Abstract
We examined the expression in breast cancer stem cells (BCSCs) of Globo H, a potential tumor-associated antigen for immunotherapy of epithelial cancers including breast cancer. Flow cytometry revealed Globo H expression in 25/41 breast cancer specimens (61.0%). Non-BCSCs from 25/25 and BCSCs from 8/40 (20%) expressed Globo H. We showed the expression of stage-specific embryonic antigen 3 (SSEA3), the pentasaccharide precursor of Globo H, in 31/40 (77.5%) tumors. Non-BCSCs from 29/31 and BCSCs from 25/40 (62.5%) expressed SSEA3. Like Globo H, SSEA3 expression in normal tissues was predominately at the secretory borders of epithelium, where access to the immune system is restricted. Immunization of mice with Globo H-KLH and α-GalCer induced antibodies reactive with Globo H and SSEA3, suggesting that a Globo H-based vaccine will target tumor cells expressing Globo H or SSEA3. We next sought to reduce Globo H expression by siRNA targeting fucosyltransferase (FUT) 1 and 2, which mediate alpha-1,2 linkage of fucose to SSEA3 to generate Globo H. We showed both genes to be involved in the biosynthesis of Globo H. Moreover, FUT2 expression in BCSCs was significantly lower than in non-BCSCs harvested from a primary human breast cancer in NOD/SCID mouse, whereas FUT1 was slightly lower in BCSCs. Thus, the lower expression of Globo H in BCSCs may be attributed to less FUT2/FUT1, and to reduced SSEA3 in BCSCs compared with non-BCSCs. Our findings provide insight into further development of a Globo H-based vaccine and FUT1/FUT2-targeted therapy for breast cancer.
Stem cells are defined as a group of cells with the capacity for self-renewal and for differentiation into different types of cells and tissues (1). As both malignant tumors and normal tissues contain heterogeneous populations of cells, the existence of cancer stem cells that might play a key role in tumor growth and maintaining tumor heterogeneity has been proposed (2). After the initial discovery of leukemia stem cells in 1997 by Bonnet et al. (3), cancer stem cells have been identified from a variety of solid tumors, such as brain, breast, colon, and prostate cancers (4–7). Breast cancer stem cells (BCSCs) were first shown to reside in the CD24−CD44+ subpopulation of breast cancer by Al-Hajj et al. (4), based on their ability to generate tumors with phenotypic diversity on xenotransplantation into NOD/SCID mice (4). These CD24−CD44+ BCSCs were noted to be more resistant to radiation than non-BCSCs (8). Furthermore, the majority of early disseminated cancer cells in the bone marrow of breast cancer patients displayed the phenotype of CD24−CD44+ (9), suggesting that BCSCs were capable of metastasis. Based on their capability for growth, differentiation, and metastasis and their resistance to radiation, BCSCs have now become the hotly pursued target for therapy of breast cancer (10, 11).
To design therapy against cancer stem cells, it will be desirable to seek molecular targets of cancer stem cells that are absent from normal cells. One of such potential targets is Globo H, a hexasaccharide (Fucα 1→2Galβ1→3GalNAc β1→3Galα 1→4Galβ1→4Glcβ1), which was isolated from the human breast cancer cell line MCF-7 (12, 13). Globo H is overexpressed on a variety of epithelial cell tumors such as colon, ovarian, gastric, pancreatic, lung, prostate, and breast cancers, with the use of anti-Globo H monoclonal antibodies MBr1 (12–14) or VK-9 (15). Immunohistochemical staining of small cell lung carcinomas (SCLC) with MBr1 revealed that patients with Globo H-positive tumors showed a shorter survival in comparison to patients with Globo H-negative tumors. Furthermore, primary SCLC tumors showed less reactivity with MBr1 than local or distant metastatic lesions (16). In breast cancer, Globo H expression was observed in >60% of ductal, lobular, and tubular carcinoma, but not in nonepithelial breast tumors (17). Globo H is not expressed in normal tissue except for weak expression in the apical epithelial cells at lumen borders, a site that appears to be inaccessible to the immune system (17–19). Thus, Globo H has been considered as an ideal target for immunotherapy of many epithelial cancers and indeed two phase I trials of a Globo H-based vaccine in breast and prostate cancer, respectively, have shown promising results (20, 21). With the recent revelation of cancer stem cells in breast cancer, it becomes important to address the issue of whether Globo H-based therapy will target BCSCs or not.
In addition to the vaccine strategy, Globo H-targeted therapy may be achieved by targeting the enzymes involved in its biosynthesis. The exact gene(s) involved in the biosynthesis of Globo H remains to be elucidated. Among the 13 human fucosyl transferase genes cloned, FUT1 (22) and FUT2 (23) have been shown to be responsible for the α1,2- linkage of fucose. Using synthetic acceptors and purified enzyme fractions from a prostate cancer cell line (24), Matta et al. demonstrated that Globo H was generated by adding fucose to the terminal galactose moiety of Galβ1, 3GalNAcβ1, 3Galα, which was catalyzed by the fractions containing α1, 2-fucosyltransferase (FUT) activity. However, it remains unclear whether FUT1 or FUT2 or both enzymes are involved in the synthesis of Globo H and whether they are expressed by the BCSCs.
In the present study, we compared the expression of Globo H and its precursor molecule, SSEA3, in BCSCs and non-BCSCs. We further investigated the involvement of FUT1 and FUT2 in the biosynthesis of Globo H. The findings provide insight into Globo H-targeted therapy for breast cancer.
Results
Globo H Is Expressed in BCSCs, but at Lower Frequency than in Non-BCSCs.
The clinical characteristics of 53 patients with breast cancer are summarized in supporting information (SI) Table S1. The median age was 48 years (ranging from 31 to 81 years). They consisted of 17 stage I, 26 stage II, 8 stage III, and 1 unknown stage. A majority of the tumor specimens included in our study had the pathology of infiltrating ductal carcinoma (77.3%), with 56.6% positive for ER and 49.1% positive for HER-2 (Table S1). Primary tumor cells isolated from 41 of 53 enrolled patients by enzymatic digestion were stained with specific antibodies to CD45, CD24, CD44, and Globo H, and CD45+ cells were first gated out to eliminate the leukocytes. Overall, Globo H was detected in 25/41 (61.0%) of the tumors (Table 1), and there was no significant correlation between expression level of Globo H on tumors and various clinicopathological factors, such as stage (P = 0.3498), ER (P = 0.075), or HER-2 (P = 0.6468) (Table S1). To compare the Globo H expression between BCSCs and non-BCSCs, we further separated CD45− tumor cells into BCSCs and non-BCSCs based on their expressions of surface markers. The BCSCs were identified as CD45−/CD24−/CD44+ cells; the rest of the CD45− population were considered as non-BCSCs. The expression of Globo H within each of these two gated populations varied among tumor samples. For example, tumor from patient BC0145 contained 17.1% BCSCs that were negative for Globo H, whereas 43.8% of the non-BCSCs expressed Globo H. In patient BC0240, Globo H expression was detected in 66.4% of non-BCSCs and 23% of BCSCs (Fig. S1A). Using this approach, we evaluated the expression of Globo H on BCSCs and non-BCSCs in 41 tumor specimens. As summarized in Table 1, among the 25/41 (61.0%) samples expressing Globo H, the percentage of positive cells ranged from 14.3% to 75.2%. Importantly, the non-BCSCs isolated from these 25 tumors all expressed Globo H, with the percentage of positive cells ranging from 24.4% to 79.2%. In comparison, BCSCs from 8 of 40 (20.0%) tumors showed positive staining for Globo H, with the percentage of positive cells ranging from 9.7% to 71.0%. A comparison of the extent of Globo H expression in BCSCs and non-BCSCs in these 8 cases revealed that the percentage of Globo H-positive cells in 7 of 8 BCSCs was lower than that of non-BCSCs (Table S2). Furthermore, the mean fluorescence intensity (MFI) of positive cells in 7 of 8 Globo H-positive BCSCs (MFI 6.3∼45.9) was also lower than that of non-BCSCs (MFI 11.9∼107.9). Taken together, these results indicate that BCSCs express Globo H, albeit at a lower level and frequency than non-BCSCs.
Table 1.
A comparison of Globo H and SSEA3 expression in BCSCs and non-BCSCs
| Glycan and population | No. of patients | Positive |
||
|---|---|---|---|---|
| No. | Range* | % of total | ||
| Globo H | ||||
| Entire | 41 | 25 | 14.3–75.2 | 61.0 |
| Non-BCSCs | 41 | 25 | 24.4–79.2 | 61.0 |
| BCSCs | 40† | 8 | 9.7–71.0 | 20.0 |
| SSEA3‡ | ||||
| Entire | 40 | 31 | 5.9–66.4 | 77.5 |
| Non-BCSCs | 40 | 29 | 24.3–70.4 | 77.5 |
| BCSCs | 40 | 25 | 5.0–58.4 | 62.5 |
Globo H or SSEA3 expression was determined by flow cytometry as described in Materials and Methods. BCSCs were defined as CD45−CD24−CD44+ cells, and non-BCSCs were defined as the remaining populations of CD45− cells.
*Range was calculated as percentage of positive cells in total cells.
†Tumor cells from one of the 41 patients showed an absence of CD24−CD44+ subpopulation.
‡Among the 53 tumor samples, 28 were examined for the expression of both Globo H and SSEA3, 13 were tested for Globo H only, and the remaining 12 were tested for SSEA3 only.
Higher Frequency of SSEA3 Expression than Globo H Expression in Breast Cancer and BCSCs.
We next examined the expression of SSEA3, which is the pentasaccharide precursor of Globo H, in 40 of 53 cases. Flow cytometric analysis revealed variable expression among different tumor specimens, as well as between BCSCs and non-BCSCs of each tumor. For instance, BCSCs of patient BC0264, which accounted for 5.7% of the total isolated tumor cells, were negative for SSEA3, whereas 70.4% of the non-BCSCs expressed SSEA3. For patient BC0266, SSEA3 expression was detected in 60.1% of non-BCSCs and 50.3% of BCSCs (Fig. S1B). Altogether, SSEA3 was detected in 31 of 40 (77.5%) breast cancer specimens tested, with the percentage of positive cells ranging from 5.9% to 66.4% (Table 1). The expression of SSEA3 was noted in 16 of 17 Globo H-positive tumors and 7 of 11 Globo H-negative tumors (Table 2). There was a significant positive correlation between the expression of SSEA3 and Globo H in these 28 cases based on Pearson correlation coefficient analysis (Fig. S2) (r = 0.4917 and P = 0.0079). Subpopulation analysis revealed that SSEA3 was detected in 29 non-BCSCs from all 31 SSEA3-expressing tumors, with the percentage of positive cells ranging from 24.3% to 70.4%. SSEA3 was present in BCSCs from 25 of 40 (62.5%) tumors tested, with the percentage of positive cells ranging from 5.0% to 58.4% (Table 2). Within the BCSC subpopulation, SSEA3 expression was noted in 7 of 11 (63.6%) Globo H-negative tumors and 16 of 17 (94.1%) Globo H-positive tumors. The latter included all 5 tumors that expressed Globo H in BCSCs and 7 of 12 (58.3%) tumors that did not (Table 2). These results document that SSEA3 was expressed in breast cancer as well as breast cancer stem cells, and that its expression was more prevalent than Globo H (77.5% for SSEA3 and 61.0% for Globo H). BCSCs also expressed SSEA3 at higher frequency than Globo H (62.5% for SSEA3 and 20.0% for Globo H), although both were expressed to a lesser extent compared to their expression in non-BCSCs. Moreover, the lower frequency of SSEA3 expression in BCSCs may have accounted for the lack of Globo H expression in Globo H-negative cases or the absence of Globo H on BCSCs in Globo H-positive cases.
Table 2.
SSEA3 expression in Globo H-negative and Globo H-positive specimens
| Frequency of SSEA3 expression in breast cancer |
||||
|---|---|---|---|---|
| Total population | Non-BCSCs | BCSCs | ||
| Globo H− | 63.6% (7/11) | 54.5% (6/11) | 45.5% (5/11) | |
| Globo H+ | 94.1% (16/17) | 94.1% (16/17) | Globo H− | Globo H+ |
| 58.3% (7/12) | 100% (5/5) | |||
Globo H and SSEA3 expression was determined by flow cytometry as described in Materials and Methods. BCSCs were defined as CD45−CD24−CD44+ cells, and non-BCSCs were defined as the remaining population of CD45− cells.
SSEA3 Expression in Normal Tissues.
Globo H expression has been reported to be restricted to the luminal surface of glandular tissues, such as lung, breast, or prostate (19), but information about the distribution of SSEA3 in normal tissues is limited to a report of SSEA3 being present in the extract of normal kidney tissue (25). Using tissue microarray, we analyzed the SSEA3 or Globo H expression among 20 different organs. Globo H was expressed on the epithelial cells of several glandular tissues, such as breast, gastrointestinal tract, pancreas, prostate, and uterine cervix (Table 3), and the positive staining of Globo H was restricted to apical portion of epithelial cells (Fig. S3). These findings were consistent with those of a previous report (14). The distribution of SSEA3 was similar to that of Globo H except for its absence in normal breast tissues but presence in kidney, rectum, testis, and thymus, which were negative for Globo H (Table 3). Similar to Globo H, SSEA3 expression was confined mainly to the cytoplasm or apical surface of epithelial cells (Fig. S3), which were essentially inaccessible to the immune system.
Table 3.
Expression of Globo H and SSEA3 in normal tissues
| Normal tissue | Antigen |
|||
|---|---|---|---|---|
| Globo H | SSEA3 | |||
| Brain | 0/5 | 0/5 | ||
| Bone | 0/5 | 0/5 | ||
| Lymph node | 0/5 | 0/5 | ||
| E | C | E | C | |
| Breast | 1/5 | 0/5 | 0/5 | 0/5 |
| Colon* | 1/4 | 0/4 | 2/4 | 0/4 |
| Esophagus | 5/5 | 0/5 | 2/5 | 0/5 |
| Small intestine | 3/5 | 0/5 | 2/5 | 0/5 |
| Kidney | 0/5 | 0/5 | 2/5 | 0/5 |
| Liver | 0/5 | 0/5 | 0/5 | 0/5 |
| Lung | 0/5 | 0/5 | 0/5 | 0/5 |
| Ovary | 0/5 | 0/5 | 0/5 | 0/5 |
| Pancreas | 0/5 | 0/5 | 0/5 | 0/5 |
| Prostate | 5/5 | 0/5 | 3/5 | 0/5 |
| Rectum | 1/5 | 0/5 | 1/5 | 0/5 |
| Skin | 0/5 | 0/5 | 4/5 | 0/5 |
| Spleen | 0/5 | 0/5 | 0/5 | 0/5 |
| Stomach | 0/5 | 0/5 | 0/5 | 0/5 |
| Testis | 1/5 | 0/5 | 2/5 | 0/5 |
| Thymus gland | 0/5 | 0/5 | 3/5 | 0/5 |
| Uterine cervix | 0/5 | 0/5 | 1/5 | 0/5 |
| 1/5 | 0/5 | 3/5 | 0/5 | |
Globo H and SSEA3 expression was detected by immunohisto-chemical staining of tissue array as described in Materials and Methods. E, epithelial; C, connective tissues.
*One of five colon tissue spots was lost during the dewaxing step.
Globo H Vaccine Induces Antibodies Against Both Globo H and SSEA3.
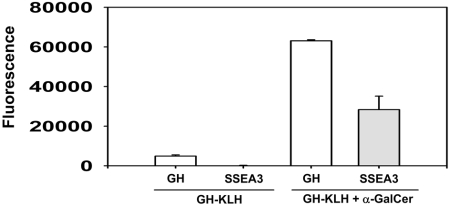
A synthetic Globo H–KLH conjugate has been used as a therapeutic vaccine for breast cancer and prostate cancer in two phase I trials and has been shown to provoke antibody responses to Globo H (20, 21) with little toxicity. In view of our demonstration of SSEA3 expression in breast cancer and its restricted distribution in normal tissues, SSEA3 may also serve as an ideal target for immunotherapy. We thus investigated the possible induction of anti-SSEA3 antibody on immunization with Globo H–KLH (see SI). Sera from immunized mice were collected at Day 10 after last immunization and were assayed for anti-Globo H and anti-SSEA3 reactivities by glycochip. A low level of anti-Globo H-specific IgG antibody was detected in Globo H–KLH-treated mice (Fig. 1). On the addition of α-galactosylceramide (α-GalCer) as an adjuvant, there was a dramatic increase in the anti-Globo H level. Moreover, sera from these mice harbored significant amounts of anti-SSEA3 antibodies (Fig. 1). These findings indicated that Globo H-KLH in combination with α-GalCer is an effective vaccine for inducing both anti-Globo H and anti-SSEA3 antibodies.
Fig. 1.
Production of anti-Globo H and anti-SSEA3 by Globo H-KLH vaccine. Mice were immunized s.c. with 0.6 μg Globo H-KLH with or without 2 μg α-GalCer, weekly for 3 weeks. The mouse sera were obtained at Day 10 after the third immunization, and anti-Globo H (GH) or anti-SSEA3 antibody was detected with glycochip assay as described in SI Materials and Methods.
Both FUT1 and FUT2 Are Involved in the Biosynthesis of Globo H in Breast Cancer Cell Lines.
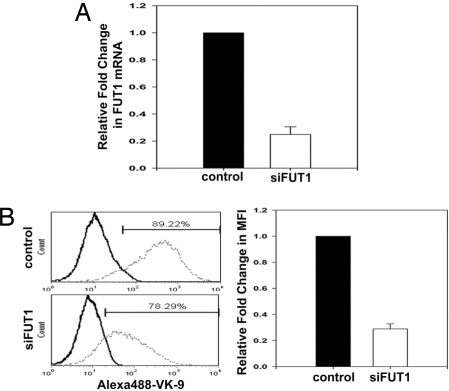
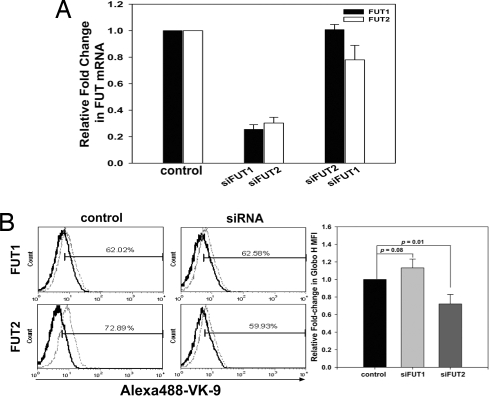
In addition to vaccine, Globo H-targeted therapy may also be achieved by targeting the enzymes involved in its biosynthesis. Globo H is a glycolipid with α1,2-linked fucose as its terminal sugar, and FUT1 and FUT2 are the only FUTs currently known to be responsible for attaching the terminal fucose via a α1,2 linkage. Although α1,2-FUTs are critical for the biosynthesis of the Lewis antigen, the exact contributions of FUT1 and FUT2 to Globo H biosynthetic process have yet to be delineated. We thus examined the expression of FUT1 and FUT2 in various Globo H-expressing breast cancer cell lines. The human breast cancer cell line MCF-7 has been shown to highly express the Globo H antigen (91.7%) and was used here as a positive control. Two other breast cancer cell lines MB157 and T-47D were found to stain positively for Globo-H in 94.2% and 77.7% of the cells, respectively, although the fluorescent intensity of T047D was considerably weaker (Fig. S4B). Next, the level of FUT1 and FUT2 mRNA expressions in all three cell lines was determined by qRT-PCR. The ΔCt value of FUT1 in MCF-7 was used to normalize the ΔCt value of the target gene in each cell line. No significant difference in the transcription of FUT1 was found among these three cell lines (Fig. S4A). However, the levels of FUT2 expression were barely detectable in MCF-7 and MB157 cells, as reflected by their high Ct values (37–38) (data not shown), suggesting that FUT1 was responsible for Globo H biosynthesis in these two cell lines. As for the T47D cell line, FUT2 expression was >6,000-fold greater than in the other two cell lines (Fig. S4A), although its Globo H expression was weaker. It is noteworthy that these three breast cancer cell lines displayed a similarly high level of SSEA3 (data not shown). The differential expression of FUT1, FUT2 mRNA, and Globo H with similar expression of SSEA3 suggests that both FUT1 and FUT2 might be involved in the generation of Globo H. We next investigated the individual contribution of FUT1 and FUT2 to Globo H biosynthesis, using the siRNA technology to specifically knock down the FUT1 or FUT2. MB157 cells that expressed only FUT1 were infected with lentiviral vectors encoding the siFUT1 construct and examined for any alterations in Globo H expression on the cell surface by flow cytometry. Delivery of lentiviral vectors encoding the siFUT1 construct into MB-157 cells resulted in a decrease in FUT1 expression to 25% of cells infected with control vector (Fig. 2A). After siRNA silencing of the FUT1 gene in MB-157 cells, the expression of Globo H antigen declined from 89.2 to 78.3% of cells, along with a 70% decrease in MFI (Fig. 2B). These findings suggest that FUT1 is involved in the generation of Globo H in MB157 breast cancer cells. The high level of FUT2 mRNA expression in T-47D cells, which stained positively for Globo H, suggests that FUT2 is also involved in the biosynthesis of Globo H in breast cancer cells. Transfection of T-47D cells with siFUT2 or siFUT1 resulted in 70% or 75% inhibition of FUT2 or FUT1 mRNA expression, respectively (Fig. 3A). It was noteworthy that silencing of FUT2 led to a decrease of Globo H antigen expression from 72.9% to 60% of cells, with 30% reduction in MFI (P = 0.01). However, FUT1 silencing did not significantly affect the percentage or MFI of Globo H expression in T-47D cells (Fig. 3B). These findings suggest that FUT2 is also involved in Globo H antigen biosynthesis in generating the terminal Fucα (1, 2)-Galβ- epitope in breast cancer cells.
Fig. 2.
siRNA-induced knockdown of the FUT1 gene resulted in diminished expression of Globo H antigen in MB157 cells. Knockdown of FUT1 by siRNA was used to examine the involvement of FUT1 in Globo H biosynthesis. (A) The efficiency of siFUT1 in MB157 cells was evaluated by qRT-PCR analysis. Total RNA was extracted 72 h after infection with control lentivirus (control) or siFUT1-encoding vector (siFUT1). The level of FUT1 mRNA expressions was determined and plotted as fold-change relative to the control. (B) (Left) Globo H expression was determined by flow cytometry with the AlexaFluor488-VK-9 antibodies. (Right) The fold-change in MFI of Globo H relative to the control. AlexaFluor488-conjugated-mIgG3 was used as the isotype control in all experiments.
Fig. 3.
Silencing of FUT2 but not FUT1 mRNA in T-47D cells reduced the level of Globo H expression. Knockdown of FUT1 or FUT2 by siRNA was used to examine the involvement of these two FUTs in Globo H biosynthesis. (A) The efficiency of siFUT1 and siFUT2 in T-47D cells was evaluated by qRT-PCR analysis. Total RNA was extracted 72 h after infection with control lentivirus (control), siFUT1-encoding vector (siFUT1), or siFUT2-encoding vector (siFUT2). The levels of FUT1 and FUT2 mRNA expressions were determined and plotted as fold-change relative to the control. Infection of cells with siFUT1 lentivirus did not affect FUT2 expression, nor did siFUT2 alter FUT1 expression. (B) Left: Alteration in the expression of Globo H antigen was examined by flow cytometry with the AlexaFluor488-VK-9 antibodies. Right: The fold-change in MFI of Globo H relative to the control. AlexaFluor488-conjugated-mIgG3 was used as the isotype control in all experiments. P value was calculated by using the student t test.
Expression of FUT2 Was Lower in BCSCs.
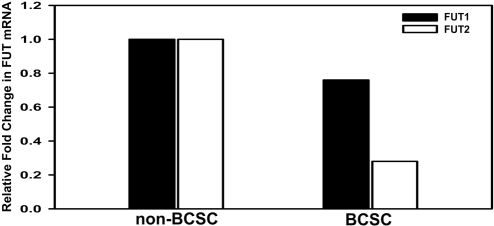
Our findings thus far revealed that both FUT1 and FUT2 may participate in the biosynthesis of Globo H antigen in human breast cancer cell lines. We next explored the issue of whether the reduced expression of Globo H in BCSCs is attributable to differential expressions of FUT1 or FUT2 in BCSCs and non-BCSCs. Such analysis is logistically difficult, if not impossible, given the limited size of freshly removed primary tumors available for research. We therefore attempted to engraft human primary breast cancer cells obtained from patients into NOD/SCID mice by injecting primary tumor cells into their mammary fat pads (see SI Materials and Methods). Primary tumor cells obtained from patient BC0145 were successfully engrafted in NOD/SCID mice and maintained by serial passages (Fig. S5A). Similar to the primary tumor cells of BC0145 (Fig. S1A), tumor cells derived from the xenografts also contained CD24−/CD44+ cells (Fig. S5B). The engrafted tumors displayed a similar histopathological profile as the parental tumors, as well as immunohistochemical staining for the expressions of ER, PR, and HER2 (Fig. S5C). After the tumor cells were harvested from the NOD/SCID mice, the cells of murine origin were depleted by gating out the H2Kd+ cells with anti-H2Kd antibody, and only those of human origins (negative for the murine MHC molecule H2Kd) were separated into BCSCs (H2Kd−/CD24−/CD44+) and non-BCSCs by cell sorter. As determined by qRT-PCR, the expression of FUT2 in BCSCs was only 28% of that in the non-BCSCs, whereas the level of FUT1 expression BCSC population was slightly lower (76% of the non-BCSC) (Fig. 4). These findings suggest that decreased expressions of FUT1, and particularly FUT2, may have contributed to the lack of Globo H expression on BCSCs of BC0145 tumor (Fig. S1A).
Fig. 4.
BCSCs express lower level of FUT2 relative to non-BCSCs. Total RNA was extracted from BCSCs and non-BCSCs isolated from the engrafted tumors and reverse-transcribed to cDNA. Expressions of FUT1 and FUT2 were determined by q-PCR. Levels of FUT1 and FUT2 mRNA in BCSCs were then normalized to that of the corresponding mRNA in non-BCSCs.
Discussion
In the present study, we evaluated the expression of the Globo H antigens on 41 primary human breast cancer specimens and the CD24−/CD44+ subpopulation containing BCSCs by flow cytometric analysis. We showed that Globo H was expressed in ≈60% of tumor samples, with less frequent and lower level of expression in the BCSC subpopulation as compared to non-BCSCs. The overall expression of SSEA3, the precursor of Globo H, was higher (≈80%) than Globo H in breast cancer, and it was much more frequently detected on BCSCs (62.5%) when compared with Globo H. These results demonstrate SSEA3 expression in breast cancer, and a differential expression of Globo H and SSEA3 between BCSCs and non-BCSCs.
We also explored the relationship between Globo H expression and various clinicopathologic factors and found no statistically significant correlation. These findings are consistent with the results of previous studies that used immunohistochemical staining of tumor tissues (17, 18). Nevertheless, there appears to be a trend for higher expression of Globo H in ER-positive breast cancer (Table S2), although the correlation was not statistically significant (P = 0.075). This tendency for a correlation of Globo H expression with ER positivity in our study was consistent with the previous report by Perrone et al. (P = 0.06) (18).
SSEA3, which is also referred to as Gb5 or globopentaosylceramide, has been reported to be expressed in normal kidney (25) and the ascites fluids from patients of hepatoma or pancreatic cancer (26), but its distribution among other normal organs or in breast cancer has not been investigated. Using immunohistochemical staining of 20 different normal organs and flow cytometric analysis of 40 primary breast cancer cells, we demonstrated the expression of SSEA3 in breast cancer, including BCSCs and various normal tissues, in addition to kidney. Furthermore, the expression of SSEA3 was mostly restricted to the cytoplasm or apical surface of epithelial cells, similar to that of Globo H. In light of the confined distribution of SSEA3 to largely immune system-inaccessible sites in normal tissues and the prevalence of SSEA3 expression in breast cancer (77.5%) and BCSCs (62.5%), SSEA3 may serve as another potential candidate for a carbohydrate-based breast cancer vaccine.
A Globo H–KLH conjugate has been used as a therapeutic vaccine for breast cancer in a phase I clinical trial with promising results (20). A recent report showed that polyclonal antibodies responses of globo series glycans, such as Globo H, SSEA3 (pentasaccharide), and trisaccharide, were observed in the serum of breast cancer patients (28), suggesting that a Globo H vaccine might also induce the production of the antibodies reactive with oligosaccharides other than Globo H. We examined this in an animal study, in which immunization of mice with Globo H-KLH and α-GalCer as an adjuvant led to the induction of both anti-SSEA3 and anti-Globo H antibodies (Fig. 1). Study results also demonstrated that NKT-stimulatory glycolipids such as α-GalCer (29, 30) may serve as an effective adjuvant for carbohydrate antigen. Taken together, our findings of expression of Globo H and SSEA3 in BCSCs and the ability of a Globo H vaccine to generate polyclonal antibodies reactive with both Globo H and SSEA3 suggest that Globo H vaccine may have promising therapeutic potential for breast cancer.
Fucose is often a terminal sugar in glycans that participate in important cell–cell interactions and cell migration processes in connection with physiological and pathological processes such as fertilization, embryogenesis, lymphocyte trafficking, immune responses, and cancer metastasis (31, 32). However, the exact molecules modified by FUTs and their involvement in the above-mentioned complex cellular processes remain enigmatic. The FUT1 and FUT2 genes each encodes an α1, 2-FUT that transfers fucose via an α1,2 linkage from GDP-fucose to galactose. In humans, FUT1 functions together with FUT2 in synthesizing the H-antigen, and the secretor status is determined by FUT2. Whereas FUT1 is expressed on erythrocyte membrane and vascular endothelium (33), FUT2 is mainly expressed in the mucosal epithelium of buccal, gastrointestinal, respiratory tract, breast, and genitourinary tract, as well as in body fluids (34). Despite the fact that two α1,2-fucosyltransferases share a high degree of sequence homology, their specificities were different; the former prefers Type I (Galβ1,3GlcNAc) and Type II (Galβ1,4GlcNAc) acceptors, the latter is more active on Type I and Type III (Galβ1,3GalNAc) (35, 36). By means of a specific acceptor for measuring activity of α1, 2-FUTs, human LNCaP cells, which are of the prostate cancer origin, and MCF-7 cells have been reported to express exclusive α1, 2-FUT activity. By partial purification of α1, 2-FUTs from LNCaP cells and enzymatic transfer of [14C] fucose to Globo H backbone acceptor, it has been shown that the enzymatic activity of α1, 2-FUTs is responsible for Globo H synthesis (24). However, it was unclear whether the FUT1 or FUT2 gene is responsible for the biosynthesis of Globo H, although the preference of FUT2 for Type III acceptors implies that this enzyme may be responsible for the biosynthesis of Globo H (12). In the present study, using lentiviral delivery of siRNA targeting either FUT1 or FUT2 in human breast cancer cell lines, we have demonstrated for the first time that both FUT1 and FUT2 participate in the making of Globo H antigen and that the relative contribution of each depended on the individual cell lines. We found that MB157 and MCF-7, which did not have detectable FUT2, expressed more Globo H thanT-47D, the only cell line expressing significant levels of both FUT1 and FUT2. Furthermore, delivery of siFUT2 to T-47D cells caused a more pronounced decrease in expression of Globo H than siFUT1. These findings implied that in MB157 and MCF7, FUT1 was responsible for Globo H (Fig. 3) but when both FUT1 and FUT2 were present, such as in the case of T-47D, FUT2 played an greater role in the fucosylation of Globo H than FUT1 (Fig. 4). However, it remains unclear why T-47D displayed less Globo H than the other two cell lines. Because the level of SSEA3 expression was similar among these three cell lines (data not shown), it is possible that T-47D may contain more fucosidases or other SSEA3-reactive enzymes that compete with FUTs. Further analysis of FUT1 and FUT2 mRNA expression in BCSCs isolated from a xenografted primary human breast cancer indicated that the lower expression of Globo H on BCSCs of this tumor might arise from lower level of FUT2 expression as compared to that in non-BCSCs. Delivery of a FUT1 transgene was shown to lead to a dramatic decrease in cell surface sialyl-Lex (sLex) synthesis, with a concomitant increase in Ley and Leb expression, causing the cells to fail to interact with E-selectin (37). It remains to be explored whether there is any differential expression of FUT1 and FUT2 between nonmetastatic and metastatic breast cancers.
Blood group antigens and precursor or related antigens (H, Lewis) are expressed most abundantly in endodermal epithelial cells, where the majority of human cancers arise (38). Consequently, changes in these blood group and precursor or related antigens constitute the major tumor-associated changes of glycosylation, and many of them lead to formation of tumor-associated carbohydrate antigens (39). In addition to Globo H, there are other glycans that have also been shown to be overexpressed in breast cancer, including sLex, sialyl-Lea, sialyl-Tn, and polysialic acid (40). The expression of these glycans on BCSCs remains unclear, although some of these glycan epitiopes have been considered for vaccine development besides Globo H, such as sialyl-Tn (40). Our findings of Globo H and SSEA3 expression on BCSCs provide an impetus for similar studies to determine the expression of other tumor-associated glycans in BCSCs before further development of a carbohydrate-based breast cancer vaccine.
Materials and Methods
Isolation of Primary Tumor Cells from Human Breast Cancer Specimens.
A total of 31 human breast cancer specimens were obtained from patients who had undergone initial surgery at the Tri-Service General Hospital (Taipei, Taiwan). Samples were fully encoded to protect patient confidentiality and were used under a protocol approved by the Institutional Review Board of Human Subjects Research Ethics Committee of Academia Sinica, Taipei, Taiwan. The tumor specimens were sliced to square fragments of 1 mm2 and subjected to enzymatic digestion by incubation in RPMI1640 medium containing collagenase (1,000 U/ml), hyaluronidase (300 U/ml), and DNase I (100 μg/ml) at 37°C for 2 h. Primary breast tumor cells were collected after filtration through a 100-μm cell strainer (BD Biosciences) and resuspended in RPMI1640 medium supplemented with 5% FBS.
Flow Cytometry Analysis.
Primary breast cancer cells were prepared as 1 × 105 cells in 50 μl of PBS containing 2% FBS and 0.1% NaN3. Cells were labeled with anti-CD24-PE, anti-CD44-APC, and anti-CD45-PerCP-Cy5.5 antibody mixtures (1 μl of each). Globo H expression was detected by staining with monoclonal anti-Globo H antibody (VK-9) conjugated with Alexa488. Analyses were performed on a FACSCanto flow cytometer (Becton Dickinson). BCSCs were defined as CD45−/CD24−/CD44+ cells, and non-BCSCs were defined as other populations of CD45− cells. Globo H expression was further analyzed in the gated region.
qRT-PCR of FUT1 or FUT2.
Total RNA was extracted and reverse-transcripted to cDNA with oligo(dT) primer. RTPCR for simultaneous detection and quantification of the cDNA samples was performed on an ABI Prism 7000 Sequence Detection System and analyzed with the ABI Prism 7000 SDS software (Applied Biosystems). Fifty nanograms of cDNA sample were used for qPCR reaction as 50°C for 2 min, 95°C for 10 min, followed by 40 cycles of 95°C for 10 sec and 60°C for 1 min. The end-point used in the real-time quantification was calculated by the ABI Prism 7000 SDS software, and the threshold cycle number (Ct value) for each analyzed sample was calculated. Each target gene was normalized to that of HPRT1 to derive the change in Ct value (ΔCt).
Construction and Production of siRNA in Lentiviral Vector.
TRCN0000036074 (siFUT1) clone, TRCN0000036102 (siFUT2) clone, pLKO.1-puro vector (control), pMD.G plasmid and pCMVΔR8.91 plasmid were obtained form National RNAi Core Facility at the Institute of Molecular Biology, (Academia Sinica, Taipei, Taiwan). The siFUT1 and siFUT2 clones encode a small interfering oligonucleotide specific for human FUT1 and FUT2 gene, respectively. The production of lentivirus was described in the SI Materials and Methods.
Supplementary Material
Acknowledgments.
We thank the Tumor Bank at Tri-Service General Hospital for providing patient samples, nurse Pei-Lan Hsu for gathering samples and relevant clinical information, and the National RNAi Core of Academia Sinica for providing RNAi for FUT 1 and FUT 2. This research was supported by Academia Sinica Grant 5202402020–0 and in part by Grant 685032 from National Science Council in Taiwan.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0804979105/DCSupplemental.
References
- 1.Reya T, Morrison S-J, Clarke M-F, Weissman I-L. Stem cells, cancer, and cancer stem cells. Nature. 2001;414:105–111. doi: 10.1038/35102167. [DOI] [PubMed] [Google Scholar]
- 2.Park C-H, Bergsagel D-E, McCulloch E. Mouse myeloma tumor stem cells: Primary cell culture assay. J Natl Cancer Inst. 1971;46:411–422. [PubMed] [Google Scholar]
- 3.Bonnet D, Dick J-E. Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat Med. 1997;3:730–737. doi: 10.1038/nm0797-730. [DOI] [PubMed] [Google Scholar]
- 4.Al-Hajj M, Wicha M-S, Benito-Hernandez A, Morrison S-J, Clarke M-F. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci USA. 2003;100:3983–3988. doi: 10.1073/pnas.0530291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Collins A-T, Berry P-A, Hyde C, Stower M-J, Maitland N-J. Prospective identification of tumorigenic prostate cancer stem cells. Cancer Res. 2005;65:10946–10951. doi: 10.1158/0008-5472.CAN-05-2018. [DOI] [PubMed] [Google Scholar]
- 6.O'Brien C-A, Pollett A, Gallinger S, Dick J-E. A human colon cancer cell capable of initiating tumor growth in immunodeficient mice. Nature. 2006;445:106–110. doi: 10.1038/nature05372. [DOI] [PubMed] [Google Scholar]
- 7.Singh S-K, et al. Identification of human brain tumor initiating cells. Nature. 2004;432:396–401. doi: 10.1038/nature03128. [DOI] [PubMed] [Google Scholar]
- 8.Phillips T-M, McBride W-H, Pajonk F. The response of CD24(-/low)/CD44+ breast cancer-initiating cells to radiation. J Natl Cancer Inst. 2006;98:1777–1785. doi: 10.1093/jnci/djj495. [DOI] [PubMed] [Google Scholar]
- 9.Balic M, et al. Most early disseminated cancer cells detected in bone marrow of breast cancer patients have a putative breast cancer stem cell phenotype. Clin Cancer Res. 2006;12:5615–5621. doi: 10.1158/1078-0432.CCR-06-0169. [DOI] [PubMed] [Google Scholar]
- 10.Dalerba P, Cho R-W, Clarke M-F. Cancer stem cells: Models and concepts. Ann Rev Med. 2007;58:18.1–18.18. doi: 10.1146/annurev.med.58.062105.204854. [DOI] [PubMed] [Google Scholar]
- 11.Tang C, Ang B-T, Pervaiz S. Cancer stem cell: Target for anti-cancer therapy. FASEB J. 2007;21:1–9. doi: 10.1096/fj.07-8560rev. [DOI] [PubMed] [Google Scholar]
- 12.Menard S, Tagliabue E, Canevari S, Fossati G, Colnaghi M-I. Generation of monoclonal antibodies reacting with normal and cancer cells of human breast. Cancer Res. 1983;43:1295–1300. [PubMed] [Google Scholar]
- 13.Bremer E-G, et al. Characterization of a glycosphingolipid antigen defined by the monoclonal antibody MBr1 expressed in normal and neoplastic epithelial cells of human mammary gland. J Biol Chem. 1984;259:14773–14777. [PubMed] [Google Scholar]
- 14.Canevari S, Fossati G, Balsari A, Sonnino S, Colnaghi M-I. Immunochemical analysis of the determinant recognized by a monoclonal antibody (MBr1) which specifically binds to human mammary epithelial cells. Cancer Res. 1983;43:1301–1305. [PubMed] [Google Scholar]
- 15.Ragupathi G, et al. A Fully Synthetic Globo H carbohydrate vaccine induces a focused humoral response in prostate cancer patients: a proof of principle. Angew Chem Int Ed. 1999;38:563–566. doi: 10.1002/(SICI)1521-3773(19990215)38:4<563::AID-ANIE563>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 16.Martignone S, et al. Relationship between CaMBr1 expression and tumor progression in small cell lung carcinomas. Tumori. 1989;75:373–377. doi: 10.1177/030089168907500414. [DOI] [PubMed] [Google Scholar]
- 17.Mariani-Costantini R, et al. Reactivity of a monoclonal antibody with tissues and tumors from the human breast. Immunohistochemical localization of a new antigen and clinicopathologic correlations. Am J Pathol. 1984;115:47–56. [PMC free article] [PubMed] [Google Scholar]
- 18.Perrone F, et al. Prognostic significance of the CaMBr1 antigen on breast carcinoma: Relevance of the type of recognised glycoconjugate. Eur J Cancer. 1993;29A:2113–2117. doi: 10.1016/0959-8049(93)90045-h. [DOI] [PubMed] [Google Scholar]
- 19.Zhang S, et al. Selection of tumor antigens as targets for immune attack using immunohistochemistry: I. Focus on gangliosides. Int J Cancer. 1997;73:42–49. doi: 10.1002/(sici)1097-0215(19970926)73:1<42::aid-ijc8>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 20.Gilewski T, et al. Immunization of metastatic breast cancer patients with a fully synthetic globo H conjugate: A phase I trial. Proc Natl Acad Sci USA. 2001;98:3270–3275. doi: 10.1073/pnas.051626298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Slovin S-F, et al. Carbohydrate vaccines in cancer: Immunogenicity of a fully synthetic globo H hexasaccharide conjugate in man. Proc Natl Acad Sci USA. 1999;96:5710–5715. doi: 10.1073/pnas.96.10.5710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rajan V-P, Larsen R-D, Ajmera S, Ernst L-K, Lowe J-B. A cloned human DNA restriction fragment determines expression of a GDP-L-fucose: Beta-D-galactoside 2-alpha-L-fucosyltransferase in transfected cells. Evidence for isolation and transfer of the human H blood group locus. J Biol Chem. 1989;264:11158–11167. [PubMed] [Google Scholar]
- 23.Rouquier S, et al. Molecular cloning of a human genomic region containing the H blood group alpha(1,2)fucosyltransferase gene and two H locus-related DNA restriction fragments. Isolation of a candidate for the human Secretor blood group locus. J Biol Chem. 1995;270:4632–4639. doi: 10.1074/jbc.270.9.4632. [DOI] [PubMed] [Google Scholar]
- 24.Chandrasekaran E-V, Chawda R, Locke R-D, Piskorz C-F, Matta K-L. Biosynthesis of the carbohydrate antigenic determinants, Globo H, blood group H, and Lewis b: A role for prostate cancer cell alpha1,2-L-fucosyltransferase. Glycobiology. 2002;12:153–162. doi: 10.1093/glycob/12.3.153. [DOI] [PubMed] [Google Scholar]
- 25.Holgersson J, Jovall P-A, Samuelsson B-E, Breimer M-E. Blood group type glycosphingolipids of human kidneys. Structural characterization of extended globo-series compounds. Glycoconj J. 1991;8:424–433. doi: 10.1007/BF00731294. [DOI] [PubMed] [Google Scholar]
- 26.Taki T, Kojima S, Seto H, Yamada H, Matsumoto M. Glycolipid composition of ascitic fluids from patients with cancer. J Biochem. 1984;96:1257–65. doi: 10.1093/oxfordjournals.jbchem.a134944. [DOI] [PubMed] [Google Scholar]
- 27.Hakomori S. Tumor-associated carbohydrate antigens defining tumor malignancy: Basis for development of anti-cancer vaccines. Adv Exp Med Biol. 2001;491:369–402. doi: 10.1007/978-1-4615-1267-7_24. [DOI] [PubMed] [Google Scholar]
- 28.Huang C-Y, et al. Carbohydrate microarray for profiling the antibodies interacting with Globo H tumor antigen. Proc Natl Acad Sci USA. 2006;103:15–20. doi: 10.1073/pnas.0509693102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ko S-Y, et al. alpha-Galactosylceramide can act as a nasal vaccine adjuvant inducing protective immune responses against viral infection and tumor. J Immunol. 2005;175:3309–3317. doi: 10.4049/jimmunol.175.5.3309. [DOI] [PubMed] [Google Scholar]
- 30.Chang Y-J, et al. Potent immune-modulating and anticancer effects of NKT cell stimulatory glycolipids. Proc Natl Acad Sci USA. 2007;104:10299–10304. doi: 10.1073/pnas.0703824104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Becker D-J, Lowe J-B. Fucose: Biosynthesis and biological function in mammals. Glycobiology. 2003;13:41R–53R. doi: 10.1093/glycob/cwg054. [DOI] [PubMed] [Google Scholar]
- 32.Listinsky J-J, Listinsky C-M, Alapati V, Siegal G-P. Cell surface fucose ablation as a therapeutic strategy for malignant neoplasms. Adv Anat Pathol. 2001;8:330–337. doi: 10.1097/00125480-200111000-00003. [DOI] [PubMed] [Google Scholar]
- 33.Mollicone R, Cailleau A, Oriol R. Molecular genetics of H, Se, Lewis and other fucosyltransferase genes. Transfus Clin Biol. 1995;2:235–242. doi: 10.1016/s1246-7820(05)80089-8. [DOI] [PubMed] [Google Scholar]
- 34.Avent N-D. Human erythrocyte antigen expression: Its molecular bases. Br J Biomed Sci. 1997;54:16–37. [PubMed] [Google Scholar]
- 35.Kyprianou P, Betteridge A, Donald A-S, Watkins W-M. Purification of the blood group H gene associated alpha-2-L-fucosyltransferase from human plasma. Glycoconj J. 1990;7:573–588. doi: 10.1007/BF01189078. [DOI] [PubMed] [Google Scholar]
- 36.Sarnesto A, Kohlin T, Thurin J, Blaszczyk-Thurin M. Purification of H gene-encoded beta-galactoside alpha 1, 2 fucosyltransferase from human serum. J Biol Chem. 1990;265:15067–15075. [PubMed] [Google Scholar]
- 37.Mathieu S, et al. Transgene expression of alpha(1,2)-fucosyltransferase-I (FUT1) in tumor cells selectively inhibits sialyl-Lewis x expression and binding to E-selectin without affecting synthesis of sialyl-Lewis a or binding to P-selectin. Am J Pathol. 2004;164:371–383. doi: 10.1016/s0002-9440(10)63127-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Szulman A-E. Chemistry, distribution, and function of blood group substances. Annu Rev Med. 1966;17:307–322. doi: 10.1146/annurev.me.17.020166.001515. [DOI] [PubMed] [Google Scholar]
- 39.Hakomori S. Aberrant glycosylation in tumors and tumor-associated carbohydrate antigens. Adv Cancer Res. 1989;52:257–331. doi: 10.1016/s0065-230x(08)60215-8. [DOI] [PubMed] [Google Scholar]
- 40.Dube D-H, Bertozzi C-R. Glycans in cancer and inflammation—Potential for therapeutics and diagnostics. Nat Rev Drug Discov. 2005;4:477–488. doi: 10.1038/nrd1751. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.