Abstract
It has long been known that adipose tissue in obesity is in a heightened state of inflammation. Recently, our understanding of this has been transformed by the knowledge that immune cells such as macrophages and T cells can infiltrate adipose tissue and are responsible for the majority of inflammatory cytokine production. These seminal findings have opened up a new area in biology that is garnering the interest of scientists involved in research relating to cell motility, inflammation, obesity, physiology, diabetes and cardiovascular disease. Some important general questions relevant to this field are: how are macrophages recruited to adipose tissue in obesity? What are the physiological consequences of macrophage—adipocyte interactions? Do these inflammatory macrophages contribute to pathophysiological conditions associated with obesity, such as insulin resistance, dyslipidemia, diabetes and cardiovascular disease? This review focuses on the first of these important questions.
Keywords: activation, adipose tissue, chemokines, crown-like structures, hypoxia, inflammation, insulin resistance, leptin, lymphocytes, macrophages
During the past two decades, the complex nature of adipose tissue (AT) has become an area of intense investigation. This is in part due to the growing worldwide obesity epidemic, and in part to the identification of leptin as an adipokine secreted from AT. Since the discovery of leptin, many other adipokines, such as adiponectin, resistin, visfatin and omentin, have also been identified. These discoveries have led to the first revolution in the field of AT biology, the identification of AT as an endocrine organ. More recently, it has been discovered that not only adipocytes, but also immune cells, such as macrophages [1,2] and T lymphocytes [3–5], reside in AT, and that these cells may induce insulin resistance by promoting a proinflammatory milieu within the AT. This discovery has led to the second revolution in the field of AT biology, the identification of AT as an organ at the interface of inflammation and insulin resistance. In this review, we focus on what has been discovered in the past 5 years regarding macrophage infiltration into AT. In particular, we will discuss the continuum of AT physiology, from initiation and propagation of macrophage recruitment to AT, ultimately leading to the remodeling of AT.
Adipose tissue structure & function
Although adipocytes make up the bulk of AT mass, many other cell types are also found in AT. These include preadipocytes, vascular cells, such as endothelial cells (ECs), and immune cells. The major functions of AT include insulating and cushioning internal organs as well as storing excess energy in the form of triglycerides. This process is highly regulated, such that plasma nonesterified fatty acid levels are tightly controlled during fasting and feeding [6]. In addition to these functions, AT has an endocrine role, secreting many different adipokines and cytokines into the circulation that impact whole body physiology in significant ways.
History of macrophage infiltration into adipose tissue
The presence of macrophages in AT was first reported less than 8 years ago [7]. Subsequent studies have demonstrated that macrophage infiltration into white AT is increased in obesity [1,2]. Xu et al. demonstrated increased levels of macrophage markers in AT of leptin-deficient (Lepob/ob), leptin receptor-deficient (LepRdb/db), and diet-induced obese (DIO) mice compared with lean controls [1]. Ferrante and colleagues demonstrated a similar relationship between obesity and macrophage infiltration into AT in lethal yellow Agouti (Ay), Lepob/ob and DIO mice [2]. Using bone marrow transplantation, they demonstrated that the recruited AT macrophages (ATMs) were bone marrow derived [2]. These original discoveries have subsequently been confirmed and expanded on by other groups [8–18].
Consequences of macrophage recruitment into white adipose tissue
In the seminal papers by Xu [1] and Weisberg [2], important observations were made regarding the consequences of macrophage infiltration into AT. First, both groups showed that expression of inflammatory cytokines such as TNF-α and macrophage inflammatory protein (MIP)-1α in AT was almost entirely derived from macrophages rather than adipocytes [1,2]. Xu et al. demonstrated that macrophage infiltration into AT temporally preceded elevations in plasma insulin levels [1]. Based on this, they proposed that ATMs contribute to whole-body insulin resistance by inducing inflammation and insulin resistance starting locally within the AT. This hypothesis has gained momentum and been supported by additional studies in recent years. For example, mice with an absence of TNF-α expression from ATMs following bone marrow transplantation of TNF-α-deficient bone marrow, exhibited reduced epididymal fat pad weight and enhanced insulin sensitivity compared with control mice receiving TNF-α sufficient marrow [19]. Using in vitro studies, Suganami et al. demonstrated that macrophages and adipocytes interact in a paracrine manner, whereby TNF-α secretion from macrophages interferes with adipocyte insulin signaling and induces fatty acid lipolysis. These fatty acids then further exacerbate the inflammatory pheno-type of the macrophages, creating a vicious cycle of inflammation and insulin resistance [20]. Thus, it is widely believed that macrophage infiltration into AT leads to increased local inflammation, which in turn reduces insulin sensitivity both in the AT and systemically.
Initiation of macrophage recruitment
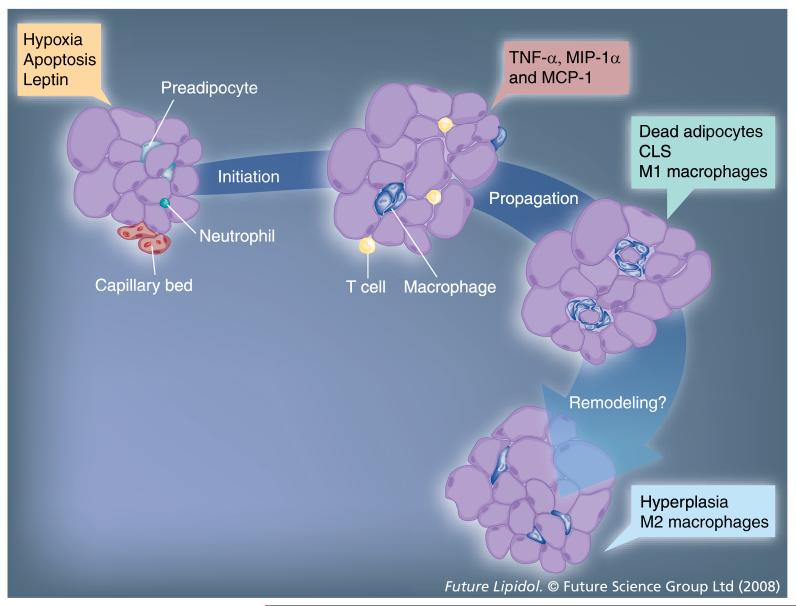
Both adipocyte hyperplasia and hypertrophy can contribute to AT expansion; however, in adults, hypertrophy appears to predominate. Some of the consequences of hypertrophy include fatty acid flux, vascularization, increased leptin secretion, hypoxia and adipocyte cell death. These adipocyte-related consequences of AT expansion are all possible contributors to the initiation of macrophage recruitment. All stages of macrophage recruitment to AT in obesity are depicted in (Figure 1).
Figure 1. Stages of macrophage infiltration into adipose tissue obesity.
During weight gain, macrophages are recruited to the expanding adipose tissue (AT) bed. Factors that initiate this process are thought to be derived from the adipocytes or capillary endothelial cells within AT and include adipocyte secretion of leptin, hypoxia, adipocyte cell death and initial infiltration of other immune cells, such as neutrophils and T cells. Adipose tissue macrophages (ATMs) are highly inflammatory, secreting cytokines such as TNF-α, and likely contribute to propagation of the recruitment of additional macrophages by secreting chemokines such as MCP-1 and MIP-1α. A dramatic increase in ATMs results in the formation of CLS that surround the dead adipocytes. These macrophages appear to contribute to remodeling of AT, during which fewer CLS are present and AT expansion may occur via hyperplasia more than hypertrophy. In addition, a repolarization from an M1 toward an M2 phenotype may occur.
CLS: Crown-like structures; MCP-1: Monocyte chemotactic protein-1; MIP-1α: Macrophage inflammatory protein 1α.
Fatty acid flux
Implicit when discussing weight gain and AT expansion is the idea of overnutrition. As previously mentioned, excess nutrients are ultimately stored as triglycerides in the AT; thus, fatty acid flux within AT may be an important regulator of inflammation and macrophage recruitment to AT. Furthermore, the fatty acid content of the diet may contribute to this effect, and many of the models used to induce ATM accumulation involve the use of diets rich in saturated fatty acids [1,2,13,21,22]. Toll-like receptor (TLR) 4 has recently been shown to be a receptor for saturated fatty acids and to mediate inflammatory cytokine production in macrophages exposed to fatty acids [23–25]. Furthermore, polyunsaturated fatty acids protect against saturated fatty acid-induced inflammatory cytokine production [23–25]. Suganami et al. have shown in vitro that macrophage TLR4 expression mediates their inflammatory responses to fatty acids released from adipocytes [26]. In addition, several groups have used TLR4-deficient mice to demonstrate in vivo that TLR4 plays a role in AT expansion and inflammation to varying degrees depending on the mouse strain, type of high-fat diet and length of feeding [25,27–30]. Our own data have extended these findings to demonstrate that deficiency of myeloid TLR4 can result in reduced macrophage infiltration and inflammation in AT under certain dietary conditions [Hasty et al., Unpublished Data]. In contrast to saturated fatty acids, polyunsaturated fatty acids, such as fish oils, have been shown to reduce macrophage infiltration into AT [31,32]. Thus, dietary fatty acid composition can potentially impact the initiation of macrophage recruitment to AT.
Endothelial cell-mediated adhesion
Infiltration of immune cells into tissues occurs by a process of adherence to blood vessel ECs, rolling along the EC layer and extravasation into the underlying tissue; thus, capillary ECs likely influence the transport of immune cells into AT. Adhesion molecules such as intercellular adhesion molecule (ICAM)-1 have been shown to be expressed in murine AT [33]. Furthermore, it has been shown in mice that within 3 weeks of high-fat feeding, AT expression of ICAM-1 was increased, and after 6 months of feeding, soluble ICAM-1 in plasma correlated with bodyweight and fat mass [34]. Most recently, Nishimura et al. used in vivo imaging to show that leukocyte—EC interactions are increased in the microcirculation of the subcutaneous AT of obese mice, and that administration of antibody to ICAM-1 normalized these interactions [35]. By contrast, it has also been shown that macrophage infiltration into AT is not altered in ICAM-1-deficient mice [33]. Despite these conflicting studies, the cumulative in vivo and in vitro evidence supports a role for capillary ECs in the initiation of macrophage recruitment to AT, although further studies are required to define the role of ECs and adhesion molecules more clearly.
Leptin
Although leptin deficiency causes morbid obesity in mice and humans, what is more commonly found in obesity is an increase in plasma leptin levels, indicating the presence of central leptin resistance. The impact of hyperleptinemia on peripheral cells and tissues is not completely understood; however, there is some evidence to suggest that leptin itself may initiate the recruitment of macrophages to AT. For example, Curat et al. demonstrated that leptin can increase adhesion molecule expression in ECs [36]. Leptin has been previously shown to be a chemoattractant for neutrophils, smooth muscle cells, ECs and cancer cells [37–41], and we recently demonstrated that leptin can act as a potent chemoattractant for monocytes and macrophages in vitro [42]. In vivo support of this concept derives from the papers of Xu and Weisberg showing that while Lepob/ob and LepRdb/db mice were obese and had increased macrophage infiltration into AT compared with lean mice [1,2], the degree of macrophage infiltration was less than what would be expected for their bodyweight. In fact, Xu and colleagues suggested a possible role for leptin in mediating macrophage recruitment to AT based upon this observation [1]. Thus, although further in vivo evidence is warranted, leptin may contribute to the initiation of macrophage recruitment to white AT.
Hypoxia
At least three different obese mouse models (DIO, KKAy and Lepob/ob) have been used to demonstrate that hypoxia occurs in obese AT [43,44]. Decreased vascular density, which has been observed in obese mice [17,45], may contribute to this. Furthermore, protein and mRNA levels of hypoxia-inducible factor (HIF)-1α are elevated in AT from obese mice, as are mRNA levels for other hypoxia inducible genes [43,44]. Ye et al. have demonstrated in vitro that hypoxia may contribute to AT inflammation by showing that exposure of primary adipocytes and macrophages to hypoxia increases their expression of multiple inflammatory genes [44].
Recently, Wang, Wood and Trayhurn investigated the effect of hypoxia on human adipocytes and preadipocytes [46,47]. Like murine adipocytes, human adipocytes also have increased expression of HIF-1α and other hypoxia-related genes when exposed to low oxygen levels (1%) [47]. Human preadipocytes exposed to hypoxia had elevated HIF-1α mRNA, increased secretion of vascular endothelial growth factor, and although leptin expression and secretion was nearly absent in preadipocytes under normal oxygen conditions, hypoxia stimulated their expression and secretion of leptin [46]. Therefore, hypoxia is potentially involved in several of the initiating factors of macrophage recruitment including leptin secretion and adipocyte death (discussed below), as well as an initial upregulation of inflammatory genes.
Adipocyte death
A prominent hypothesis regarding the reasons for which macrophages enter the AT is that they are recruited to phagocytose dead or dying adipocytes present in the expanding AT depot. Whereas adipocyte death is rare in lean humans and mice, it is a common hallmark of obesity and is positively correlated with adipocyte hypertrophy [48–50]. In fact, hormone-sensitive lipase-deficient mice, which have adipocyte hypertrophy but are not obese, also exhibit increased adipocyte death [51]. Within AT, crown-like structures (CLS), composed mostly of macrophages [48], surround dead adipocytes, identified by the absence of perilipin staining [48–50]. CLS stain positive for F4/80, MAC-2, TNF-α and IL-6, indicative of their macrophage content and inflammatory properties [48–50]. Both adipocyte death and secretion of inflammatory cytokines are increased in visceral and epididymal fat depots relative to inguinal (subcutaneous) fat depots [49,50], and these two fat pads also have more ATM accumulation than subcutaneous AT [9,52,53]. Thus, it is likely that adipocyte cell death, which may be caused by the hypoxic conditions that occur during rapid AT expansion (reviewed in [54]), is a signal that attracts phagocytic macrophages.
Neutrophils
Since the recruitment of neutrophils generally occurs prior to the recruitment of macrophages during inflammatory responses, Elgazar-Carmon and colleagues evaluated AT for the presence of neutrophils at early time points following high-fat diet feeding in mice [55]. They found that neutrophils transiently infiltrated abdominal AT. In human studies, Leik et al. demonstrated that hypertensive pregnant women have an increase in neutrophils adhered to endothelium in their AT compared with control patients [56]. Thus, there is some evidence that neutrophils can also infiltrate AT and may be one of the initiating events in the recruitment of macrophages.
T lymphocytes
It was recently shown that the number of CD3+ T cells is increased in AT in obesity [3–5]. Wu et al. demonstrated that CD3+ T cells infiltrate white AT of DIO mice [5]. Based on the upregulation of the chemokine RANTES and its receptor, CCR5, in the AT of obese mice as well as the results of in vitro migration experiments, they suggested that adipocyte secretion of RANTES may contribute to the recruitment of T cells to the AT [5]. Obese humans also have increased gene expression of RANTES and CCR5 in subcutaneous and visceral AT, and this expression is positively correlated with CD3 and CD11b, a macrophage marker [5].
To understand the function of T cells in AT some investigators have further characterized them as either CD4+ or CD8+. Rausch et al. found a three- to four-fold increase in cytotoxic CD3+/CD8+/CD4- T lymphocytes in both DIO and Lepob/ob mice [4]. However, in patients with Type 2 diabetes, Kintscher et al. detected only moderate expression of CD8 in AT, but demonstrated the presence of CD4+ T cells, which positively correlated with bodyweight [3]. Furthermore, they found that the majority of the macrophages were human leukocyte antigen-DR-positive, suggesting that they may have been activated by IFN-γ, a cytokine released by CD4+ helper T cells [3].
How T lymphocytes contribute to the sequence of changes in AT that occur as it expands is still in question. As mentioned above, macrophage infiltration of the AT has been shown to temporally precede systemic insulin resistance in DIO mice [1]. In a different study, T-cell accumulation in AT, as well as impaired glucose tolerance and insulin sensitivity, preceded macrophage accumulation in AT in DIO mice [3]. Thus, the sequence in which immune cells infiltrate AT, and whether they are a cause or consequence of insulin resistance is not yet clearly defined. Fully understanding the contribution of T cells to the inflammatory nature of obese AT remains an intriguing challenge for continued study.
Propagation of macrophage recruitment
While capillary ECs and adipocytes contribute to the initiation of macrophage recruitment, ATMs themselves likely contribute to propagation of the signals that promote further attraction of new macrophages. In support of this notion, we have recently utilized the Ay mouse model and shown that ATM content was highly correlated with AT mass when total fat mass ranged from 2 to 15 g [21]. When total fat mass was greater than 15 g, the expression level of macrophage marker F4/80 spanned a fourfold range but was no longer positively correlated with total fat mass, indicating that something other than fat mass must contribute to continued recruitment of macrophages to AT. Therefore, it is possible that factors secreted by macrophages, rather than adipocytes, may have a more important role in the propagation of ATM accumulation. Owing to the relative abundance of studies on the roles of chemokines in macrophage infiltration, this review focuses on their contribution; however, the literature indicates that other molecules are also important in this process.
Chemokines
Chemokines are small, 8–10 kDa, chemotactic cytokines that are well established to play a role in macrophage mobilization out of bone marrow and into many different tissues during the inflammatory process. Although they can be secreted by adipocytes, studies in which adipocytes were separated from stromal vascular fractions have demonstrated that the majority of chemokine secretion in AT is from the stromal vascular population [1,9]. Thus, expression of chemokines from ATMs likely contributes to propagation of macrophage accumulation in the AT.
Mouse studies
Numerous chemokines exhibit elevated mRNA and protein levels in the AT and plasma of genetically or diet-induced obese mice. Expression of the macrophage chemokines monocyte chemotactic protein (MCP)-1 and MIP-1α have been shown to be increased in the white AT of LepRdb/db, Lepob/ob and DIO mice [1,10,13,57–60]. Other chemokines upregulated in AT from DIO and/or Lepob/ob mice are MCP-2 [12], MCP-3 [12], RANTES [5] and CXCL14 [60]. Receptors for these chemokines, such as CCR2, CCR3 and CCR5, are also elevated in the white AT of DIO mice [5,12]. Furthermore, Chen et al. have performed microarray analyses of epididymal and inguinal white AT from DIO mice, and have shown altered gene expression of 60 inflammatory genes, which included MCP-1 and MCP-3 as the two inflammatory genes that were most highly upregulated [10].
To date, MCP-1 and its receptor CCR2 are the best studied of the chemokine ligands and receptors for their role in macrophage accumulation in white AT and obesity-induced insulin resistance. Yu et al. demonstrated that MCP-1 was upregulated at the mRNA and protein levels in the mesenteric, epididymal, subcutaneous and perirenal white AT of DIO mice, with the highest upregulation being in the mesenteric fat [59]. They likewise observed increased gene expression of the macrophage markers F4/80 and CD68 in AT beds [59]. In addition, they performed in vitro migration experiments showing increased macrophage migration to mesenteric AT-conditioned media, which was blocked in response to an antibody for MCP-1 [59].
Two different groups have engineered transgenic mice using the aP2 promoter to over-express MCP-1 in AT [13,58]. Both groups found increased numbers of macrophages within the AT of the MCP-1-overexpressing mice, and these mice were insulin resistant and glucose intolerant, suggesting that MCP-1 may promote macrophage infiltration and insulin resistance during obesity. On the other hand, experiments with MCP-1-deficient (-/-) mice have yielded inconsistent results. Kanda et al. found that DIO MCP-1-/- mice had fewer ATMs and were more insulin sensitive and glucose tolerant, although they demonstrated no differences in bodyweight relative to wild-type controls [13]. By contrast, Inouye et al. observed significant increases in bodyweight in the DIO MCP-1-/- mice and possibly a slight increase in macrophage accumulation in AT combined with decreased insulin sensitivity [57]. Kirk et al. found similar results to Inouye et al. except that they observed no differences in insulin or glucose tolerance [61]. The role of MCP-1 has also been examined using a dominant-negative approach, where improved insulin sensitivity and glucose tolerance were found in both LepRdb/db and DIO mice [13].
Similar to MCP-1 studies, experiments with CCR2-/- mice resulted in opposing conclusions regarding the importance of CCR2 in macrophage infiltration of AT. Weisberg et al. observed decreased macrophage accumulation in the epididymal, subcutaneous and perirenal AT of CCR2-/- mice on a high-fat diet, decreased expression of TNF-α in epididymal AT, as well as improved insulin sensitivity and glucose tolerance relative to CCR2+/+ controls [12]. When they treated wild-type DIO mice with the CCR2 antagonist INCB3344 for 2 weeks, they noted improved insulin sensitivity and a small but significant decrease in immunohistochemical staining for F4/80 in the AT [12]. Chen et al. did not observe any differences in the amount of ATMs or in plasma insulin or glucose levels in CCR2-/-mice [10]; however, the mice from this study were on a DBA1/J background, which may make them less susceptible to diet-induced obesity, in contrast to the mice in Weisberg’s studies, which were on a C57BL/6J background.
A chemokine outside of the CC subfamily, CXCL14, is also upregulated in white and brown AT in genetic and diet-induced obesity [60]. DIO mice deficient in CXCL14 had fewer ATMs, were more insulin sensitive and had a faster glucose disposal rate [60]. This supports CXCL14 having a role in macrophage accumulation and insulin resistance in diet-induced obesity. In conclusion, it is clear that obese mice have elevated chemokines in their AT and that many of these chemokines are secreted by macrophages. However, there appears to be a significant amount of redundancy in their functions, such that alteration of only one chemokine or chemokine receptor at a time may have only minor effects on ATM accumulation. More work needs to be done to clarify which chemokines are important in the recruitment of macrophages and the mechanisms by which they induce insulin resistance.
Human studies
Multiple human studies have demonstrated an elevation in gene expression of various chemokines in AT in obesity, including MCP-1, MIP-1α, MIP-1β, MCP-2, MCP-4, MIP-2α and pulmonary and activation-regulated chemokine [15,62,63]. The expression of MCP-1 from the stromal vascular fraction of fat has been shown to be greater than its expression from the adipocyte fraction, and MCP-1 expression is positively correlated with the expression of macro-phage/monocyte markers CD68 and CD14 [9], providing evidence that macrophages are the main source of this chemokine in human AT. Westerbacka et al. have demonstrated that insulin-resistant humans have increased expression of macrophage markers in AT in comparison with insulin-sensitive individuals, and that AT expression of MCP-1 and MIP-1α is elevated during hyperinsulinemic euglycemic clamps in people with insulin resistance [63]. Furthermore, a significant positive correlation has been observed between gene expression of the chemokines MCP-1, MIP-1α and MCP-2 and fasting serum insulin as well as whole-body glucose disposal rate [62]. Finally, a recent study by Stulnig and colleagues demonstrated that expression of six different chemokines and their receptors was higher in obese compared with lean subjects, and the expression of CCL3 (MIP1-α) and CCL5 (RANTES) positively correlated with fasting plasma insulin levels independent of waist circumference [64]. Thus, in agreement with murine studies, analysis of human AT has demonstrated a potential role for macrophage-derived chemokines in the propagation of macrophage recruitment and in insulin resistance.
Adipose tissue remodeling
Although much attention has been given to the inflammatory nature of AT in obesity, some evidence indicates that the number of ATMs can decrease in association with AT remodeling and repair [50]. A similar phenomenon has been observed in renal inflammation and in athero-sclerotic lesions, where macrophages have a role in both inflammation and tissue repair [65–67]. The capability of macrophages to secrete both pro- and anti-inflammatory cytokines contributes to their dual role, and, in fact, ingestion of apoptotic cells has been shown to reprogram macrophages to become anti-inflammatory [68].
Alternatively & classically activated macrophages
Like T cells, macrophages are now understood to be a heterogeneous population of cells that can be polarized toward an M1 ‘classically activated’ or an M2 ‘alternatively activated’ phenotype [69,70]. The M1 macrophage phenotype is induced by inflammatory stimuli and results in secretion of pro-inflammatory cytokines, whereas the M2 phenotype is induced by exposure to IL-4 and IL-13 and results in the secretion of anti-inflammatory cytokines. One hallmark feature of M2 macrophages is the production of arginase, which blocks inducible nitric oxide synthase (iNOS) activity and is thought to allow M2 macrophages to contribute to tissue repair [71]. Interestingly, M1 and M2 macrophages are also present in AT. Recent publications by Saltiel and colleagues [22,72] have demonstrated that ATMs in lean mice express arginase-1 and IL-10, indicating that they are M2 or alternatively activated. Upon high-fat feeding, expression of these genes was decreased, while expression of TNF-α and iNOS was increased, indicating that the ATMs were of the M1, or classically activated, phenotype [22]. Furthermore, using a pulse-label technique, these authors elegantly showed that the macrophages recruited to AT during high-fat feeding highly express IL-6, iNOS and CCR2, indicating that they had characteristics of migratory phagocytic cells [72]. In addition to providing evidence for the recruitment of M1 macrophages to AT, this observation provides support for the concept that macrophages are recruited to AT to phagocytose dead adipocytes.
Subsequent to these studies, Chawla and colleagues demonstrated that macrophage deficiency of PPAR-γ results in an inability to develop an alternatively activated M2 phenotype [73]. Furthermore, mice with macrophage PPARγ deficiency showed a predisposition to diet-induced weight gain, glucose intolerance and insulin resistance. Interestingly, despite the increased AT mass, total ATMs were reduced in the PPARγ-deficient mice, and this appeared to be due to a reduction in M2 macrophages. Further evidence for a role of PPARγ in macrophage activation stems from studies by Stienstra et al. demonstrating that treatment of high-fat fed mice with the PPARγ agonist rosiglitazone led to a repolarization of AT macrophages to an M2 phenotype [74]. Kang et al. demonstrated that myeloid deficiency of PPAR-δ results in a loss of M2 macrophages leading to proinflammatory cytokine production from adipocytes, increased weight gain and increased systemic insulin resistance [75]. Together, these studies indicate that M1 macrophages promote inflammation and insulin resistance in AT, while M2 macrophages are required to protect against these physiological consequences of the increasing AT mass. Thus, the repertoire of M1 and M2 macrophages appears to control the inflammatory status of AT and may contribute to tissue repair and remodeling of AT during its expansion.
As is commonly observed in biology, human macrophage populations cannot always be classified simply as ‘M1’ or ‘M2’. Zeyda et al. and Bourlier et al. have recently demonstrated that human ATMs have both M1 and M2 characteristics, as evidenced by their secretion of both pro- and anti-inflammatory cytokines [76,77]. Thus, although human macrophages may undergo phenotypic changes similar to murine macrophages, it will be critical to characterize ATMs carefully to determine their inflammatory properties.
Cell death & hyperplasia
Strissel et al. have examined the progression of cell death in the AT of DIO mice and found that after 16 weeks on a high-fat diet approximately 80% of the adipocytes were dead (perilipin-negative and surrounded by CLS) in the epididymal AT [50]. An intriguing aspect to this finding was that after 20 weeks on high-fat diet, the percentage of dead cells was reduced to approximately 16%, similar to the level observed after 12 weeks on high-fat diet. Collagen deposition was also greater at week 16 than at week 20, and although the number of adipocytes increased from week 16 to 20, the cell size distribution changed to include a majority of small adipocytes (<5000 μm2) by week 20. The authors suggested that AT remodeling had occurred and, based on the differences in distribution of adipocyte size, that after the peak of dead adipocytes at week 16, the AT transitions from expanding through mostly hypertrophy to mostly hyperplasia [50]. Perhaps the dramatic increase in cell death and subsequent ingestion of dead adipocytes by macrophages is partially responsible for the trend towards decreased inflammation that Strissel et al. observed at week 20, since ingestion of dead cells can induce an M2 phenotype in macrophages. An important note regarding this study is that Strissel et al. found a negative correlation between epididymal fat mass and hepatic mass after the AT was remodeled [50], suggesting that the apparently beneficial changes within the AT may be associated with ectopic lipid storage.
Adipose tissue vascularization
As mentioned earlier, reduced vascular density has been observed in the AT of obese mice [17,45]. Pang et al. demonstrated that ATMs significantly contribute to the elevated expression of the angiogenic factor platelet-derived growth factor in obese Lepob/ob mice, however in lean mice most of this expression comes from preadipocytes [45]. Therefore, the increased presence of macrophages in obese AT may assist in AT remodeling by stimulating angiogenesis [45], a suggestion which Bourlier et al. has further supported with in vitro evidence [77]. It will be informative to find out whether macrophages have an important in vivo role in the vascularization of AT in obese humans.
Matrix metalloproteinases & tissue inhibitors of metalloproteinases
As in other tissues, AT remodeling likely involves the matrix metalloproteinase system. Expression studies in AT from DIO, Lepob/ob and LepRdb/db mice demonstrated an increase in mRNA levels of matrix metalloproteinases (MMPs), such as MMP-2, -3, -11, -12, -14 and -19 in obese compared with lean animals [78,79]. By contrast, MMPs such as -7 and -9 were downregulated [78,79]. Furthermore, tissue inhibitors of MMPs (TIMPS) are also differentially regulated in AT of obese mouse models [78,79]. A more direct role for MMPs and TIMPs has been shown in gene knockout studies. For example, MMP-2-/- mice gained less weight and had smaller fat pads when placed on a high-fat diet compared with MMP-2+/+ controls [80]. TIMP-1-/- mice also had a similar phenotype [81]. Finally, studies utilizing inhibitors of MMPs have shown a reduction in AT mass in mice that have reduced MMP activity [82,83]. Interestingly, dietary fish oils have been shown to inhibit the increase in MMPs noted in high-fat fed mice [84]. These data indicate that the MMP/TIMP system may be activated in obesity to allow for adipocyte hypertrophy, AT expansion and remodeling.
Human studies also point to a potential role of MMPs in obesity as evidenced by an increase in plasma levels of MMP-2 and MMP-9 in obese compared with lean individuals [85]. Furthermore, TIMP-1 protein levels were found to be significantly higher in obese individuals, and plasma TIMP-1 levels correlated positively with BMI, plasma free fatty acids, cholesterol, leptin and IL-6 levels [86].
Conclusion
There are many different molecules and processes that have been shown to play a role in macrophage recruitment to AT. In this review, we have broken macrophage infiltration into three primary stages (Figure 1):
Initiation
Propagation
Remodeling
However, this process most certainly occurs on a continuum, such that certain characteristics of the macrophages and adipocytes are similar in all three stages. In addition, there appears to be a great degree of compensation among the factors responsible for attracting macrophages to the AT, such that removal of one player generally has only minimal effects on the cumulative accrual of macrophages. Although knowledge in the field continues to expand, much work is needed to identify the role of each key contributor.
Future perspective
Despite the advances that have been made in the field regarding initiation and propagation of macrophage recruitment to AT, there are possible factors that remain to be analyzed. For example, it has not been proven in vivo, whether fatty acid flux, leptin or hypoxia play a role in initiating macrophage recruitment to AT. Furthermore, it is not known whether macrophage-derived chemokines other than MCP-1 might be involved in propagating the signals that stimulate the influx of additional macrophages. Finally, the area of AT remodeling is very new, and future studies will certainly highlight the role of the remodeling process in AT physiology.
In addition to understanding the mechanisms by which macrophages are recruited to AT, it is imperative that we understand the physiological relevance of the paracrine loop between macrophages and adipocytes. Furthermore, our knowledge of whether the macrophages contribute to the pathophysiological consequences of obesity is very rudimentary and must be expanded. Finally, although it has clearly been shown that inflammatory macrophages infiltrate AT in humans, the model we propose is based primarily on studies performed in mice. Thus, the continued translation of this research from rodents into humans is necessary to turn this basic biological knowledge into useful therapeutic options for inflammation and insulin resistance associated with obesity.
Executive summary.
Initiation of macrophage recruitment to adipose tissue
Uncontrolled fatty acid flux may contribute to macrophage recruitment by increasing the proinflammatory status of the adipose tissue (AT).
Immune cells must enter tissue via blood vessels, and changes in endothelial cells may increase macrophage adhesion and migration into AT.
Leptin secretion from AT is increased in obesity and could contribute to macrophage recruitment by increasing adhesion molecule expression on endothelial cells or by acting as a chemoattractant.
Rapid expansion of AT mass could lead to hypoxia, which could ultimately promote adipocyte cell death and the infiltration of macrophages to phagocytose the debris.
Other immune cells, such as neutrophils and T cells, may enter the AT first and contribute to macrophage recruitment.
Propagation of macrophage recruitment to adipose tissue
After a certain degree of AT expansion, AT macrophage (ATM) content no longer correlates with bodyweight or AT mass. Thus, chemokine secretion from ATMs themselves likely contributes to propagation of macrophage recruitment to AT.
Remodeling of adipose tissue
M1 ‘classically activated’ and M2 ‘alternatively activated’ macrophages are both present in AT and help control inflammatory status and remodeling as AT expands.
Phagocytosis of dead adipocytes may promote ATM polarization toward an M2 phenotype.
Adipose tissue vascularization and the matrix metalloproteinase/tissue inhibitor of matrix metalloproteinase system probably contribute to AT remodeling.
Acknowledgement
We would like to thank the members of our laboratory for their helpful comments and suggestions on this article.
Footnotes
Financial & competing interests disclosure
Alyssa H Hasty is supported by a Career Development Award from the American Diabetes Association (1-07-CD-10) and by NIH grant HL089466. Bonnie K Surmi is supported by a predoctoral fellowship from the American Heart Association (0815231E). The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Contributor Information
Bonnie K Surmi, Vanderbilt University Medical Center, Department of Molecular Physiology & Biophysics, Nashville, TN 37232, USA Tel.: +1 615 322 5972; Fax: +1 615 322 8973; bonnie.k.wasson@vanderbilt.edu.
Alyssa H Hasty, Vanderbilt University Medical Center, 702 Light Hall, Nashville, TN 37232-0615, USA Tel.: +1 615 322 5177; Fax: +1 615 322 8973; alyssa.hasty@vanderbilt.edu.
Bibliography
Papers of special note have been highlighted as either of interest (•) or of considerable interest (••) to readers.
- 1.Xu H, Barnes GT, Yang Q, et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J. Clin. Invest. 2003;112:1821–1830. doi: 10.1172/JCI19451.•• Describes the observation that macrophages infiltrate adipose tissue in obesity. In addition, these authors demonstrate that the presence of adipose tissue macrophages temporally precedes systemic hyperinsulinemia.
- 2.Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW., Jr Obesity is associated with macrophage accumulation in adipose tissue. J. Clin. Invest. 2003;112:1796–1808. doi: 10.1172/JCI19246.•• Demonstrated increased macrophage infiltration into white adipose tissue in obese mice and humans. The authors demonstrate that the adipose tissue macrophages are responsible for the majority of inflammatory cytokine production and that these cells are derived from the bone marrow.
- 3.Kintscher U, Hartge M, Hess K, et al. T-lymphocyte infiltration in visceral adipose tissue. A primary event in adipose tissue inflammation and the development of obesity-mediated insulin resistance Arterioscler. Thromb. Vasc. Biol DOI: 10.1161/ATVBAHA.108.165100 2008. (Epub ahead of print). [DOI] [PubMed] [Google Scholar]
- 4.Rausch ME, Weisberg S, Vardhana P, Tortoriello DV. Obesity in C57BL/6J mice is characterized by adipose tissue hypoxia and cytotoxic T-cell infiltration. Int. J. Obes. (Lond.) 2008;32:451–463. doi: 10.1038/sj.ijo.0803744. [DOI] [PubMed] [Google Scholar]
- 5.Wu H, Ghosh S, Perrard XD, et al. T-cell accumulation and regulated on activation, normal T cell expressed and secreted upregulation in adipose tissue in obesity. Circulation. 2007;115:1029–1038. doi: 10.1161/CIRCULATIONAHA.106.638379. [DOI] [PubMed] [Google Scholar]
- 6.Zechner R, Strauss JG, Haemmerle G, Lass A, Zimmermann R. Lipolysis: pathway under construction. Curr. Opin. Lipidol. 2005;16:333–340. doi: 10.1097/01.mol.0000169354.20395.1c. [DOI] [PubMed] [Google Scholar]
- 7.Bornstein SR, Abu-Asab M, Glasow A, et al. Immunohistochemical and ultrastructural localization of leptin and leptin receptor in human white adipose tissue and differentiating human adipose cells in primary culture. Diabetes. 2000;49:532–538. doi: 10.2337/diabetes.49.4.532. [DOI] [PubMed] [Google Scholar]
- 8.Christiansen T, Richelsen B, Bruun JM. Monocyte chemoattractant protein-1 is produced in isolated adipocytes, associated with adiposity and reduced after weight loss in morbid obese subjects. Int. J. Obes. (Lond.) 2003;29:146–450. doi: 10.1038/sj.ijo.0802839. [DOI] [PubMed] [Google Scholar]
- 9.Bruun JM, Lihn AS, Pedersen SB, Richelsen B. Monocyte chemoattractant protein-1 release is higher in visceral than subcutaneous human adipose tissue (AT): implication of macrophages resident in the AT. J. Clin. Endocrinol. Metab. 2005;90:2282–2289. doi: 10.1210/jc.2004-1696. [DOI] [PubMed] [Google Scholar]
- 10.Chen A, Mumick S, Zhang C, et al. Diet induction of monocyte chemoattractant protein-1 and its impact on obesity. Obes. Res. 2005;13:1311–1320. doi: 10.1038/oby.2005.159. [DOI] [PubMed] [Google Scholar]
- 11.Cinti S, Mitchell G, Barbatelli G, et al. Adipocyte death defines macrophage localization and function in adipose tissue of obese mice and humans. J. Lipid Res. 2005;46(11):2347–2355. doi: 10.1194/jlr.M500294-JLR200.• First to demonstrate that macrophage crown-like structures surround dead adipocytes in white adipose tissue in obesity.
- 12.Weisberg SP, Hunter D, Huber R, et al. CCR2 modulates inflammatory and metabolic effects of high-fat feeding. J. Clin. Invest. 2006;116:115–124. doi: 10.1172/JCI24335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kanda H, Tateya S, Tamori Y, et al. MCP-1 contributes to macrophage infiltration into adipose tissue, insulin resistance, and hepatic steatosis in obesity. J. Clin. Invest. 2006;116:1494–1505. doi: 10.1172/JCI26498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cancello R, Tordjman J, Poitou C, et al. Increased infiltration of macrophages in omental adipose tissue is associated with marked hepatic lesions in morbid human obesity. Diabetes. 2006;55:1554–1561. doi: 10.2337/db06-0133. [DOI] [PubMed] [Google Scholar]
- 15.Dahlman I, Kaaman M, Olsson T, et al. A unique role of monocyte chemoattractant protein 1 among chemokines in adipose tissue of obese subjects. J. Clin. Endocrinol. Metab. 2005;90:5834–5840. doi: 10.1210/jc.2005-0369. [DOI] [PubMed] [Google Scholar]
- 16.Lee YH, Nair S, Rousseau E, et al. Microarray profiling of isolated abdominal subcutaneous adipocytes from obese vs non-obese Pima Indians: increased expression of inflammation-related genes. Diabetologia. 2005;48:1776–1783. doi: 10.1007/s00125-005-1867-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Voros G, Maquoi E, Demeulemeester D, Clerx N, Collen D, Lijnen HR. Modulation of angiogenesis during adipose tissue development in murine models of obesity. Endocrinology. 2005;146:4545–4554. doi: 10.1210/en.2005-0532. [DOI] [PubMed] [Google Scholar]
- 18.Aprovian CM, Bigomia S, Mott M, et al. Adipose macrophage infiltration is associated with insulin resistance and vascular endothelial dysfunction in obese subjects Arterioscler. Thromb. Vasc. Biol DOI: 10.1161/ATVBAHA.108.170316 2008. (Epub ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.De Taeye BM, Novitskaya T, McGuinness OP, et al. Macrophage TNF-α contributes to insulin resistance and hepatic steatosis in diet-induced obesity. Am. J. Physiol. Endocrinol. Metab. 2007;293:E713–E725. doi: 10.1152/ajpendo.00194.2007. [DOI] [PubMed] [Google Scholar]
- 20.Suganami T, Nishida J, Ogawa Y. A paracrine loop between adipocytes and macrophages aggravates inflammatory changes. Role of free fatty acids and tumor necrosis factor α. Arterioscler. Thromb. Vasc. Biol. 2005;25(10):2062–2068. doi: 10.1161/01.ATV.0000183883.72263.13. [DOI] [PubMed] [Google Scholar]
- 21.Coenen KR, Gruen ML, Chait A, Hasty AH. Diet-induced increases in adiposity, but not plasma lipids, promote macrophage infiltration into white adipose tissue. Diabetes. 2007;56:564–573. doi: 10.2337/db06-1375. [DOI] [PubMed] [Google Scholar]
- 22.Lumeng CN, Bodzin JL, Saltiel AR. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J. Clin. Invest. 2007;117:175–184. doi: 10.1172/JCI29881.•• Demonstrates for the first time that M1 classically activated and M2 alternatively activated macrophages exist in adipose tissue.
- 23.Lee JY, Sohn KH, Rhee SH, Hwang D. Saturated fatty acids, but not unsaturated fatty acids, induce the expression of cyclooxygenase-2 mediated through Toll-like receptor 4. J. Biol. Chem. 2001;276:16683–16689. doi: 10.1074/jbc.M011695200. [DOI] [PubMed] [Google Scholar]
- 24.Lee JY, Plakidas A, Lee WH, et al. Differential modulation of Toll-like receptors by fatty acids: preferential inhibition by n-3 polyunsaturated fatty acids. J. Lipid Res. 2003;44:479–486. doi: 10.1194/jlr.M200361-JLR200. [DOI] [PubMed] [Google Scholar]
- 25.Shi H, Kokoeva MV, Inouye K, Tzameli I, Yin H, Flier JS. TLR4 links innate immunity and fatty acid-induced insulin resistance. J. Clin. Invest. 2006;116(11):3015–3025. doi: 10.1172/JCI28898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Suganami T, Tanimoto-Koyama K, Nishida J, et al. Role of the Toll-like receptor 4/NF-κB pathway in saturated fatty acid-induced inflammatory changes in the interaction between adipocytes and macrophages. Arterioscler. Thromb. Vasc. Biol. 2007;27:84–91. doi: 10.1161/01.ATV.0000251608.09329.9a. [DOI] [PubMed] [Google Scholar]
- 27.Suganami T, Mieda T, Itoh M, Shimoda Y, Kamei Y, Ogawa Y. Attenuation of obesity-induced adipose tissue inflammation in C3H/HeJ mice carrying a Toll-like receptor 4 mutation. Biochem. Biophys. Res. Commun. 2007;354:45–49. doi: 10.1016/j.bbrc.2006.12.190. [DOI] [PubMed] [Google Scholar]
- 28.Poggi M, Bastelica D, Gual P, et al. C3H/HeJ mice carrying a toll-like receptor 4 mutation are protected against the development of insulin resistance in white adipose tissue in response to a high-fat diet. Diabetologia. 2007;50:1267–1276. doi: 10.1007/s00125-007-0654-8. [DOI] [PubMed] [Google Scholar]
- 29.Tsukumo DM, Carvalho-Filho MA, Carvalheira JB, et al. Loss-of-function mutation in Toll-like receptor 4 prevents diet-induced obesity and insulin resistance. Diabetes. 2007;56:1986–1998. doi: 10.2337/db06-1595. [DOI] [PubMed] [Google Scholar]
- 30.Davis JE, Gabler NK, Walker-Daniels J, Spurlock ME. Tlr-4 deficiency selectively protects against obesity induced by diets high in saturated fat. Obesity (Silver Spring) 2008;16:1248–1255. doi: 10.1038/oby.2008.210. [DOI] [PubMed] [Google Scholar]
- 31.Todoric J, Loffler M, Huber J, et al. Adipose tissue inflammation induced by high-fat diet in obese diabetic mice is prevented by n-3 polyunsaturated fatty acids. Diabetologia. 2006;49:2109–2119. doi: 10.1007/s00125-006-0300-x. [DOI] [PubMed] [Google Scholar]
- 32.Saraswathi V, Gao L, Morrow JD, Chait A, Niswender KD, Hasty AH. Fish oil increases cholesterol storage in white adipose tissue with concomitant decreases in inflammation, hepatic steatosis, and atherosclerosis in mice. J. Nutr. 2007;137:1776–1782. doi: 10.1093/jn/137.7.1776. [DOI] [PubMed] [Google Scholar]
- 33.Robker RL, Collins RG, Beaudet AL, Mersmann HJ, Smith CW. Leukocyte migration in adipose tissue of mice null for ICAM-1 and Mac-1 adhesion receptors. Obes. Res. 2004;12:936–940. doi: 10.1038/oby.2004.114. [DOI] [PubMed] [Google Scholar]
- 34.Brake DK, Smith EO, Mersmann H, Smith CW, Robker RL. ICAM-1 expression in adipose tissue: effects of diet-induced obesity in mice. Am. J. Physiol. Cell Physiol. 2006;291:C1232–C1239. doi: 10.1152/ajpcell.00008.2006. [DOI] [PubMed] [Google Scholar]
- 35.Nishimura S, Manabe I, Nagasaki M, et al. In vivo imaging in mice reveals local cell dynamics and inflammation in obese adipose tissue. J. Clin. Invest. 2008;118:710–721. doi: 10.1172/JCI33328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Curat CA, Miranville A, Sengenes C, et al. From blood monocytes to adipose tissue-resident macrophages: induction of diapedesis by human mature adipocytes. Diabetes. 2004;53:1285–1292. doi: 10.2337/diabetes.53.5.1285. [DOI] [PubMed] [Google Scholar]
- 37.Frankenberry KA, Somasundar P, McFadden DW, Vona-Davis LC. Leptin induces cell migration and the expression of growth factors in human prostate cancer cells. Am. J. Surg. 2004;188:560–565. doi: 10.1016/j.amjsurg.2004.07.031. [DOI] [PubMed] [Google Scholar]
- 38.Goetze S, Bungenstock A, Czupalla C, et al. Leptin induces endothelial cell migration through Akt, which is inhibited by PPARγ-ligands. Hypertension. 2002;40:748–754. doi: 10.1161/01.hyp.0000035522.63647.d3. [DOI] [PubMed] [Google Scholar]
- 39.Oda A, Taniguchi T, Yokoyama M. Leptin stimulates rat aortic smooth muscle cell proliferation and migration. Kobe J. Med. Sci. 2001;47:141–150. [PubMed] [Google Scholar]
- 40.Ottonello L, Gnerre P, Bertolotto M, et al. Leptin as a uremic toxin interferes with neutrophil chemotaxis. J. Am. Soc. Nephrol. 2004;15:2366–2372. doi: 10.1097/01.ASN.0000139321.98029.40. [DOI] [PubMed] [Google Scholar]
- 41.Wolk R, Deb A, Caplice NM, Somers VK. Leptin receptor and functional effects of leptin in human endothelial progenitor cells. Atherosclerosis. 2005;183:131–139. doi: 10.1016/j.atherosclerosis.2005.03.048. [DOI] [PubMed] [Google Scholar]
- 42.Gruen ML, Hao M, Piston DW, Hasty AH. Leptin requires canonical migratory signaling pathways for induction of monocyte and macrophage chemotaxis. Am. J. Physiol. Cell Physiol. 2007;293:C1481–C1488. doi: 10.1152/ajpcell.00062.2007. [DOI] [PubMed] [Google Scholar]
- 43.Hosogai N, Fukuhara A, Oshima K, et al. Adipose tissue hypoxia in obesity and its impact on adipocytokine dysregulation. Diabetes. 2007;56:901–911. doi: 10.2337/db06-0911. [DOI] [PubMed] [Google Scholar]
- 44.Ye J, Gao Z, Yin J, He Q. Hypoxia is a potential risk factor for chronic inflammation and adiponectin reduction in adipose tissue of ob/ob and dietary obese mice. Am. J. Physiol. Endocrinol. Metab. 2007;293:E1118–E1128. doi: 10.1152/ajpendo.00435.2007. [DOI] [PubMed] [Google Scholar]
- 45.Pang C, Gao Z, Yin J, Zhang J, Jia W, Ye J. Macrophage infiltration into adipose tissue may promote angiogenesis for adipose tissue remodeling in obesity. Am. J. Physiol. Endocrinol. Metab. 2008;295:E313–E322. doi: 10.1152/ajpendo.90296.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang B, Wood I, Trayhurn P. Hypoxia induces leptin gene expression and secretion in human preadipocytes: differential effects of hypoxia on adipokine expression by preadipocytes. J. Endocrinol. 2008;198:127–134. doi: 10.1677/JOE-08-0156. [DOI] [PubMed] [Google Scholar]
- 47.Wang B, Wood IS, Trayhurn P. Dysregulation of the expression and secretion of inflammation-related adipokines by hypoxia in human adipocytes. Pflugers Arch. 2007;455:479–492. doi: 10.1007/s00424-007-0301-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cinti S, Mitchell G, Barbatelli G, et al. Adipocyte death defines macrophage localization and function in adipose tissue of obese mice and humans. J. Lipid Res. 2005;46:2347–2355. doi: 10.1194/jlr.M500294-JLR200. [DOI] [PubMed] [Google Scholar]
- 49.Murano I, Barbatelli G, Parisani V, et al. Dead adipocytes, detected as crown-like structures (CLS), are prevalent in visceral fat depots of genetically obese mice. J. Lipid Res. 2008;49:1562–1568. doi: 10.1194/jlr.M800019-JLR200. [DOI] [PubMed] [Google Scholar]
- 50.Strissel KJ, Stancheva Z, Miyoshi H, et al. Adipocyte death, adipose tissue remodeling, and obesity complications. Diabetes. 2007;56:2910–2918. doi: 10.2337/db07-0767. • Demonstrates that as adipose tissue expands it goes through a phase where the majority of adipocytes are dead, followed by a remodeling process that involves adipocyte hyperplasia.
- 51.Osuga J, Ishibashi S, Oka T, et al. Targeted disruption of hormone-sensitive lipase results in male sterility and adipocyte hypertrophy, but not in obesity. Proc. Natl Acad. Sci. USA. 2000;97:787–792. doi: 10.1073/pnas.97.2.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Okamoto Y, Higashiyama H, Rong JX, et al. Comparison of mitochondrial and macrophage content between subcutaneous and visceral fat in db/db mice. Exp. Mol. Pathol. 2007;83:73–83. doi: 10.1016/j.yexmp.2007.02.007. [DOI] [PubMed] [Google Scholar]
- 53.Murano I, Barbatelli G, Parisani V, et al. Dead adipocytes, detected as crown-like structures (CLS), are prevalent in visceral fat depots of genetically obese mice. J. Lipid Res. 2008;49:1562–1568. doi: 10.1194/jlr.M800019-JLR200. [DOI] [PubMed] [Google Scholar]
- 54.Goossens GH. The role of adipose tissue dysfunction in the pathogenesis of obesity-related insulin resistance. Physiol. Behav. 2008;94:206–218. doi: 10.1016/j.physbeh.2007.10.010. [DOI] [PubMed] [Google Scholar]
- 55.Elgazar-Carmon V, Rudich A, Hadad N, Levy R.Neutrophils transiently infiltrate intra-abdominal fat early in the course of high fat feeding J. Lipid Res DOI: 10.1194/jlr.M800132-JLR200 2008. (Epub ahead of print). [DOI] [PubMed] [Google Scholar]
- 56.Leik CE, Walsh SW. Neutrophils infiltrate resistance-sized vessels of subcutaneous fat in women with preeclampsia. Hypertension. 2004;44:72–77. doi: 10.1161/01.HYP.0000130483.83154.37. [DOI] [PubMed] [Google Scholar]
- 57.Inouye KE, Shi H, Howard JK, et al. Absence of CC chemokine ligand 2 does not limit obesity-associated infiltration of macrophages into adipose tissue. Diabetes. 2007;56:2242–2250. doi: 10.2337/db07-0425. [DOI] [PubMed] [Google Scholar]
- 58.Kamei N, Tobe K, Suzuki R, et al. Overexpression of monocyte chemoattractant protein-1 in adipose tissues causes macrophage recruitment and insulin resistance. J. Biol. Chem. 2006;281:26602–26614. doi: 10.1074/jbc.M601284200. [DOI] [PubMed] [Google Scholar]
- 59.Yu R, Kim CS, Kwon BS, Kawada T. Mesenteric adipose tissue-derived monocyte chemoattractant protein-1 plays a crucial role in adipose tissue macrophage migration and activation in obese mice. Obesity (Silver Spring) 2006;14:1353–1362. doi: 10.1038/oby.2006.153. [DOI] [PubMed] [Google Scholar]
- 60.Nara N, Nakayama Y, Okamoto S, et al. Disruption of CXC motif chemokine ligand-14 in mice ameliorates obesity-induced insulin resistance. J. Biol. Chem. 2007;282:30794–30803. doi: 10.1074/jbc.M700412200. [DOI] [PubMed] [Google Scholar]
- 61.Kirk EA, Sagawa ZK, McDonald TO, O’Brien KD, Heinecke JW. Macrophage chemoattractant protein-1 deficiency fails to restrain macrophage infiltration into adipose tissue. Diabetes. 2008;57:1254–1261. doi: 10.2337/db07-1061. [DOI] [PubMed] [Google Scholar]
- 62.Murdolo G, Hammarstedt A, Sandqvist M, et al. Monocyte chemoattractant protein-1 in subcutaneous abdominal adipose tissue: characterization of interstitial concentration and regulation of gene expression by insulin. J. Clin. Endocrinol. Metab. 2007;92:2688–2695. doi: 10.1210/jc.2006-2814. [DOI] [PubMed] [Google Scholar]
- 63.Westerbacka J, Corner A, Kolak M, et al. Insulin regulation of MCP-1 in human adipose tissue of obese and lean women. Am. J. Physiol. Endocrinol. Metab. 2008;294:E841–E845. doi: 10.1152/ajpendo.00653.2006. [DOI] [PubMed] [Google Scholar]
- 64.Huber J, Kiefer FW, Zeyda M, et al. CC chemokine and CC chemokine receptor profiles in visceral and subcutaneous adipose tissue are altered in human obesity J. Clin. Endocrinol. Metab DOI: 10.1210/jc.2007-2630 2008. (Epub ahead of print). [DOI] [PubMed] [Google Scholar]
- 65.Ferenbach D, Kluth DC, Hughes J. Inflammatory cells in renal injury and repair. Semin. Nephrol. 2007;27:250–259. doi: 10.1016/j.semnephrol.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 66.Johnson JL. Matrix metalloproteinases: influence on smooth muscle cells and atherosclerotic plaque stability. Expert Rev. Cardiovasc. Ther. 2007;5:265–282. doi: 10.1586/14779072.5.2.265. [DOI] [PubMed] [Google Scholar]
- 67.Libby P. Inflammatory mechanisms: the molecular basis of inflammation and disease. Nutr. Rev. 2007;65:S140–S146. doi: 10.1111/j.1753-4887.2007.tb00352.x. [DOI] [PubMed] [Google Scholar]
- 68.Savill J, Dransfield I, Gregory C, Haslett C. A blast from the past: clearance of apoptotic cells regulates immune responses. Nat. Rev. Immunol. 2002;2:965–975. doi: 10.1038/nri957. [DOI] [PubMed] [Google Scholar]
- 69.Mantovani A, Sica A, Sozzani S, Allavena P, Vecchi A, Locati M. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. 2004;25:677–686. doi: 10.1016/j.it.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 70.Gordon S, Taylor PR. Monocyte and macrophage heterogeneity. Nat. Rev. Immunol. 2005;5:953–964. doi: 10.1038/nri1733. [DOI] [PubMed] [Google Scholar]
- 71.Satriano J. Arginine pathways and the inflammatory response: interregulation of nitric oxide and polyamines: review article. Amino Acids. 2004;26:321–329. doi: 10.1007/s00726-004-0078-4. [DOI] [PubMed] [Google Scholar]
- 72.Lumeng CN, Deyoung SM, Bodzin JL, Saltiel AR. Increased inflammatory properties of adipose tissue macrophages recruited during diet-induced obesity. Diabetes. 2007;56:16–23. doi: 10.2337/db06-1076. [DOI] [PubMed] [Google Scholar]
- 73.Odegaard JI, Ricardo-Gonzalez RR, Goforth MH, et al. Macrophage-specific PPARγ controls alternative activation and improves insulin resistance. Nature. 2007;447:1116–1120. doi: 10.1038/nature05894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Stienstra R, Duval C, Keshtkar S, van der Laak J, Kersten S, Muller M. PPARγ activation promotes infiltration of alternatively activated macrophages into adipose tissue. J. Biol. Chem. 2008;283:22620–22627. doi: 10.1074/jbc.M710314200. [DOI] [PubMed] [Google Scholar]
- 75.Kang K, Reilly SM, Volkan K, et al. Adipocyte-derived Th2 cytokines and myeloid PPARδ regulate macrophage polarization and insulin sensitivity. Cell Metab. 2008;7:485–495. doi: 10.1016/j.cmet.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zeyda M, Farmer D, Todoric J, et al. Human adipose tissue macrophages are of an anti-inflammatory phenotype but capable of excessive pro-inflammatory mediator production. Int. J. Obes. (Lond.) 2007;31:1420–1428. doi: 10.1038/sj.ijo.0803632. [DOI] [PubMed] [Google Scholar]
- 77.Bourlier V, Zakaroff-Girard A, Miranville A, et al. Remodeling phenotype of human subcutaneous adipose tissue macrophages. Circulation. 2008;117:806–815. doi: 10.1161/CIRCULATIONAHA.107.724096. [DOI] [PubMed] [Google Scholar]
- 78.Chavey C, Mari B, Monthouel MN, et al. Matrix metalloproteinases are differentially expressed in adipose tissue during obesity and modulate adipocyte differentiation. J. Biol. Chem. 2003;278:11888–11896. doi: 10.1074/jbc.M209196200. [DOI] [PubMed] [Google Scholar]
- 79.Maquoi E, Munaut C, Colige A, Collen D, Lijnen HR. Modulation of adipose tissue expression of murine matrix metalloproteinases and their tissue inhibitors with obesity. Diabetes. 2002;51:1093–1101. doi: 10.2337/diabetes.51.4.1093. [DOI] [PubMed] [Google Scholar]
- 80.Van Hul M, Lijnen HR. A functional role of gelatinase A in the development of nutritionally induced obesity in mice. J. Thromb. Haemost. 2008;6:1198–1206. doi: 10.1111/j.1538-7836.2008.02988.x. [DOI] [PubMed] [Google Scholar]
- 81.Lijnen HR, Demeulemeester D, Van Hoef B, Collen D, Maquoi E. Deficiency of tissue inhibitor of matrix metalloproteinase-1 (TIMP-1) impairs nutritionally induced obesity in mice. Thromb. Haemost. 2003;89:249–255. [PubMed] [Google Scholar]
- 82.Demeulemeester D, Collen D, Lijnen HR. Effect of matrix metalloproteinase inhibition on adipose tissue development. Biochem. Biophys. Res. Commun. 2005;329:105–110. doi: 10.1016/j.bbrc.2005.01.103. [DOI] [PubMed] [Google Scholar]
- 83.Lijnen HR, Maquoi E, Hansen LB, Van Hoef B, Frederix L, Collen D. Matrix metalloproteinase inhibition impairs adipose tissue development in mice. Arterioscler. Thromb. Vasc. Biol. 2002;22:374–379. doi: 10.1161/hq0302.104522. [DOI] [PubMed] [Google Scholar]
- 84.Huber J, Loffler M, Bilban M, et al. Prevention of high-fat diet-induced adipose tissue remodeling in obese diabetic mice by n-3 polyunsaturated fatty acids. Int. J. Obes. (Lond.) 2007;31:1004–1013. doi: 10.1038/sj.ijo.0803511. [DOI] [PubMed] [Google Scholar]
- 85.Derosa G, Ferrari I, D’Angelo A, et al. Matrix metalloproteinase-2 and -9 levels in obese patients. Endothelium. 2008;15:219–224. doi: 10.1080/10623320802228815. [DOI] [PubMed] [Google Scholar]
- 86.Kralisch S, Bluher M, Tonjes A, et al. Tissue inhibitor of metalloproteinase-1 predicts adiposity in humans. Eur. J. Endocrinol. 2007;156:257–261. doi: 10.1530/eje.1.02328. [DOI] [PubMed] [Google Scholar]