Abstract
The past decade has witnessed a quantum leap in our understanding of the origins, diffusion, and impact of early agriculture in the Mediterranean Basin. In large measure these advances are attributable to new methods for documenting domestication in plants and animals. The initial steps toward plant and animal domestication in the Eastern Mediterranean can now be pushed back to the 12th millennium cal B.P. Evidence for herd management and crop cultivation appears at least 1,000 years earlier than the morphological changes traditionally used to document domestication. Different species seem to have been domesticated in different parts of the Fertile Crescent, with genetic analyses detecting multiple domestic lineages for each species. Recent evidence suggests that the expansion of domesticates and agricultural economies across the Mediterranean was accomplished by several waves of seafaring colonists who established coastal farming enclaves around the Mediterranean Basin. This process also involved the adoption of domesticates and domestic technologies by indigenous populations and the local domestication of some endemic species. Human environmental impacts are seen in the complete replacement of endemic island faunas by imported mainland fauna and in today's anthropogenic, but threatened, Mediterranean landscapes where sustainable agricultural practices have helped maintain high biodiversity since the Neolithic.
Keywords: archaeology, livestock
The transition from foraging and hunting to farming and herding is a significant threshold in human history. Domesticates and the agricultural economies based on them are associated with radical restructuring of human societies, worldwide alterations in biodiversity, and significant changes in the Earth's landforms and its atmosphere. Given the momentous outcomes of this transition it comes as little surprise that the origin and spread of domesticates and the emergence of agriculture remain topics of enduring interest to both the scholarly community and the general public.
The past decade has seen remarkable analytical advances in documenting domestication (1), particularly in tracking the domestication of four major Near Eastern livestock species (sheep, goats, cattle, and pigs) and their subsequent dispersal throughout the Mediterranean Basin. New morphometric methods are tracking changes in human prey strategies that mark the transition from hunting to herding. Genetic analyses bring fresh insights into initial livestock domestication and their dispersal. Small-sample atomic mass spectrometry radiocarbon dating provides refined chronological frameworks for these developments. These recent analytical advances, in turn, have produced an explosion of new information that is calling into question prevailing hypotheses about the origin and early spread of animal domesticates and the Neolithic lifeways of which they were a part. Here, I bring together these different sources of information to consider the origins, diffusion, and impacts of domesticates and agriculture in the Mediterranean Basin, outlining our current understanding of these developments and highlighting promising areas for future study.
Initial Animal Domestication in the Fertile Crescent
Until the late 1990s archaeozoologists relied on morphological changes in target species to identify where and when wild prey animals were transformed into herded livestock (2). A proposed sharp and rapid reduction in overall body size among archaeological prey populations was the most widely accepted morphological marker of this threshold (3, 4). Based on this size reduction criterion, the established consensus was that animal domestication (beginning with goats and then sheep) occurred at ca. 10,000–9,500 B.P.†, ≈1,000 years after the domestication of crop plants in the southern Levant (3, 5). Domestication of these two animal species was thought to have occurred somewhere to the north and east of the heartland of plant domestication (5), although a second, independent domestication of goats was proposed for the southern Levant (6).
The utility of this size reduction marker, and indeed of all morphological markers, has come under increasing scrutiny (7). My own work on both modern skeletal collections and archaeological caprine (sheep and goat) remains from the Near East finds little support for the almost axiomatic acceptance that domestication results in an automatic overall reduction in body size in managed animals (ref. 8 and references therein). Rather than domestic status, sex is the primary factor affecting body size in these ungulates, manifested by a marked and consistent difference between larger males and smaller females in essentially all skeletal elements. Environment also strongly influences body size, with increasing heat and aridity positively correlated with smaller size. What archaeozoologists had originally interpreted as body size reduction associated with initial domestication can now be attributed to differences in the culling strategies of herders as opposed to hunters. In most prey species, hunters focus on large adult animals (particularly males) to maximize return, and the bones of these larger animals generally dominate in prey assemblages generated by hunters. Archaeological assemblages generated by herders, on the other hand, are usually dominated by the bones of smaller females slaughtered after their prime reproductive years. Excess males not needed for herd propagation were harvested at young ages and their more friable bones are usually less well represented in these assemblages.
Although the linkage between domestication and body size was called into question by this research, the marked degree of sexual dimorphism in caprines it documented offered another approach to tracking the transition from hunting to herding. Pronounced differences in the size of male and female skeletal elements make it possible to separate archaeological assemblages into sex-specific subpopulations, which, based on a refined understanding of the sequence and timing of long bone fusion (9), can be used to generate high-resolution harvest profiles for male and female animals capable of distinguishing the prey strategies of hunters from the harvest strategies of herders.
Application of this approach to archaeological assemblages from Iraq and Iran has identified the clear signature of a managed herd of goats (harvesting of young males and prolonged survivorship of females) at the site of Ganj Dareh in highland Iran (8). Directly dated to 9,900 B.P., the goats from this site show no evidence of size reduction or any other domestication-induced morphological change. Smaller body size and changes in the size and shape of horns [a morphological change clearly linked to domestication (2)] appear 500–1,000 years later than this demographic shift, when managed animals were moved from the natural habitat of wild goats and introduced into hotter and more arid lowland Iran. These follow-on morphological changes likely reflect responses to new selective pressures, plus the now more limited opportunities for introgression between managed and wild animals or the restocking of herds with wild animals.
Looking back before Ganj Dareh, unusual demographic profiles detected in sheep bone assemblages from northeastern Iraq (10) and southeastern Anatolia (11) dating to 12,000 B.P. may reflect early attempts at manipulating herd demographics to maximize returns. These assemblages show an almost exclusive focus on 2- to 3-year-old males, which is older than expected with herd management but younger than expected with hunting. This pattern is argued to result from a prime male hunting strategy developed under conditions of intensive pressure on local wild herds during the Younger Dryas climatic downturn (11). The proposed scenario suggests that, rather than broadening the prey strategy to include both males and females, hunters conserved female breeding stock while at the same time relying on a steady immigration of younger males drawn from surrounding territories to fill the vacuum left by the kill-off of local prime-age males.
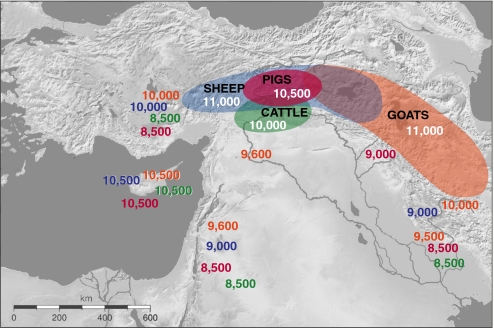
Lower-resolution demographic methods used by archaeozoologists working elsewhere in the Fertile Crescent are detecting parallel patterns to those documented in the Zagros. Changes in the age of harvested caprines, and possibly demographically driven changes in size consistent with early herd management, are found in southeastern Anatolia at ca. 10,500 B.P. (12). Sheep seem to be the initial early focus of the transition from hunting to herding in this region, with managed goats arriving from outside the area at ca. 10,200 B.P. (12). Similarly, demographic evidence for the management of morphologically unaltered caprines (mostly sheep) is found in Central Anatolia between 10,400 and 9,400 B.P. (13). These results suggest that both sheep and goats were brought under domestication (probably independently of one another and possibly multiple times) in the region that stretches from the northern Zagros to southeastern Anatolia between ca. 11,000 and 10,500 B.P., and perhaps even earlier (Fig. 1). Morphologically wild, but managed, goats appear to have been moved relatively rapidly through the region, reaching the southernmost tips of both the eastern and western arms of the Fertile Crescent by ca. 9,500 B.P. Domestic sheep were spread more slowly and first appear in these regions ≈500–1,000 years later than managed goats (10, 12).
Fig. 1.
The origin and dispersal of domestic livestock species in the Fertile Crescent. Shaded areas show the general region and the approximate dates in calibrated years B.P. in which initial domestication is thought to take place. Dates outside of the shaded areas show the approximate date when the domesticate first appears in a region. Orange, goats (Capra hircus); blue, sheep (Ovis aries); green, cattle (Bos taurus); fuscia, pigs (Sus scrofa).
Recent research has also clarified the spatial and temporal context of the domestication of two other major livestock species in the Near East: pigs and cattle. Archaeological evidence now suggests that pigs were first domesticated somewhere in southeastern Anatolia by 10,500–10,000 B.P. and that the timing of their geographical expansion as domesticates was similar, although perhaps slower, to that of sheep (Fig. 1) (10, 14). Morphologically altered domestic pigs are not found in the southern Levant or lowland Iran until ca. 8,500–8,000 B.P. Recent demographic evidence suggests that taurine cattle were initially domesticated somewhere in the upper Eu-phrates Valley between ca. 11,000 and 10,000 B.P. (15), but, like sheep and pigs, they arrived relatively late in more distant parts of the Fertile Crescent (Fig. 1). Morphologically altered domestic pigs and cattle are not found in Central Anatolia until after 8,500 B.P. (16).
Genetic data from modern and archaeological specimens both support and enhance this picture of initial animal domestication. Recent work has succeeded in definitively identifying the progenitors of both domestic sheep and goat as belonging to species found in the Fertile Crescent (Ovis orientalis and Capra aegagrus, respectively) (17, 18). Moreover, in both of these livestock species there are at least four and, in the case of goats, as many as six (19), genetically distinguishable domestic lineages, or haplotypes. It is not entirely clear, however, whether these different lineages represent spatially and temporally discrete “domestication events” in which different populations of animals were brought under domestication independently of one another (20). Genetic data for taurine cattle have identified five different domestic haplotypes, at least three and possibly four of which originated in the Fertile Crescent (21). Similarly, as many as four of the many different lineages of domestic pigs originated in the Near East (22, 23).
Animal domestication in the Near East can then be seen as arising from a period of prolonged human interaction with the ancestors of core livestock species that unfolded across much of the Fertile Crescent. Over time hunting strategies aimed at maximizing local availability of wild ungulates developed into active management, with all four major livestock species coming under management over a period from ca. 11,000 to 10,000 B.P. Even species like gazelle, which are behaviorally unsuited to domestication, may have been auditioned for management in the southern and northern Levant, where they were the most abundant wild ungulate (11). Clear-cut morphological responses to domestication (i.e., changes in horns in bovids and tooth size in pigs) are not evident in these four livestock species until ca. 9,500–9,000 B.P.
As is the case with animal domestication in the Near East, the leading edge of plant domestication in the region is now recognized as an extended process (24, 25). Evidence from multiple locations point to a prolonged period of human manipulation of morphologically wild, but possibly cultivated, plants which, in certain species, resulted in the development of morphologically altered domesticated crops (26–28). This period of intensified plant management dates at least as far back as ca. 12,000 B.P., with morphological markers of crop domestication (i.e., nonshattering seed heads in cereals) not well established until ca. 10,500 B.P. (24, 25, 29). Agricultural economies reliant on a mix of domesticated crops and livestock apparently do not fully crystallize in the region until ca. 9,500–9,000 B.P. (5, 10).
The Diffusion of Animal Domesticates in the Mediterranean Basin
The last two decades has witnessed the rise and fall of a number of models of Neolithic expansion across the Mediterranean Basin. In the early 1980s Ammerman and Cavalli-Sforza (30) combined archaeological and human genetic data to frame their “wave and advance” model. This model attributed the westward spread of the Neolithic to Near Eastern colonists who, driven by agriculture-fueled population growth, slowly pushed aside indigenous hunter–gatherers at a predicted average pace of ≈1 km per year.
Objecting to the passive role this model assigned to indigenous Mesolithic people, a number of researchers subsequently countered with alternative models that awarded local populations a starring role in Mediterranean Neolithic emergence. Early models within this indigenist perspective argued for autochonous domestication of crops and livestock in a process parallel to, but independent of, the Near East (31–33). The presence of wild oats, barley, and lentils in Upper Paleolithic and Mesolithic levels at Francthi Cave on the eastern coast of Greece, followed by the appearance of fully domesticated barley and lentils in later Neolithic levels, was interpreted as evidence for the local crop domestication (34). Legumes recovered from Mesolithic cave deposits in southern France were seen as evidence of incipient cultivation, if not domestication, of local wild plants (35). Evidence for indigenous animal domestication was based on the identification of wild sheep in Pleistocene age deposits in southern France and the presence of domestic sheep and goat remains in Mesolithic contexts in France and Spain (36, 37). Reports of domesticated pig and cattle remains in Mesolithic (pre-8,000 B.P.) levels from sites in southern Spain (38) were also cited as evidence for the local domestication of these species.
Genetic studies have subsequently ruled out European ancestry for domestic wheat, barley, and pulses, confirming the Near East as the source of these crops (26, 39). Morphological, cytological, hemoglobin, and, most recently, genetic studies have shown that the “wild” sheep and goats found on Mediterranean islands, once argued to be the descendents of the progenitors of indigenous domestic caprines, are instead the feral descendents of Near Eastern caprines (for a review in chronological order, see refs. 40, 41, 17, and 18).
Ruling out the indigenous domestication of caprines and major crop plants did not, however, lead to an embrace of colonist expansion diffusion models for the emergence of Neolithic lifeways in the Mediterranean. Instead, researchers subscribing to the indigenist perspective, especially those working in the western Mediterranean, argued that cultural and not demic diffusion was the primary engine driving this transition. Specifically, proponents maintained that the selective adoption of various elements of the Neolithic package by indigenous populations around the Mediterranean could have happened through trade and technology transfer alone without any direct contact between indigenous hunter–gatherers and colonizing farming populations from the east (41–46).
The shortcomings of these different single-agent models have become increasingly apparent as new archaeological data come to light and older collections are reanalyzed by using new methods and new perspectives. Recent genetic analyses of livestock species and their progenitors have also contributed important new insights into this process. As a result, a much more complex, and more interesting, scenario is emerging for the Neolithic transition across the Mediterranean Basin.
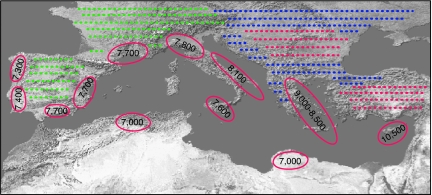
Beginning in the early 1990s a number of sites have been discovered and excavated on Cyprus that have radically transformed our understanding of Neolithic emergence in the Mediterranean Basin (47). Until the early 1990s Cyprus was thought to have been colonized ca. 8,500 B.P. by a derived offshoot of fully established Neolithic mainland cultures (48). The new sites, however, date 2,000 years earlier (10,500–9,000 B.P.) and document the arrival of early pioneers hypothesized to have originated somewhere in the Northern Levant (Figs. 1 and 2) (47, 49). Traveling the 60 k to Cyprus by boat, these colonists transported the full complement of economically important mainland fauna (50). including all four major livestock species (sheep, goat, cattle, and pig). Early colonists also imported mainland game animals like fallow deer and fox that, although perhaps kept in captivity (48), were never truly domesticated. None of these animals are endemic to Cyprus. Although imported livestock species did not show any of the morphological features traditionally used to mark domestic status when they arrived on the island, demographic profiles of these animals are consistent with human management. In contrast, demographic profiles of the fallow deer are indicative of hunting, suggesting that early colonists were engaged in game stocking and herd management (13, 48). Deep wells constructed at one of these early sites yielded abundant evidence of domesticated einkorn and emmer wheat and lentils, none of which are native to Cyprus, and domestic barley, which in the wild is endemic to the island (26, 51). Other introduced plants include pistachios and flax, as well as figs possibly domesticated in the Levant by this time (28). Thus the initial diffusion of the nascent Neolithic package out of the Fertile Crescent to Cyprus involved the transplant of all aspects of daily life (i.e., subsistence resources, technologies, and, most likely, social networks and belief systems) by seafaring colonists who, for unclear reasons, were seeking a fresh start in a new land (52). Far from being an isolated event, the colonization of Cyprus provides a clear and valuable template for the subsequent diffusion of the Neolithic across the rest of the Mediterranean Basin.
Fig. 2.
An integrated model of the Neolithic expansion in the Mediterranean Basin. The location of colonist farming enclaves is shown in the red ellipses. Approximate dates of these enclaves are given inside the ellipses in calibrated years B.P. Red dots represent areas that are proposed to have been settled by colonist farmers; green dots indicate areas where indigenous foragers adopted elements of the Neolithic package; and blue dots indicate areas of proposed integration of colonist farmers with indigenous foraging groups. Data were complied from refs. 52, 54, 56, 57, and 65 and figure 7.1 of ref. 74.
Recent archaeological evidence from the Aegean, for example, no longer supports a model of gradual in-place transition of ancestral Mesolithic cultures into Neolithic cultures (53–55). Instead, there appears to have been a sharp decline in Late Mesolithic population levels, combined with the sudden appearance of radically different Neolithic settlements in previously unoccupied locations. As on Cyprus, recent work in the Aegean argues for the arrival of maritime colonists who, at ca. 9,000 to 8,000 B.P., carried many components of the full Neolithic package (plant and animal domesticates, new lithic traditions, and, perhaps a bit later, pottery) (Fig. 2). Following a leapfrog pattern, these seafaring pioneers established farming communities that selectively focused on favorable environments in coastal Greece and on various Aegean Islands.
Based on a careful reevaluation of archaeological evidence, especially available radiocarbon dates, researchers now see major discontinuities between Mesolithic and Neolithic cultures in Italy (56, 57). They argue that Neolithic lifeways were introduced into the Italian peninsula ca. 8,000 B.P. by maritime colonists who first established farming villages on the Apulian “boot heel” region of southeastern Italy (Fig. 2). These traditions appear in northwest coastal Italy ≈200–300 years later (ca. 7,800–7,600 B.P.). In southern France, a compelling case can be made for a marked geographic, ecological, and cultural break between interior Mesolithic settlements and coastal Neolithic colonies (58) Recent excavation of a coastal settlement in southern France, dating to 7,700–7,600 B.P. and characterized as a beach-head colony of seafaring migrant farmers from mainland Italy, has yielded pottery, domestic sheep, einkorn, and emmer wheat (59).
Questions have also been raised about the evidence for the early occurrence of domestic animals and pottery in Mesolithic contexts in the western Mediterranean, which had formed a primary foundation of earlier culture diffusion models. Based on a reappraisal of the complicated cave stratigraphy of Iberian sites and a reanalysis of their associated radiocarbon dates, Zilhão (60–62) argues that the pottery and domesticated caprines recovered from Mesolithic levels actually derive from higher Neolithic levels. Domestic sheep reported as recovered from Mesolithic and earlier deposits in southern France can also be argued to have been derived from overlying Neolithic contexts, or, in the case of higher elevation sites, represent misidentified native chamois (Rupicapra rupicapra) and European ibex (Capra ibex) (41, 58, 63). Similarly, Rowley-Conwy's (64) reexamination of the argument for the domestic status of pigs and cattle in Mesolithic contexts in southern Spain suggests that the smaller size of these animals is not a sign of their domestication as originally argued, but is instead a reflection of body size responses to different climatic regimes among native wild animals.
Having discounted evidence for piecemeal cultural diffusion of various elements of Neolithic economy and their selective adoption by indigenous Mesolithic populations in the western Mediterranean, Zilhão (61, 62) has gone on to demonstrate that, as in other parts of the Mediterranean Basin, the Late Mesolithic of the Iberian Peninsula was a period of population decline and relocation. Also as elsewhere, Neolithic settlements with apparently fully formed agro-pastoral economic systems suddenly appear in the Iberian Peninsula as coastal enclaves occupying limestone-based soils abandoned by earlier Mesolithic peoples. The initial establishment of these colonies follows a familiar pattern, with farming enclaves appearing in favorable coastal locals around the periphery of the Iberian Peninsula at a steady and quite rapid pace, appearing first on the eastern and southern coasts of Spain at ca. 7,700–7,600 B.P. and on the Atlantic coast of Portugal ca. 7,400–7,300 B.P. (Fig. 2).
However, Zilhão's (61, 62) work in Portugal has also shown that Mesolithic cultures focusing on the intensive exploitation of estuary resources persist for several hundred years after the establishment of these farming enclaves and that the subsequent spread of agricultural economies into the interior likely proceeded through a combined process of colonist expansion, selective local adoption of Neolithic technologies, and the integration of colonist and indigenous populations. Similar patterns of development are hinted at in interior and northern Italy, which seem to lag several hundred years behind coastal areas in the appearance of plant and animal domesticates and other markers of Neolithic adaptations (56, 57). In southern France, the initial, essentially exclusive, focus on domestic livestock evidenced at the early coastal pioneering sites stands in stark contrast to subsistence strategies of later interior sites that show persistence of hunting along with the utilization of domesticates, a pattern that points to the blending of Neolithic and Mesolithic traditions after initial colonization (65). Farther east, the disjunction between later Neolithic sites and their Mesolithic and early Neolithic predecessors in the Aegean signals a similar process of dispersal, adoption, and integration (54) (Fig. 2).
Genetic studies of modern and ancient DNA from Mediterranean Basin livestock species and their progenitors adds further support, and nuance, to this emerging picture. A study of ancient mtDNA has shown that two haplotypes of domestic goats (the A and C lineages) had arrived in southern France by 7,300 B.P., suggesting their dispersal out of the Near East as a single package (66). Among modern goat breeds in Portugal researchers have found both the ubiquitous A haplotype and the much more restricted haplotype C (67). Three domestic lineages were found among modern breeds of sheep in Portugal, including those previously found only in the Middle East and Asia (68). Both Portuguese sheep and goat show a much higher degree of within-breed genetic diversity than expected at the westernmost periphery of sheep and goat expansion. This diversity is attributed to multiple introductions of caprines into the Iberian Peninsula, not only through maritime colonization from Italy and France, but through subsequent introductions out of Africa or overland through Europe.
Genetic data also support a pattern of multiple introductions of cattle into the region. The T3 haplotype of domestic cattle, which dominates among modern and ancient European cattle, seems to have followed a relatively rapid path of expansion around the Mediterranean Basin without any significant introgression with female European aurochsen (refs. 21, 69, and 70 and contra ancient DNA evidence reported in ref. 71). Modern DNA, however, indicates that T and T2 haplotype cattle were included in migratory movements into the Balkans and Central Europe (71). T1 cattle, which dominate among modern North African taurine cattle, were initially argued to represent a separate North African domestication event (21). This lineage now seems more likely to have been brought under domestication with other T haplogroup cattle in the Near East (72) and subsequently radiated across North Africa through trade and human migration. The patchy occurrence of the TI haplotype among modern cattle in the Iberian Peninsula, Sicily and central Italy, and the Balkans suggests that T1 cattle entered southern Europe out of North Africa through multiple points of entry (71). It is also possible that T1 cattle traveled overland across the Dardanelles into Eastern Europe. The high-diversity T haplogroup taurine cattle found among modern Tuscan cattle has been linked to a post-Neolithic migration of Etruscans who, based on both historical evidence and modern human genetic data, are believed to have been of Eastern Mediterranean origin (73).
Pigs tell a different story. Research by Larson et al. (22) has shown that current-day domestic pigs in Europe bear no trace of Middle Eastern ancestry, but instead are most closely related to European wild boar. Subsequent analysis by the same team of mtDNA extracted from archaeological remains has found convincing evidence for the dispersal of Near Eastern pigs into and across Europe between 7,500 and 5,000 B.P. (23). Surprisingly, subsequent to this initial diffusion, Near Eastern swine are later replaced by domestic pigs of European maternal ancestry, even within the Near East. Indigenous domestication of European boar also apparently happened several times, with two major European clades indicative of two separate domestication events, and an additional clade, currently restricted to Italy and Sardinia, representing another (22, 23).
Thus it appears that none of the earlier models for Neolithic emergence in the Mediterranean accurately or adequately frame the transition. Clearly there was a movement of people westward out of the Near East all of the way to the Atlantic shores of the Iberian Peninsula. But this demic expansion did not follow the slow and steady, all-encompassing pace of expansion predicted by the wave and advance model. Instead the rate of dispersal varied, with Neolithic colonists taking 2,000 years to move from Cyprus to the Aegean, another 500 to reach Italy, and then only 500–600 years to travel the much greater distance from Italy to the Atlantic (52). Moreover, rather than entirely replacing or engulfing indigenous foraging populations, these colonists seem to have been restricted to scattered coastal farming enclaves established around the Mediterranean Basin. Although cultural diffusion can no longer be argued to provide a universal explanation for Neolithic expansion into the Mediterranean, it is clear that the movement of Neolithic lifeways out of these beachhead settlements involved selective adoption and adaptation of elements of the Neolithic package by indigenous peoples. Moreover, although caprines, cattle, and primary crop plants were most certainly not independently domesticated in Europe, recent genetic data for pigs points to indigenous domestication of local wild boar, possibly occurring multiple times in geographically separate subpopulations. Genetic studies of rye and oats also indicate that the modern varieties of these major crop plants have a European and not Near Eastern ancestry (24). Future interpretive frameworks will have to take a more integrated approach, which recognizes colonization, diffusion, and independent domestication as all playing a role in Neolithic expansion across the Mediterranean (65, 74) (Fig. 2).
Environmental Impacts
The impact of Neolithic economies on the biotic communities of the Mediterranean Basin is most clearly seen on the large islands scattered across the region, where highly endemic and disharmonic faunas were replaced by a mixture of domestic and wild mainland fauna (75–77). Although humans are clearly the agent of island introductions of mainland faunas, their role in the extirpation of endemic island faunas is unclear.
Once again, Cyprus reflects a general pattern for the Mediterranean Basin. The endemic mammalian fauna of Cyprus was impoverished and unbalanced, limited to pygmy hippopotamus (Phanourios minutus), a pygmy elephant (Elephas cypriotes), a genet (Genetta plesictoides), the only carnivore on the island), and a mouse (Mus cyprinacus, the only endemic to survive to the present day) (76, 78). None of the larger endemics are represented among the imported mainland fauna associated with the sites of colonists of the 11th millennium B.P. (50). It is now clear that these pioneer settlers were not the first humans on Cyprus and that mainland hunters made periodic visits to the island 1,000 years earlier during the Younger Dryas climatic downturn (79) (Table 1). Simmons (79) argues that a large accumulation of pygmy hippopotamus remains found in a collapsed seaside rock shelter is directly associated with an overlying, but apparently contemporaneous, stratum containing stone tools and hearths dated to ca. 11,775 B.P. Other researchers, although ac-cepting the evidence for early visits of hunter–gatherers to the island, question the evidence for human predation on the endemic mammals discovered at the site (refs. 80 and 81, but see ref. 82).
Table 1.
The extinction of Late Pleistocene large endemic mammals and human colonization of Mediterranean islands
| Time period | Island/mammal |
|||
|---|---|---|---|---|
| Gymnesic Islands/Myotragus balearicus | Tyrrhenian Islands/Megaloceros cazioti | Crete/Candiacervus sp. | Cyprus/Phanourios minutus | |
| Most recent endemic | 5,000* | 10,000† | > 9,000 | 11,500*† |
| Earliest human presence | 7,000 | 10,000 | None | 11,500 |
| Neolithic colonization | 4,000 | 7,600 | 9,000 | 10,500 |
Large endemic mammals on Crete included pygmy hippopotamus (Hippopotamus creutzburgi), elephants (Elephas creutzburgi), and several species of megalocerine deer (Candiacervus sp.) (76). Dating the last appearance of these species has proven problematic (83), although it appears that the Cretian hippopotamuses and elephants became extinct before the endemic deer. There is as yet no evidence for a temporal overlap between humans and larger endemic mammals on Crete. As on Cyprus, Cretian endemics do not occur among the faunal remains recovered from the earliest known Neolithic settlement on the island, which dates to ca. 9,000 B.P. (84) (Table 1). It is possible that the larger endemic mammals of Crete became extinct long before Neolithic farmers and herders colonized the island, thus limiting the island's appeal to earlier mainland hunting parties. It is also possible, however, that the ephemeral camps of these hunters have yet to be discovered.
A closer connection between humans and now extinct endemic island faunas has been proposed for the islands of Sardinia and Corsica. The case for an overlap between humans and indigenous megalocerine deer (Megaloceros cazioti) on Sardinia in the Upper Paleolithic (ca. 20,000 B.P.) (85) is contested (86). But there is firmer evidence that Mesolithic hunter–gatherers at least visited (if not colonized) Sardinia by ca. 10,000 B.P. and that they encountered endemic deer and, possibly, the island's sole carnivore, a small fox-size canid (Cynotherium sardous) (refs. 76 and 86 and Table 1). Neither of these species survived much beyond this initial contact, however, and there is no evidence of overlap between Mesolithic hunter–gatherers and these two endemics on Corsica (76). A number of sites on both islands attest to heavy utilization of smaller endemic mammals, especially a rat-size endemic lagomorph (Prolagus sardus), which seems to be the primary source of animal protein until Neolithic colonizers brought domestic livestock to these islands at ca. 7,600 B.P. (87). All of these smaller endemics, however, were extripated sometime between the Late Roman period and the early Middle Ages, probably through combined pressures associated with the introduction of Rattus rattus during the Early Roman colonization of the island and subsequent episodes of deforestation and agricultural intensification (75, 88).
The apparent exception to this pattern of rapid extinction of endemic island fauna just before, or slightly after, substantial human colonization was, until recently, thought to be a small derived caprine species (Mytotragus balearicus), found only on the Gymnesic islands off the eastern coast of Spain (89). Here initial dating argued for a >3,000-year overlap between initial human presence on Mallorca at 8,000 B.P. and the last documented Myotragus specimens on the island dated at ca. 5,000 B.P.. A case was even mounted for domestication of this species before its extinction (90), which, if true, made Myotragus the only domestic animal to undergo extinction. Recent reevaluation of the primary arguments for Myotragus domestication has, however, overturned this hypothesis (89, 91). A reexamination of the dates for earliest human occupation of these islands and the most recent evidence for Myotragus, moreover, has all but eliminated any overlap between humans and Myotragus (89). The earliest evidence of substantial human settlement of Mallorca dates to ca. 4,000 B.P., whereas the most recent solid evidence for Myotragus on the island dates to ca. 5,000 B.P., with Myotragus extinction and initial human colonization happening sometime between these two dates (Table 1).
Even lacking definitive evidence for humans involvement in the extirpation of larger endemic mammals on Mediterranean islands, the progressive east-to-west disappearance of larger endemics coincident with human settlement of Mediterranean islands (Table 1) clearly suggests that humans played some role in their extinction. Having evolved in the absence of major predators, these larger herbivores probably lacked protective behaviors, making them especially vulnerable to sustained human predation. Moreover, all of the species that became extinct around the time of initial human settlement likely had low reproductive rates, a trait commonly found among island endemics, which further limited their ability to withstand any degree of hunting pressure (76). Extermination of these island endemics by humans could have happened within a generation. Smaller endemic mammals seem to have survived initial human colonization somewhat better, perhaps as a result of their higher reproductive rates and because they were less attractive prey species. However, they, too, eventually could not withstand the combination of overhunting, loss of habitat, and competition with invasive mainland imports. The almost complete turnover of island faunas throughout the region [involving essentially 100% of mammal species, plus many avian and herpetological species (77)] and their replacement with domestic livestock, game species, and an array of anthropolophilous small vertebrate species (76) clearly implicates humans in these island extinctions.
The impact of the Neolithic transition on mainland environments throughout the Mediterranean, although perhaps less dramatic, is no less pronounced. Blondel and Aronson (92), in fact, argue that the entire Mediterranean Basin is characterized by highly anthropogenic environments shaped by thousands of years of human landscape management, species introductions, and associated responses by indigenous faunas and floras, all dating to the Neolithic emergence. They also note that the various forms of traditional human landscape engineering in the Mediterranean have created viable, sustainable ecosystems, which have, in fact, been highly beneficial to Mediterranean biodiversity. This balanced system is undergoing increasing threat from urban growth and agricultural intensification, however, threats that can only be met with a clear understanding of the long-term role of human management in shaping current-day biodiversity in the Mediterranean Basin.
Future Research
Recent research on the initial development and subsequent expansion of domestication and agricultural economies in the Mediterranean Basin provides a clear roadmap for future research. This is especially true for the Fertile Crescent where recent advances are transforming our understanding of the origins of plant and animal domestication in this key heartland region. Traditional approaches to documenting domestication relied on the appearance of genetically driven morphological change (i.e., the development of nonshattering seed heads in cereals and body size reduction in animals). The development of new analytical approaches has, however, provided a window into the preceding processes of human interaction with target plant and animal species and the genetic responses to this interaction that eventually resulted in morphological change. Researchers in the Fertile Crescent are detecting early signs of human ecosystem engineering aimed at encouraging plant production (24, 25); they are able to document the manipulation of herd structure to promote a secure and predictable yield of animal products (7, 8, 10, 11). In both plants and animals these new indicators precede the manifestation of traditional morphological markers of domestication by hundreds, if not thousands, of years. Estimating exactly when during this extended coevolutionary process a plant or animal species crossed the domestic threshold is now more a semantic issue than a substantive research question (20, 93). Although some researchers may require the appearance of specific morphological traits before conferring domestic status, others may be more willing to consider a managed animal or a cultivated plant as having achieved this status. Regardless of where one chooses to draw the line between wild and domestic, recent advances have provided researchers with powerful new tools capable of examining the entire process of domestication, not just its morphological impacts which, if they occur at all, only appear after the process is well underway.
Just how far back this process of active human resource management goes or how widespread it was in the Fertile Crescent is, at this point, an open question. The early dispersal of an integrated economy based on crop plants and managed animals to Cyprus at least 2,000 years before the apparent crystallization of agricultural economies on the mainland, however, suggests that our understanding of plant and animal domestication and agricultural emergence on the mainland is, at best, incomplete. Researchers working in this area are only just beginning to realize the potential of these newly available analytic tools. Additional even more effective analytical approaches will almost certainly be developed in the future as researchers embrace this broader concept of domestication and begin to exploit the many opportunities available in the Fertile Crescent region for documenting it.
Recent research has also shown that the dispersal of domesticates and the Neolithic way of life west across the Mediterranean Basin was much more complex and multifaceted than previous prime mover models could accommodate. To varying degrees, in different areas, this process involved elements of demic diffusion, local adoption, and independent domestication. But the outlines of this complex process are just beginning to come into focus. Maritime colonization of the Mediterranean clearly involved not one, but multiple unrelated seaborne migrations (52). The cultural context of these migratory movements, their causes, their routes, their timing, and their tempo all call out for additional investigation. The southern margin of the Mediterranean Basis along coastal North Africa is essentially terra incognita for understanding the course of Neolithic emergence and seems an especially promising region for future research (60).
The subsequent inland transfer of domesticates, agriculture, and associated Neolithic lifeways from newly arrived colonists to indigenous populations around the Mediterranean Basin is another intriguing research area that will benefit from recent advances in our ability to detect and date domesticates in the archaeological record. Careful analysis of increasingly more precise radiocarbon dates will continue to be critical in discriminating between demic diffusion and selective adoption of Neolithic components in different parts of the Mediterranean (e.g., ref. 94). New demographic techniques for profiling prey strategies, morphometric techniques capable of tracking genetic and plastic responses to human management, isotopic analysis, and the increasing success of ancient DNA studies of domesticates will enhance our understanding of the ways in which both colonists and local populations adapted management strategies to these new environments.
There are also obvious opportunities for those interested in understanding the independent domestication of European wild species. Larson et al. (23), for example, suggest that European wild boar were domesticated only after the introduction of Near Eastern domestic swine, representing a case of apparent technology transfer rather than truly independent domestication. Local, culturally independent domestication of indigenous wild pigs, however, still cannot be ruled out. Application of enhanced archaeological and genetic techniques for detecting and dating domestication to both extant and yet-to-be-recovered assemblages is key to understanding patterns of indigenous domestication around the Mediterranean Basin.
Finally, although we may never be able to detect the final coup de grâce for endemic island faunas, the future holds considerable promise for a much fuller understanding of the human impact on Mediterranean biodiversity. As it has in the Gymnesic islands and on Cyprus, “carpet-dating” large numbers of archaeological materials by the small-sample atomic mass spectrometry radiocarbon method will certainly help refine the chronology of the disappearance of endemic island faunas and the arrival of human colonists. Application of demographic profiling techniques to the remains of these animals may make it possible to distinguish between natural-death accumulations and prey assemblages resulting from human predation. Similarly, recognition of the broader role of humans in shaping post-Neolithic environments is central to understanding how Mediterranean biodiversity evolved and how we might best work to conserve it. The archaeobiological sciences have a valuable role to play in providing greater time depth to biodiversity studies by monitoring the creation of anthropogenic ecosystems and tracing the development and impacts of both environmentally sustainable and destructive agricultural economies over thousands of years of human occupation.
Acknowledgments.
This work has benefited from the comments of Greger Larson, Bruce Smith, and Jean-Denis Vigne and from the artistry of Marcia Bakry, who drew the maps.
Footnotes
This article grew out of a presentation given by M.Z. at the Calpe 2007 Symposium: People in the Mediterranean–A History of Interaction, September 27–30, 2007, the Gibraltar Museum, Gibraltar.
The author declares no conflict of interest.
This article is a PNAS Direct Submission.
All dates are reported in calibrated years before present.
References
- 1.Zeder MA, Emshwiller E, Smith BD, Bradley DG, editors. Documenting Domestication. Berkeley: Univ of California Press; 2006. [DOI] [PubMed] [Google Scholar]
- 2.Zeder MA. Archaeological approaches to documenting animal domestication. In: Zeder MA, Emshwiller E, Smith BD, Bradley DG, editors. Documenting Domestication. Berkeley: Univ of California Press; 2006. pp. 171–180. [Google Scholar]
- 3.Uerpmann H-P. Supplements to the Tuebingen Atlas of the Near Orient, series B. Weisbaden: Ludwig Reichert; 1979. Problems of the Neolithization of the Mediterranean Region (original in German) [Google Scholar]
- 4.Meadow RH. Osteological evidence for the process of animal domestication. In: Clutton-Brock J, editor. The Walking Larder. London: Unwin Hyman; 1989. pp. 80–90. [Google Scholar]
- 5.Bar-Yosef O, Meadow RH. The origins of agriculture in the Near East. In: Price TD, Gebauer A-B, editors. Last Hunters, First Farmers. Santa Fe, NM: School of American Research Press; 1995. pp. 39–94. [Google Scholar]
- 6.Horwitz LK. The development of ovicaprine domestication during the PPNB of the southern Levant. In: Buitenhuis H, Clason AT, editors. Archaeozoology of the Near East I. Leiden, The Netherlands: Universal Book Service; 1993. pp. 27–36. [Google Scholar]
- 7.Vigne J-D, Peters J, Helmer D. New archaeozoological approaches to trace the first steps of animal domestication. In: Vigne J-D, Peters J, Helmer D, editors. The First Steps of Animal Domestication. Oxford: Oxbow Books; 2005. pp. 1–16. [Google Scholar]
- 8.Zeder MA. A critical examination of markers of initial domestication in goats (Capra hircus) In: Zeder MA, Emshwiller E, Smith BD, Bradley DG, editors. Documenting Domestication. Berkeley: Univ of California Press; 2006. pp. 181–208. [Google Scholar]
- 9.Zeder MA. Reconciling rates of long bone fusion and tooth eruption and wear in sheep (Ovis) and goat (Capra) In: Ruscillo D, editor. Ageing and Sexing Animals from Archaeological Sites. Oxford: Oxbow Press; 2006. pp. 87–118. [Google Scholar]
- 10.Zeder MA. The Neolithic (macro)-revolution. J Archaeol Res. 2009 in press. [Google Scholar]
- 11.Redding RW. Breaking the mold, a consideration of variation in the evolution of animal domestication. In: Vigne J-D, Peters J, Helmer D, editors. The First Steps of Animal Domestication. Oxford: Oxbow Books; 2005. pp. 41–48. [Google Scholar]
- 12.Peters J, von den Driesch A, Helmer D. The upper Euphrates-Tigris Basin, cradle of agro-pastoralism? In: Vigne J-D, Peters J, Helmer D, editors. The First Steps of Animal Domestication. Oxford: Oxbow Books; 2005. pp. 96–124. [Google Scholar]
- 13.Vigne J-D, Buitenhuis H, Davis S. The first steps in animal domestication into the east of the Euphrates: Cypres and Central Anatolia (original in French) Paléorient. 1999;25/2:49–62. [Google Scholar]
- 14.Ervynck A, Dobney K, Hongo H, Meadow RH. Born free! Paléorient. 2001;27/2:47–73. [Google Scholar]
- 15.Helmer D, Gourichon L, Monchot H, Peters J, Saña Segui M. Identifying early domestic cattle from prepottery Neolithic sites on the middle Euphrates using sexual dimorphism. In: Vigne J-D, Peters J, Helmer D, editors. The First Steps of Animal Domestication. Oxford: Oxbow Books; 2005. pp. 86–95. [Google Scholar]
- 16.Martin L, Russell N, Carruthers D. Animal remains from the central Anatolian Neolithic. In: Gérard F, Thissen L, editors. The Neolithic of Central Anatolia. İstanbul, Turkey: Ege Yayınları; 2002. pp. 193–206. [Google Scholar]
- 17.Bruford M, Townsend SJ. Mitochondrial DNA diversity in modern sheep: Implications for domestication. In: Zeder MA, Emshwiller E, Smith BD, Bradley DG, editors. Documenting Domestication. Berkeley: Univ California Press; 2006. pp. 306–316. [Google Scholar]
- 18.Luikart G, Fernández H, Mashkour M, England PR, Taberlet P. Origins and diffusion of domestic goats inferred from DNA markers. In: Zeder MA, Emshwiller E, Smith BD, Bradley DG, editors. Documenting Domestication. Berkeley: Univ of California Press; 2006. pp. 294–305. [Google Scholar]
- 19.Naderi S, et al. Large-scale mitochondrial DNA analysis of the domestic goat reveals six haplogroups with high diversity. PLos One. 2007;10:1–23. doi: 10.1371/journal.pone.0001012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dobney K, Larson G. Genetics and animal domestication. J Zool. 2006;286:261–271. [Google Scholar]
- 21.Bradley DG. Genetics and the origins of domestic cattle. In: Zeder MA, Emshwiller E, Smith BD, Bradley DG, editors. Documenting Domestication. Berkeley: Univ of California Press; 2006. pp. 317–328. [Google Scholar]
- 22.Larson G, et al. Worldwide pylogeography of wild boar reveals multiple centers of pig domestication. Science. 2005;307:1618–1621. doi: 10.1126/science.1106927. [DOI] [PubMed] [Google Scholar]
- 23.Larson G, et al. Ancient DNA, pig domestication, and the spread of the Neolithic into Europe. Proc Natl Acad Sci USA. 2007;104:15276–15281. doi: 10.1073/pnas.0703411104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Weiss E, Kislev ME, Hartmann A. Autonomous cultivation before domestication. Science. 2006;312:1608–1610. doi: 10.1126/science.1127235. [DOI] [PubMed] [Google Scholar]
- 25.Wilcox G, Fornite S, Herveux L. Early Holocene cultivation before domestication in northern Syria. Vegetative History Archaeobot. 2007 doi: 10.1007/s00334-007-0121-y. [DOI] [Google Scholar]
- 26.Wilcox G. Geographical variation in major cereal components and evidence for independent domestication events in Western Asia. In: Cappers RTJ, Bottema S, editors. The Dawn of Farming in the Near East. Berlin: Ex Oriente; 2002. pp. 133–140. Studies in Near Eastern Production, Subsistence, and Environment no. 6. [Google Scholar]
- 27.Wilcox G. The distribution, natural habitats, and availability of wild cereals in relation to their domestication in the Near East. Vegetative History Archaeobot. 2005;14:534–541. [Google Scholar]
- 28.Kislev ME, Hartmann A, Bar-Yosef O. Early domesticated fig in the Jordan Valley. Science. 2006;312:1375–1374. doi: 10.1126/science.1125910. [DOI] [PubMed] [Google Scholar]
- 29.Nesbitt M. When and where did domesticated cereals first occur in Southwest Asia? In: Cappers RTJ, Bottema S, editors. The Dawn of Farming in the Near East. Berlin: Ex Oriente; 2002. pp. 113–132. Studies in Near Eastern Production, Subsistence, and Environment no. 6. [Google Scholar]
- 30.Ammerman AJ, Cavalli-Sforza LL. The Neolithic Transition and the Genetics of Populations in Europe. Princeton: Princeton Univ Press; 1984. [Google Scholar]
- 31.Higgs E, Jarman MR. The origins of agriculture. Antiquity. 1969;43:31–41. [Google Scholar]
- 32.Dennell R. European Economic Prehistory. London: Academic; 1983. [Google Scholar]
- 33.Barker G. Prehistoric Farming in Europe. Cambridge, UK: Cambridge Univ Press; 1985. [Google Scholar]
- 34.Hansen JM, Renfrew JM. Paleolithic-Neolithic seed remains at Francthi Cave, Greece. Nature. 1978;271:349–352. [Google Scholar]
- 35.Vaquer J, Geddes D, Barbaza M, Erroux J. Mesolithic plant exploitation at the Balma Abeurador (France) Oxford J Arch. 1986;5:1–18. [Google Scholar]
- 36.Ducos P. Some evidence for the origin of domestication in France (original in French) In: Guilaine J, editor. The Prehistory of France. Paris: Centre National de la Recherche Scientifique; 1976. pp. 165–177. (original in French) [Google Scholar]
- 37.Estévez J. Study of the faunal remains (original in Spanish) In: Olaria C, editor. Cova Fosca. Castellón, Spain: Servico de Publicaciones Diputación de Castellón; 1988. pp. 281–337. [Google Scholar]
- 38.Boessneck J, von den Driesch A. Finds of animal bones from four caves in southern Spain (original in German) In: Boessneck J, von den Driesch A, editors. Studies on finds of early animal bones from the Iberian Peninsula 7. Munich: Institut für Palaeoanatomir, Domestikationsforschung und Geschichte de Tiermedizin der Universität München; 1980. pp. 1–83. [Google Scholar]
- 39.Zohary D. The mode of domestication of the founder crops of Southwest Asian agriculture. In: Harris D, editor. The Origins and Spread of Agriculture and Pastoralism in Eurasia. Washington DC: Smithsonian Institution Press; 1996. pp. 142–158. [Google Scholar]
- 40.Geddes DS. Mesolithic domestic sheep in west Mediterranean Europe. J Archaeol Sci. 1985;12:25–48. [Google Scholar]
- 41.Poplin F, et al. The beginning of herding in France (original in French) In: Demoule J-P, Guilaine J, editors. The French Neolithic. Paris: Picard; 1986. pp. 37–51. (original in French) [Google Scholar]
- 42.Guilaine J. First Herders and Farmers in the Occidental Mediterranean. Paris: Mouton; 1976. (original in French) [Google Scholar]
- 43.Price TD. The Mesolithic of Western Europe. J World Prehist. 1987;1:225–305. [Google Scholar]
- 44.Zvelebil M. On the transition to farming in Europe, or what was spreading with the Neolithic. Antiquity. 1989;63:379–383. [Google Scholar]
- 45.Dennell R. The origin of crop agriculture in Europe. In: Cowan CW, Watson PJ, editors. The Origins of Agriculture. Washington, DC: Smithsonian Institution Press; 1992. pp. 71–100. [Google Scholar]
- 46.Lewthwaite J. The transition to food production. In: Zvelebil M, editor. Hunters in Transition: Mesolithic Societies in Temperate Eurasia and Their Transition to Farming. Cambridge, UK: Cambridge Univ Press; 1986. pp. 53–66. [Google Scholar]
- 47.Guilaine J, LeBrun, editors. The Neolithic of Cyprus. Athens: École Français d'Athèns; 2003. (original in French) Bull Corr Héllenique Suppl 43. [Google Scholar]
- 48.LeBrun A, Cluzan S, Davis SJM, Hansen J, Renault-Mislkovsky The pre-ceramic Neolithic of Cyprus (original in French) L'Anthropologie. 1987;91:283–316. [Google Scholar]
- 49.Peltenburg E. Introduction. In: Peltenburg E, Wasse A, editors. Neolithic Revolution. Chippenham, UK: Anthony Row; 2004. pp. xi–xx. [Google Scholar]
- 50.Vigne J-D, Carrére I, Guilaine J. Unstable status of early domestic ungulates in the Near East. In: Guilaine J, LeBrun A, editors. The Neolithic of Cyprus. Athens: École Français d'Athèns; 2003. pp. 239–251. (original in French) Bull Corr Héllenique, Suppl 43. [Google Scholar]
- 51.Colledge S. Reappraisal of the archaeobotanical evidence for the emergence and dispersal of the “founder crops.”. In: Peltenburg E, Wasse A, editors. Neolithic Revolution. Chippenham, UK: Anthony Row; 2004. pp. 49–60. [Google Scholar]
- 52.Guilaine J. The Neolithization in the Mediterranean and France. In: Ammerman A, Biagi P, editors. The Widening Harvest. Boston: Archaeological Institute of America; 2003. pp. 189–206. [Google Scholar]
- 53.Broodbank C. Colonization and configuration in the insular Neolithic of the Aegean. In: Halstead P, editor. Neolithic Society in Greece. Sheffield, UK: Sheffield Academic; 1999. pp. 15–41. [Google Scholar]
- 54.Pérles C. The Early Neolithic in Greece. Cambridge, UK: Cambridge Univ Press; 2001. [Google Scholar]
- 55.Runnels C. The origins of the Greek Neolithic. In: Ammerman A, Biagi P, editors. The Widening Harvest. Boston: Archaeological Institute of America; 2003. pp. 121–132. [Google Scholar]
- 56.Biagi P. A review of the late Mesolithic in Italy and its implication for the Neolithic transition. In: Ammerman A, Biagi P, editors. The Widening Harvest. Boston: Archaeological Institute of America; 2003. pp. 133–156. [Google Scholar]
- 57.Skeates R. Radiocarbon dating and interpretations of the Mesolithic-Neolithic transition in Italy. In: Ammerman A, Biagi P, editors. The Widening Harvest. Boston: Archaeological Institute of America; 2003. pp. 157–188. [Google Scholar]
- 58.Binder D. Mesolithic and Neolithic interaction in southern France and northern Italy. In: Price TD, editor. Europe's First Farmers. Cambridge, UK: Cambridge Univ Press; 2000. pp. 117–145. [Google Scholar]
- 59.Guilaine J, Manen C, Vigne J-D. New Insights into the Neolithization of Mediterranean France. Toulouse, France: Centre d'Anthropologie; 2008. (original in French) in press. [Google Scholar]
- 60.Zilhão J. The spread of agro-pastoral economies across Mediterranean Europe. J Med Archaeol. 1993;6:5–63. [Google Scholar]
- 61.Zilhão J. From the Mesolithic to the Neolithic in the Iberian Peninsula. In: Price TD, editor. Europe's First Farmers. Cambridge, UK: Cambridge Univ Press; 2000. pp. 144–183. [Google Scholar]
- 62.Zilhão J. Radiocarbon evidence for maritime pioneer colonization at the origins of farming in west Mediterranean Europe. Proc Natl Acad Sci USA. 2001;98:14180–14185. doi: 10.1073/pnas.241522898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Guilaine J, et al. Dourgne. Toulouse/Carcassonne, France: Centre d'Anthropologie de Societes Rurales, Archeologie en Terre d'Aude; 1993. [Google Scholar]
- 64.Rowley-Conwy P. Early domestic animals in Europe. In: Ammerman A, Biagi P, editors. The Widening Harvest. Boston: Archaeological Institute of America; 2003. pp. 99–120. [Google Scholar]
- 65.Tresset A, Vigne J-D. Substitution of species, techniques and symbols at the Mesolithic-Neolithic transition in western Europe. Proc Br Acad. 2007;144:189–210. [Google Scholar]
- 66.Fernández H, et al. Divergent mtDNA of goats in an Early Neolithic site, far from the initial domestication areas. Proc Natl Acad Sci USA. 2006;103:15375–15379. doi: 10.1073/pnas.0602753103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pereira F, Pereira L, Van Asch B, Bradley DG, Amorim A. The mtDNA catalogue of all Portuguese autochothonous goat (Capra hircus) breeds. Mol Ecol. 2005;14:2313–2318. doi: 10.1111/j.1365-294X.2005.02594.x. [DOI] [PubMed] [Google Scholar]
- 68.Pereira F, et al. Genetic signatures of a Mediterranean influence in Iberian Peninsula sheep husbandry. Mol Biol Evol. 2006;23:1420–1426. doi: 10.1093/molbev/msl007. [DOI] [PubMed] [Google Scholar]
- 69.Edwards C, et al. Mitochondrial DNA analysis shows a Near Eastern origin for domestic cattle and no indication of domestication of European aurochs. Proc R Soc London Ser B. 2007;274:1377–1385. doi: 10.1098/rspb.2007.0020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cymbron T, et al. Microsatellite diversity suggests different histories for Mediterranean and Norther Eurpean cattle populations. Proc R Soc London Ser B. 2005;272:1837–1843. doi: 10.1098/rspb.2005.3138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Beja-Pereira A, et al. The origin of European cattle. Proc Natl Acad Sci USA. 2006;103:8113–8118. doi: 10.1073/pnas.0509210103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Achilli A, et al. Mitochrondrial genomes of extinct aurochs survive in domestic cattle. Curr Biol. 2008;18:157–158. doi: 10.1016/j.cub.2008.01.019. [DOI] [PubMed] [Google Scholar]
- 73.Pellecchia M, et al. The mystery of Etruscan origins. Proc R Soc London Ser B. 2007;274:1175–1179. doi: 10.1098/rspb.2006.0258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zvelebil M. The social context of the agricultural transition to Europe. In: Renfrew C, Boyle K, editors. Archaeogenetics: DNA and the Population Prehistory of Europe. Oxford: McDonald Institute Monographs, Oxbow; 2000. pp. 57–80. [Google Scholar]
- 75.Blondel J, Vigne J-D. Space, time, and man as determinants of diversity of birds and mammals in the Mediterranean Region. In: Ricklefs RE, Schluter D, editors. Species Diversity in Ecological Communities. Chicago: Univ Chicago Press; 1993. pp. 135–146. [Google Scholar]
- 76.Vigne J-D. The large “true” Mediterranean islands as a model for the Holocene human impact on the European vertebrate fauna? In: Benneke N, editor. The Holocene History of the European Vertebrate Fauna. Eurasien-Abteilung, Berlin: Deutsches Archäologisches Institut; 1999. pp. 295–322. [Google Scholar]
- 77.Alcover JA, Seguí B, Bover P. Extinctions and local disappearances of vertebrates in the western Mediterranean islands. In: McPhee RDE, editor. Extinctions in Near Time. New York: Kluwer; 1999. pp. 165–188. [Google Scholar]
- 78.Cucchi T, et al. A new endemic species of the subgeneus Mus (Rodentia Mammalia) on the island of Cyprus. Zootaxa. 1241:1–36. [Google Scholar]
- 79.Simmons A. Faunal Extinction in an Island Society. New York: Kluwer; 1999. [Google Scholar]
- 80.Bunimovitz S, Barkai R. Ancient bones and modern myths. J Med Arch. 1996;9:85–96. [Google Scholar]
- 81.Ammerman A, Noller J. New light on Aeotokremnos. World Archaeol. 2005;37:533–543. [Google Scholar]
- 82.Simmons A, Mandel R. Not such a new light. World Archaeol. 2007;39:475–482. [Google Scholar]
- 83.Reese DS, Belluomini G, Ikeya M. Absolute dates for the Pleistocene fauna of Crete. In: Reese DS, editor. Pleistocene and Holocene Fauna of Crete and its First Settlers. Madison, WI: Prehistory Press; 1996. pp. 47–51. [Google Scholar]
- 84.Broodbank C, Strasser TF. Migrant farmers and the Neolithic colonization of Crete. Antiquity. 1991;65:233–245. [Google Scholar]
- 85.Sondaar PY, Martini F, Ulzega A, Klein G, Hofmeijer Pleistocene Man in Sardinia (original in French) L'Anthropologie. 1991;95:181–200. [Google Scholar]
- 86.Vigne J-D. The first evidence of modern humans in Corsica and Sardinia (original in French) In: Hedges J, Hedges E, editors. Human Migration and Environmental Variation in the Quaternary. Oxford: Archaeopress; 2005. pp. 139–145. (original in French) BAR International Series 1352. [Google Scholar]
- 87.Vigne J-D, Desse-Berset N. The exploitation of animal resources in the Mediterranean Islands during the Preneolithic, the example of Corsica. In: Fisher A, editor. Man and Sea in the Mesolithic. Oxford: Oxbow Books; 1995. pp. 309–318. [Google Scholar]
- 88.Vigne J-D. Small mammal fossil assemblages as indicators of environment change in northern Corsica during the last 2,500 years. J Archaeol Sci. 1996;23:199–215. [Google Scholar]
- 89.Bover P, Alcover JA. Understanding Late Quaternary extinctions. J Biogeog. 2003;30:771–781. [Google Scholar]
- 90.Waldren WH. Balearic Prehistoric Ecology and Culture. Oxford: Archaeopress; 1982. British Archaeological Reports IS 149. [Google Scholar]
- 91.Ramis D, Bover P. A review of the evidence for domestication of Myotragus balearicus Bate 1909 (Artiodactyla, Caprinae) in the Balearic Islands. J Archaeol Sci. 2001;28:265–282. [Google Scholar]
- 92.Blondel J, Aronson J. Biology and Wildlife of the Mediterranean Region. Oxford: Oxford Univ Press; 1999. [Google Scholar]
- 93.Zeder MA. Central questions in the domestication of plants and animals. Evol Anthropol. 2006;15:105–117. [Google Scholar]
- 94.Gkiastra M, Russell T, Shennan, Steele J. Neolithic transition in Europe. Antiquity. 2003;77:45–62. [Google Scholar]