Abstract
The cellular inhibitor of apoptosis 1 and 2 (cIAP1 and cIAP2) proteins have been implicated in the activation of NF-κB by TNFα; however, genetic deletion of either cIAP1 or 2 did not support a physiologically relevant role, perhaps because of functional redundancy. To address this, we used combined genetic and siRNA knockdown approaches and report that cIAP1 and 2 are indeed critical, yet redundant, regulators of NF-κB activation upon TNFα treatment. Whereas NF-κB was properly activated by TNFα in cultured and primary cells deficient in either cIAP1 or 2, removal of both cIAPs severely blunted its activation. After treatment with TNFα, cIAP1 and 2 were rapidly recruited to the TNF receptor 1, along with the adapter protein TNF receptor associated factor 2. Importantly, either cIAP1 or 2 was required for proper TNF receptor 1 signalosome function. In their combined absence, polyubiquitination of receptor interacting protein 1, an upstream event necessary for NF-κB signaling, was attenuated. As a result, phosphorylation of the inhibitor of κB kinase β was diminished, and signal transduction was severely blunted. Consequently, cells missing both cIAP1 and 2 were sensitized to TNFα-mediated apoptosis. Collectively, these data demonstrate that either cIAP1 or 2 is required for proper Rip1 polyubiquitination and NF-κB activation upon TNFα treatment.
Keywords: apoptosis, Receptor Interacting Protein (RIP1)
The cellular inhibitor of apoptosis 1 and 2 (cIAP1 and 2) proteins were identified based on their homology to a family of viral and cellular IAP proteins known to bind to caspases and inhibit their activity (1). The cIAP1 and 2 genes reside in tandem on chromosome 11q22, and given their high degree of similarity, likely arose by gene duplication. Both cIAP1 and 2 contain three characteristic baculovirus IAP repeat domains that facilitate binding to caspases and other proteins (2, 3). In addition, they contain a caspase recruitment domain of unknown function and a RING finger domain that functions as an E3 ubiquitin ligase (4). The precise biologic roles of cIAP1 and 2 are currently not known. Biochemical data have indicated that cIAP1 and 2, initially thought to be caspase inhibitors (2), can bind to caspases but do not directly inhibit them (5). Instead, accumulating evidence suggests that cIAP1 and 2 are involved in various signal transduction pathways, including NF-κB activation in response to TNFα (3, 6–12).
TNFα is a pleiotropic cytokine involved in numerous biologic functions, such as the development and proper functioning of the immune system, and tissue regeneration in response to injury (13). In most cells, TNFα signals through its receptor TNF-R1, exerting its effect primarily by activating the proinflammatory transcription factor NF-κB. Upon TNFα occupancy, TNF-R1 rapidly recruits the TNFR-associated death domain (TRADD) protein and the receptor-interacting protein 1 (Rip1). TRADD binding in turn recruits the TNFR-associated factor 2 (TRAF2) to form a large membrane complex. When this occurs, Rip1 is modified with large polyubiquitin chains conjugated at lysine 63 (K63) residues of ubiquitin, a crucial event for propagation of the signal (14, 15). The E3 ligase responsible for targeting Rip1 in this setting is not known, although it has been speculated to be TRAF2 (16). The polyubiquitin chain on Rip1 serves as a docking site for both the TGF-β-activated kinase 1 (Tak1) and Tak1 binding proteins 2/3 (Tab2/3) heterocomplex, and the inhibitor of κB (IκB) kinase (IKK) α/β/γ heterocomplex (14, 15). Upon assembly of this complex, Tak1 phosphorylates IKKβ on its activation loop. Phosphorylated IKKβ in turn phosphorylates IκBα, an inhibitory protein that normally prevents NF-κB dimers (typically p50/p65; hereafter referred to as NF-κB) from translocating to the nucleus. The phosphorylation of IκBα signals for its ubiquitination and proteasomal degradation, which allows NF-κB to enter the nucleus and regulate target genes. In addition to a wide range of inflammatory and immune proteins, NF-κB activates the transcription of a number of prosurvival genes, most important among these being FLICE inhibitory protein (c-FLIP), cIAP2, and manganese superoxide dismutase (6, 17, 18). These gene products serve as a “checkpoint” for proper NF-κB activation by inhibiting a caspase-8-mediated apoptotic pathway that is concomitantly engaged by TNFα (19). Thus, in the absence of proper NF-κB activation, TNFα strongly induces cell death by apoptosis.
Evidence implicating cIAP1 and 2 in TNFα-mediated NF-κB activation originated from studies demonstrating that they can interact with TRAF2 and can bind to TNF receptors (8, 9). Subsequent overexpression studies showed that cIAP2 has the capacity to activate NF-κB (6) and that a RING-deficient version of cIAP1 can cooperate in the activation of NF-κB by TRAF2 (3). More recently, however, their involvement in NF-κB signaling has been called into question, because studies in cIAP1 and 2 null mice have not supported such a role (20, 21). Mice with whole-body deletion of cIAP1 are asymptomatic, and the primary cells tested from these animals display normal TNFα-induced NF-κB activation and are not sensitized to TNFα-mediated cell death (21). Similarly, cIAP2 null mice do not display an overt phenotype, although their macrophages are sensitive to LPS-mediated apoptosis (20). This is not due to a defect in LPS-induced NF-κB activation, however, which is normal in cIAP2 null primary macrophages. Unfortunately, a confounding variable in both the cIAP1 and 2 null mice is the presence of the other cIAP, which may be able to compensate. In fact, primary cells from the cIAP1 null mouse have substantially elevated levels of cIAP2, and biochemical experiments have revealed that cIAP2 is a direct target for cIAP1-mediated ubiquitination and degradation (21).
To investigate whether cIAP1 and 2 are biologically relevant components of TNFα-induced NF-κB signaling, we combined genetic knockout with siRNA-mediated knockdown to evaluate the effect of their single and combined elimination in various primary and cultured cell lines. Using this approach, we demonstrate that both cIAP1 and 2 can regulate NF-κB signaling in response to TNFα and that they are redundant in this function in a tissue-specific manner. Our data show that either cIAP1 or 2 is required for proper polyubiquitination of Rip1, activation of NF-κB, and resistance to apoptosis in response to TNFα, thereby revealing a physiologically relevant role for these proteins in the control of TNFα-mediated signal transduction.
Results
Tissue Specific Elevation of cIAP2 in the cIAP1 Null Mouse.
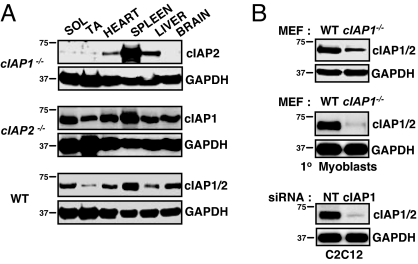
NF-κB activation has been found to be unaltered in splenocytes, thymocytes, and embryonic fibroblasts (MEFs) derived from cIAP1 null mice, and in macrophages derived from cIAP2 null mice (20, 21). However, each of these cell types expresses considerable levels of the other cIAP, and the lack of NF-κB dysfunction may be due to functional redundancy. To investigate this, we began by screening various tissues from the cIAP1 and 2 null mice for the expression of the other cIAP (Fig. 1A). In the cIAP1 null mouse, the level of cIAP2 was highly expressed in the spleen (Fig. 1A), as previously reported, presumably due to the loss of cIAP1-mediated degradation of cIAP2 (21). In the heart and liver from cIAP1 null mice, cIAP2 was moderately expressed. Strikingly, there was very little cIAP2 in both slow (soleus) and fast (tibialis anterior) twitch skeletal muscle, and none in the brain (Fig. 1A). Thus, the expression of cIAP2 in the cIAP1 null mouse is highly tissue specific. In contrast, cIAP1 was observed in every tissue examined from the cIAP2 null mouse, having highest expression in the spleen and moderate levels in the heart, liver, muscles, and brain (Fig. 1A).
Fig. 1.
Tissue-specific elevation of cIAP2 in the cIAP1 null mouse. (A) Soleus (SOL), tibialis anterior (TA), heart, spleen, liver, and brain samples were taken from 3-month-old WT and cIAP1 and 2 null mice, homogenized, and protein lysates were immunoblotted for cIAP1 and 2 levels using our rabbit anti-rat IAP1 polyclonal antibody. (B) (Top) Primary mouse skeletal myoblasts were cultured, and cIAP1 and 2 levels were assessed by immunoblotting (MEFs were used as a control). (Bottom) Murine C2C12 myoblasts were treated with siRNA targeting cIAP1, and cIAP1 and 2 levels were assessed by immonoblotting.
Given the low levels of cIAP2 in skeletal muscle from the cIAP1 null mouse, we isolated primary myoblasts and examined cIAP2 expression. For all experiments, we used our rabbit anti-rat IAP1 (RIAP1) polyclonal antibody to simultaneously detect both cIAP1 and 2 (22), because in our hands it is the only antibody that reliably recognizes cIAP1 and 2 in mouse tissue. Mouse cIAP1 and 2 are very close in size, with cIAP1 migrating <2 kDa smaller than cIAP2, a distinction not easily visualized [but see for example supporting information (SI) Fig. S1]. Consistent with our observations in the tissue, cIAP2 was not observed at appreciable levels in cultured primary muscle cells (Fig. 1B). We also cultured murine C2C12 skeletal myoblasts and treated them with siRNA targeting cIAP1. Again, cIAP2 was not observed at appreciable levels (Fig. 1B). In contrast, MEFs derived from cIAP1 null mice display moderate levels of cIAP2 [note the small shift in migration between cIAP1 and 2 (Fig. 1B)]. Taken together, both C2C12 and primary myoblasts express cIAP1 but do not express cIAP2, consistent with our observations in skeletal muscle tissue. These data demonstrate that murine skeletal myoblasts are a useful cell system in which to investigate cIAP1 function individually and raise the possibility that cIAP1 may be particularly relevant to skeletal muscle biology.
TNFα-Mediated NF-κB Signaling in Skeletal Myoblasts Depends on cIAP1.
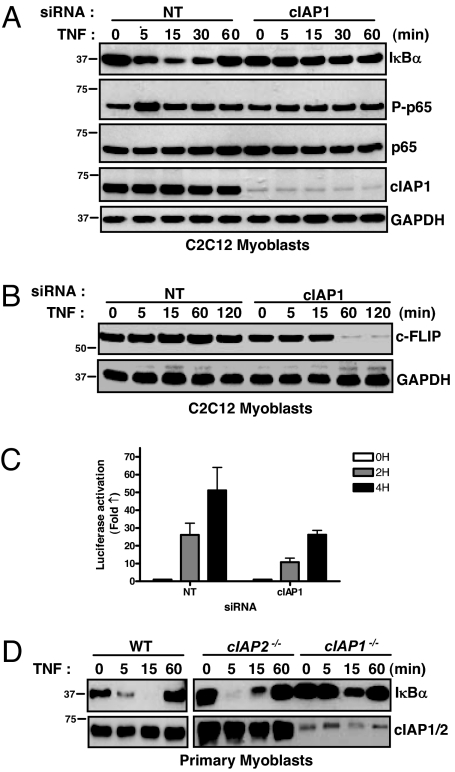
To test whether cIAP1 was involved in TNFα-mediated NF-κB signaling in skeletal myoblasts, we used siRNA to silence it in C2C12 cells before treating with TNFα. In C2C12 cells treated with nontargeting siRNA, TNFα induced rapid and transient degradation of IκBα, indicative of NF-κB activation (Fig. 2A). Additionally, the NF-κB protein p65 was rapidly and transiently phosphorylated, further indication of NF-κB activation. In sharp contrast, cIAP1 knockdown rendered C2C12 cells refractory to TNFα-mediated NF-κB activation: IκBα levels remained constant, and p65 phosphorylation was unaltered (Fig. 2A). To examine the consequences of blunted NF-κB activation, we assessed the levels of c-FLIP, which requires proper NF-κB signaling to avoid being degraded in response to TNFα (23). In C2C12 cells treated with cIAP1-specific siRNA, c-FLIP levels declined rapidly in response to TNFα (Fig. 2B). cIAP1 knockdown also blunted luciferase activation in C2C12 cells stably expressing an NF-κB responsive luciferase reporter construct (Fig. 2C).
Fig. 2.
TNFα-mediated NF-κB signaling in skeletal myoblasts depends on cIAP1. (A) C2C12 myoblasts were treated with siRNA for 24 h, treated with TNFα for the indicated times, and immunoblotted for members of the NF-κB signaling pathway. (B) C2C12 myoblasts were treated with siRNA for 24 h, treated with TNFα, and immunoblotted for c-FLIP. (C) C2C12 myoblasts stably expressing an NF-κB luciferase reporter construct were treated with siRNA for 24 h, followed by TNFα for the indicated amounts of time before luciferase activity was measured. Data are expressed mean fold change ± SD, n = 4. (D) Primary skeletal myoblasts were extracted, cultured for 24 h, and treated with TNFα. Protein lysates were collected and immunoblotted for IκBα.
To confirm the importance of cIAP1 in primary muscle cells, we extracted myoblasts from WT and cIAP1 and 2 null mice. In both WT and cIAP2 null myoblasts, TNFα treatment induced IκBα degradation, with kinetics similar to that observed in C2C12 myoblasts (Fig. 2D). However, in cIAP1 null myoblasts, in which levels of cIAP2 are barely detectable, IκBα degradation was substantially attenuated. The related family member X-linked IAP (XIAP) has also been previously implicated in NF-κB signaling in some studies (24), and we therefore evaluated for its role in TNFα-mediated NF-κB activation. Unlike cIAP1 knockdown, siRNA targeting of XIAP had no bearing on TNFα-mediated NF-κB activation (Fig. S2). Collectively, these data indicate that cIAP1 is required for TNFα-mediated NF-κB signaling in skeletal myoblasts.
cIAP1 and 2 Redundantly Regulate TNFα-Mediated NF-κB Signaling in Fibroblasts and Hepatocytes.
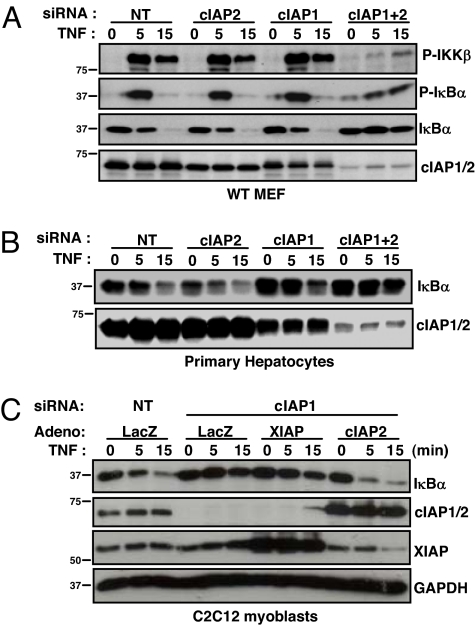
To examine the role of cIAP2 in TNFα-induced NF-κB signaling, we used siRNA to knockdown cIAP1 and/or 2 in WT MEFs. TNFα strongly activated NF-κB in WT MEFs treated with nontargeting siRNA, as assessed by the rapid and transient phosphorylation of IKKβ and IκBα, followed by the degradation of IκBα (Fig. 3A). In WT MEFs treated with cIAP1 or 2 siRNA, substantial levels of the other cIAP are observed, and TNFα activated NF-κB with similar strength and kinetics as in the nontargeting condition (Fig. 3A). In contrast, dual siRNA-mediated knockdown of cIAP1 and 2 severely attenuated TNFα-mediated phosphorylation of IKKβ and IκBα, and the degradation of IκBα was completely blocked, indicating defective NF-κB activation (Fig. 3A). Qualitatively similar results were obtained in cIAP1 null MEFs treated with siRNA targeting cIAP2, and C2C12 cells targeting cIAP1 (Fig. S3). To examine cIAP2 in a primary cell line, we isolated hepatocytes from WT animals. TNFα-induced IκBα degradation occurred similarly in WT hepatocytes treated with nontargeting siRNA, or that targeting cIAP1 or 2 (Fig. 3B). However, IκBα degradation was attenuated in WT hepatocytes treated with siRNA targeting both cIAPs. Identical results were obtained in primary fibroblasts, in which TNFα-induced IκBα degradation was similar in cells derived from WT and cIAP1 and 2 null mice (Fig. S4). In contrast, siRNA-mediated knockdown of the remaining cIAP in these cells resulted in blunted IκBα degradation to a degree that correlated with the efficiency of the knockdown. Given the obvious functional redundancy between cIAP1 and 2 observed, we asked whether cIAP2 could rescue the NF-κB defect in C2C12 cells (which do not express appreciable levels of cIAP2) incurred by the loss of cIAP1. As might be predicted, ectopic expression of human cIAP2 rescued TNFα-mediated IκBα degradation, whereas ectopic expression of XIAP did not (Fig. 3C). These data demonstrate that cIAP2 is required for proper TNFα-induced NF-κB signal transduction in MEFs and hepatocytes missing cIAP1. Together with the results obtained in skeletal myoblasts, these data show that both cIAP1 and 2 are important components of the TNFα-mediated NF-κB signaling pathway and that they are redundant in some tissues.
Fig. 3.
cIAP1 and 2 redundantly regulate TNFα-mediated NF-κB activation in MEF cells and hepatocytes. (A) MEFs were treated with siRNA for 24 h, protein lysates extracted at the indicated times after TNFα treatment, and immunoblots were performed. (B) Primary hepatocytes were extracted from WT mice, treated with siRNA for 24 h, treated with TNFα for the indicated times, and immunoblotted. (C) C2C12 myoblasts were treated with siRNA and 4 h later infected with adenovirus (Adeno). Twenty hours later, cells were treated with TNFα, protein lysates collected, and immunoblots were performed.
Both cIAP1 and 2 Are Recruited to TNF-R1 upon TNFα Treatment and Are Required for Proper Rip1 Polyubiquitination.
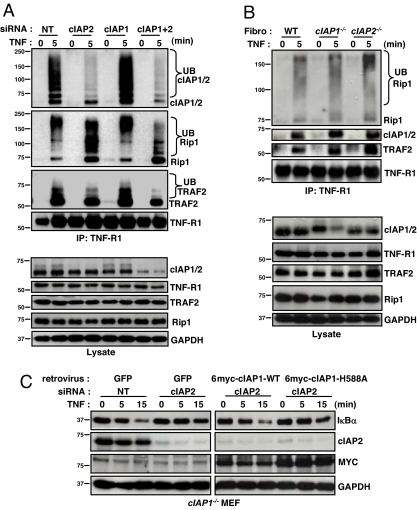
Previous research has shown that both cIAP1 and 2 exist in a complex with TRAF2 and that cIAP1 is rapidly recruited to TNF-R1 when stimulated with TNFα (8, 9). It was also reported that cIAP2 was not recruited to TNF-R1 (8), but given the observed functional redundancy between these two proteins in TNFα-mediated NF-κB signaling, we decided to revisit this question. To do so, we treated WT MEFS with siRNA toward cIAP1 and/or 2, and immunoprecipitated endogenous TNF-R1 before and after TNFα treatment for 5 min. Similar to cIAP1, cIAP2 was rapidly recruited to TNF-R1 upon TNFα treatment (Fig. 4A). Moreover, cIAP2 was greatly modified at the receptor, whereas cIAP1 was modified to a much lesser degree. These higher-molecular-weight modifications are typical of polyubiquitination, although confirmation of this awaits further testing. These data demonstrate that, like cIAP1, cIAP2 is rapidly recruited to TNF-R1 upon TNFα occupancy.
Fig. 4.
Both cIAP1 and 2 are recruited to TNF-R1 upon TNFα treatment and are required for Rip1 polyubiquitination. (A) WT MEFs were treated with siRNA for 24 h, and endogenous TNF-R1 was immunoprecipitated before and 5 min after TNFα treatment and immunoblotted with the indicated antibodies. (B) Primary fibroblasts were cultured and treated with TNFα. TNF-R1 was immunoprecipitated, and immunoblots were performed as outlined. (C) cIAP1−/− MEFs were treated with siRNA and 8 h later infected with retrovirus. Forty-eight hours after siRNA treatment, cells were treated with TNFα and immunoblots were performed.
To test whether endogenous cIAP1 and 2 are involved in TNFα-mediated Rip1 polyubiquitination, we immunoblotted TNF-R1 immunoprecipitation samples for Rip1. In control MEFs treated with nontargeting siRNA, Rip1 was rapidly recruited to TNF-R1 and seemed to be largely polyubiquitinated (Fig. 4A). Similarly, in MEFs treated with siRNA targeting either cIAP1 or 2, Rip1 recruited to TNF-R1 was largely polyubiquitinated, albeit slightly less so when cIAP2 was knocked down. In contrast, when MEFs were treated with siRNA targeting both cIAP1 and 2, the majority of TNF-R1 bound Rip1 was nonubiquitinated, with only a small percentage being ubiquitinated (Fig. 4A). These data indicate that proper Rip1 polyubiquitination upon TNFα treatment requires either cIAP1 or 2. Interestingly, TRAF2 was also modified at TNF-R1, and its modification also depended on the expression of either cIAP1 or 2 (Fig. 4A). Importantly, the protein levels of both Rip1 and TRAF2 remained constant in response to TNFα, suggesting that their modifications are not targeting them for degradation (Fig. 4A and Fig. S5). Additionally, the protein levels of TNF-R1 were not altered by siRNA-mediated knockdown of cIAP1 and/or 2 (Fig. 4A and Fig. S6).
To validate these siRNA experiments, we cultured fibroblasts extracted from skeletal muscle of WT and cIAP1 and 2 null mice, and performed TNF-R1 immunoprecipitations before and 5 min after TNFα treatment. Again, both cIAP1 and 2 were recruited to TNF-R1 after TNFα treatment, and Rip1 polyubiquitination was evident (Fig. 4B). In contrast, when the remaining cIAP was knocked down by siRNA in these cells, the Rip1 recruited to TNF-R1 was largely “collapsed” into its unmodified 74-kDa protein form (Fig. S7). The correlation between the degree of knockdown and the amount of unmodified Rip1 was evident in this experiment, indicating defective polyubiquitination.
Given that cIAP1 and 2 are involved in Rip1 ubiquitination, we asked whether their RING finger domain was required to facilitate TNFα-mediated NF-κB signaling. To answer this question, we performed retroviral-mediated rescue experiments using both WT and E3 mutant cIAP1 (H588A point mutation rendering inactive the E3 ligase activity) in cIAP1 null MEFS treated with siRNA targeting cIAP2. Although ectopic expression of WT cIAP1 was able to rescue the NF-κB activation defect incurred by dual loss of cIAP1 and 2, the E3 ligase mutant cIAP1 was unable to do so (Fig. 4C). These data indicate that the E3 ligase function is crucial for cIAP1-mediated regulation of TNFα-mediated NF-κB signaling.
Either cIAP1 or 2 Is Required to Protect Cells Against TNFα-Induced Apoptosis.
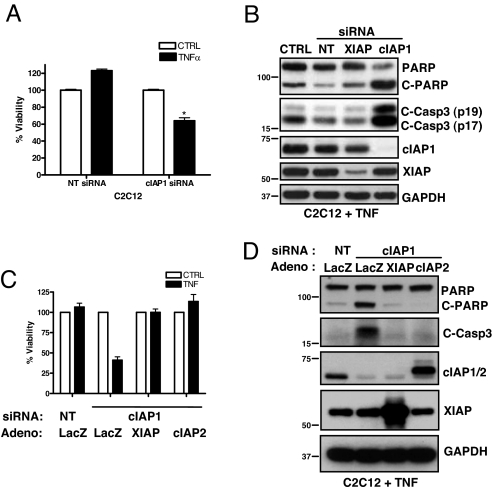
Cell survival in response to TNFα depends on proper NF-κB induction (19). Given the important role for cIAP1 and 2 in TNFα-mediated Rip1 polyubiquitination and NF-κB activation, we reasoned that cells missing both cIAP1 and 2 would be sensitized to TNFα-induced apoptosis. To investigate this, we introduced siRNA targeting cIAP1 into C2C12 myoblasts and treated them with TNFα for 24 h. C2C12 cells treated with nontargeting duplexes remained viable when treated with TNFα (Fig. 5A), and caspase-3 and poly(ADP-ribose) polymerase (PARP, a marker of caspase-3 activation) cleavage were barely detected (Fig. 5B). In contrast, C2C12 cells (which lack cIAP2 expression) treated with siRNA targeting cIAP1 were sensitized to TNFα-induced apoptosis, as evidenced by a decrease in cell viability and elevated cleavage of both caspase-3 and PARP. XIAP knockdown did not sensitize C2C12 cells to TNFα-induced apoptosis, nor did it increase the cIAP1 knockdown-mediated sensitization to TNFα (Fig. 5B and data not shown). We then decided to test this in cIAP1 null MEFs because, although these cells were demonstrated to be resistant to TNFα-mediated apoptosis (21), recent data in SV40-transformed MEFs derived from cIAP1 null mice demonstrated high sensitization to TNFα-mediated killing (11). Consistent with the earlier report (21), we observed that cIAP1 null MEFs were not sensitive to apoptosis when treated with TNFα (Fig. S8). However, siRNA-mediated knockdown of cIAP2 greatly sensitized cIAP1 null MEFs to TNFα-mediated cell death (Fig. S8). As expected, the initiating caspases-8 was also cleaved (Fig. S8), indicating activation of the extrinsic apoptosis pathway. Finally, we tested whether cIAP2 could protect C2C12 myoblasts treated with siRNA targeting cIAP1 against TNFα-mediated apoptosis. Ectopic expression of cIAP2 completely rescued the cIAP1 knockdown-mediated phenotype, to the same extent as XIAP, which strongly inhibits apoptosis when overexpressed (Fig. 5 C and D). Collectively, these data reveal crucial roles for cIAP1 and 2 in the protection against TNFα-induced apoptosis.
Fig. 5.
Either cIAP1 or 2 is required to protect against TNFα-induced apoptosis. (A) C2C12 myoblasts were treated with siRNA for 24 h, followed by TNFα treatment for 24 h, and cell viability was assessed. Data are expressed as % viability ± SD relative to no TNF treatment controls (set at 100%), n = 4 per condition. (B) C2C12 myoblasts treated with siRNA (24 h) followed by TNFα (24 h) were immunoblotted for markers of caspase activation. (C) C2C12 myoblasts were treated with siRNA and 4 h later infected with adenovirus (Adeno). After 24 h of knockdown, cells were treated with TNFα for 24 h, and cell viability was assessed (as above). (D) C2C12 cells were treated as in C, and protein lysates were immunoblotted for the indicated proteins.
Discussion
Both cIAP1 and 2 Can Facilitate TNFα-Mediated NF-κB Activation.
The role of cIAP1 and 2 in TNFα-mediated NF-κB signaling has been controversial. Early studies implied that cIAP1 and 2 are involved in NF-κB signaling because they were found to interact with TRAF2 and bind to TNF receptors (8, 9). In addition, it was shown that cIAP1 and 2 can activate, or cooperate in the activation of, NF-κB when overexpressed (3, 6). In contrast, more recent studies in cIAP1 and 2 null mice have cast doubts about their physiologic relevance (20, 21). In an attempt to resolve these discrepant data, we tested the role of cIAP1 and 2 in TNFα-mediated NF-κB activation with an eye to the fact that both display wide tissue distribution and may be functionally redundant. Using combined genetic knockout and siRNA-mediated knockdown techniques, we show that the loss of both cIAP1 and 2 greatly attenuates NF-κB signaling by TNFα. Moreover, we demonstrate that the expression of either cIAP1 or 2 is sufficient for proper signal transduction. Although dual knockdown of cIAP1 and 2 did not completely eliminate TNFα-mediated NF-κB activation, it dramatically altered its kinetics and strength. As a likely consequence, FLIP levels declined rapidly, and apoptosis ensued in response to TNFα. We conclude, therefore, that both cIAP1 and 2 are biologically relevant components of the TNFα-mediated NF-κB signal transduction pathway. This is consistent with data from Santoro et al. (25), who reported that cIAP1 and 2 regulate TNFα-mediated NF-κB activation in endothelial cells, which die in response to TNFα in their combined absence. Given that each cell examined from the knockout mice had considerable levels of the other cIAP (20, 21), our data explain why NF-κB dysfunction was not observed in the cIAP knockout studies.
Proper TNF-R1 Signalosome Function Requires Either cIAP1 or 2.
Ubiquitination events are required for the proper assembly and functioning of the TNF-R1 signalosome, notably the K63-mediated polyubiquitination of Rip1, which is necessary for the recruitment of the Tab2/3-Tak1 and the IKKα/β/γ complexes (14, 15). Although previous knockdown studies have suggested that TRAF2 is the E3 ubiquitin ligase that targets Rip1 (16), it is conceivable that TRAF2 may be indirectly involved by recruiting another E3 ligase to the TNF-R1 complex. We show that either cIAP1 or 2, known binding partners of TRAF2 (9), is required for the polyubiquitination of Rip1, given that in their combined absence Rip1 polyubiquitination is dramatically reduced upon TNFα treatment. The RING-mediated E3 ligase function of cIAP1 and 2 is likely responsible for their role in Rip1 polyubiquitination, because the cIAP1 E3 ligase mutant was not able to rescue NF-κB signaling. As such, one model explaining the role of cIAP1 and 2 in Rip1 polyubiquitination is that TRAF2 serves as an adapter protein to recruit cIAP1 and 2 to the TNF-R1 signalosome, which then target Rip1 directly for polyubiquitination. In support of this, cIAP2 has been shown to directly facilitate K63-linked polyubiquitin chains on Rip1 in vitro (cIAP1 was not examined) (15), and TRAF2 has been shown to enhance the interaction between cIAP1/2 and Rip1 (7). Alternatively, a second model is that cIAP1 and 2 facilitate Rip1 polyubiquitination indirectly by promoting TRAF2-mediated polyubiquitination of Rip1, consistent with the fact that cIAP1 and 2 were involved in TRAF2 modification at TNF-R1. Further investigation is necessary to dissect out the precise role(s) of cIAP1, cIAP2, and TRAF2 in TNFα-mediated Rip1 ubiquitination.
Redundant and Specialized Roles of cIAP1 and 2.
We have shown that cIAP1 and 2 can both facilitate TNFα-mediated NF-κB activation. However, our anti-RIAP1 polyclonal antibody does not distinguish between cIAP1 and 2, and therefore we do not know what the individual roles of cIAP1 and 2 are in TNFα-mediated NF-κB activation in WT cells. Several observations, nevertheless, suggest that they may have specialized roles therein. First, cIAP1 is more abundantly and ubiquitously expressed in cells, and it negatively regulates the levels of cIAP2 by targeting it for ubiquitination (21). This suggests that cIAP1 may be the primary regulator of TNFα-mediated NF-κB activation, whereas cIAP2 may serve as a built-in “back-up” in situations in which cIAP1 levels are challenged. Second, the cIAP2 promoter is highly responsive to classical NF-κB activation, whereas the cIAP1 promoter is not (6). This suggests that cIAP1 may be primarily involved in the initial activation of NF-κB in response to TNFα, whereas cIAP2 may participate in fine-tuning the NF-κB response in a “feed-forward” manner. The development of cIAP1- and 2-specific antibodies that reliably detect the endogenous proteins are clearly needed to elucidate their precise roles in this pathway.
Diverse Roles of cIAP1 and 2 in NF-κB Signaling: Implications for Cancer Therapy.
Recently, several groups reported that cIAP1 and 2 are negative regulators of both alternative (stimulus-independent and stimulus-dependent) and classical (stimulus-independent) NF-κB signaling (11, 12). These studies demonstrated that cIAP1 and 2 constitutively target the NF-κB inducing kinase (NIK) for K48-linked ubiquitination and proteasomal degradation, thereby repressing alternative NF-κB activity. The mechanism responsible for cIAP1- and 2-mediated repression over stimulus-independent classical NF-κB activity was not well defined, although the authors suggested that it was due to the ability of cIAP1 to negatively regulate the recruitment of Rip1 to TNF-R1 (11). Given that we now show that cIAP1 and 2 regulate TNFα-mediated (i.e., stimulus-dependent) NF-κB activation, it is apparent that these IAP proteins are integrated into multiple NF-κB pathways. Intriguingly, cIAP1 and 2 have the capacity to either positively or negatively regulate NF-κB signaling, and this seems to depend on whether they participate in K48- or K63-linked ubiquitination events. How these decisions are made is the subject of intense investigation.
Notably, the role of cIAP1 and 2 as negative regulators of stimulus-independent classical and alternative NF-κB signaling (11, 12) was uncovered in studies using small-molecule IAP antagonist compounds. These compounds were designed to mimic the interaction between XIAP and its antagonist Smac (second mitochondria-derived activator of caspases) and show great promise for the treatment of cancer (26). Smac-mimetics were intended to de-repress XIAP-mediated inhibition of apoptosis in cancer cells. However, both Vince et al. (11) and Varfolomeev et al. (12), and several other groups (27, 28), have demonstrated that the ability of Smac-mimetics to kill cancer cells is due to cIAP1 and 2 degradation rather than XIAP antagonism. Mechanistically, Smac-mimetics induce autoubiquitination and degradation of cIAP1 and 2, which relieves the repression by cIAP1 and 2 on stimulus-independent classical and alternative NF-κB signaling. Consequently, these NF-κB pathways are activated, which leads to the production of TNFα in a subset of the cancer cells. The newly synthesized TNFα is in turn secreted and activates its TNF receptors. The authors showed that this autocrine TNFα signaling loop strongly induces caspase 8-dependent apoptosis in the absence of cIAP1 and 2, although the mechanism of action was not determined. Our data offer insight into this mechanism: we show that cIAP1 and 2 are critical regulators of TNFα-mediated Rip1 polyubiquitination and NF-κB activation. Therefore, in their dual absence, TNFα would not activate the prosurvival NF-κB pathway but rather would default to the prodeath extrinsic apoptosis pathway. Of note, our data demonstrate that although normal cells do not die in response to dual cIAP1 and 2 knockdown (presumably because they do not generate TNFα), they are greatly sensitized to apoptosis upon exposure to exogenous TNFα, which can occur ectopically in patients with cancer. As such, the pursuit of this very promising new treatment strategy should proceed cautiously, to define the therapeutic window and identify systemic and regional levels of TNFα and tissue-specific toxic effects.
Materials and Methods
Materials.
The following antibodies were used for immunoblot analyses: GAPDH (Advanced ImmunoChemical; clone 6C5), phopsho-IκBα (Cell Signaling Technology; 2859), IκBα (Cell Signaling Technology; 4812), phospho-p65 (Cell Signaling Technology; 3033), p65 (Cell Signaling Technology; 3034), c-FLIP (Alexis Biochemicals; clone Dave-2), Rip1 (BD Transduction Laboratories; clone 38), TRAF2 (Stressgen Biotechnologies; AAP-422), TNF-R1 (Abcam; AB19139), PARP (Cell Signaling Technology; 9542), caspase 3 (Cell Signaling Technology; 9661), caspase 8 (Cell Signaling Technology; 4927), and myc-tag (Stressgen Biotechnologies; MSA-110). Our rabbit anti-rat IAP1 and IAP3 (RIAP1 and 3) polyclonal antibodies were used to detect cIAP1/2 and XIAP, respectively. For immunoprecipitations, we used a goat anti-TNF-R1 (R&D Systems; AF425). Recombinant mouse TNFα was purchased from R&D Systems and used at a concentration of 10 ng/ml.
Mouse Tissue and Primary Cell Extraction.
Three-month-old WT, cIAP1 null, and cIAP2 null mice were killed by cervical dislocation, and tissues were extracted quickly and flash-frozen in liquid nitrogen. Tissues were homogenized in buffer (50 mM Tris, pH 7.4; 150 mM NaCl; 1% Triton X-100; 1% Nonidet P-40; 0.1% SDS) using a rotor-stator homogenizer. Primary myoblasts, fibroblasts, and hepatocytes were cultured using standard procedures, as outlined in SI Text.
Cell Culture.
Murine C2C12 cells (ATCC) were cultured in complete media (DMEM supplemented with 10% FCS, penicillin, and streptomycin). C2C12 cells stably expressing an NF-κB luciferase reporter were purchased from Panomics Laboratories. Immortalized (3T3-like) MEFs were cultured in complete media supplemented with nonessential amino acids. Chemically synthesized siRNA duplexes were purchased from Invitrogen. We used Lipofectamine RNAiMAX reagent for siRNA knockdown, according to the manufacturer's instructions.
Retrovirus and Adenovirus Rescue Experiments.
For retrovirus rescue experiments, WT and E3 ligase mutant (H588A) cIAP1 constructs were subcloned into a pBMN-GFP retroviral vector (Orbigen) and transfected into Phoenix Ampho cells using lipofectamine 2000 (Invitrogen). After 72 h, >90% of cells were GFP positive, and we then collected the media, spun down cells and debris (5 min at 300 × g), and stored the virus-containing media supernatant at −80°C. For rescue experiments, cIAP1 null MEFs were seeded at 2 × 104 per 35-mm well, and the next day treated with siRNA targeting cIAP2. Eight hours later, the media was replaced with 1 ml of virus-containing media. Cells were then incubated for 48 h, at which time the media were changed and experiments were conducted. For adenovirus rescue experiments, C2C12 myoblasts were seeded at 5 × 104 per 35-mm well and treated with siRNA targeting cIAP1 the next morning. Four hours later the media were replaced, and adenovirus expressing LacZ, XIAP, or cIAP1 was added at an MOI of 100. Twenty hours later, the media were replaced and experiments were conducted.
Immunoblots, immunoprecipitations, viability assays, and luciferase assays were performed using standard procedures, as outlined in the SI Text.
Supplementary Material
Acknowledgments.
We thank G. Nolan for Phoenix Ampho cells. R.G.K. is supported by the Canadian Institutes of Health Research (CIHR), the Muscular Dystrophy Association of America, and the Howard Hughes Medical Institute (HHMI). R.G.K. is an HHMI International Research Scholar. D.J.M. was supported by postdoctoral fellowships from the Natural Sciences and Engineering Research Council of Canada and the CIHR.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0711122105/DCSupplemental.
References
- 1.Liston P, et al. Suppression of apoptosis in mammalian cells by NAIP and a related family of IAP genes. Nature. 1996;379:349–353. doi: 10.1038/379349a0. [DOI] [PubMed] [Google Scholar]
- 2.Roy N, Deveraux QL, Takahashi R, Salvesen GS, Reed JC. The c-IAP-1 and c-IAP-2 proteins are direct inhibitors of specific caspases. EMBO J. 1997;16:6914–6925. doi: 10.1093/emboj/16.23.6914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Samuel T, et al. Distinct BIR domains of cIAP1 mediate binding to and ubiquitination of tumor necrosis factor receptor-associated factor 2 and second mitochondrial activator of caspases. J Biol Chem. 2006;281:1080–1090. doi: 10.1074/jbc.M509381200. [DOI] [PubMed] [Google Scholar]
- 4.Yang Y, Fang S, Jensen JP, Weissman AM, Ashwell JD. Ubiquitin protein ligase activity of IAPs and their degradation in proteasomes in response to apoptotic stimuli. Science. 2000;288:874–877. doi: 10.1126/science.288.5467.874. [DOI] [PubMed] [Google Scholar]
- 5.Eckelman BP, Salvesen GS. The human anti-apoptotic proteins cIAP1 and cIAP2 bind but do not inhibit caspases. J Biol Chem. 2006;281:3254–3260. doi: 10.1074/jbc.M510863200. [DOI] [PubMed] [Google Scholar]
- 6.Chu ZL, et al. Suppression of tumor necrosis factor-induced cell death by inhibitor of apoptosis c-IAP2 is under NF-kappaB control. Proc Natl Acad Sci USA. 1997;94:10057–10062. doi: 10.1073/pnas.94.19.10057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Park SM, Yoon JB, Lee TH. Receptor interacting protein is ubiquitinated by cellular inhibitor of apoptosis proteins (c-IAP1 and c-IAP2) in vitro. FEBS Lett. 2004;566:151–156. doi: 10.1016/j.febslet.2004.04.021. [DOI] [PubMed] [Google Scholar]
- 8.Shu HB, Takeuchi M, Goeddel DV. The tumor necrosis factor receptor 2 signal transducers TRAF2 and c-IAP1 are components of the tumor necrosis factor receptor 1 signaling complex. Proc Natl Acad Sci USA. 1996;93:13973–13978. doi: 10.1073/pnas.93.24.13973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rothe M, Pan MG, Henzel WJ, Ayres TM, Goeddel DV. The TNFR2-TRAF signaling complex contains two novel proteins related to baculoviral inhibitor of apoptosis proteins. Cell. 1995;83:1243–1252. doi: 10.1016/0092-8674(95)90149-3. [DOI] [PubMed] [Google Scholar]
- 10.Wang CY, Mayo MW, Korneluk RG, Goeddel DV, Baldwin AS., Jr NF-kappaB antiapoptosis: Induction of TRAF1 and TRAF2 and c-IAP1 and c-IAP2 to suppress caspase-8 activation. Science. 1998;281:1680–1683. doi: 10.1126/science.281.5383.1680. [DOI] [PubMed] [Google Scholar]
- 11.Vince JE, et al. IAP antagonists target cIAP1 to induce TNFalpha-dependent apoptosis. Cell. 2007;131:682–693. doi: 10.1016/j.cell.2007.10.037. [DOI] [PubMed] [Google Scholar]
- 12.Varfolomeev E, et al. IAP antagonists induce autoubiquitination of c-IAPs, NF-kappaB activation, and TNFalpha-dependent apoptosis. Cell. 2007;131:669–681. doi: 10.1016/j.cell.2007.10.030. [DOI] [PubMed] [Google Scholar]
- 13.Wajant H, Pfizenmaier K, Scheurich P. Tumor necrosis factor signaling. Cell Death Differ. 2003;10:45–65. doi: 10.1038/sj.cdd.4401189. [DOI] [PubMed] [Google Scholar]
- 14.Ea CK, Deng L, Xia ZP, Pineda G, Chen ZJ. Activation of IKK by TNFalpha requires site-specific ubiquitination of RIP1 and polyubiquitin binding by NEMO. Mol Cell. 2006;22:245–257. doi: 10.1016/j.molcel.2006.03.026. [DOI] [PubMed] [Google Scholar]
- 15.Wu CJ, Conze DB, Li T, Srinivasula SM, Ashwell JD. Sensing of Lys 63-linked polyubiquitination by NEMO is a key event in NF-kappaB activation. Nat Cell Biol. 2006;8:398–406. doi: 10.1038/ncb1384. and correction 8:24. [DOI] [PubMed] [Google Scholar]
- 16.Lee TH, Shank J, Cusson N, Kelliher MA. The kinase activity of Rip1 is not required for tumor necrosis factor-alpha-induced IkappaB kinase or p38 MAP kinase activation or for the ubiquitination of Rip1 by Traf2 J. Biol Chem. 2001;279:33185–33191. doi: 10.1074/jbc.M404206200. [DOI] [PubMed] [Google Scholar]
- 17.Micheau O, Lens S, Gaide O, Alevizopoulos K, Tschopp J. NF-kappaB signals induce the expression of c-FLIP. Mol Cell Biol. 2001;21:5299–5305. doi: 10.1128/MCB.21.16.5299-5305.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kamata H, et al. Reactive oxygen species promote TNFalpha-induced death and sustained JNK activation by inhibiting MAP kinase phosphatases. Cell. 2005;120:649–661. doi: 10.1016/j.cell.2004.12.041. [DOI] [PubMed] [Google Scholar]
- 19.Luo JL, Kamata H, Karin M. IKK/NF-kappaB signaling: Balancing life and death—a new approach to cancer therapy. J Clin Invest. 2005;115:2625–2632. doi: 10.1172/JCI26322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Conte D, et al. Inhibitor of apoptosis protein cIAP2 is essential for lipopolysaccharide-induced macrophage survival. Mol Cell Biol. 26:699–708. doi: 10.1128/MCB.26.2.699-708.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Conze DB, et al. Posttranscriptional down-regulation of c-IAP2 by the ubiquitin protein ligase c-IAP1 in vivo. Mol Cell Biol. 2005;25:3348–3356. doi: 10.1128/MCB.25.8.3348-3356.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Holcik M, Lefebvre CA, Hicks K, Korneluk RG. Cloning and characterization of the rat homologues of the Inhibitor of Apoptosis protein 1, 2, and 3 genes. BMC Genomics. 2002;3:5. doi: 10.1186/1471-2164-3-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chang L, et al. The E3 ubiquitin ligase itch couples JNK activation to TNFalpha-induced cell death by inducing c-FLIP(L) turnover. Cell. 2006;124:601–613. doi: 10.1016/j.cell.2006.01.021. [DOI] [PubMed] [Google Scholar]
- 24.Lu M, et al. XIAP induces NF-kappaB activation via the BIR1/TAB1 interaction and BIR1 dimerization. Mol Cell. 2007;26:689–702. doi: 10.1016/j.molcel.2007.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Santoro MM, Samuel T, Mitchell T, Reed JC, Stainier DY. Birc2 (cIap1) regulates endothelial cell integrity and blood vessel homeostasis. Nat Genet. 2007;39:1397–1402. doi: 10.1038/ng.2007.8. [DOI] [PubMed] [Google Scholar]
- 26.Zobel K, et al. Design, synthesis, and biological activity of a potent Smac mimetic that sensitizes cancer cells to apoptosis by antagonizing IAPs. ACS Chem Biol. 2006;1:525–533. doi: 10.1021/cb600276q. [DOI] [PubMed] [Google Scholar]
- 27.Gaither A, et al. A Smac mimetic rescue screen reveals roles for inhibitor of apoptosis proteins in tumor necrosis factor-alpha signaling. Cancer Res. 2007;67:11493–11498. doi: 10.1158/0008-5472.CAN-07-5173. [DOI] [PubMed] [Google Scholar]
- 28.Petersen SL, et al. Autocrine TNFalpha signaling renders human cancer cells susceptible to Smac-mimetic-induced apoptosis. Cancer Cell. 12:445–456. doi: 10.1016/j.ccr.2007.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.