used in Figs. 3 A and 6) contained 130 mM KCl, 0.1 mM EGTA, 10 mM Hepes, 5 mM K2ATP, 1 mM NaCl, 2 mM MgCl2, pH = 7.3 with KOH. K-gluconate pipette solution (used in Figs. 1, 2, and 4 and 5) contained 120 mM K-gluconate, 10 mM KCl, 0.1 mM EGTA, 10 mM Hepes, 5 mM K2ATP, 1 mM NaCl, 2 mM MgCl2, pH = 7.3 with KOH. K-methylsulphate pipette solution (used in Fig. 3 B) contained 120 mM K-MeSO4, 10 mM KCl, 0.1 mM EGTA, 10 mM Hepes, 5 mM K2ATP, 1 mM NaCl, 2 mM MgCl2, pH = 7.3 with KOH. Tetrodotoxin (TTX) was obtained from Tocris Cookson. All other chemicals were from Sigma.
Data collection and analysis.
For patch–clamp recordings, living orexin-eGFP neurons were visualized in brain slices using an Olympus BX50WI upright microscope equipped with infrared gradient contrast optics (28), a mercury lamp, and filters for visualizing eGFP-containing cells. Whole-cell voltage– and current–clamp recordings from orexin-eGFP neurons were performed using an EPC-9 amplifier (Heka) at 35°C, as described (17). Patch pipettes were pulled from borosilicate glass and had tip resistances of 4–7 MΩ (when measured with K-gluconate pipette solution). Series resistances were in the range of 5–10 MΩ and were not compensated. Data were sampled and filtered using Pulse/Pulsefit software (Heka) and analyzed with Pulsefit and Origin (Microcal) software.
To monitor membrane resistance together with changes in membrane potential (Fig. 6 A and B), cells were injected every 20–40 s with a fixed-amplitude (10–40 pA, 1-s duration) square pulse of hyperpolarizing current (22). The amplitude of the resulting downward deflections in membrane potential is proportional to membrane resistance (Ohm's law).
Current–voltage relationships (Fig. 6 C and D) were obtained by performing voltage–clamp ramps from 0 to −140 mV at a rate of 0.1 mV/ms ramp, which is sufficiently slow to allow leak-like K+ currents to reach steady-state at each potential (29). In Fig. 6D, the net current–voltage relationship was fitted with the GHK current equation (30) in the following form:
Here, I is current (in A), V is membrane potential (in V), z is the charge of a potassium ion (+1), [K+]i is the pipette K+ concentration (in mM), [K+] is the ACSF K+ concentration (in mM), T is temperature (in Kelvin), and R and F have their usual meanings (30). PK (a constant reflecting the K+ permeability of the membrane) was the only free parameter during fits. The value of PK used to obtain the fit shown in Fig. 6D was 1.389 × 10−17 cm s−1. Values are presented as means ±SEM.
Immunohistochemistry.
Postrecording immunofluorescence detection of orexin neurons in wild-type mice (Fig. 4 A and B) was performed as in our previous studies (22). Neurobiotin tracer (0.2%) (Vector Laboratories) was added to the pipette solution. Neurons were maintained in the whole-cell configuration for 5–10 min to record the electrical fingerprint and allow thorough cytosolic infusion of neurobiotin, after which the patch pipette was gently withdrawn via the outside-out configuration. Slices were then fixed in 4% paraformaldehyde (Sigma) in PBS (pH 7.4) for 1 h at room temperature, incubated with primary antibodies overnight, and then with fluorescent secondary antibodies for 3 h. The primary antibodies and labels were in [volume:volume] dilutions: neurobiotin, rabbit anti-orexin-A [1:250] (Phoenix Pharmaceuticals). The corresponding secondary antibodies and labels were in [volume:volume] dilutions: streptavidin-Cy2 [1:500] (Amersham Biosciences), goat anti-rabbit-Cy5 [1:500] (Amersham Biosciences). Slices were analyzed using a Leica TCS SP2 confocal laser-scanning microscope. Cy2 was excited with an Argon laser at 488 nm and fluorescence emission collected at 505–530 nm. Cy5 was excited with a HeNe laser at 633 nm and emission detected at 650–700 nm. To achieve optimal separation of fluorescent signals, the sequential “between lines” scanning mode of the microscope was used.
Acknowledgments.
We thank Dr. Antonio Gonzalez for assistance with data analysis and comments on the manuscript. This work was supported by Diabetes U.K. (grant to D.B.), Biotechnology and Biological Sciences Research Council (grant to D.B. and studentship to R.H.W.), and the Royal Society (Dorothy Hodgkin fellowship to D.B.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
References
- 1.Saper CB, Chou TC, Elmquist JK. The need to feed: Homeostatic and hedonic control of eating. Neuron. 2002;36:199–211. doi: 10.1016/s0896-6273(02)00969-8. [DOI] [PubMed] [Google Scholar]
- 2.de Lecea L, et al. The hypocretins: Hypothalamus-specific peptides with neuroexcitatory activity. Proc Natl Acad Sci USA. 1998;95:322–327. doi: 10.1073/pnas.95.1.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sakurai T, et al. Orexins and orexin receptors: A family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell. 1998;92:573–585. doi: 10.1016/s0092-8674(00)80949-6. [DOI] [PubMed] [Google Scholar]
- 4.Sutcliffe JG, de Lecea L. The hypocretins: Setting the arousal threshold. Nat Rev Neurosci. 2002;3:339–349. doi: 10.1038/nrn808. [DOI] [PubMed] [Google Scholar]
- 5.Sakurai T. The neural circuit of orexin (hypocretin): Maintaining sleep and wakefulness. Nat Rev Neurosci. 2007;8:171–181. doi: 10.1038/nrn2092. [DOI] [PubMed] [Google Scholar]
- 6.Adamantidis AR, Zhang F, Aravanis AM, Deisseroth K, de Lecea L. Neural substrates of awakening probed with optogenetic control of hypocretin neurons. Nature. 2007;450:420–424. doi: 10.1038/nature06310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lin L, et al. The sleep disorder canine narcolepsy is caused by a mutation in the hypocretin (orexin) receptor 2 gene. Cell. 1999;98:365–376. doi: 10.1016/s0092-8674(00)81965-0. [DOI] [PubMed] [Google Scholar]
- 8.Chemelli RM, et al. Narcolepsy in orexin knockout mice: Molecular genetics of sleep regulation. Cell. 1999;98:437–451. doi: 10.1016/s0092-8674(00)81973-x. [DOI] [PubMed] [Google Scholar]
- 9.Harris GC, Wimmer M, Aston-Jones G. A role for lateral hypothalamic orexin neurons in reward seeking. Nature. 2005;437:556–559. doi: 10.1038/nature04071. [DOI] [PubMed] [Google Scholar]
- 10.Borgland SL, Taha SA, Sarti F, Fields HL, Bonci A. Orexin A in the VTA is critical for the induction of synaptic plasticity and behavioral sensitization to cocaine. Neuron. 2006;49:589–601. doi: 10.1016/j.neuron.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 11.Boutrel B, et al. Role for hypocretin in mediating stress-induced reinstatement of cocaine-seeking behavior. Proc Natl Acad Sci USA. 2005;102:19168–19173. doi: 10.1073/pnas.0507480102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yamanaka A, et al. Hypothalamic orexin neurons regulate arousal according to energy balance in mice. Neuron. 2003;38:701–713. doi: 10.1016/s0896-6273(03)00331-3. [DOI] [PubMed] [Google Scholar]
- 13.Routh VH. Glucose-sensing neurons: Are they physiologically relevant? Physiol Behav. 2002;76:403–413. doi: 10.1016/s0031-9384(02)00761-8. [DOI] [PubMed] [Google Scholar]
- 14.Burdakov D, Luckman SM, Verkhratsky A. Glucose-sensing neurons of the hypothalamus. Philos Trans R Soc London Ser B. 2005;360:2227–2235. doi: 10.1098/rstb.2005.1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Burdakov D, et al. Tandem-pore K+ channels mediate inhibition of orexin neurons by glucose. Neuron. 2006;50:711–722. doi: 10.1016/j.neuron.2006.04.032. [DOI] [PubMed] [Google Scholar]
- 16.Carpenter R. Neurophysiology. London: Arnold; 2003. [Google Scholar]
- 17.Williams RH, Jensen LT, Verkhratsky A, Fugger L, Burdakov D. Control of hypothalamic orexin neurons by acid and CO2. Proc Natl Acad Sci USA. 2007;104:10685–10690. doi: 10.1073/pnas.0702676104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Silver IA, Erecinska M. Extracellular glucose concentration in mammalian brain: Continuous monitoring of changes during increased neuronal activity and upon limitation in oxygen supply in normo-, hypo-, and hyperglycemic animals. J Neurosci. 1994;14:5068–5076. doi: 10.1523/JNEUROSCI.14-08-05068.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Miki T, et al. ATP-sensitive K+ channels in the hypothalamus are essential for the maintenance of glucose homeostasis. Nat Neurosci. 2001;4:507–512. doi: 10.1038/87455. [DOI] [PubMed] [Google Scholar]
- 20.Burdakov D, Ashcroft FM. Cholecystokinin tunes firing of an electrically distinct subset of arcuate nucleus neurons by activating A-Type potassium channels. J Neurosci. 2002;22:6380–6387. doi: 10.1523/JNEUROSCI.22-15-06380.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Burdakov D, Alexopoulos H, Vincent A, Ashcroft FM. Low-voltage-activated A-current controls the firing dynamics of mouse hypothalamic orexin neurons. Eur J Neurosci. 2004;20:3281–3285. doi: 10.1111/j.1460-9568.2004.03815.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Burdakov D, Gerasimenko O, Verkhratsky A. Physiological changes in glucose differentially modulate the excitability of hypothalamic melanin-concentrating hormone and orexin neurons in situ. J Neurosci. 2005;25:2429–2433. doi: 10.1523/JNEUROSCI.4925-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Harris GC, Aston-Jones G. Arousal and reward: A dichotomy in orexin function. Trends Neurosci. 2006;29:571–577. doi: 10.1016/j.tins.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 24.Barry PH, Lynch JW. Liquid junction potentials and small cell effects in patch-clamp analysis. J Membr Biol. 1991;121:101–117. doi: 10.1007/BF01870526. [DOI] [PubMed] [Google Scholar]
- 25.Eggermann E, Bayer L, Serafin M, Saint-Mleux B, Bernheim L, Machard D, Jones BE, Muhlethaler M. The wake-promoting hypocretin-orexin neurons are in an intrinsic state of membrane depolarization. J Neurosci. 2003;23:1557–1562. doi: 10.1523/JNEUROSCI.23-05-01557.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Flier JS. Obesity wars: Molecular progress confronts an expanding epidemic. Cell. 2004;116:337–350. doi: 10.1016/s0092-8674(03)01081-x. [DOI] [PubMed] [Google Scholar]
- 27.Franklin K, Paxinos G. The Mouse Brain in Stereotoxic Coordinates. New York: Academic; 1997. [Google Scholar]
- 28.Dodt HU, Eder M, Schierloh A, Zieglgansberger W. Infrared-guided laser stimulation of neurons in brain slices. Sci STKE. 2002;2002:PL2. doi: 10.1126/stke.2002.120.pl2. [DOI] [PubMed] [Google Scholar]
- 29.Meuth SG, et al. Contribution of TWIK-related acid-sensitive K+ channel 1 (TASK1) and TASK3 channels to the control of activity modes in thalamocortical neurons. J Neurosci. 2003;23:6460–6469. doi: 10.1523/JNEUROSCI.23-16-06460.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hille B. Ion Channels of Excitable Membranes. Sunderland, MA: Sinauer; 2001. [Google Scholar]