Abstract
The endoplasmic reticulum (ER) has emerged as a key to understanding the development and consequences of hepatic fat accumulation in nonalcoholic fatty liver disease (NAFLD). An essential function of this organelle is the proper assembly of proteins that are destined for intracellular organelles and the cell surface. Recent evidence suggests that chemical chaperones that enhance the functional capacity of the ER improve liver function in obesity and NAFLD. These chaperones may therefore provide a novel potential therapeutic strategy in NAFLD.
Keywords: Insulin resistance, nonalcoholic fatty liver disease, obesity, steatosis, unfolded protein response
Introduction
Nonalcoholic fatty liver disease (NAFLD) is a newly emerging obesity-related disorder characterized by fatty infiltration (steatosis) of the liver in the absence of chronic alcohol consumption. In some individuals, steatosis progresses to nonalcoholic steatohepatitis (NASH), which is characterized by steatosis, inflammation and fibrosis, and end-stage liver disease [1]. NAFLD is now recognized as the most common cause of chronic liver enzyme elevations and cryptogenic cirrhosis [2]. The prevalence of NAFLD has nearly doubled since 1980, and current US estimates indicate that NAFLD may affect up to 25% of the general population and 80% of obese and diabetic individuals [3]. NAFLD has also emerged as a common pediatric disease, afflicting 3 to 9% of all children in the US and up to 50% of obese children [4].
The original working model explaining the pathogenesis of NAFLD, the ‘two-hit’ hypothesis, was first proposed by Day et al, in 1998 [5]. According to this hypothesis, steatosis ‘first hit’, represents and increases the the vulnerability of the liver to various ‘second hits’ that in turn lead to the inflammation, fibrosis characteristic of NASH. Since features of the metabolic syndrome such as obesity, insulin resistance and hypertriglyceridemia are not only predisposing factors for NAFLD, but are also risk factors for disease progression, more severe fibrosis and advanced disease [6], this hypothesis continues to undergo modification.
There is no proven, effective therapy for NAFLD. Improvement in liver function and hepatomegaly has been observed in obese patients with NAFLD after gradual, sustained weight reduction of 10% [1]. Lifestyle modifications, similar to those recommended for obesity, remain therefore the primary treatment option. There are several promising drug candidates for NAFLD, including antioxidants, metformin, thiazolidinediones, gemfibrozil and probucol; however, none have been rigorously tested in large sample populations [1]. Thus, there is currently no specific treatment for NAFLD, and the primary treatment option, lifestyle modification, has proven to be ineffective over the long term. In the present review, the putative role of the endoplasmic reticulum (ER) in NAFLD, and its potential as a therapeutic target, are discussed.
The endoplasmic reticulum and the unfolded protein response
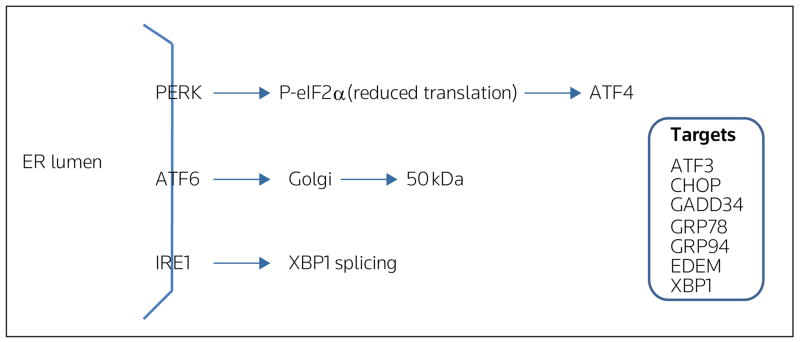
The ER is one of the largest cellular organelles, its membranes representing as much as half of the total membranes in the cell. An essential function of the ER is the proper assembly of proteins that are ultimately destined for intracellular organelles and the cell surface [7]. The status of protein assembly and folding is monitored and relayed to the cytosol and nucleus by the unfolded protein response (UPR). Cellular perturbations, such as loss of the luminal oxidizing environment, imbalance in calcium homeostasis and aberrant N-linked glycosylation, and cellular death, can disrupt ER homeostasis and lead to the accumulation of unfolded proteins and protein aggregates in the ER lumen, both of which can be detrimental to cell function and survival. Disruption of ER homeostasis, termed ER stress, activates the UPR [8,9]. In mammals, ER stress is sensed and the UPR activated by three ER trans- membrane proteins: PERK (RNA-dependent protein kinase-like ER eukaryotic initiation factor-2α kinase), ATF6 (activating transcription factor 6), and IRE1 (inositol-requiring ER-to-nucleus signaling protein 1) (Figure 1).
Figure 1. Simplified illustration of three proximal stress sensors, and putative targets involved in the unfolded protein response.
In mammals, stress in the endoplasmic reticulum (ER) is sensed and the unfolded protein response (UPR) is activated by three ER transmembrane proteins: PERK (RNA-dependent protein kinase-like ER eukaryotic initiation factor-2α kinase), ATF6 (activating transcription factor 6), and IRE1 (inositol-requiring ER-to-nucleus signaling protein 1). Activation, as a result of UPR initiation, leads to increased translation and expression of ATF4 (activating transcription factor 4) through phosphorylation of eIF2α (eukaryotic initiation factor 2α). ATF6 is transported to the Golgi, where it is cleaved into a 50-kDa fragment, which then migrates to the nucleus and activates target genes. IRE1 activation leads to splicing of XBP1 (X-box-binding protein-1) and subsequent transcription of molecular chaperones [eg, GRP78 (78-kDa glucose-regulated protein)]. ATF3 activating transcription factor 3, CHOP CCAAT/enhancer binding homologous protein, EDEM ER degradation-enhancing α-mannosidase, GADD34 growth arrest and DNA damage-inducible transcript 34, GRP94 94-kDa glucose-regulated protein. (Reproduced with permission from Elsevier and Gentile CL, Pagliassotti MJ: The role of fatty acids in the development and progression of nonalcoholic fatty liver disease. J Nutr Biochem (2008) 19(9):567-576 © 2008 Elsevier.)
PERK activation leads to phosphorylation of the α-subunit of the translation initiation factor eIF2 (eukaryotic initiation factor 2) and subsequently attenuates the initiation of translation, and increases the expression and selective translation of ATF4. Increased expression of GADD34 (growth arrest and DNA damage-inducible transcript 34), a member of the growth arrest and DNA damage family of proteins, is involved in dephosphorylation of eIF2α and, therefore, reversal of translational attenuation. Upon activation of the UPR, ATF6 is transported to the Golgi where it is cleaved and it subsequently migrates to the nucleus, as a 50-kDa fragment, where it activates transcription of UPR target genes. Activation of IRE1 promotes the splicing of X-box-binding protein-1 (XBP1) mRNA and subsequent transcription of molecular chaperones, for example, GRP78 (78-kDa glucose-regulated protein), and genes involved in ER-associated degradation, for example, EDEM (ER degradation-enhancing α-mannosidase) [8,9]. Thus, activation of the UPR serves to attenuate global protein synthesis and to enhance the capacity for protein folding and degradation. Failure of the UPR to mitigate ER stress and re-establish ER homeostasis can lead to cell dysfunction and death [8,10].
A putative role for the UPR in diabetes, obesity, and NAFLD
Several studies have linked ER dysfunction and the UPR to impairments in glucose homeostasis and diabetes. For example, PERK −/− mice develop diabetes due to a rapid and progressive decline in endocrine and exocrine pancreatic function [11]. Conversely, mice with a homozygous mutation of serine 51 on eIF2α die within 18 hours of birth as a result of hypoglycemia and impaired induction of genes involved in hepatic gluconeogenesis [12]. Programmed cell death in response to ER stress is mediated, in part, through transcriptional activation of CHOP (CCAAT/enhancer binding homologous protein) [13,14]. Targeted disruption of the CHOP gene in Akita mice, a mouse line that spontaneously develops hyperglycemia with reduced β-cell mass, delayed the onset of diabetes [15]. It has therefore been proposed that chronic disruption of ER homeostasis may contribute to the attrition of β-cell function and to impaired regulation of glucose homeostasis in diabetes [16–18].
Obesity is characterized by lipid accumulation in both adipose and non-adipose tissues, including the liver. ER stress and activation of the UPR in liver and adipose tissue have been observed in both genetic and dietary murine models of obesity [19]. Thus, it has been proposed that obesity provokes ER stress, presumably by increasing the load of unfolded proteins in the ER. Insulin resistance is a characteristic feature of obesity and NAFLD, and has been causally linked to hepatic steatosis [20,21]. Interestingly, when UPR signaling was compromised by a partial loss-of-function mutation in XBP1, or a mutation in the ER chaperone, ORP150 (oxygen-regulated protein 150 kDa), both of which further increased ER stress, increased insulin resistance was observed in obese mice [19,22]. Studies have also documented increased phosphorylation of eIF2α in livers from patients with NAFLD and NASH compared with individuals with the metabolic syndrome and normal liver histology [23], and activation of the UPR in adipose tissue of obese patients [24].
The ER transmembrane kinase/endoribonuclease IRE1 initiates a novel mRNA splicing mechanism that modifies XBP1 mRNA to encode a potent basic leucine zipper transcription factor, termed spliced XBP1 (XBP1s). Evidence has not only linked XBP1s to phospholipid biosynthesis and ER biogenesis, but also to the regulation of hepatic lipogenesis [25,26]. XBP1s regulation of lipid synthesis in the liver appears to be independent of ER stress and thus implies that not only are there alternative mechanisms of regulation of this transcription factor, but also that components of the UPR may contribute to the development of hepatic steatosis [26,27]. In addition, ER homeostasis can be perturbed, and the UPR can be activated in response to changes in the membrane and circulating lipid environment. For example, cholesterol loading activates the UPR and induces apoptosis in macrophages, suggesting that the UPR senses changes in membrane cholesterol concentration esterified [28]. Increased non-fatty acids, in particular long chain saturated fatty acids, which are present in obesity and diabetes, induce ER stress and activate the UPR in a number of cell types, including hepatocytes [29,30–34]. Notably, ER stress in response to increased hepatic lipids appears to decrease the ability of the liver to secrete triglycerides by limiting apoB secretion, potentially worsening steatosis [34]. These data suggest that UPR-mediated signaling contributes to the development of steatosis, which in turn induces ER stress and potential complications arising from sustained ER stress, such as cell dysfunction and death.
Molecular and chemical chaperones
If ER stress and/or protein misfolding are implicated in NAFLD pathogenesis, it is likely that strategies that promote protein folding may lead to effective therapies. Molecular chaperones, such as protein disulfide isomerases, calreticulin, and GRP78 promote protein folding by decreasing energy barriers among the stages of protein maturation and preventing aggregation of hydrophobic protein surfaces [35]. Chemical chaperones, such as glycerol, trimethylamine-N-oxide, methyl-β-cyclodextrin, and 4-phenyl butyric acid (PBA), represent a group of low molecular weight compounds that can stabilize protein conformation, improve ER folding capacity, and facilitate the appropriate trafficking of mutant proteins [36,37]. Endogenous bile acids and bile acid derivatives, such as ursodeoxycholic acid and taurine-conjugated ursodeoxycholic acid (TUDCA), can also modify ER function [38]. A number of misfolded proteins have been rescued by chemical or pharmacological chaperone intervention [39].
Several studies have demonstrated that chemical chaperones alleviate ER stress in model systems used to study lysosomal storage disease, hereditary hemochromatosis and cholangiocarcinoma [40–42]. Oral administration of PBA or TUDCA to ob/ob mice (leptin-deficient mice; a model of severe obesity and insulin resistance) normalized blood glucose levels, improved insulin sensitivity, reduced hepatic steatosis, normalized liver enzymes and reduced biochemical markers of ER stress in liver and adipose tissue [37]. Pretreatment of McA liver cells with PBA significantly reduced biochemical markers of ER stress in response to increased fatty acid delivery [34]. Although the exact mechanism(s) that lead to hepatic ER stress in models of obesity and NAFLD are unclear, these data suggest that one trigger involves an increased demand on the protein synthetic machinery and/or impairment in protein folding capacity/degradation. Therefore, the use of chemical chaperones to enhance the functional capacity of the ER may be a novel intervention strategy in the treatment of hepatic abnormalities that arise in obesity and NAFLD [37].
Efficacy of chemical chaperones
Chemical chaperones ameliorate ER stress and reduce liver damage in murine and cellular models of obesity and NAFLD. The chemical chaperone sodium phenylbutyrate is an orally administered agent that was developed to promote waste nitrogen excretion in the treatment of urea-cycle disorders [43,44]. In 1995, there was over 100 patient-years of experience with this drug with no untoward effects. In a long-term trial involving eleven patients with homozygous β-thalassemia and one patient with sickle cell-β-thalassemia treated with sodium phenylbutyrate for 41 to 460 days, weight gain and edema caused by an increase in salt load were observed in two of twelve individuals, transient epigastric discomfort in seven of twelve, and abnormal body odor in three of twelve [43]. Several trials of ursodeoxycholic acid (UDCA) in primary biliary cirrhosis showed improvement in biochemical parameters [45]. UDCA-induced normalization of serum bilirubin was associated with clinical benefits [46]. UDCA appears to be safe and has few side effects: some patients experience weight gain, hair loss, and in rare cases, diarrhea [45].
The effectiveness of UDCA in NAFLD is equivocal. Three trials, one that studied 24 patients over a 12-month period, one that studied 15 patients over a 3-month period, and one that studied 29 patients for 6 months, suggested that UDCA treatment (10 to 15 mg/kg/d) produced beneficial effects on liver enzymes [47–49]. In contrast, two trials, one that studied 14 women for a 1.5-month period and one that studied 80 patients for a 24-month period, suggested that UDCA (13 to 15 mg/kg/d) did not have a significant effect on biochemical or imaging markers of liver disease over that observed with weight reduction, or was not better than placebo, respectively [50,51]. Thus, UDCA may not be effective in NASH, or may require a higher dose to be effective in some patients with NASH.
Nonetheless, based on these data as a whole, and given the potential for chemical chaperones to reduce hepatic impairments associated with obesity and NAFLD, further clinical studies in patients with NAFLD that incorporate chemical chaperones appear warranted.
Conclusions
NAFLD is currently the most common liver disorder in the developed world, affecting up to a third of individuals. It is closely associated with features of the metabolic syndrome, including obesity, and therefore therapy is being directed at treating components of the metabolic syndrome. The ER in the liver appears to be a target for inflammatory mediators, such as excess fatty acids and cytokines. Recent evidence has demonstrated that impaired ER function and ER stress in the liver is present in murine models of obesity and NAFLD, and in human NAFLD. To the extent that ER stress in these conditions results from the accumulation of misfolded proteins, a major cause of ER stress, future studies that investigate the mechanisms of action of chemical chaperones and their efficacy in large human trials appear warranted.
References
•• of outstanding interest
• of special interest
- 1.Calamita G, Portincasa P. Present and future therapeutic strategies for nonalcoholic fatty liver disease. Expert Opin Ther Targets. 2007;11(9):1231–1249. doi: 10.1517/14728222.11.9.1231. [DOI] [PubMed] [Google Scholar]
- 2.Clark JM, Diehl A. Nonalcoholic fatty liver disease: An under-recognized cause of cryptogenic cirrhosis. J Am Med Assoc. 2003;289(22):3000–3004. doi: 10.1001/jama.289.22.3000. [DOI] [PubMed] [Google Scholar]
- 3.Wieckowska A, McCullough AJ, Feldstein AE. Noninvasive diagnosis and monitoring of nonalcoholic steatohepatitis: Present and future. Hepatology. 2007;46(2):582–589. doi: 10.1002/hep.21768. [DOI] [PubMed] [Google Scholar]
- 4.Papandreou D, Rousso I, Mavromichalis I. Update on nonalcoholic fatty liver disease in children. Clin Nutr. 2007;26(4):409–415. doi: 10.1016/j.clnu.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 5.Day CP, James O. Steatohepatitis: A tale of two ‘hits’? Gastroenterology. 1998;114(4):842–845. doi: 10.1016/s0016-5085(98)70599-2. [DOI] [PubMed] [Google Scholar]
- 6.Gill HK, Wu GY. Nonalcoholic fatty liver disease and the metabolic syndrome: Effects of weight loss and a review of popular diets. Are low carbohydrate diets the answer? World J Gastroenterol. 2006;12(3):345–353. doi: 10.3748/wjg.v12.i3.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pahl HL. Signal transduction from the endoplasmic reticulum to the cell nucleus. Physiol Rev. 1999;79(3):683–701. doi: 10.1152/physrev.1999.79.3.683. [DOI] [PubMed] [Google Scholar]
- 8••.Kaufman RJ. Stress signaling from the lumen of the endoplasmic reticulum: Coordination of gene transcriptional and translational controls. Genes Dev. 1999;13(10):1211–1233. doi: 10.1101/gad.13.10.1211. An exceptional review of the unfolded protein response. [DOI] [PubMed] [Google Scholar]
- 9.Kaufman RJ. Orchestrating the unfolded protein response in health and disease. J Clin Invest. 2002;110(10):1389–1398. doi: 10.1172/JCI16886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rutkowski DT, Kaufman RJ. A trip to the ER: Coping with stress. Trends Cell Biol. 2004;14(1):20–28. doi: 10.1016/j.tcb.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 11.Harding HP, Zeng H, Zhang Y, Jungries R, Chung P, Plesken H, Sabatini DD, Ron D. Diabetes mellitus and exocrine pancreatic dysfunction in Perk −/− mice reveals a role for translational control in secretory cell survival. Mol Cell. 2001;7(6):1153–1163. doi: 10.1016/s1097-2765(01)00264-7. [DOI] [PubMed] [Google Scholar]
- 12••.Scheuner D, Song B, McEwen E, Liu C, Laybutt R, Gillespie P, Saunders T, Bonner-Weir S, Kaufman RJ. Translational control is required for the unfolded protein response and in vivo glucose homeostasis. Mol Cell. 2001;7(6):1165–1176. doi: 10.1016/s1097-2765(01)00265-9. Among the first papers to demonstrate a link between the unfolded protein response and glucose homeostasis. [DOI] [PubMed] [Google Scholar]
- 13.Kim SH, Hwang CI, Park WY, Lee JH, Song YS. GADD153 mediates celecoxib-induced apoptosis in cervical cancer cells. Carcinogenesis. 2006;27(10):1961–1969. doi: 10.1093/carcin/bgl027. [DOI] [PubMed] [Google Scholar]
- 14.Oyadomari S, Mori M. Roles of CHOP/GADD153 in endoplasmic reticulum stress. Cell Death Differ. 2004;11(4):381–389. doi: 10.1038/sj.cdd.4401373. [DOI] [PubMed] [Google Scholar]
- 15.Oyadomari S, Koizumi A, Takeda K, Gotoh T, Akira S, Araki E, Mori M. Targeted disruption of the CHOP gene delays endoplasmic reticulum-stress mediated diabetes. J Clin Invest. 2002;109(4):525–532. doi: 10.1172/JCI14550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shi Y, Taylor SI, Tan S-L, Sonenberg N. When translation meets metabolism: Multiple links to diabetes. Endocr Rev. 2003;24(1):91–101. doi: 10.1210/er.2002-0018. [DOI] [PubMed] [Google Scholar]
- 17.Zhao L, Ackerman SL. Endoplasmic reticulum stress in health and disease. Curr Opin Cell Biol. 2006;18(4):444–452. doi: 10.1016/j.ceb.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 18.Wang D, Wei Y, Schmoll D, Maclean KN, Pagliassotti MJ. Endoplasmic reticulum stress increases glucose-6-phosphatase and glucose cycling in liver cells. Endocrinology. 2006;147(1):350–358. doi: 10.1210/en.2005-1014. [DOI] [PubMed] [Google Scholar]
- 19••.Ozcan U, Cao Q, Yilmaz E, Lee A-H, Iwakoshi NN, Ozdelen E, Tuncman G, Görgün CZ, Glimcher LH, Hotamisligil GS. Endoplasmic reticulum stress links obesity, insulin action and type 2 diabetes. Science. 2004;306(5695):457–461. doi: 10.1126/science.1103160. This paper was among first to document the presence of ER stress in obesity and the participation of the unfolded protein response in the regulation of insulin action. [DOI] [PubMed] [Google Scholar]
- 20.Festi D, Colecchia A, Sacco T, Bondi M, Roda E, Marchesini G. Hepatic steatosis in obese patients: Clinical aspects and prognostic significance. Obes Rev. 2004;5(1):27–42. doi: 10.1111/j.1467-789x.2004.00126.x. [DOI] [PubMed] [Google Scholar]
- 21.Samuel VT, Liu Z-X, Qu X, Elder BD, Bilz S, Befroy D, Romanelli AJ, Shulman GI. Mechanism of hepatic insulin resistance in nonalcoholic fatty liver disease. J Biol Chem. 2004;279(31):32345–32353. doi: 10.1074/jbc.M313478200. [DOI] [PubMed] [Google Scholar]
- 22.Nakatani Y, Kaneto H, Kawamori D, Yoshiuchi K, Hatazaki M, Matsuoka TA, Ozawa K, Ogawa S, Hori M, Yamasaki Y, Matsuhisa M. Involvement of endoplasmic reticulum stress in insulin resistance and diabetes. J Biol Chem. 2005;280(1):847–851. doi: 10.1074/jbc.M411860200. [DOI] [PubMed] [Google Scholar]
- 23••.Puri P, Mirshahi F, Cheung O, Natarajan R, Maher JW, Kellum JM, Sanyal AJ. Activation and dysregulation of the unfolded protein response in nonalcoholic fatty liver disease. Gastroenterology. 2008;134(2):568–576. doi: 10.1053/j.gastro.2007.10.039. The only documented report of endoplasmic reticulum stress in humans with nonalcoholic fatty liver disease to date. [DOI] [PubMed] [Google Scholar]
- 24.Das SK, Chu WS, Mondal AK, Sharma NK, Kern PA, Rasouli N, Elbein SC. Endoplasmic reticulum stress response is not reduced with pioglitazone in human adipose tissue or human hepatocyte HepG2 cell lines. Am Diabetes Assoc. 2008;68:Abs 1931-P. [Google Scholar]
- 25••.Sriburi R, Jackowski S, Mori K, Brewer JW. XBP1: A link between the unfolded protein response, lipid biosynthesis, and biogenesis of the endoplasmic reticulum. J Cell Biol. 2004;167(1):35–41. doi: 10.1083/jcb.200406136. This paper was among the first to protein-1 in lipid biosynthesis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26••.Lee A-H, Scapa EF, Cohen DE, Glimcher LH. Regulation of hepatic lipogenesis by the transcription factor XBP1. Science. 2008;320(5882):1492–1496. doi: 10.1126/science.1158042. This paper demonstrated that deletion of X-box-binding protein-1 in the liver resulted in hypocholesterolemia and hypotriglyceridemia, secondary to decreased production of lipids by the liver. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Oyadomari S, Harding H, Zhang Y, Oyadomari M, Ron D. Dephosphorylation of translation initiation factor 2α enhances glucose tolerance and attenuates hepatosteatosis in mice. Cell Metab. 2008;7(6):520–532. doi: 10.1016/j.cmet.2008.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Feng B, Yao PM, Li Y, Devlin CM, Zhang D, Harding HP, Sweeney M, Rong JX, Kuriakose G, Fisher EA, Marks AR, et al. The endoplasmic reticulum is the site of cholesterol-induced cytotoxicity in macrophages. Nat Cell Biol. 2003;5(9):781–792. doi: 10.1038/ncb1035. [DOI] [PubMed] [Google Scholar]
- 29.Yang L, Jhaveri R, Huang J, Qi Y, Diehl AM. Endoplasmic reticulum stress, hepatocytes CD1d and NK T-cell abnormalities in murine fatty livers. Lab Invest. 2007;87(9):927–937. doi: 10.1038/labinvest.3700603. [DOI] [PubMed] [Google Scholar]
- 30.Kharroubi I, Ladriere L, Cardozo AK, Dogusan Z, Cnop M, Eizirik DL. Free fatty acids and cytokines induce pancreatic β-cell apoptosis by different mechanisms: Role of nuclear factor-κB and endoplasmic reticulum stress. Endocrinology. 2004;145(11):5087–5096. doi: 10.1210/en.2004-0478. [DOI] [PubMed] [Google Scholar]
- 31••.Borradaile NM, Han X, Harp JD, Gale SE, Ory DS, Schaffer JE. Disruption of endoplasmic reticulum structure and integrity in lipotoxic cell death. J Lipid Res. 2006;47(12):2726–2737. doi: 10.1194/jlr.M600299-JLR200. An important paper that demonstrated the sensitivity of the endoplasmic reticulum to saturated fatty acids. [DOI] [PubMed] [Google Scholar]
- 32.Wang D, Wei Y, Pagliassotti MJ. Saturated fatty acids promote endoplasmic reticulum stress and liver injury in rats with hepatic steatosis. Endocrinology. 2006;147(2):943–951. doi: 10.1210/en.2005-0570. [DOI] [PubMed] [Google Scholar]
- 33.Wei Y, Wang D, Topczewski F, Pagliassotti MJ. Saturated fatty acids induce endoplasmic reticulum stress and apoptosis independently of ceramide in liver cells. Am J Physiol Endocrinol Metab. 2006;291(2):E275–E281. doi: 10.1152/ajpendo.00644.2005. [DOI] [PubMed] [Google Scholar]
- 34.Ota T, Gayet C, Ginsberg HN. Inhibition of apolipoprotein B100 secretion by lipid-induced hepatic endoplasmic reticulum stress in rodents. J Clin Invest. 2008;118(1):316–332. doi: 10.1172/JCI32752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Welch WJ, Brown CR. Influence of molecular and chemical chaperones on protein folding. Cell Stress Chaperones. 1996;1(2):109–115. doi: 10.1379/1466-1268(1996)001<0109:iomacc>2.3.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kolter T, Wendeler M. Chemical chaperones – a new concept in drug research. Chembiochem. 2003;4(4):260–264. doi: 10.1002/cbic.200390045. [DOI] [PubMed] [Google Scholar]
- 37••.Ozcan U, Yilmaz E, Ozcan L, Furuhashi M, Vaillancourt E, Smith RO, Görgün CZ, Hotamisligil GS. Chemical chaperones reduce ER stress and restore glucose homeostasis in a mouse model of type 2 diabetes. Science. 2006;313(5790):1137–1140. doi: 10.1126/science.1128294. This paper demonstrated that chemical chaperones enhance the adaptive capacity of the endoplasmic reticulum and can act as potent anti-diabetic agents with potential application in the treatment of type 2 diabetes and nonalcoholic fatty liver disease. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xie Q, Khaoustov VI, Chung CC, Sohn J, Krishnan B, Lewis DE, Yoffe B. Effect of tauroursodeoxycholic acid on endoplasmic reticulum stress-induced caspase-12 activation. Hepatology. 2002;36(3):592–601. doi: 10.1053/jhep.2002.35441. [DOI] [PubMed] [Google Scholar]
- 39.Loo TW, Clarke DM. Chemical and pharmacological chaperones as new therapeutic agents. Expert Rev Mol Med. 2007;9(16):1–18. doi: 10.1017/S1462399407000361. [DOI] [PubMed] [Google Scholar]
- 40.Wei H, Kim SJ, Zhang Z, Tsai PC, Wisniewski KE, Mukherjee AB. ER and oxidative stresses are common mediators of apoptosis in both neurodegenerative and non-neurodegenerative lysosomal storage disorders and are alleviated by chemical chaperones. Hum Mol Genet. 2008;17(4):469–477. doi: 10.1093/hmg/ddm324. [DOI] [PubMed] [Google Scholar]
- 41.Alpini G, Kanno N, Phinizy JL, Glaser S, Francis H, Taffetani S, LeSage G. Tauroursodeoxycholate inhibits human cholangiocarcinoma growth via Ca2+-, PKC-, and MAPK-dependent pathways. Am J Physiol Gastrointest Liver Physiol. 2004;286(6):G973–G982. doi: 10.1152/ajpgi.00270.2003. [DOI] [PubMed] [Google Scholar]
- 42.de Almeida SF, Picarote G, Fleming JV, Carmo-Fonseca M, Azevedo JE, de Sousa M. Chemical chaperones reduce endoplasmic reticulum stress and prevent mutant HFE aggregate formation. J Biol Chem. 2007;282(38):27905–27912. doi: 10.1074/jbc.M702672200. [DOI] [PubMed] [Google Scholar]
- 43.Collins AF, Pearson HA, Giardina P, McDonagh KT, Brusilow SW, Dover GJ. Oral sodium phenylbutyrate therapy in homozygous β thalassemia: A clinical trial. Blood. 1995;85(1):43–49. [PubMed] [Google Scholar]
- 44.Maestri NE, Brusilow SW, Clissold DB, Bassett SS. Long-term treatment of girls with ornithine transcarbamylase deficiency. N Engl J Med. 1996;335(12):855–859. doi: 10.1056/NEJM199609193351204. [DOI] [PubMed] [Google Scholar]
- 45.Kaplan MM, Gershwin ME. Primary biliary cirrhosis. N Engl J Med. 2005;353(12):1261–1273. doi: 10.1056/NEJMra043898. [DOI] [PubMed] [Google Scholar]
- 46.Bonnand AM, Heathcote EJ, Lindor KD, Poupon RE. Clinical significance of serum bilirubin levels under ursodeoxycholic acid therapy in patients with primary biliary cirrhosis. Hepatology. 1999;29(1):39–43. doi: 10.1002/hep.510290140. [DOI] [PubMed] [Google Scholar]
- 47.Laurin J, Lindor KD, Crippin JS, Gossard A, Gores GJ, Ludwig J, Rakela J, McGill DB. Ursodeoxycholic acid or clofibrate in the treatment of non-alcohol-induced steatohepatitis: A pilot study. Hepatology. 1996;23(6):1464–1467. doi: 10.1002/hep.510230624. [DOI] [PubMed] [Google Scholar]
- 48.Santos VN, Lanzoni VP, Szejnfeld J, Shigueoka D, Parise ER. A randomized double-blind study of the short-time treatment of obese patients with nonalcoholic fatty liver disease with ursodeoxycholic acid. Braz J Med Biol Res. 2003;36(6):723–729. doi: 10.1590/s0100-879x2003000600007. [DOI] [PubMed] [Google Scholar]
- 49.Ersoz G, Gunsar F, Karasu Z, Akay S, Batur Y, Akarca US. Management of fatty liver disease with vitamin E and C compared to ursodeoxycholic acid treatment. Turk J Gastroenterol. 2005;16(3):124–128. [PubMed] [Google Scholar]
- 50.Méndez-Sánchez N, González V, Chávez-Tapia N, Ramos MH, Uribe M. Weight reduction and ursodeoxycholic acid in subjects with nonalcoholic fatty liver disease. A double-blind, placebo-controlled trial. Ann Hepatol. 2004;3(3):108–112. [PubMed] [Google Scholar]
- 51.Lindor KD, Kowdley KV, Heathcote EJ, Harrison ME, Jorgensen R, Angulo P, Lymp JF, Burgart L, Colin P. Ursodeoxycholic acid for treatment of nonalcoholic steatohepatitis: Results of a randomized trial. Hepatology. 2004;39(3):770–778. doi: 10.1002/hep.20092. [DOI] [PubMed] [Google Scholar]