Abstract
The primary function of fruit is to attract animals that disperse viable seeds, but the nutritional rewards that attract beneficial consumers also attract consumers that kill seeds instead of dispersing them. Many of these unwanted consumers are microbes, and microbial defense is commonly invoked to explain the bitter, distasteful, occasionally toxic chemicals found in many ripe fruits. This explanation has been criticized, however, due to a lack of evidence that microbial consumers influence fruit chemistry in wild populations. In the present study, we use wild chilies to show that chemical defense of ripe fruit reflects variation in the risk of microbial attack. Capsaicinoids are the chemicals responsible for the well known pungency of chili fruits. Capsicum chacoense is naturally polymorphic for the production of capsaicinoids and displays geographic variation in the proportion of individual plants in a population that produce capsaicinoids. We show that this variation is directly linked to variation in the damage caused by a fungal pathogen of chili seeds. We find that Fusarium fungus is the primary cause of predispersal chili seed mortality, and we experimentally demonstrate that capsaicinoids protect chili seeds from Fusarium. Further, foraging by hemipteran insects facilitates the entry of Fusarium into fruits, and we show that variation in hemipteran foraging pressure among chili populations predicts the proportion of plants in a population producing capsaicinoids. These results suggest that the pungency in chilies may be an adaptive response to selection by a microbial pathogen, supporting the influence of microbial consumers on fruit chemistry.
Keywords: directed deterrence, frugivory, fruit chemistry, secondary metabolite, Capsicum chacoense
The evolution of fruit, a reward for animal dispersal of seeds, is a commonly cited example of a key innovation in the radiation of angiosperms (1–3). However, the nutritional qualities of fruit pulp that are responsible for attracting beneficial dispersers also attract consumers that are detrimental to plant fitness. These consumers range from vertebrate and invertebrate seed predators to microbial consumers of fruits and seeds that reduce the likelihood of dispersal and the viability of seeds (4). Fruit chemistry is commonly thought to mediate these interactions, either by deterring seed predators (4–6) or reducing microbial attack of fruits and seeds (4, 7, 8). These mechanisms are not mutually exclusive, but chemicals that deter fruit consumption often affect a wide range of species (7, 9), and defensive chemistry in ripe fruit must be sufficiently targeted toward detrimental organisms to allow consumption by vertebrate seed dispersers. Fruit secondary compounds that deter microbial consumers without reducing seed dispersal by vertebrates are thought to be far more plausible than secondary compounds that selectively deter vertebrate predators (7), because microbial fruit consumers are uniformly negative in their impacts on plant fitness (4) and are farther removed in their morphology, physiology, and mode of consumption from vertebrate seed dispersers than are other unwanted consumers (4, 7).
Microbial deterrence is thus a primary hypothesis explaining the presence of noxious, bitter, and sometimes toxic chemicals in many ripe fruits; the negative effects these chemicals often have on vertebrate dispersers are assumed to be balanced by the benefits of deterring microbial consumers. Unfortunately, this hypothesis remains largely untested, because no work to date has shown that variance in microbial pathogen pressure is related to variance in the chemistry of ripe fruits in wild populations. A strong test would require a species in which fruit chemistry is well known, likely to protect against microbial pathogens, unique to the fruit, and highly variable. The most famous plants with these qualities are chilies (genus Capsicum). Chilies were one of the first plants domesticated in the New World (10), and they are now consumed by one in four humans daily (11), largely because of the pungency produced by capsaicinoids. Capsaicinoids are well characterized (9) and broadly antimicrobial (12–14). In fact, early humans likely selected chilies for use and domestication expressly because of their antimicrobial properties (12, 15). Finally, because capsaicinoids are found only within the fruit of Capsicum species and their concentrations increase during fruit ripening (16), the function of these chemicals is likely restricted in the fruit itself, not attributable to alternative functions in other parts of the plant (17).
Chilies thus provide an exceptionally clear window into the function of fruit chemistry, and our recent rediscovery of a polymorphism for capsaicinoid production in wild populations of multiple chili species (18) provides the variability we need to explicitly examine the function of these chemicals in wild populations. We have studied this polymorphism most intensively in Capsicum chacoense Hunz., which is native to the Chaco region of Bolivia, Argentina, and Paraguay (19). In polymorphic populations, C. chacoense plants producing fruits that contain capsaicinoids grow alongside plants with fruits that are nutritionally similar (20) but completely lack capsaicinoids (18) [see supporting information (SI)]. In addition, the proportion of plants producing capsaicinoids varies widely among populations. At the southwestern end of our 300-km-long study area in southeastern Bolivia, the polymorphism is virtually absent; most populations contain only pungent plants. To the north and east of this area, nonpungent plants gradually increase in frequency, until >70% of individuals lack capsaicinoids, and the few plants that do produce pungent fruit have capsaicinoid concentrations barely one-third the level found in completely pungent populations (18).
We use this geographic gradient as a tool to study the impact of microbial pathogens on fruit chemistry, and we made the following predictions: (i) Microbial fruit pathogens will have a large negative impact on nonpungent chilies, (ii) capsaicinoids will reduce microbial damage to chili fruits and seeds, and (iii) among populations, the proportion of plants producing capsaicinoids will increase as the intensity of microbial attack increases.
Impact of Microbial Pathogens.
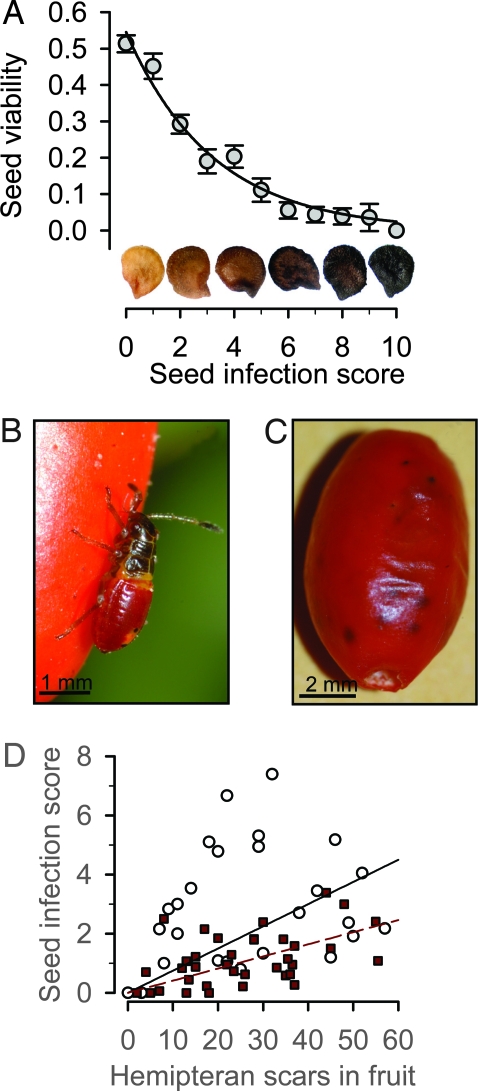
Across all populations in this system, the only significant cause of predispersal fruit and seed damage is microbial infection. This damage appears to be caused primarily by a single fungal species, Fusarium semitectum Berkeley and Ravenel (hereafter Fusarium). Fusarium infection of seeds causes discoloration that is easy to score, and we found Fusarium infection in >90% of all ripe fruits sampled across our populations (n = 305 fruits). The vast majority (95%) of these infections were provisionally attributable to Fusarium, which rots chili fruits and kills seeds. Even at low levels of infection, Fusarium causes substantial reductions in seed survival (Fig. 1A). Its entry into fruits is facilitated by hemipteran bugs that pierce the pericarp of fruits with their proboscises (Fig. 1B). This piercing introduces Fusarium into the fruit and seeds, leaving visible scars on the fruit surface, which turns black as the fungus invades (Fig. 1 B and C). We randomly selected single ripe fruits from pungent and nonpungent plants in our primary study site (called San Julian), counted foraging scars on the fruit, and scored all seeds in each fruit for degree of Fusarium infection. Fungal infection of seeds increased with the number of foraging scars on the fruit (F1,67 = 8.0, P = 0.006; Fig. 1D), and seeds from fruits without signs of insect damage showed no signs of fungal infection (odds ratio 7.3, Cochran's χ2 = 10.8, P = 0.001; Fig. 1D).
Fig. 1.
Fitness impacts and mechanics of fungal infection. (A) Seed survival (proportion of seeds germinating or still viable at end of germination trials ± 1 SE) as a function of Fusarium infection score (survival = 0.53−0.31 × fungal score, r2 = 0.97, P < 0.0005; n = 3414 seeds). Fusarium seed infection was scored from 0 (no infection) to 10 (uniformly black on both sides of the seed) using the seed standard pictured above the abscissa. (B) Acroleucus coxalis (Stäl) (Lygaeidae) nymph, the most common hemipteran foraging on chilies, piercing a chili fruit. (C) Ripe fruit with fungal infection spreading under surface of fruit at holes (hemipteran foraging scars). (D) Mean infection score on seeds in mature fruit as a function of hemipteran foraging scars on each fruit. Open symbols = nonpungent fruits; dark red symbols = pungent fruits. Regression forced through the origin (nonpungent F1,26 = 29, B = 0.075, P < 0.001; pungent F1,35 = 80, B = 0.041, P < 0.001).
Capsaicinoids and Microbial Damage.
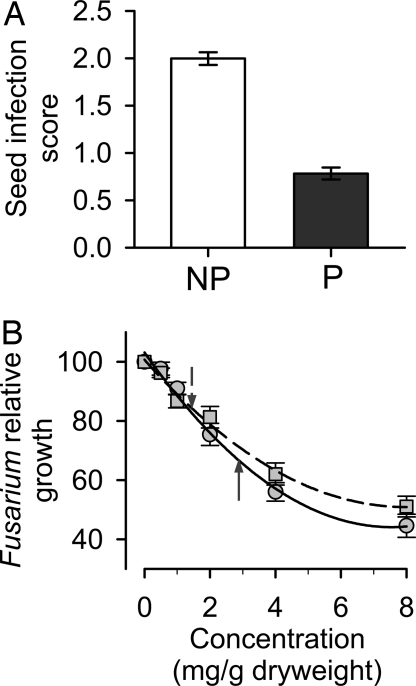
The same data gathered to assess the impact of hemipteran foraging on fungal infection also suggest a strong antifungal role for capsaicinoids. Although fungal infection of seeds increased with the number of hemipteran-foraging scars in both nonpungent and pungent fruits, the slope of this relationship was significantly steeper in nonpungent fruits (F2,66 = 55.81, P < 0.0001; Fig. 1D). Thus, for a given level of hemipteran foraging pressure, seed infection rates in nonpungent fruits are almost twice as high as in pungent fruit (F1,67 = 12.4, P = 0.001; Fig. 1D). We experimentally verified this susceptibility of nonpungent fruits to Fusarium by placing cages over randomly selected pungent and nonpungent plants in the same polymorphic population such that birds were prevented from removing fruits, but Fusarium-transmitting hemipterans had free access. We let these fruits mature naturally, then removed and scored their seeds for Fusarium infection. Degree of infection was more than twice as high on seeds from nonpungent plants than on seeds from pungent plants (F1,33 = 6.2, P = 0.018; Fig. 2A).
Fig. 2.
Effects of capsaicinoids on Fusarium infection. (A) Seed infection scores (mean ± 1 SE) in fruit from nonpungent (white) and pungent (red) plants (n = 10 pungent and 10 nonpungent per year). On each plant we evaluated five random fruits each year. Seed infection scores were assigned using the standard series shown in Fig. 1A. (B) Relative growth rate of Fusarium as a function of capsaicin (circles, solid line) and dihydrocapsaicin (squares, dashed line) concentrations. All experiments were conducted in media that mimicked the nutritional composition of wild chili fruits. Growth was measured relative to growth in a control media that lacked capsaicinoids (± 1 SE, four isolates, six replicates per isolate per treatment). Lines are quadratic functions of capsaicinoid concentration (r2 > 0.99, P < 0.0005). Mean capsaicin and dihydrocapsaicin concentrations in fruit at our primary study, where we assessed fungal loads, were 2.85 mg/g dry mass (solid up arrow), and 1.43 mg/g dry mass (dashed down arrow), respectively.
Nonpungent and pungent fruits are visually indistinguishable in the field, and their nutritional profiles are virtually identical (20). Nonetheless, the large difference in seed infection we observed between pungent and nonpungent plants could be because of a factor other than the presence or absence of capsaicinoids. Fungal loads in pungent fruit were 45–55% lower than in nonpungent fruit (Figs. 1D and 2A). If this reduction were caused by the presence of capsaicinoids in the pungent fruit, we should be able to generate a similar effect size in a more controlled experimental setting where capsaicinoid content is the sole independent variable. To test this we created artificial fruit media that mimicked the nutritional composition of C. chacoense fruit (20), differing only in the presence and concentration of the two primary capsaicinoids, capsaicin and dihydrocapsaicin. Inoculating these media with Fusarium isolates cultured from C. chacoense seeds from the same population showed that both capsaicin and dihydrocapsaicin cause strong dose-dependent inhibition of Fusarium growth (Fig. 2B, quadratic fits, r2 > 0.9). More importantly, at the capsaicinoid levels found in our focal population (Fig. 2B, arrows), capsaicin reduced Fusarium growth by 33%, and dihydrocapsaicin reduced Fusarium growth by 16%. Together, these chemicals fully account for the observed reduction in Fusarium seed infection in pungent fruit (predicted reduction based on capsaicinoid concentrations = 49%, the 95% CI of observed reduction = 41%–80%).
Fruit Chemistry and Fungal Selection.
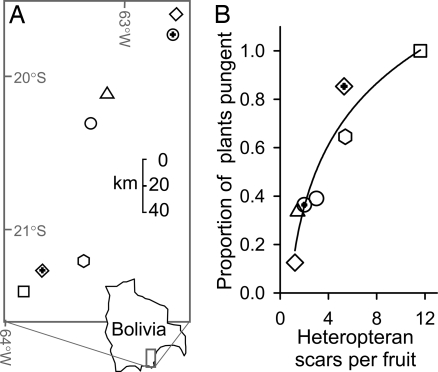
Capsaicinoids thus protect chili fruits and seeds from a fungal pathogen that severely reduces seed viability. If this process shapes the chemistry of chili fruits, changes in fungal selection pressure among chili populations should lead to parallel changes in the chemical defense of chili fruits, explaining among-population variation in capsaicinoid production. This prediction was supported. We surveyed seven chili populations, distributed across a 1,600 km2 area in eastern Bolivia (Fig. 3A). In each population, we randomly selected fruit and counted hemipteran-foraging scars. Because foraging pressure is positively correlated with fungal attack on seeds (Fig. 1D), the number of scars provides an index of variation in Fusarium pressure on fruits across populations. As predicted, the mean number of hemipteran scars on fruits in a population was a strong predictor of the proportion of plants producing capsaicinoids (r2 = 0.91, n = 7, F1,5 = 39, P = 0.0008; Fig. 3B).
Fig. 3.
Heteropteran foraging, fungal pressure, and capsaicinoid production. (A) Locations of the seven populations in which we examined the relationship between hemipteran foraging frequency and fruit chemistry. (B) Hemipteran foraging frequency on chili fruits, an index of fusarium infection pressure, is a strong nonlinear predictor of the proportion of plants producing capsaicinoids (y = alnx + b, r2 = 0.91, F1,5 = 39, P = 0.0008).
Insect-mediated fungal attack on seeds thus appears at least partially responsible for driving phenotypic evolution in chili fruits; as fungal pressure increases, selection for protection against fungal attack should also increase, favoring pungent phenotypes. Yet pungency does not appear to come free of costs: Our previous work shows that tradeoffs between capsaicinoid production and seed-coat thickness can favor nonpungent plants because seeds from these plants have thicker seed coats and are better protected as they pass through the digestive tract of seed dispersers (21). Teasing apart the relative importance of these potential selection pressures will require direct experimental evidence for specific adaptive functions (22). We suggest that the ratio of pungent to nonpungent plants in a given population reflects a long-term averaging of multiple benefits (6) and costs (21) of capsaicinoid production, creating a mosaic of evolutionary outcomes (23).
These findings provide strong support for the role of microbes in shaping fruit chemistry in wild species (4, 7, 8, 24) as increases in microbial pressure are met by concomitant increases in the frequency of chemically protected fruit. Though the focus of our work has been the chemical response of chilies to microbial attack, the antimicrobial properties of capsaicinoids extend well beyond Fusarium and have captured the interests of food scientists, ethnopharmacologists, and evolutionary biologists interested in historical and geographic patterns of how chilies are used by humans (12–14). For example, it has been postulated that the capsaicinoids in chilies may have had a profound influence on the domestication and use of chilies as a spice because of humans harnessing capsaicinoids' antimicrobial benefits for food preservation (12, 15). Before the advent of refrigeration, microbial contamination of food was a common cause of illness and death in many cultures (25), and the consumption of chilies with food may have reduced the risk of microbial infection (12–14, 25), providing an adaptive reason to eat pungent food. If the antimicrobial properties of chilies are truly responsible for their early domestication and spread, our research provides an evolutionary foundation for this relationship—human use of chilies may mirror the evolutionary function of these compounds in the fruits that produce them.
Materials and Methods
Fungal Identification.
We isolated fungal lines in sterile culture and used both morphological traits and DNA sequence data (i.e., compared DNA sequence data to related sequences in the National Center for Biotechnology Information GenBank database using BLAST) to identify fungal isolates.
Scoring Seeds for Fungal Attack.
All scoring of fungal infection on seeds was done blind; observers had no knowledge of seed source or pungency. We scored all seeds on both sides from 0 (no obvious infection) to 5 (highest level of infection) and summed each seed's two scores, creating a single score from 0 to 10.
Foraging Scars and Fungal Pressure.
We counted the number of hemipteran foraging scars on randomly selected ripe fruit, then removed all seeds and scored them for fungal infection. For analysis, we used an ANCOVA design with regressions fit through the origin (Fig. 1D).
Pungent Versus Nonpungent Fungal Loads.
Randomly selected unripe fruits were marked on pungent and nonpungent plants in the same population and left on plants until they had fully ripened. Seeds were then removed and scored for fungal infection. We used a linear mixed model to compare fungal loads between pungent and nonpungent fruit, blocking on year, plant, and fruit within plant. Pungency was a fixed effect, and Fusarium infection score was the dependent variable.
Fusarium and Seed Survival.
Seeds from pungent and nonpungent plants were stored in the field through the dormancy season (April to October). Germination trials were conducted in the field, using moist filter paper on natural soil. Seeds were scored for Fusarium infection as described. Seed survival (Ss) was assessed as Ss = g + (1 − g × v), for each level of Fusarium infection (0—10), where g is the percentage of seeds germinating after six weeks, and v is the percentage of ungerminated seeds testing positive for metabolically active tissue with tetrazolium chloride (26). We used a total of 3414 seeds for these trials. See the SI for detailed methods and results.
Artificial Fruit Media.
To mirror the nutrient profile of ripe C. chacoense fruits (20), we created 11 batches of artificial fruit media (see the SI for the recipe), added one of five concentrations of capsaicin or dihydrocapsaicin (0.25, 0.5, 1, 2, and 4 mg/g) dissolved in methanol to 10 of the batches, and added an equal amount of methanol as a control to the 11th batch. We then poured sterile media into 12-well plates (n = 24 plates, 288 wells) and inoculated media in the center with a small plug of Fusarium taken from one of four isolates, replicating each isolate in each treatment six times. Radial mycelial growth was measured at 72 h, and growth on treatment media was standardized relative to growth on control media, lacking capsaicinoids.
Complete Methods and associated references are available in the online version of this article.
Supplementary Material
Acknowledgments.
Field assistance was provided by Melissa Simon and Rob Dobbs. Research was supported by National Science Foundation and National Geographic Society grants (to J.J.T. and D.J.L.) and by a National Science Foundation Graduate Research Fellowship (to D.C.H.). Logistical support in Bolivia was provided by Fundación Amigos de la Naturaleza and the Wildlife Conservation Society.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0802691105/DCSupplemental.
References
- 1.Tiffney BH. Seed size, dispersal syndromes, and the rise of the angiosperms: Evidence and hypothesis. Ann Miss Bot Garden. 1984;71:551–576. [Google Scholar]
- 2.Smith JF. High species diversity in fleshy-fruited tropical understory plants. Am Nat. 2001;157:646–653. doi: 10.1086/320625. [DOI] [PubMed] [Google Scholar]
- 3.Tiffney BH. Vertebrate dispersal of seed plants through time. Ann Rev Ecol Evol Syst. 2004;35:1–29. [Google Scholar]
- 4.Herrera CM. Defense of ripe fruit from pests: Its significance in relation to plant-disperser interactions. Am Nat. 1982;120:218–241. [Google Scholar]
- 5.Janzen DH. Seed-eaters versus seed size number toxicity and dispersal. Evolution. 1969;23:1–27. doi: 10.1111/j.1558-5646.1969.tb03489.x. [DOI] [PubMed] [Google Scholar]
- 6.Tewksbury JJ, Nabhan GP. Seed dispersal: Directed deterrence by capsaicin in chillies. Nature. 2001;412:403–404. doi: 10.1038/35086653. [DOI] [PubMed] [Google Scholar]
- 7.Cipollini ML, Levey DJ. Secondary metabolites of fleshy vertebrate-dispersed fruits: Adaptive hypotheses and implications for seed dispersal. Am Nat. 1997;150:346–372. doi: 10.1086/286069. [DOI] [PubMed] [Google Scholar]
- 8.Gershenzon J. The cost of plant chemical defense against herbivory: A biochemical perspective. In: Bernays EA, editor. Insect-Plant Interactions. Boca Raton: CRC; 1994. pp. 105–173. [Google Scholar]
- 9.Levey DJ, Tewksbury JJ, Izhaki I, Tsahar E, Haak D. Evolutionary ecology of secondary compounds in ripe fruit: Case studies with capsaicin and emodin. In: Dennis AJ, Schupp EW, Green RA, Westcott DA, editors. Seed Dispersal: Theory and Its Application in a Changing World. Oxfordshire: CABI; 2007. pp. 37–58. [Google Scholar]
- 10.Perry L, Dickau R, Zarrillo S, Holst I, Pearsall DM, Piperno DR, Berman MJ, Cooke RG, Rademaker K, Ranere AJ, et al. Starch fossils and the domestication and dispersal of chili peppers (Capsicum spp. L) in the Americas. Science. 2007;315:986–988. doi: 10.1126/science.1136914. [DOI] [PubMed] [Google Scholar]
- 11.Cordell GA, Araujo OE. Capsaicin: Identification, nomenclature, and pharmacotherapy. Ann Pharmacotherapy. 1993;27:330–336. doi: 10.1177/106002809302700316. [DOI] [PubMed] [Google Scholar]
- 12.Billing J, Sherman PW. Antimicrobial functions of spices: Why some like it hot. Quart Rev Biol. 1998;73:3–49. doi: 10.1086/420058. [DOI] [PubMed] [Google Scholar]
- 13.Molina-Torres J, García-Chávez A, Ramírez-Chávez E. Antimicrobial properties of alkamides present in flavouring plants traditionally used in Mesoamerica: Affinin and capsaicin. J Ethnopharmacology. 1999;64:241–248. doi: 10.1016/s0378-8741(98)00134-2. [DOI] [PubMed] [Google Scholar]
- 14.Xing FB, Cheng GX, Yi KK. Study on the antimicrobial activities of the capsaicin microcapsules. J Appl Polym Sci. 2006;102:1318–1321. [Google Scholar]
- 15.Sherman PW, Billing J. Darwinian gastronomy: Why we use spices. Bioscience. 1999;49:453–463. [Google Scholar]
- 16.Suzuki T, Iwai K. Constituents of red pepper species: Chemistry, biochemistry, pharmacology, and food science of the pungent principle of Capsicum species. In: Cordell GA, editor. The Alkaloids. New York: Academic; 1984. pp. 227–299. [Google Scholar]
- 17.Eriksson O, Ehrlen J. Secondary metabolites in fleshy fruits: Are adaptive explanations needed? Am Nat. 1998;152:905–907. doi: 10.1086/286217. [DOI] [PubMed] [Google Scholar]
- 18.Tewksbury JJ, Manchego C, Haak DC, Levey DJ. Where did the chili get its spice? Biogeography of capsaicinoid production in ancestral wild chili species. Chem Ecol. 2006 doi: 10.1007/s10886-005-9017-4. [DOI] [PubMed] [Google Scholar]
- 19.McLeod MJ, Guttman SI, Eshbaugh WH. Early evolution of chili peppers (Capsicum) Econ Bot. 1982;36:361–386. [Google Scholar]
- 20.Levey DJ, Tewksbury JJ, Cipollini M, Carlo T. A field test of the directed deterrence hypothesis in two species of wild chili. Oecologia. 2006;150:51–68. doi: 10.1007/s00442-006-0496-y. [DOI] [PubMed] [Google Scholar]
- 21.Tewksbury JJ, Levey DJ, Huizinga M, Haak D, Travaset A. Costs and benefits of capsaicin-mediated control of gut retention in dispersers of wild chilies. Ecology. 2008;89:107–117. doi: 10.1890/07-0445.1. [DOI] [PubMed] [Google Scholar]
- 22.Nuismer SL, Gandon S. Moving beyond common-garden and transplant designs: Insight into the causes of local adaptation in species interactions. Am Nat. 2008;171:658–668. doi: 10.1086/587077. [DOI] [PubMed] [Google Scholar]
- 23.Thompson JN. The Geographic Mosaic of Coevolution. Chicago: University of Chicago Press; 2005. [Google Scholar]
- 24.Janzen DH. Why fruits rot, seeds mold, and meat spoils. Am Nat. 1977;111:691–713. [Google Scholar]
- 25.Sherman PW, Flaxman SM. Protecting ourselves from food: Spices and morning sickness may shield us from toxins and microorganisms in the diet. American Sci. 2001;89:142–151. [Google Scholar]
- 26.Cottrell HJ. Tetrazolium salt as a seed germination indicator. Nature. 1947;159 doi: 10.1038/159748a0. 748.mbf. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.