Abstract
The amygdala is a key limbic area involved in fear responses and pavlovian conditioning with the potential to directly respond to endocrine signals associated with fear or stress. To gain insights into the molecular mechanisms and subregional specificity of fear conditioning, we disrupted type II glucocorticoid receptors (GRs) in the central nucleus of the amygdala (CeA) by delivering lentiviral vectors containing Cre-recombinase into floxed-GR mice. GR deletion in the CeA (CeAGRKO mice) prevented conditioned fear behavior. In contrast, forebrain disruption of GRs excluding the CeA did not. The conditioned fear deficit in CeAGRKO mice was associated with decreases in cFos and corticotropin-releasing hormone (CRH) expression. Moreover, intracerebroventricular delivery of CRH rescued the conditioned fear deficit in CeAGRKO mice. We conclude that fear conditioning involves a neuroendocrine circuit by using GR activation in the CeA for acute CRH induction and long-lasting behavioral modulation.
Keywords: adrenal, animal behavior, knockout mice, lentivirus, stress
The amygdala is essential for proper adaptation to stress and specific modulation of emotional learning and memory (1). In rats, lesions of the amygdala complex lead to impaired emotional conditioning but normal spatial memory (2), whereas in humans, functional MRI studies have shown that activation of the amygdala occurs during conditioned fear (3). One of the best-studied roles for the amygdala is in pavlovian fear conditioning. In this form of learning, neutral conditioned stimuli (CS; e.g., physical context and auditory cue) are paired with a noxious unconditioned stimulus (US; e.g., foot shock). Upon reexposure to a CS, the organism will exhibit a US-stereotyped response (e.g., freezing behavior).
Much research has focused on the basolateral nucleus of the amygdala (BLA) as the main area for acquisition and consolidation of fear memory (4, 5). Pharmacological or electrolytic lesions of the BLA prevent fear expression during testing when the lesion occurs either before conditioning or between training and testing (6). Conversely, lesions of hypothalamic and brainstem projections from the amygdala have implicated the central nucleus of the amygdala (CeA) in the expression of autonomic and behavioral correlates of conditioned fear (7). In addition, recent work using muscimol to temporarily inactivate the CeA or anisomycin to prevent protein synthesis in the CeA has revealed the important role of this nucleus in fear acquisition and consolidation (8, 9). One of the drawbacks of permanent lesion or temporary inactivation studies, however, is that they include effects in axon tracts passing through the amygdala.
The use of receptor-specific antagonists and agonists has allowed a more detailed characterization of the molecules involved in normal conditioning. For instance, the CeA has been shown to respond to hormonal changes associated with stress and fear. During stress, activation of the hypothalamic pituitary adrenal (HPA) axis causes a release of glucocorticoids (e.g., corticosterone), which bind to glucocorticoid receptors (GRs) throughout the CNS to alter behavior. Experimental manipulation of glucocorticoids can dramatically influence the acquisition and consolidation of fear conditioning (10, 11). Notably, different anatomical populations of GRs in the CNS have been hypothesized to have unique roles in modulating glucocorticoid release and behavior. For instance, whereas application of corticosterone to the hippocampus inhibits HPA axis activation (12), electrical or hormonal stimulation of the amygdala promotes glucocorticoid release (13). Behaviorally, GR modulation of pavlovian conditioning has focused on the BLA (14), leaving the role of GRs in the CeA less understood.
GRs have a number of characteristics that suggest they may be involved in the CeA's role in pavlovian conditioning. First, GRs in the CeA are poised to modulate the protein synthesis necessary for CeA modulation of conditioning (8) through their established role as transcription factors. Second, corticotropin-releasing hormone (CRH), a modulator itself of conditioning (15) likely via BLA CRH receptors (16), is increased in the CeA after conditioned fear (17) and with CeA corticosterone application (18). This suggests that corticosterone release during conditioned fear training may act to modulate CRH expression in the CeA to alter downstream targets of CeA output. Unfortunately, the observations that GR activation in distinct anatomical areas can have opposing effects on hormone release and behavior have made data from traditional gene deletion approaches difficult to interpret (19).
In the present study, we sought to define the specific role of GRs within the CeA in modulating conditioned fear. We delivered lentiviral vectors (LVs) containing Cre-recombinase into bilateral CeA of floxed-GR mice. In this way, we can specifically and quantitatively disrupt GR expression to determine the effect of the CeA-localized GR population in the modulation of freezing behavior in fear conditioning. Our findings indicate that CeA GR signaling is required in pavlovian fear conditioning and that CeA CRH may mediate GR action.
Results
To evaluate the role of GRs in amygdala-based fear conditioning, we used a viral delivery–conditional inactivation approach to disrupt a loxP-flanked GR allele (20). Two LVs were generated and used to deliver either Cre-recombinase or GFP to cells [supporting information (SI) and Fig. S1A]. Efficacy of CeA stereotaxic targeting was assessed in Rosa-26 reporter mice with injection of LV-Cre, but not LV-GFP, inducing specific and restricted LacZ expression in the CeA (Fig. S1B).
Deletion of GRs in the CeA.
The results of in vitro transduction of CHO cells and in vivo transduction of CeA neurons in Rosa-26 mice suggested that viral mediated Cre delivery would be an efficient method to target disruption of GRs in the CeA. To further analyze this system, we delivered LV-Cre to floxed-GR mice. Two weeks after viral delivery, coronal sections were immunostained for GRs and Cre-recombinase. Essentially all Cre-positive cells were GR-negative (Fig. S1C). To assess the neuronal specificity of our LV, we evaluated coexpression of Cre-recombinase and the neuronal marker, NeuN, in LV-Cre-injected floxed-GR mice. Consistent with the known neurotropism of lentivirus (21), quantitation of Cre-positive cells coexpressing NeuN indicated that 93.0 ± 0.76% (n = 3) of the cells expressing Cre were neurons.
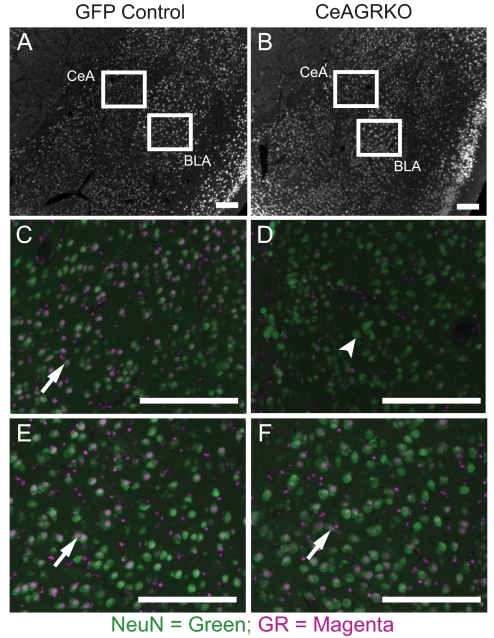
To determine the amount of GRs deleted in neurons, we immunolocalized GRs and NeuN in coronal sections from floxed-GR mice that had been bilaterally injected with LV-GFP (GFP controls) or LV-Cre (CeAGRKO). Quantitation of the percent of NeuN-positive GR-expressing cells indicated that we were able to disrupt GRs in a significant number of neurons in the CeA (percent NeuN-positive GR-expressing cells in the CeA; GFP controls, n = 6, 95.0 ± 0.48%; CeAGRKO, n = 18, 34.8 ± 1.97%; P < 0.0001) while avoiding deletion in the nearby BLA (percent NeuN-positive GR-expressing cells in the BLA; GFP controls, n = 6, 76.2 ± 0.79%; CeAGRKO, n = 18, 78.0 ± 0.95%; P > 0.05; Fig. 1).
Fig. 1.
GR is deleted in the CeA of CeAGRKO mice but not in the BLA. Immunohistochemical analysis of GR and NeuN expression in the CeA and BLA of floxed GR mice injected into the CeA with LV-GFP (A, C, and E) or LV-Cre (B, D, and F). Panels show low magnification image of amygdala areas (A and B) with immunoreactivity for NeuN (white). Panels show magnified immunoreactivity for GR (magenta) and NeuN (green) in the CeA (C and D) and BLA (E and F). GR is deleted in NeuN-positive cells in the CeA of LV-Cre-injected floxed-GR mice but not LV-GFP-injected mice. Note significant overlap of NeuN and GR (white arrows) in all panels except the CeAGRKO CeA (D), which shows a significant number of NeuN-positive, GR-negative cells (white arrowhead). Scale bars represent 200 μm.
Disruption of CeA GR Attenuates Fear Conditioning.
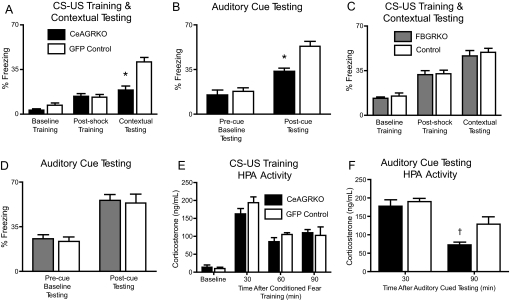
We hypothesized that GR activation in the CeA has a prominent role in the protein synthesis-dependent actions of the CeA essential for conditioned fear. Disruption of GR expression in the CeA (CeAGRKO) did not induce any significant change in freezing behavior during training (preshock and postshock) compared with GFP control mice (Fig. 2A). However, deletion of GRs in the CeA attenuated the freezing response during contextual fear testing (Fig. 2A; P = 0.0002). Furthermore, although no differences existed between CeAGRKO mice and GFP controls during cued testing, novel context baseline freezing, CeAGRKO mice exhibited a significant deficit in freezing behavior in the presence of the auditory cue (postcue freezing; P = 0.0003; Fig. 2B).
Fig. 2.
CeAGRKO mice, but not FBGRKO or control mice, show behavioral and HPA axis changes in conditioned fear. (A) CeAGRKO mice (n = 9) show a deficit in contextual freezing but no change in baseline or postshock freezing compared with GFP control mice (n = 9), representative of two independent experiments. (B) CeAGRKO mice show an attenuation of auditory cued freezing but no change in precue (baseline) freezing compared with GFP control mice. (C) FBGRKO mice (n = 6) show no changes in baseline, postshock, or contextual freezing compared with littermate controls (n = 6). (D) FBGRKO mice show no changes compared with littermate control mice during precue (baseline) or postcue auditory testing. (E) CeAGRKO mice (n = 6–9 at each time point) show no changes in plasma corticosterone concentration under basal conditions or at 30, 60, or 90 min following conditioned fear training compared with GFP controls (n = 7–9 at each time point). (F) CeAGRKO mice (n = 5 at each time point) show less sustained corticosterone at 90 min following auditory cued testing compared with GFP controls (n = 5 at each time point). (*, P < 0.001 vs. GFP control; †, P < 0.05 vs. GFP control.)
To further establish the specificity of GR action in the amygdala, we compared the conditioned fear results from CeAGRKO mice with those from forebrain-specific GR KO mice (FBGRKO). FBGRKO mice exhibit disruption of GR throughout the forebrain with the exception of the CeA, which shows normal GR expression (20). As with the CeAGRKO mice, we observed no significant changes during training in FBGRKO mice compared with littermate controls (Fig. 2C). In contrast to CeAGRKO mice, FBGRKO animals showed no differences in contextual fear testing (Fig. 2C) or auditory cued testing (Fig. 2D; precue [baseline] and postcue freezing) compared with controls.
Damage associated with the physical penetration of the needle (CeAGRKO and GFP controls) or deletion of a small amount of GR along the needle tract (CeAGRKO only) could potentially impact the motor output of the striatum. To further evaluate possible changes in locomotor behavior, we compared CeAGRKO and GFP control mice in open-field testing and a sensory-motor battery. No changes were observed between the groups in the anxiety-like parameters (time in center of open-field arena: GFP controls, n = 9, 14.7 ± 1.6 sec; CeAGRKO, n = 9, 13.1 ± 1.8 sec; P > 0.05) or locomotor parameters (total distance traveled: GFP controls, n = 9, 54.4 ± 8.2 m; CeAGRKO, n = 9, 50.8 ± 6.4 m; P > 0.05) measured in open field or in any of the sensory-motor tests analyzed (data not shown).
HPA Axis Evaluation After CeA GR Deletion.
It has been hypothesized that the CeA may increase HPA-driven glucocorticoid output. If this HPA activity-promoting role for the CeA is dependent on GR activity, then deletion of CeA GR may affect HPA drive under circadian conditions or during stress. To evaluate this possibility, we measured plasma corticosterone and adrenocorticotrophic hormone (ACTH) levels at circadian nadir and 30, 60, and 90 min after conditioned fear training. No differences in corticosterone (Fig. 2E) or ACTH (data not shown) were found between CeAGRKO mice and GFP controls at any time point evaluated. Thus, it appears that changes in glucocorticoid levels before or following conditioned fear training are not sufficient to explain the behavioral changes observed in the CeAGRKO mice.
We also evaluated corticosterone levels following auditory cued testing to determine if disruption of CeA GR is required for neuroendocrine fear responses in addition to behavior responses. We found reduced corticosterone in CeAGRKO mice at 90 min (P < 0.05) but not at 30 min following auditory cued testing compared with GFP controls (Fig. 2F).
Conditioned Fear Deficit Is Associated with Changes in cFos Expression.
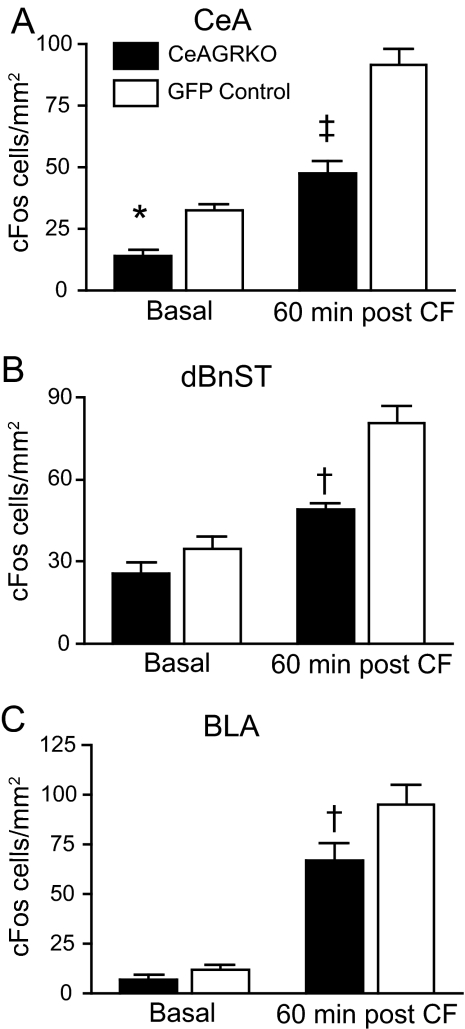
A well known role for activated GRs is in modulating gene transcription through direct DNA binding or through protein–protein interactions, which in turn can alter protein synthesis. Moreover, protein synthesis and neuronal activation are important components in the CeA's role in conditioned fear (8). To assess the effects of CeA GR deletion on protein synthesis and neuronal activation, we evaluated cFos at baseline and 60 min after conditioned fear. cFos expression, as measured by the number of immunopositive cells in an area, was reduced in CeAGRKO mice at baseline (P < 0.05) and after conditioned fear training (P < 0.001) in the CeA compared with GFP controls (Fig. 3A and Fig. S2A). However, in the bed nucleus of the stria terminalis (BnST; Fig. 4B and Fig. S2B) and BLA (Fig. 4C and Fig. S2C), cFos expression was significantly reduced in the KO mice after conditioned fear training (P < 0.01) but not at baseline.
Fig. 3.
cFos expression is altered in CeAGRKO mice in the CeA, BnST, and BLA. (A) In the CeA, CeAGRKO mice (n = 7) exhibit a reduced number of cFos-positive cells under basal conditions and 60 min following conditioned fear (CF) compared with GFP controls (n = 8). (B) In the dorsal BnST (dBnST), CeAGRKO mice exhibit normal basal cFos expression but reduced cFos expression following conditioned fear compared with GFP controls. (C) In the BLA, CeAGRKO mice exhibit normal basal cFos expression but reduced cFos expression following conditioned fear compared with GFP controls. (*, P < 0.05 vs. basal GFP group; †, P < 0.01 vs. GFP control group after conditioning; ‡, P < 0.001 vs. GFP control group after conditioning.)
Fig. 4.
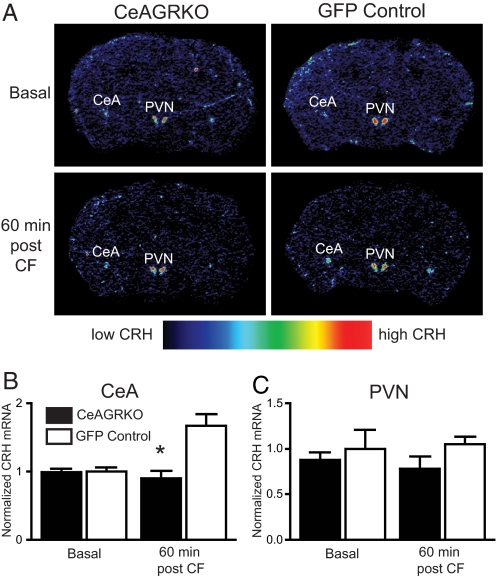
CRH mRNA is decreased in CeAGRKO mice after conditioned fear (CF). (A) Representative sections of CeAGRKO mice (Left) and GFP control mice (Right) under basal conditions (Top) or 60 min following conditioned fear training (Bottom) showing CRH expression as measured by in situ hybridization. (B) Although basal levels of CRH mRNA in CeA are equivalent between groups, CRH mRNA is significantly up-regulated in GFP controls (n = 6–8 per time point) but not in CeAGRKO mice (n = 6–7) after conditioned fear. (C) There are no significant changes in CRH expression in the PVN in either group under basal conditions or after conditioned fear training. (*, P < 0.001 vs. GFP control group after conditioned fear.)
Conditioned Fear Deficit Is Associated with Changes in CRH Expression.
The results of our evaluation of cFos expression suggest that deletion of GRs in the CeA may have an effect on new protein synthesis after conditioned fear training. Therefore, we analyzed the expression of CRH following conditioned fear in comparison with basal levels because CRH has been shown to be an important modulator of conditioned fear (15, 16) and can be regulated by glucocorticoids (13). Using in situ hybridization, we found that GFP control mice showed a significant up-regulation of CRH (P < 0.001) in the CeA that was absent in the CeAGRKO mice 60 min following conditioned fear training (Fig. 4 A and B). However, we found no significant changes in CRH mRNA levels at baseline in the CeA or at any time point in the paraventricular nucleus of the hypothalamus (PVN) when comparing CeAGRKO and GFP control mice (Fig. 4 A and B).
CRH Injection Rescues Behavioral Deficits.
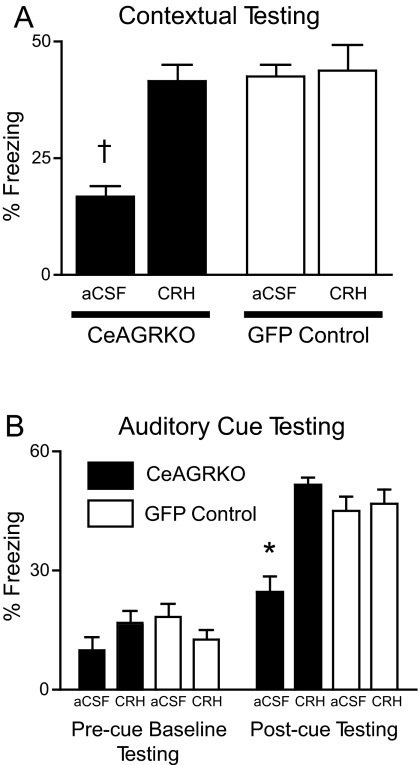
The results of the CRH mRNA in situ hybridization experiments provide evidence that CRH signaling may be an important contributor to GR modulation of conditioned fear behavior. We tested the hypothesis that restoration of CRH alone could rescue the CeAGRKO behavioral deficit. We delivered CRH or vehicle—artificial cerebrospinal fluid (aCSF)—intracerebroventricularly (ICV) immediately before conditioned fear training in CeAGRKO and GFP control mice, or we delivered CRH between training and testing in both groups of mice. Pretraining ICV injection of CRH or aCSF caused equivalent increased arousal in all groups during both baseline and postshock training (data not shown). During subsequent contextual fear testing, CeAGRKO mice given CRH before training showed a significant increase in freezing compared with CeAGRKO mice given control aCSF injections (P < 0.001; Fig. 5A). Similarly, although there were no significant differences between groups in precue freezing (i.e., novel context baseline) during auditory cued testing, CeAGRKO mice given CRH before training showed a significant increase in freezing compared with CeAGRKO mice given control aCSF injections following auditory CS onset (Fig. 5B; P < 0.01). CeAGRKO mice given CRH between training and testing continued to show a deficit in testing compared with GFP controls (Fig. S3 A and B).
Fig. 5.
CRH rescues attenuation of conditioned fear in CeAGRKO mice. (A) ICV delivery of CRH before training causes contextual test freezing in CeAGRKO mice equivalent to GFP control mice levels. (B) ICV delivery of CRH before training causes an increase in postcue freezing in CeAGRKO mice. Equivalent precue (baseline) freezing occurs in all groups. (*, P < 0.01 vs. all other groups; †, P < 0.001 vs. all other groups.)
Discussion
Although multiple lines of evidence have suggested that the amygdala is a crucial anatomical component of fear-based learning and memory, key questions regarding the molecular details and subregion specificity of the amygdala's role in pavlovian conditioning remain unanswered. Here, we report that disruption of GR specifically within the CeA causes an attenuation of freezing during both contextual fear and auditory cued testing that is associated with decreased expression of cFos and CRH. Furthermore, the attenuation of both contextual and auditory cued freezing can be reversed via ICV delivery of CRH before conditioning. These findings provide additional evidence that the CeA, like the BLA, is a site of memory acquisition and consolidation, and that at least part of this role can be attributed to signaling through glucocorticoid-stimulated GRs.
Our viral-mediated deletion approach has a number of advantages over GR antagonists that have been used to define the role of GR in a variety of situations. First, our lentivirus approach provided long-term disruption of GR in contrast to the shorter-term disruption with GR antagonists. This allowed us to look at the effect of deleting GR on both basal changes and changes following conditioning in the same animals without having to perform multiple injections. Second, we confirmed that our CeAGRKO model specifically disrupted GRs in the CeA while leaving nearby GR populations in the BLA intact.
Although none of our animals included in behavioral analysis showed significant deletion in the BLA, it is possible that a small amount of GR was deleted in the BLA or along the injection needle tract in the striatum. However, based on our data from FBGRKO mice, we think this small number of GR-negative cells outside the CeA is not sufficient to explain the behavioral deficit in conditioned fear in the CeAGRKO mice. FBGRKO mice show efficient disruption of GR in the cortex, hippocampus, BLA, and striatum, but normal expression of GR in the CeA, thalamus, and PVN (22). Our results with FBGRKO mice suggest that striatum or BLA deletion of GRs is not sufficient to induce the conditioned fear deficits seen in CeAGRKO mice. These results contrast with the results of pharmacological blockade of GRs in the BLA, which has been shown to impair conditioning (23). The difference in phenotype may be related to adaptive processes that occur in the KO mice after long-term loss of GRs in the BLA (20).
Another possible mechanism for the CeAGRKO auditory and contextual fear deficit stems from the hypothesized role of the CeA in promoting the release of glucocorticoids. We found no differences in corticosterone between CeAGRKO mice and GFP control mice after training, suggesting that the behavioral and molecular changes observed in CeAGRKO mice were caused by reduced activation of GRs in the CeA by normal levels of glucocorticoids. We also sought to find whether CeA GR action was required for conditioning of HPA axis responses during testing. We found sustained corticosterone elevation after testing in control mice that was impaired in CeAGRKO mice. This finding could reflect either reduced HPA drive because of the failure to learn the CS or a direct role for CeA GRs in regulating HPA axis activity during testing.
We found reduced cFos in the CeA at baseline and following conditioned fear training in the CeAGRKO mice compared with control mice. In the BnST and BLA, we found normal baseline cFos with reduced cFos following conditioned fear training. Our interpretation of the CeAGRKO behavioral deficit is that, during conditioned fear training, a reduced number of GRs in the CeA are activated, which causes reduced protein expression and subsequently weaker fear-based conditioning through reduced activation of downstream CeA targets. Potentially, at baseline, normal GR in the BnST and BLA is able to compensate for any reduced input from the CeA. Reduced baseline CeA cFos in CeAGRKO mice is consistent with reduced basal protein expression seen in GR neural KO mice (24) and is not likely to be representative of global CeA hypoactivation because lesioning of the CeA causes behavioral (25) and HPA axis activity changes (26) not observed in our experiments.
Two other previous models of GR alteration have been used to study the role of GR in modulating conditioned fear behavior. YGR transgenic mice overexpress GR throughout the nervous system and pituitary gland and have normal conditioned fear behavior despite an increase in GR expression (27). Notably, these mice exhibit lowered corticosterone levels following restraint stress. Similarly, GR null heterozygotes show normal conditioning along with increased corticosterone after restraint stress (27). These data, along with our present findings, suggest that, in both models, altered corticosterone release following conditioned fear training offsets the predicted effects of transgenic manipulation of GR on conditioned fear behavior.
As a final analysis of the molecular underpinnings of the CeAGRKO behavioral deficit, we analyzed the role of CRH in the GR modulation of conditioned fear. Previous work has shown that CRH-positive neurons in the CeA may comprise a central node in the network of areas involved in conditioned fear (16). However, it remains unproven whether physiological levels of glucocorticoids released during conditioning and binding to GRs are indeed responsible for the up-regulation of CRH. We hypothesized that glucocorticoid release during pavlovian conditioning activates GRs in the CeA to induce CRH expression and release onto downstream targets. In support of this model, we found increased CRH mRNA in the CeA of GFP control mice after conditioned fear but not in CeAGRKO mice. To determine whether ICV delivery of CRH was sufficient to restore normal fear conditioning, we delivered CRH or vehicle to CeAGRKO and GFP control mice before CS/US training. CRH infusion induced an increase in freezing in the CeAGRKO mice with regard to both types of conditioning. This increase was not a result of nonspecific effects of ICV injections as CeAGRKO mice receiving vehicle continued to show a deficit in conditioned fear. Furthermore, the effect of the CRH was not likely to be related to a nonspecific anxiogenic effect because both CeAGRKO and GFP control mice given ICV CRH or aCSF show equivalent freezing during CS/US training, and CRH delivered between training and testing did not rescue the behavioral deficit. Because the infusion of CRH occurs 1 week before contextual or auditory cued testing, it is likely that the observed rescue occurs as a result of a modifying role on CS/US acquisition or consolidation rather than on the actual expression of fear. Our results differ from those found in conventional CRH KO mice, which show no alteration in fear conditioning (28). This contrast may be explained by developmental adaptations to the lack of CRH or opposing actions of CRHR1 or R2 receptors (15, 28).
Our results have interesting implications for understanding contextual versus auditory cued conditioning. First, although contextual and auditory conditioning are classically separated into hippocampal- and amygdala-dependent processes, respectively, our data indicate that GRs in the CeA play an important role in both contextual and auditory fear testing apart from any discernible alterations in hippocampal function. Second, although adrenalectomy only impairs contextual conditioning (10), application of glucocorticoids can facilitate both contextual and auditory conditioning (11). The disparity between our work and the adrenalectomy data may be related to the time course of conditioning. Compared with the 24-h separation between training and auditory cued testing used by others (10), our protocol involves a 1-week separation, providing a more stringent test of long-term memory.
Overall, these results demonstrate the critical role of GR populations in the CeA in mediating pavlovian fear responses. Further delineation of GR and CRH action in nearby targets of the CeA including the BnST and BLA will offer additional support for the important modulatory role of stress hormones on emotional and fear-based conditioning and will have important implications for the pathogenesis and therapy of psychiatric and stress-related disorders.
Methods
Animals.
Characterization of CeAGRKO Mice.
Mice homozygous for the GRloxP allele (29) are inbred on a C57BL/6 background, and 3–4-month-old male mice are stereotaxically injected with either LV-GFP or LV-Cre-recombinase. Briefly, anesthetized mice were mounted in a standard stereotaxic frame (Kopf Instruments). A small hole was drilled on each side of midline over the CeA with the following coordinates: bregma, 1.25; lateral, ±2.75; ventral, 4.75. A 32-gauge needle was lowered into the hole and left in place for 1 min before injection of virus, and 4 × 105 infectious viral particles were injected into each CeA. All behavioral testing with mice occurred at least 2 weeks after recovery from surgery.
After behavior testing, correct targeting of LV-GFP in GFP controls to the CeA was verified by determining whether >95% of the GFP-positive cell bodies were in the CeA (see SI Text). LV-Cre-infected mice (i.e., CeAGRKO) were analyzed with immunohistochemistry with antibodies recognizing GR (1:200; Santa Cruz Biotechnology) and NeuN (1:200; Chemicon), GR and Cre (1:200; Novagen), or Cre and NeuN (see SI Text for immunohistochemical details). For quantitation of the number of GR and NeuN double-positive cells divided by the total number of NeuN positive cells in a region, at least three matched sections per mouse covering the anterior–posterior extent of the CeA were counted for NeuN and GR in bilateral CeAs and BLAs.
Generation of FBGRKO.
Mice homozygous for the previously described GRloxPneo allele were crossed with mice expressing Cre-recombinase under the control of the calcium/calmodulin-dependent kinase II promoter (20). Male littermate KO (Cre-positive) and control (Cre-negative) mice 4–6 months of age were used for indicated experiments. Mice were of a mixed C57BL/6 × 129 × CBA background.
Lentivirus Production.
Viral vectors were derived from the HIV-based lentivirus backbone pLV-EF1a-GFP (30), which allows for viral transduction and expression of GFP in neurons. We developed the Cre-recombinase-containing vector by cloning in Cre-recombinase into the GFP backbone. Production of replication-deficient viral particles was done as previously described (31). See SI Text for details.
Behavioral Analysis.
All behavioral analyses were performed by an observer blinded to genotype and treatment. A general motor battery including the inclined screen, ledge, and platform tests was performed as described in ref. 32. Anxiety and locomotion were measured with an open field apparatus. Conditioned fear was analyzed using a single CS/US training protocol including an auditory cue (white noise) and foot shock (2 sec, 0.7 mA). Testing occurred 6 or 7 days after training in the context alone or in a novel context with or without the auditory cue. See SI Text for a full description of behavioral testing paradigms.
Microinjections for Evaluation of the Role of CRH in Conditioned Fear.
CRH (Mouse/rat/human CRH, 1 mg/ml stock in 10 mM acetic acid; Bachem) was diluted to a final concentration of 50 ng/μl in 1× aCSF. Vehicle solution was prepared by diluting 10 mM acetic acid in aCSF in an identical manner. ICV injections into awake-behaving mice were done as previously described (33). CRH or aCSF was delivered at a rate of 1 μl/min for 2 min into CeAGRKO and GFP controls. Injection of drug occurred either 5 min before conditioned fear training or on day 3 after training. Remaining behavioral experiment (training and testing) was identical to conditioned fear described earlier. Targeting of ventricles was verified by posthoc injection of fast green (Sigma) through a hole in the skull.
Radioimmunoassays.
Plasma concentrations of corticosterone and ACTH were determined by RIA from blood collected by retroorbital phlebotomy at circadian nadir (60 min after lights on) or during conditioned fear as described in ref. 20.
Immunohistochemistry.
Brains were collected under basal conditions or 60 min after the end of conditioned fear training and processed with an antibody recognizing cFos (1:20,000; Calbiochem). See SI Text for details.
In Situ Hybridization.
Brains were collected under basal conditions or 60 min after the end of conditioned fear training and processed as previously described to evaluate CRH mRNA expression (22) (see SI Text for details). Densitometric analysis of bilateral CeA and PVN in situ signal (two sections per mouse) was performed using National Institutes of Health Image software.
Data Analysis.
Results are expressed as mean ± SEM. Statistical comparisons were performed with the use of the Student t test or one- or two-way ANOVA with posthoc Bonferroni tests.
Supplementary Material
Acknowledgments.
We thank Jeffrey Medin (University of Toronto) for reagents and Yarimar Carrasquillo, John Cirrito, Alana Conti, Shannon Macauley, Christine Ratajczak, Lindsay Wieczorek, and Zhongqiu Zhao for assistance. This work was supported by National Institutes of Health Neuroscience Blueprint Core Grant P30NS057105, National Institutes of Health grant AG18876 (to L.J.M.) and National Institutes of Health grant F31MH075250 (to B.J.K.). We dedicate this article to Dr. Enriqueta Bond on the occasion of her retirement as president of the Burroughs Wellcome Fund for fostering the career development of biomedical scientists.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0803216105/DCSupplemental.
References
- 1.Kim JJ, Jung MW. Neural circuits and mechanisms involved in pavlovian fear conditioning: A critical review. Neurosci Biobehav Rev. 2006;30:188–202. doi: 10.1016/j.neubiorev.2005.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sutherland RJ, McDonald RJ. Hippocampus, amygdala, and memory deficits in rats. Behav Brain Res. 1990;37:57–79. doi: 10.1016/0166-4328(90)90072-m. [DOI] [PubMed] [Google Scholar]
- 3.Hyman SE. A new image for fear and emotion. Nature. 1998;393:417–418. doi: 10.1038/30855. [DOI] [PubMed] [Google Scholar]
- 4.Amorapanth P, LeDoux JE, Nader K. Different lateral amygdala outputs mediate reactions and actions elicited by a fear-arousing stimulus. Nat Neurosci. 2000;3:74–79. doi: 10.1038/71145. [DOI] [PubMed] [Google Scholar]
- 5.Campeau S, Davis M. Involvement of the central nucleus and basolateral complex of the amygdala in fear conditioning measured with fear-potentiated startle in rats trained concurrently with auditory and visual conditioned stimuli. J Neurosci. 1995;15:2301–2311. doi: 10.1523/JNEUROSCI.15-03-02301.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maren S, Aharonov G, Fanselow MS. Retrograde abolition of conditional fear after excitotoxic lesions in the basolateral amygdala of rats: Absence of a temporal gradient. Behav Neurosci. 1996;110:718–726. doi: 10.1037//0735-7044.110.4.718. [DOI] [PubMed] [Google Scholar]
- 7.LeDoux JE, Iwata J, Cicchetti P, Reis DJ. Different projections of the central amygdaloid nucleus mediate autonomic and behavioral correlates of conditioned fear. J Neurosci. 1988;8:2517–2529. doi: 10.1523/JNEUROSCI.08-07-02517.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wilensky AE, Schafe GE, Kristensen MP, LeDoux JE. Rethinking the fear circuit: The central nucleus of the amygdala is required for the acquisition, consolidation, and expression of pavlovian fear conditioning. J Neurosci. 2006;26:12387–12396. doi: 10.1523/JNEUROSCI.4316-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zimmerman JM, Rabinak CA, McLachlan IG, Maren S. The central nucleus of the amygdala is essential for acquiring and expressing conditional fear after overtraining. Learn Mem. 2007;14:634–644. doi: 10.1101/lm.607207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pugh CR, Tremblay D, Fleshner M, Rudy JW. A selective role for corticosterone in contextual-fear conditioning. Behav Neurosci. 1997;111:503–511. [PubMed] [Google Scholar]
- 11.Hui GK, Figueroa IR, Poytress BS, Roozendaal B, McGaugh JL, Weinberger NM. Memory enhancement of classical fear conditioning by post-training injections of corticosterone in rats. Neurobiol Learn Mem. 2004;81:67–74. doi: 10.1016/j.nlm.2003.09.002. [DOI] [PubMed] [Google Scholar]
- 12.Kovacs KJ, Makara GB. Corticosterone and dexamethasone act at different brain sites to inhibit adrenalectomy-induced adrenocorticotropin hypersecretion. Brain Res. 1988;474:205–210. doi: 10.1016/0006-8993(88)90435-0. [DOI] [PubMed] [Google Scholar]
- 13.Shepard JD, Barron KW, Myers DA. Stereotaxic localization of corticosterone to the amygdala enhances hypothalamo-pituitary-adrenal responses to behavioral stress. Brain Res. 2003;963:203–213. doi: 10.1016/s0006-8993(02)03978-1. [DOI] [PubMed] [Google Scholar]
- 14.Roozendaal B, McGaugh JL. Amygdaloid nuclei lesions differentially affect glucocorticoid-induced memory enhancement in an inhibitory avoidance task. Neurobiol Learn Mem. 1996;65:1–8. doi: 10.1006/nlme.1996.0001. [DOI] [PubMed] [Google Scholar]
- 15.Radulovic J, Ruhmann A, Liepold T, Spiess J. Modulation of learning and anxiety by corticotropin-releasing factor (crf) and stress: Differential roles of crf receptors 1 and 2. J Neurosci. 1999;19:5016–5025. doi: 10.1523/JNEUROSCI.19-12-05016.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Roozendaal B, Brunson KL, Holloway BL, McGaugh JL, Baram TZ. Involvement of stress-released corticotropin-releasing hormone in the basolateral amygdala in regulating memory consolidation. Proc Natl Acad Sci USA. 2002;99:13908–13913. doi: 10.1073/pnas.212504599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thompson BL, Erickson K, Schulkin J, Rosen JB. Corticosterone facilitates retention of contextually conditioned fear and increases crh mrna expression in the amygdala. Behav Brain Res. 2004;149:209–215. doi: 10.1016/s0166-4328(03)00216-x. [DOI] [PubMed] [Google Scholar]
- 18.Shepard JD, Barron KW, Myers DA. Corticosterone delivery to the amygdala increases corticotropin-releasing factor mrna in the central amygdaloid nucleus and anxiety-like behavior. Brain Res. 2000;861:288–295. doi: 10.1016/s0006-8993(00)02019-9. [DOI] [PubMed] [Google Scholar]
- 19.Howell MP, Muglia LJ. Effects of genetically altered brain glucocorticoid receptor action on behavior and adrenal axis regulation in mice. Front Neuroendocrinol. 2006;27:275–284. doi: 10.1016/j.yfrne.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 20.Boyle MP, et al. Acquired deficit of forebrain glucocorticoid receptor produces depression-like changes in adrenal axis regulation and behavior. Proc Natl Acad Sci USA. 2005;102:473–478. doi: 10.1073/pnas.0406458102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Blomer U, et al. Highly efficient and sustained gene transfer in adult neurons with a lentivirus vector. J Virol. 1997;71:6641–6649. doi: 10.1128/jvi.71.9.6641-6649.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Boyle MP, Kolber BJ, Vogt SK, Wozniak DF, Muglia LJ. Forebrain glucocorticoid receptors modulate anxiety-associated locomotor activation and adrenal responsiveness. J Neurosci. 2006;26:1971–1978. doi: 10.1523/JNEUROSCI.2173-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Donley MP, Schulkin J, Rosen JB. Glucocorticoid receptor antagonism in the basolateral amygdala and ventral hippocampus interferes with long-term memory of contextual fear. Behav Brain Res. 2005;164:197–205. doi: 10.1016/j.bbr.2005.06.020. [DOI] [PubMed] [Google Scholar]
- 24.Revest JM, et al. The mapk pathway and egr-1 mediate stress-related behavioral effects of glucocorticoids. Nat Neurosci. 2005;8:664–672. doi: 10.1038/nn1441. [DOI] [PubMed] [Google Scholar]
- 25.Peinado-Manzano A. Effects of bilateral lesions of the central and lateral amygdala on free operant successive discrimination. Behav Brain Res. 1988;29:61–71. doi: 10.1016/0166-4328(88)90053-8. [DOI] [PubMed] [Google Scholar]
- 26.Van de Kar LD, Piechowski RA, Rittenhouse PA, Gray TS. Amygdaloid lesions: Differential effect on conditioned stress and immobilization-induced increases in corticosterone and renin secretion. Neuroendocrinology. 1991;54:89–95. doi: 10.1159/000125856. [DOI] [PubMed] [Google Scholar]
- 27.Ridder S, et al. Mice with genetically altered glucocorticoid receptor expression show altered sensitivity for stress-induced depressive reactions. J Neurosci. 2005;25:6243–6250. doi: 10.1523/JNEUROSCI.0736-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Weninger SC, et al. Stress-induced behaviors require the corticotropin-releasing hormone (crh) receptor, but not crh. Proc Natl Acad Sci USA. 1999;96:8283–8288. doi: 10.1073/pnas.96.14.8283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brewer JA, et al. T-cell glucocorticoid receptor is required to suppress cox-2-mediated lethal immune activation. Nat Med. 2003;9:1318–1322. doi: 10.1038/nm895. [DOI] [PubMed] [Google Scholar]
- 30.Yoshimitsu M, et al. Bioluminescent imaging of a marking transgene and correction of Fabry mice by neonatal injection of recombinant lentiviral vectors. Proc Natl Acad Sci USA. 2004;101:16909–16914. doi: 10.1073/pnas.0407572101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hofling AA, Devine S, Vogler C, Sands MS. Human cd34+ hematopoietic progenitor cell-directed lentiviral-mediated gene therapy in a xenotransplantation model of lysosomal storage disease. Mol Ther. 2004;9:856–865. doi: 10.1016/j.ymthe.2004.03.013. [DOI] [PubMed] [Google Scholar]
- 32.Ho N, et al. Impaired synaptic plasticity and camp response element-binding protein activation in ca2+/calmodulin-dependent protein kinase type iv/gr-deficient mice. J Neurosci. 2000;20:6459–6472. doi: 10.1523/JNEUROSCI.20-17-06459.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Haley TJ, McCormick WG. Pharmacological effects produced by intracerebral injection of drugs in the conscious mouse. Br J Pharmacol. 1957;12:12–15. doi: 10.1111/j.1476-5381.1957.tb01354.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.