Abstract
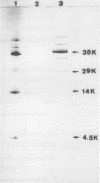
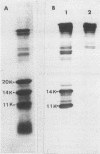
The complete amino acid sequence of the streptokinase (SKase) of Streptococcus pyogenes M type 12 strain A374, isolated from a patient with poststreptococcal glomerulonephritis (PSGN), was determined. The epitope domain for the monoclonal antibody N-59, which cross-reacts with SKases of both the PSGN-associated strain and S. equisimilis H46A (a non-PSGN-associated strain), was predicted to be localized in residues 370 to 374. The epitope domain specific for monoclonal antibody RU-1, which reacts only with the PSGN-associated SKase, was localized to residues 164 to 236.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bajaj A. P., Castellino F. J. Activation of human plasminogen by equimolar levels of streptokinase. J Biol Chem. 1977 Jan 25;252(2):492–498. [PubMed] [Google Scholar]
- DILLON H. C., Jr, WANNAMAKER L. W. PHYSICAL AND IMMUNOLOGICAL DIFFERENCES AMONG STREPTOKINASES. J Exp Med. 1965 Mar 1;121:351–371. doi: 10.1084/jem.121.3.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerlach D., Köhler W. Untersuchungen zur Heterogenität von Streptokinasen IV. Mitteilung: Der Nachweis von Isostreptokinasen bei Streptococcus pyogenes Typ 1. Zentralbl Bakteriol A. 1981 Feb;248(4):446–454. [PubMed] [Google Scholar]
- Gerlach D., Köhler W. Untersuchungen zur Heterogenität von Streptokinasen verschiedener Herkunft. Zentralbl Bakteriol Orig A. 1977 Jul;238(3):336–349. [PubMed] [Google Scholar]
- Holm S. E., Bergholm A. M., Johnston K. H. A streptococcal plasminogen activator in the focus of infection and in the kidneys during the initial phase of experimental streptococcal glomerulonephritis. APMIS. 1988 Dec;96(12):1097–1108. doi: 10.1111/j.1699-0463.1988.tb00987.x. [DOI] [PubMed] [Google Scholar]
- Holm S. E. The pathogenesis of acute post-streptococcal glomerulonephritis in new lights. Review article. APMIS. 1988 Mar;96(3):189–193. doi: 10.1111/j.1699-0463.1988.tb05289.x. [DOI] [PubMed] [Google Scholar]
- Huang T. T., Malke H., Ferretti J. J. Heterogeneity of the streptokinase gene in group A streptococci. Infect Immun. 1989 Feb;57(2):502–506. doi: 10.1128/iai.57.2.502-506.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston K. H., Zabriskie J. B. Purification and partial characterization of the nephritis strain-associated protein from Streptococcus pyogenes, group A. J Exp Med. 1986 Mar 1;163(3):697–712. doi: 10.1084/jem.163.3.697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klippenstein G. L., Holleman J. W., Klotz I. M. The primary structure of Golfingia gouldii hemerythrin. Oder of peptides in fragments produced by tryptic digestion of succinylated hemerythrin. Complete amino acid sequence. Biochemistry. 1968 Nov;7(11):3868–3878. doi: 10.1021/bi00851a012. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Malke H., Roe B., Ferretti J. J. Nucleotide sequence of the streptokinase gene from Streptococcus equisimilis H46A. Gene. 1985;34(2-3):357–362. doi: 10.1016/0378-1119(85)90145-3. [DOI] [PubMed] [Google Scholar]
- Ohkuni H., Friedman J., van de Rijn I., Fischetti V. A., Poon-King T., Zabriskie J. B. Immunological studies of post-streptococcal sequelae: serological studies with an extracellular protein associated with nephritogenic streptococci. Clin Exp Immunol. 1983 Oct;54(1):185–193. [PMC free article] [PubMed] [Google Scholar]
- Ohkuni H., Todome Y., Yoshimura K., Yamamoto T., Suzuki H., Yokomuro K., Johnston K. H., Zabriskie J. B. Detection of nephritis strain-associated streptokinase by monoclonal antibodies. J Med Microbiol. 1991 Jul;35(1):60–63. doi: 10.1099/00222615-35-1-60. [DOI] [PubMed] [Google Scholar]
- Tillett W. S., Edwards L. B., Garner R. L. FIBRINOLYTIC ACTIVITY OF HEMOLYTIC STREPTOCOCCI. THE DEVELOPMENT OF RESISTANCE TO FIBRINOLYSIS FOLLOWING ACUTE HEMOLYTIC STREPTOCOCCUS INFECTIONS. J Clin Invest. 1934 Jan;13(1):47–78. doi: 10.1172/JCI100579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsugita A., Uchida T., Mewes H. W., Ataka T. A rapid vapor-phase acid (hydrochloric acid and trifluoroacetic acid) hydrolysis of peptide and protein. J Biochem. 1987 Dec;102(6):1593–1597. doi: 10.1093/oxfordjournals.jbchem.a122209. [DOI] [PubMed] [Google Scholar]
- WEINSTEIN L. Antigenic dissimilarity of streptokinases. Proc Soc Exp Biol Med. 1953 Aug-Sep;83(4):689–691. doi: 10.3181/00379727-83-20460. [DOI] [PubMed] [Google Scholar]
- Walter F., Siegel M., Malke H. Nucleotide sequence of the streptokinase gene from a Streptococcus pyogenes type 1 strain. Nucleic Acids Res. 1989 Feb 11;17(3):1261–1261. doi: 10.1093/nar/17.3.1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter F., Siegel M., Malke H. Nucleotide sequence of the streptokinase gene from a group-G Streptococcus. Nucleic Acids Res. 1989 Feb 11;17(3):1262–1262. doi: 10.1093/nar/17.3.1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Rijn I., Kessler R. E. Growth characteristics of group A streptococci in a new chemically defined medium. Infect Immun. 1980 Feb;27(2):444–448. doi: 10.1128/iai.27.2.444-448.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]