Abstract
Allosteric regulation of protein function is a fundamental phenomenon of major importance in many cellular processes. Such regulation is often achieved by ligand-induced conformational changes in multimeric proteins that may give rise to cooperativity in protein function. At the heart of allosteric mechanisms offered to account for such phenomenon, involving either concerted or sequential conformational transitions, lie changes in intersubunit interactions along the ligation pathway of the protein. However, structure–function analysis of such homooligomeric proteins by means of mutagenesis, although it provides valuable indirect information regarding (allosteric) mechanisms of action, it does not define the contribution of individual subunits nor interactions thereof to cooperativity in protein function, because any point mutation introduced into homooligomeric proteins will be present in all subunits. Here, we present a general strategy for the direct analysis of cooperativity in multisubunit proteins that combines measurement of the effects on protein function of all possible combinations of mutated subunits with analysis of the hierarchy of intersubunit interactions, assessed by using high-order double-mutant cycle-coupling analysis. We show that the pattern of high-order intersubunit coupling can serve as a discriminative criterion for defining concerted versus sequential conformational transitions underlying protein function. This strategy was applied to the particular case of the voltage-activated potassium channel protein (Kv) to provide compelling evidence for a concerted all-or-none activation gate opening of the Kv channel pore domain. An direct and detailed analysis of the contribution of high-order intersubunit interactions to cooperativity in the function of an allosteric protein has not previously been presented.
Keywords: activation gate, allosteric enzymes, double-mutant cycles, voltage-activated potassium channels, non-additivity
Allosteric regulation of protein function is often achieved by changes in protein conformation induced by the binding of substrate or other ligand molecules (1). In multisubunit proteins, such conformational changes may give rise to cooperativity in ligand binding. Several mechanistic models, in particular the Monod–Wymann–Changeux (MWC) model (2) and the Koshland–Némethy–Filmer (KNF) model (3), were developed in the 1960s to describe cooperativity in ligand binding. In both models, cooperativity is explained by binding-induced conformational changes in the subunits of the protein that may be concerted (MWC), sequential (KNF), or a combination of both (4). No matter the type of conformational change, cooperativity in ligand binding in all allosteric models is manifested by changes in intersubunit interactions along the ligation pathway of the protein (1).
Despite the general acceptance of intersubunit interactions playing a fundamental role in determining the magnitude of cooperativity in ligand binding by an allosteric enzyme, structure–function analysis of such proteins through mutagenesis has proven unsatisfactory in providing information on the contribution of individual subunits and their interactions to cooperativity in protein function. This is because any point mutation introduced into a homooligomeric protein will be present in all subunits. Furthermore, to gain mechanistic insight into the function of the protein of interest and to assess the magnitude of cooperativity in its ligand binding, it is common practice to fit steady-state binding data to model-based equations. These analyses, although informative, suffer from one major shortcoming, namely that they only allow for an indirect assessment of the nature and extent of cooperativity. Successful data fitting to a model-based equation is not, however, a proof for a suggested mechanism.
How then can one reveal, in a direct manner, the sequential or concerted nature of conformational transitions of an allosteric protein and further evaluate, in a rigorous manner, the contribution of individual protein subunits and mutual interactions thereof to cooperativity in protein function? Toward this end, we now describe a strategy that meets these challenges by directly exploring the nature and magnitude of cooperativity in multisubunit allosteric proteins. The approach adopted involves measuring the effects on protein function of all possible combinations of subunit mutations introduced into tandem-linked subunits of a homooligomeric protein and the calculation of the hierarchy of intersubunit interaction energies by using high-order double-mutant cycle analysis (5–7). As an example of the strength of this approach, we make use of the voltage-activated potassium channel (8–10), an allosteric protein involved in electrical signaling (11), as a model system. We show that opening of the channel's activation gate, which grants accessibility of the intracellular pool of K+ ions to the upper selectivity filter inactivation gate, occurs in a concerted all-or-none manner. Our findings thus provide a detailed example of direct assessment of the contribution of intersubunit interactions to cooperativity in the function of an allosteric enzyme.
Results
A Strategy for Direct Analysis of Cooperativity in Multisubunit Allosteric Proteins.
As outlined above, cooperativity in the function of oligomeric proteins is tightly related to changes in intersubunit interactions (1). To gain insight into possible interactions contributing to cooperativity in the function of a protein, a concatenated gene, encoding the n identical protein subunits connected by flexible linkers, each harboring unique restriction sites, is used (Fig. 1A) (12) (kindly provided by F. Sigworth, Yale University, New Haven, CT). This tandem-subunit gene construct enables one to “cut and paste” mutated subunits in any combination desired (see supporting information (SI) Text) (12). Constructs encoding tandem-linked subunits have been previously used to examine different aspects of Kv channel function, including channel inactivation and assembly (12–14). In only a few cases have such tandem-linked subunit constructs been used to ascertain the contribution of individual subunits to cooperativity in the function of the oligomeric protein (15–17). These studies, however, did not exploit the full potential of such constructs to decipher the contributions of intersubunit interactions to cooperativity in protein function. As will be elaborated below, we rigorously demonstrate how such tandem-linked gene constructs may be used to elucidate the nature of conformational transitions underlying allosteric protein function and to allow for detailed assessment of the contribution of individual subunits and, most importantly, the magnitude of interactions thereof to cooperativity in protein function. In the case of the homotetrameric allosteric protein example considered here, namely the voltage-activated potassium channel (Kv), such analysis is achieved by measuring the effects on channel opening of all possible combinations of single-, double-, triple-, and quadruple-subunit mutants (Fig. 1B), combined with application of high-order double-mutant cycle analysis (Fig. 1C) (5–7) of intersubunit coupling.
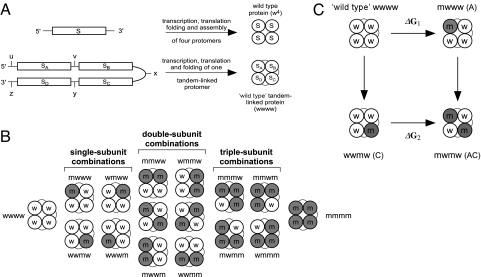
Fig. 1.
Strategy for a direct analysis of cooperativity in multisubunit allosteric proteins. (A) Schematic representations of monomeric and tandem tetrameric gene constructs giving rise to assembled tetrameric protein particles, one in which the subunits are not linked to each other and another where the identical subunits are tandem-linked. The identical subunits of the tandem tetrameric gene are separated by unique restriction sites, as indicated by the lowercase letters. (B) Such a gene design allows the construction of all possible combinations of mutated subunits. Lowercase letters w and m designate wild-type and mutated subunits, respectively. (C) Thermodynamic double-mutant cycle analysis applied to calculate the magnitude of intersubunit coupling free energy between subunit pairs. According to this analysis, the equilibrium effect on protein function upon mutating one protein subunit is evaluated once, when the other subunit is native (ΔG1) and again when it has been mutated (ΔG2). If the two subunits operate independently, then the conformational change of one subunit is independent of the conformational state of the adjacent subunit and Δ2Gi,j (= ΔG1 − ΔG2) equals zero. Otherwise, the two subunits under question can be considered as being coupled, and Δ2Gi,j ≠ 0.
Functional measurements using tandem-linked multisubunit proteins may reveal, in a direct manner, the nature of conformational transitions of the protein of interest. For instance, a mutation that dramatically disrupts intersubunit contacts can be separately introduced in one, two, three, or four protein subunits by using a tandem tetrameric gene construct. For an MWC-type allosteric protein involving concerted conformational transition of all four protein subunits (2), it is predicted that the effect of a single mutated subunit on protein function would be identical to the case where all four subunits are mutated. In other words, a mutation, introduced into only one subunit, should induce similar structural changes in all neighboring wild-type subunits. On the other hand, for a KNF-type allosteric enzyme undergoing sequential subunit transitions (3), a gradual effect on protein function is expected upon the successive addition of mutated protein subunits.
An informative and discriminative approach to discerning the type of conformational transitions underlying the function of an allosteric protein may further come from considering the hierarchy of intersubunit interactions contributing to cooperativity in protein function, as revealed by high-order thermodynamic coupling analysis (SI Text) (5–7). Such analysis is based on the powerful method of double-mutant cycles coupling analysis (Fig. 1C) (18–20) and is used here to measure the coupling free energy (Δ2Gi,j) between two subunits of an oligomeric protein (Fig. 1C). Furthermore, high-order intersubunit coupling analysis can be applied to calculate the effect of a third subunit, k, on the magnitude of coupling between the i,j subunit pair [i.e., third-order coupling, Δ3G(i,j)k] or the effect of interaction between the k,l subunit pair on the magnitude of coupling between the i,j subunit pair [i.e., fourth-order coupling, Δ4G(i,j)(k,l)] (5, 7, 21). As such, the hierarchy of cooperative intersubunit interactions contributing to protein function can be deciphered.
When one considers the MWC and KNF allosteric mechanisms described above, different high-order intersubunit coupling profiles would be expected in each case. Whereas for a tetrameric KNF-type enzyme, Δ2Gi,j < Δ3G(i,j)k < Δ4G(i,j)(k,l), for a MWC-type enzyme, Δ2Gi,j = Δ3G(i,j)k = Δ4G(i,j)(k,l). These distinct intersubunit coupling patterns result from the concerted versus sequential nature of conformational changes described by the MWC and KNF models, respectively. Because, in the case of the MWC model (2), a functionally-sensitive mutation in even one subunit triggers a similar structural change in all other subunits so as to achieve the full potential of the functional effect, mutations in the third and fourth subunits would not be expected to affect the magnitude of coupling between a subunit pair any further. On the other hand, the increase in binding affinity attained upon the successive ligand binding typical of KNF-type allosteric enzymes (3) dictates increased contributions for high-order intersubunit coupling terms. Thus, differences in high-order intersubunit coupling profiles may further serve to discriminate between possible allosteric models. What follows is a detailed example of such an analysis applied to the case of voltage-activated potassium channel protein.
Cooperativity in Pore Opening of Voltage-Activated Potassium Channels.
The voltage-activated potassium channel is an interesting example of an allosteric membrane protein comprising, primarily, an ion-conducting pore domain and a voltage-sensing domain (11). Kv channels are homotetrameric pore-forming proteins that open and close in response to changes in membrane potential, thereby regulating the flow of potassium ions across the membrane (8–10), a process underlying the generation of nerve and muscle action potentials (11). Amenable to rapid and highly-accurate functional characterization without the need for protein purification, the allosteric Kv channel protein represents an excellent model system for studying the contribution of intersubunit interactions to cooperativity in protein function. Indeed, cooperative interactions play a fundamental role in determining the voltage sensitivity of the Kv channel (16, 22–25). Based on steady-state and kinetic analyses (26, 27), a detailed mechanism of action has been suggested for the Shaker Kv channel in which transitions of the four voltage-sensor domains occur in a sequential but independent manner. Once such transitions between closed channel states are completed, a late concerted pore-opening transition ensues. This later transition can be isolated by introducing uncharged mutations into the S4 segment of the voltage-sensing domain (28). The assertion of concerted pore opening implies that no intermediates exist along the pathway leading to the last channel opening transition and that all subunits switch from the final closed to the open state in an all-or-none fashion. Earlier evidence by others, using a tandem tetrameric channel construct, offers support for such sequential voltage-sensor gating transitions (15, 16). No direct evidence, however, supporting a late concerted pore-opening transition has, thus far, been provided. Moreover, refined analyses revealed intermediate and sequential gating steps associated with selectivity filter movements during pore opening of the Shaker Kv channel, even on a time scale where (C-type) inactivation gating does not yet occur (17, 29). This observation undermines, apparently, the assertion of a concerted pore-opening transition by the Kv channel. It is possible that this assertion is a reflection of a concerted opening of the lower activation gate, in particular, when considering the major vs. subtle conformational changes associated with the activation (30) and (selectivity filter) inactivation gates (31, 32), respectively.
To probe for the nature of conformational transitions underlying activation gate opening in the archetypical Shaker Kv channel, a mutation that dramatically perturbs intersubunit contacts at this region must be chosen. Based on comparison of the crystal structures of two voltage-independent K+ channel pore domains in the closed (KcsA) (32, 33) and open (MthK) (30) states, a conformational change for gating has been proposed. The inner helix (M2) of the pore domain bends at a glycine hinge point to open the pore and straightens to close it (Fig. 2A) (30). This conformational change leads to the disassembly of the bundle crossing activation gate formed from the four M2 helices of the different channel subunits and, therefore, results in a dramatic rearrangement of intersubunit contacts (Fig. 2A). It has been shown previously that mutation of the glycine gating hinge residue of the voltage-dependent Shaker K+ channel (position 466) to any other residue but proline yields a nonactive channel, owing to a decrease in flexibility at this position (34, 35). The functional G466P mutation that confers rigidity at the gating hinge point was found to dramatically stabilize the open channel state (34). Accordingly, we chose the G466P perturbation as a reliable probe for describing intersubunit interactions contributing to activation gate opening of the Kv channel pore domain.
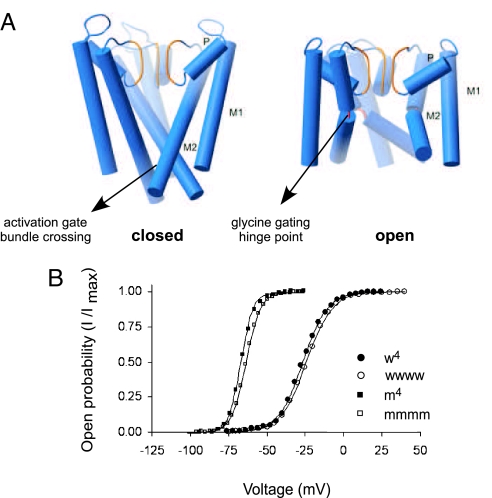
Fig. 2.
The tandem tetrameric Kv channel is properly assembled and is functionally indistinguishable from the wild-type channel. (A) The G466P mutation dramatically disrupts intersubunit contacts. Helix rods representations of the KcsA (closed) (Left) and MthK (opened) (Right) pore structures, with M1, P, and M2 labeling the outer, pore, and inner helices, respectively. (B) Voltage-activation curves of the monomeric and tandem tetrameric wild-type (w4 and wwww) or mutant (m4 and mmmm) channels. Lowercase w and m represent the wild-type or G466P mutant Shaker Kv channel subunit, respectively.
Initially, the G466P gating hinge mutation was introduced into all four subunits of a tandem tetrameric Shaker Kv channel protein and the effect on voltage-dependent gating of the channel was measured. This profile was then compared with that realized with a mutant Shaker channel assembled from four separate G466P mutant monomers. The voltage-activation curves obtained, describing the probability of the channel to be open as a function of voltage, are analogous to initial reaction velocity curves of allosteric enzymes, as a function of substrate. Fig. 2B presents voltage-activation curves from four channel proteins: The wild type (w) Shaker Kv channel assembled from four monomeric subunits (w4), the tandem-linked wild-type Kv channel (wwww), the G466P mutant (m) channel assembled from four separate mutated subunit monomers (m4) and the tandem-linked tetrameric Kv channel mutated at the G466 position in each of its four linked subunits (mmmm). In line with earlier work (12) and reaffirmed here upon comparison of the m4 and mmmm G466P mutants activation curves (indicating a dramatic open-state stabilization effect), no functional consequences were observed as a result of the linking of wild-type or mutant channel subunits. The very similar shapes of the activation curves of the corresponding wild-type or mutant pairs imply proper targeting and folding of one tandem tetrameric channel polypeptide within the membrane, as clearly demonstrated by others (12, 13, 16).
Direct Demonstration of a Concerted All-or-None Activation Gate Opening in Kv Channels.
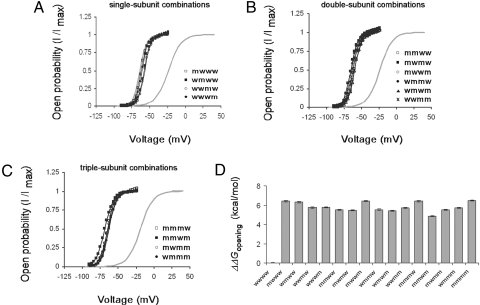
To reveal the concerted or sequential nature of the conformational transitions leading to activation gate opening, we used the tandem tetrameric channel constructs to next examine effects on voltage-dependent gating of all four possible combinations of single-subunit (G466P) point mutations (mwww, wmww,wwmw, and wwwm), of all six possible double-subunit mutant combinations (mmww, mwmw, mwwm, wmmw, wmwm, and wwmm) and of all four triple-subunit mutant combinations (mmmw, mmwm, mwmm, and wmmm) (Fig. 1B). The resulting voltage-activation curves of all three groups of single-, double-, and triple-subunit mutants were compared with those of the wild-type (wwww) and quadruple-mutant (mmmm) tandem tetrameric channels, as presented in Fig. 3 A–C, respectively. Several important observations are revealed by these curves. First, as shown in Fig. 3A, all four single-subunit mutant combinations exhibit quite similar voltage-activation curves that differ by no more than ≈2 mV from their averaged midpoint activation voltage (Table S1). This outcome further strengthens our assertion that the tandem tetrameric channel is properly assembled within the membrane, as also reported by others (12, 13, 16). Second, the activation curves of the single-subunit mutant combinations are very similar to that of the tandem tetrameric channel mutated in all of its four subunits (mmmm). An averaged change of ≈4 mV is obtained between the midpoint activation voltages of the mmmm and single-subunit mutant combinations. Third, the activation curves of all six double-subunit mutant combinations are comparable and similar to those of the single- and quadruple-subunit mutant combinations (Fig. 3B and Table S1). It is worth noting that double-subunit mutant combinations in which the two subunits are adjacent to each other or across from each other in the assembled channel structure exhibit similar activation curves. Hence, it seems that for all intents and purposes, subunit order does not significantly matter. This conclusion is further supported by the results on the single-subunit mutant combinations. Finally, the activation curves of all four possible triple-subunit mutant combinations are comparable and similar to curves of the single-, double-, and quadruple-subunit mutant combinations (Fig. 3C, Table S1). For simplicity, and as previously justified in detail (7), the effects on voltage-dependent gating of all 15 mutant subunit combinations were parameterized by using gating shifts and slopes (see SI Text). As can be clearly seen in Fig. 3D describing the free-energy change of channel opening upon mutation, all mutant subunit combinations exhibited a similar and dramatic open state-stabilizing effect on the order of 6 kcal/mol upon mutation, as compared with the wild–type tandem tetrameric channel (Table S1). Taken together, our steady-state measurements strongly imply that movements underlying activation gate opening and closing occur in a concerted all-or-none fashion, with respect to the four channel subunits. This assertion is further strengthened when one notes that overall similar activation and deactivation time constants are obtained for all mutant subunit combinations upon a fitting of the data to an oversimplifying single-exponential equation (measured over identical voltage protocol; data not shown).
Fig. 3.
The effect on voltage-dependent gating of all possible combinations of single-, double-, and triple-subunit mutations of the tandem tetrameric Shaker Kv channel. (A–C) Voltage-activation curves for the four single-subunit mutant combinations (A), six double-subunit mutant combinations (B), and four triple-subunit mutant combinations (C). Smooth curves correspond to a two-state Boltzmann function, as described in SI Text. The gray smooth curves correspond to the voltage-activation curves of the tandem tetrameric channel where all four linked subunits are either wild type (wwww) or mutant (mmmm). (D) Effects of all possible combinations of subunit mutations on the free-energy change of channel opening, shown as differences (wild type − mutant).
High-Order Thermodynamic Coupling Analysis of Intersubunit Interactions.
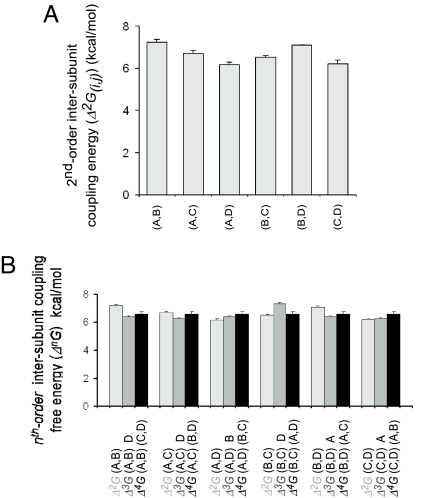
As outlined above, the pattern of high-order intersubunit couplings of an oligomeric protein, obtained through the use of double-mutant cycles analysis, can further serve to discriminate between sequential vs. concerted conformational transitions of an allosteric protein. After calculation of the free-energy change of channel gating of the wild type and the 15 possible combinations of subunit mutations, we calculated the six possible intersubunit coupling energy [Δ2G(i,j)] values describing the relation between any two subunits, by using double-mutant cycles analysis (Fig. 1B). As presented in Fig. 4A, a high value for intersubunit coupling energy is obtained in all cases, regardless of the identity and/or order of subunits examined (Table S2). The high intersubunit coupling values obtained (≈6–7 kcal/mol) are expected for conformational transitions involving large, concerted quaternary rearrangements. To reveal the pattern of hierarchy in intersubunit couplings contributing to activation gate opening, second-, third-, and fourth-order coupling free energies for the six possible subunit pairs were calculated (SI Text) by quantifying the extent to which each pairwise intersubunit interaction [Δ2G(i,j)] is affected by the presence of an adjacent subunit k [Δ3G(i,j)k] or interacting subunit pair (k,l) [Δ4G(i,j)(k,l)] (Table S3 and Table S4, respectively) (Fig. 4B). For all six intersubunit interaction pairs considered, Δ2G(i,j) ≅ Δ3G(i,j)k ≅ Δ4G(i,j)(k,l). Averaged intersubunit coupling profile of all subunit pairs is shown in Fig. S1. The high-order intersubunit coupling profile obtained by using the G466P gating hinge mutant of the Shaker Kv channel [Δ2Gi,j ≅ Δ3G(i,j)k ≅ Δ4G(i,j)(k,l)] is thus coherent with a concerted opening (and closing) of the Kv channel pore domain activation gate, as qualitatively realized above.
Fig. 4.
High-order intersubunit coupling pattern of the Shaker tandem tetrameric Kv channel is consistent with a concerted all-or-none activation gate-opening transition. (A) Pairwise intersubunit coupling free energies (Δ2Gi,j) of the six possible i,j subunit pairs. (B) Comparison of the second-, third-, and forth-order intersubunit coupling free energies associated with the subunit pairs indicated in A. Third- and fourth-order intersubunit coupling energies (Δ3G(i,j)k and Δ4G(i,j)(k,l), respectively) represent the effect of a third (k) subunit or interacting subunit pair (k,l) on the magnitude of coupling between an adjacent i,j subunit pair.
Discussion
Cooperativity in the function of homooligomeric allosteric proteins is often achieved by changes in intersubunit interactions (1). However, analysis of the contribution of individual subunits and their interactions to nonadditivity in protein function is hampered by the fact that such oligomeric proteins are assembled from several copies of a single gene product. In principle, the use of a hybrid (chimeric) protein approach in which wild-type and phenotypically-distinct mutant subunits are combined partially overcomes this limitation. This approach, however, is indirect because it usually yields all possible combinations of hybrid proteins assembled from distinct numbers of wild-type or mutant subunits. When combined with data-fitting analyses that incorporate binomial considerations for oligomeric protein assembly, this approach was found to be particularly useful in determining subunit stochiometry of an oligomeric protein (36). However, when applied to the study of allosteric systems, this approach fails to reveal the individual contribution to protein function of all possible hybrid protein particles. In only a handful of cases reported in the literature, one particular hybrid species was separated from all possible combinations, yielding valuable insight into the function of the allosteric protein under study (37, 38). The indirect nature of such a hybrid/chimeric approach, combined with the complexity in functional data interpretation, call for a rigorous and direct method for assessing the contribution of intersubunit interactions to cooperativity in protein function.
In the present study, we have described a general strategy for the direct analysis of cooperativity in multisubunit allosteric enzymes that combines measuring the effects on protein function of all possible combinations of mutated subunits introduced in the framework of a functional subunit-linked protein, together with analysis measuring high-order intersubunit thermodynamic coupling. We emphasize the assertion that the pattern of high-order intersubunit interactions can serve as a discriminative criterion for defining the concerted versus sequential nature of conformational transitions underlying protein function. The strength of this discriminate criterion is its robustness, because it takes into account all combinations of mutant subunits rather than merely relying on only a few subunit mutations that might lead to inconclusive results. In applying this strategy to the particular case of the allosteric voltage-activated potassium channel, we provide direct and compelling evidence for a concerted all-or-none transition of the Kv channel activation gate leading to pore opening. This major quaternary structural transition (Fig. 2A), involving dramatic rearrangements in intersubunit contacts, is reflected by the high (≈6–7 kcal/mol) values obtained for the various pairwise intersubunit coupling free energies. The high-order intersubunit coupling pattern [Δ2Gi,j ≅ Δ3G(i,j)k ≅ Δ4G(i,j)(k,i)] further argues for a MWC-type concerted transition for Kv channel activation gate opening.
The results presented here, moreover, highlight the important role played by the glycine gating hinge point of Kv channels in driving the conformational transitions leading to pore opening (30, 34, 35), a contribution pointed out to be only secondary to that of the lower (activation gate) PVP sequence hinge point characteristic of Kv channels (39). Only a single mutated gating hinge is sufficient to induce channel opening in a manner identical to the effect of a channel mutated in all four of its gating hinge positions (34). Thus, the glycine and PVP gating hinge points act synergistically to achieve concerted opening of the Kv channel activation gate (39). Our results thus support the notion that the concerted pore-opening transition, discussed in the elegant papers by Aldrich and Sigworth and their colleagues (26, 27), is a reflection of the concerted nature of movements associated with activation gate opening and closing.
The use of the tandem-linked Kv channel construct, also employed in numerous studies in the past, exemplifies the modular design of the Kv channel protein. Whereas introducing the voltage-sensor R365N mutation (second voltage-sensor gating charge-carrying arginine) in an increasing number of channel subunits revealed the sequential nature of conformational transition this domain undergoes (16), the G466P mutation analyzed here, in the context of the tandem tetrameric channel, revealed the concerted nature of (lower) activation gate opening. Moreover, the T449S mutation at the base of the selectivity filter also revealed sequential gating transitions probably associated with the upper selectivity filter gate (17, 29). Thus, chosen carefully, different mutation sites in the protein sequence can offer insight into the nature of conformational transitions associated with distinct domains or functional modules (e.g., channel gates).
Our results further reflect on the symmetry of Kv channels. The invariance of both the second- and third-order intersubunit couplings to the identity or order of the subunit analyzed, combined with the identical effects on voltage-dependent gating of the single-subunit mutant combination, reflect the fact that, from a functional channel gating perspective, the Kv channel is, apparently, a truly symmetric tetrameric particle. In such a case, intersubunit interaction energies involving adjacent or diagonally opposed subunit pairs are expected to be similar, as was indeed found (Fig. 4A). Interestingly, the pathway for assembly of the four Kv channel subunits is that of a dimer of dimers, giving rise to the functional fourfold symmetric Kv channel protein (13).
To summarize, we have presented a general strategy for the direct analysis of cooperativity in multisubunit allosteric proteins that relates cooperativity in protein function to the magnitude of intersubunit interactions. Moreover, we have identified an objective discriminative criterion for assessing the nature of conformational transition a protein undergoes, be it sequential or concerted. This criterion is based on the profile of high-order intersubunit interactions contributing to function of the protein under study. Principally, direct analysis of cooperativity in protein function, described here for the Kv channel protein as a test case, is general and may be applicable for the study of other allosteric proteins.
Materials and Methods
For descriptions of molecular biology techniques, high-order coupling analysis, data analysis, and coupling dataset refer to SI Text.
Essentially, electrophysiology recordings techniques were performed asdescribed (7). Briefly, K+ currents were recorded under two-electrode voltage clamp (OC725B, Warner Instruments) 1–2 days after monomeric or tandem tetrameric Shaker mRNA injection (≈50 ng) into Xenopus laevis oocytes. For all mutant subunit combinations and the m4 channel mutant, oocytes were typically held at −90 mV and stepped to different test voltages (ΔV = 3 mV) for ≈500 ms, followed by repolarization to the holding voltage. Tail-current amplitude was typically measured 2–4 ms after repolarization. All experiments were carried out at room temperature (22°C).
Supplementary Material
Acknowledgments.
We thank Prof. A. Horovitz for valuable comments on this manuscript. This work was supported by Israel Science Foundation Research Grant 323/04. O.Y. is an incumbent of the Belle and Murray Nathan Career Development Chair in Neurobiology.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0804104105/DCSupplemental.
References
- 1.Perutz MF. Mechanisms of cooperativity and allosteric regulation in proteins. Q Rev Biophys. 1989;22:139–237. doi: 10.1017/s0033583500003826. [DOI] [PubMed] [Google Scholar]
- 2.Monod J, Wyman J, Changeux JP. On the nature of allosteric transitions: A plausible model. J Mol Biol. 1965;12:88–118. doi: 10.1016/s0022-2836(65)80285-6. [DOI] [PubMed] [Google Scholar]
- 3.Koshland DE, Jr, Nemethy G, Filmer D. Comparison of experimental binding data and theoretical models in proteins containing subunits. Biochemistry. 1966;5:365–385. doi: 10.1021/bi00865a047. [DOI] [PubMed] [Google Scholar]
- 4.Eigen M. Kinetics of reaction control and information transfer in enzymes and nucleic acids. Nobel Symp. 1967;5:333–369. [Google Scholar]
- 5.Horovitz A, Fersht AR. Strategy for analyzing the co-operativity of intramolecular interactions in peptides and proteins. J Mol Biol. 1990;214:613–617. doi: 10.1016/0022-2836(90)90275-Q. [DOI] [PubMed] [Google Scholar]
- 6.Horovitz A, Fersht AR. Co-operative interactions during protein folding. J Mol Biol. 1992;224:733–740. doi: 10.1016/0022-2836(92)90557-z. [DOI] [PubMed] [Google Scholar]
- 7.Sadovsky E, Yifrach O. Principles underlying energetic coupling along an allosteric communication trajectory of a voltage-activated K+ channel. Proc Natl Acad Sci USA. 2007;104:19813–19818. doi: 10.1073/pnas.0708120104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bezanilla F. The voltage sensor in voltage-dependent ion channels. Physiol Rev. 2000;80:555–592. doi: 10.1152/physrev.2000.80.2.555. [DOI] [PubMed] [Google Scholar]
- 9.Sigworth FJ. Voltage gating of ion channels. Q Rev Biophys. 1994;27:1–40. doi: 10.1017/s0033583500002894. [DOI] [PubMed] [Google Scholar]
- 10.Yellen G. The moving parts of voltage-gated ion channels. Q Rev Biophys. 1998;31:239–295. doi: 10.1017/s0033583598003448. [DOI] [PubMed] [Google Scholar]
- 11.Hille B. Ion Channels of Excitable Membranes. Sunderland, MA: Sinauer; 2001. [Google Scholar]
- 12.Yang Y, Yan Y, Sigworth FJ. How does the W434F mutation block current in Shaker potassium channels? J Gen Physiol. 1997;109:779–789. doi: 10.1085/jgp.109.6.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tu L, Deutsch C. Evidence for dimerization of dimers in K+ channel assembly. Biophys J. 1999;76:2004–2017. doi: 10.1016/S0006-3495(99)77358-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Labro AJ, Raes AL, Snyders DJ. Coupling of voltage sensing to channel opening reflects intrasubunit interactions in kv channels. J Gen Physiol. 2005;125:71–80. doi: 10.1085/jgp.200409194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mannuzzu L, Isacoff EY. Independence and cooperativity in rearrangements of a potassium channel voltage sensor revealed by single subunit fluorescence. J Gen Physiol. 2000;115:257–268. doi: 10.1085/jgp.115.3.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tytgat J, Hess P. Evidence for cooperative interactions in potassium channel gating. Nature. 1992;359:420–423. doi: 10.1038/359420a0. [DOI] [PubMed] [Google Scholar]
- 17.Zheng J, Sigworth FJ. Intermediate conductances during deactivation of heteromultimeric Shaker potassium channels. J Gen Physiol. 1998;112:457–474. doi: 10.1085/jgp.112.4.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Carter PJ, Winter G, Wilkinson AJ, Fersht AR. The use of double mutants to detect structural changes in the active site of the tyrosyl-tRNA synthetase (Bacillus stearothermophilus) Cell. 1984;38:835–840. doi: 10.1016/0092-8674(84)90278-2. [DOI] [PubMed] [Google Scholar]
- 19.Hidalgo P, MacKinnon R. Revealing the architecture of a K+ channel pore through mutant cycles with a peptide inhibitor. Science. 1995;268:307–310. doi: 10.1126/science.7716527. [DOI] [PubMed] [Google Scholar]
- 20.Horovitz A. Double-mutant cycles: A powerful tool for analyzing protein structure and function. Fold Des. 1996;1:R121–126. doi: 10.1016/S1359-0278(96)00056-9. [DOI] [PubMed] [Google Scholar]
- 21.Chen J, Stites WE. Higher-order packing interactions in triple and quadruple mutants of staphylococcal nuclease. Biochemistry. 2001;40:14012–14019. doi: 10.1021/bi011269d. [DOI] [PubMed] [Google Scholar]
- 22.McCormack K, et al. A role for hydrophobic residues in the voltage-dependent gating of Shaker K+ channels. Proc Natl Acad Sci USA. 1991;88:2931–2935. doi: 10.1073/pnas.88.7.2931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Papazian DM, Timpe LC, Jan YN, Jan LY. Alteration of voltage-dependence of Shaker potassium channel by mutations in the S4 sequence. Nature. 1991;349:305–310. doi: 10.1038/349305a0. [DOI] [PubMed] [Google Scholar]
- 24.Schoppa NE, McCormack K, Tanouye MA, Sigworth FJ. The size of gating charge in wild-type and mutant Shaker potassium channels. Science. 1992;255:1712–1715. doi: 10.1126/science.1553560. [DOI] [PubMed] [Google Scholar]
- 25.Smith-Maxwell CJ, Ledwell JL, Aldrich RW. Role of the S4 in cooperativity of voltage-dependent potassium channel activation. J Gen Physiol. 1998;111:399–420. doi: 10.1085/jgp.111.3.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schoppa NE, Sigworth FJ. Activation of Shaker potassium channels. III: An activation gating model for wild-type and V2 mutant channels. J Gen Physiol. 1998;111:313–342. doi: 10.1085/jgp.111.2.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zagotta WN, Hoshi T, Aldrich RW. Shaker potassium channel gating. III: Evaluation of kinetic models for activation. J Gen Physiol. 1994;103:321–362. doi: 10.1085/jgp.103.2.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ledwell JL, Aldrich RW. Mutations in the S4 region isolate the final voltage-dependent cooperative step in potassium channel activation. J Gen Physiol. 1999;113:389–414. doi: 10.1085/jgp.113.3.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zheng J, Vankataramanan L, Sigworth FJ. Hidden Markov model analysis of intermediate gating steps associated with the pore gate of Shaker potassium channels. J Gen Physiol. 2001;118:547–564. doi: 10.1085/jgp.118.5.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jiang Y, et al. The open pore conformation of potassium channels. Nature. 2002;417:523–526. doi: 10.1038/417523a. [DOI] [PubMed] [Google Scholar]
- 31.Cordero-Morales JF, et al. Molecular determinants of gating at the potassium-channel selectivity filter. Nat Struct Mol Biol. 2006;13:311–318. doi: 10.1038/nsmb1069. [DOI] [PubMed] [Google Scholar]
- 32.Zhou Y, Morais-Cabral JH, Kaufman A, MacKinnon R. Chemistry of ion coordination and hydration revealed by a K+ channel-Fab complex at 2.0 Å resolution. Nature. 2001;414:43–48. doi: 10.1038/35102009. [DOI] [PubMed] [Google Scholar]
- 33.Doyle DA, et al. The structure of the potassium channel: molecular basis of K+ conduction and selectivity. Science. 1998;280:69–77. doi: 10.1126/science.280.5360.69. [DOI] [PubMed] [Google Scholar]
- 34.Magidovich E, Yifrach O. Conserved gating hinge in ligand- and voltage-dependent K+ channels. Biochemistry. 2004;43:13242–13247. doi: 10.1021/bi048377v. [DOI] [PubMed] [Google Scholar]
- 35.Ding S, Ingleby L, Ahern CA, Horn R. Investigating the putative glycine hinge in Shaker potassium channel. J Gen Physiol. 2005;126:213–226. doi: 10.1085/jgp.200509287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.MacKinnon R. Determination of the subunit stoichiometry of a voltage-activated potassium channel. Nature. 1991;350:232–235. doi: 10.1038/350232a0. [DOI] [PubMed] [Google Scholar]
- 37.Nelson SW, Honzatko RB, Fromm HJ. Hybrid tetramers of porcine liver fructose-1,6-bisphosphatase reveal multiple pathways of allosteric inhibition. J Biol Chem. 2002;277:15539–15545. doi: 10.1074/jbc.M112304200. [DOI] [PubMed] [Google Scholar]
- 38.Sakash JB, Kantrowitz ER. The contribution of individual interchain interactions to the stabilization of the T and R states of Escherichia coli aspartate transcarbamoylase. J Biol Chem. 2000;275:28701–28707. doi: 10.1074/jbc.M005079200. [DOI] [PubMed] [Google Scholar]
- 39.Webster SM, Del Camino D, Dekker JP, Yellen G. Intracellular gate opening in Shaker K+ channels defined by high-affinity metal bridges. Nature. 2004;428:864–868. doi: 10.1038/nature02468. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.