Abstract
Fibrin gels, prepared from fibrinogen and thrombin, the key proteins involved in blood clotting, were among the first biomaterials used to prevent bleeding and promote wound healing. The unique polymerization mechanism of fibrin, which allows control of gelation times and network architecture by variation in reaction conditions, allows formation of a wide array of soft substrates under physiological conditions. Fibrin gels have been extensively studied rheologically in part because their nonlinear elasticity, characterized by soft compliance at small strains and impressive stiffening to resist larger deformations, appears essential for their function as haemostatic plugs and as matrices for cell migration and wound healing. The filaments forming a fibrin network are among the softest in nature, allowing them to deform to large extents and stiffen but not break. The biochemical and mechanical properties of fibrin have recently been exploited in numerous studies that suggest its potential for applications in medicine and bioengineering.
Keywords: fibrin, gel, tissue engineering, extracellular matrix, elasticity
1. Introduction
A fibrin network is the first scaffold that a cell encounters as it performs its role in healing wounds due to trauma or other insults to normal tissue. Platelets bind and pull on the fibrin strands as soon as they assemble into a network (Kuntamukkula et al. 1978), neutrophils and macrophages (Ciano et al. 1986) attach to fibrin as they home to sites of injury to dispose of dead tissues and infectious agents that have breached the epidermal barrier, and fibroblasts first anchor to fibrin as they enter the wound site to rebuild the tissue (Brown et al. 1993; Laurens et al. 2006). Unlike the extracellular matrices and basement membranes formed by collagen, laminin and proteoglycans, which assemble slowly in an ordered manner dictated by the cells that secrete them, fibrin gels assemble rapidly by a modified polycondensation reaction (Janmey 1982) from fibrinogen, an abundant constituent of blood plasma, as soon as the protease thrombin is activated in the clotting cascade and begins to remove the part of the fibrinogen polypeptides that prevent its spontaneous polymerization. The result is a three-dimensional network of branching fibres, as shown in figure 1, that form hydrogels with large elastic moduli at very low volume fraction of polymer.
Figure 1.
Scanning electron micrograph of 1 mg l−1 human fibrinogen polymerized by 1 U ml−1 thrombin at pH 7.4 and 150 mM NaCl. Full width of the figure is 62.5 μm.
In contrast to the more permanent extracellular matrices, which are usually formed by the sequential assembly of numerous components in a spatially ordered array that is difficult or impossible to reproduce in vitro, fibrin gels formed from purified plasma proteins acquire structures and mechanical properties that are very similar to those of the blood clot. Furthermore, the fibrin clot is biodegradable, being removed in a systematic way by the fibrinolytic system (Weisel 2005). The presence of cells, especially the ones such as platelets that induce internal tensions in the fibrin network (Cohen et al. 1975), modifies the mechanical properties of blood clots in vivo, but the structures of the fibrin strands and the geometry of the networks they form are similar to those formed in vitro. Although platelets do also affect fibrin structure because their membranes contain fibrin(ogen)-binding integrins and enzyme complexes that activate thrombin, addition of isolated cells that produce internal tensions can reproduce many aspects of the native fibrin gel (Shah & Janmey 1997). Fibrin networks also specifically bind numerous proteins resident in normal blood or released into it in response to wounding, and many of these factors, such as fibronectin, growth factors and protease inhibitors, can be added back in vitro and either bind the fibrin network non-covalently or are ligated to it by the transglutaminase factor XIIIa (reviewed in Weisel 2005).
The abundance of fibrinogen, the relative ease of its purification, the ability to control gelation times by altering thrombin concentrations and the mechanical properties of fibrin gels all have advantages for aspects of wound healing and tissue engineering and have been applied in a wide range of settings (Weisel & Cederholm-Williams 1997; Laurens et al. 2006). The structure of the fibrinogen monomer, the mechanisms of its activation by thrombin and subsequent polymerization and its interaction with various cell types are relatively well characterized and have been reported in several extensive recent reviews (Mosesson 2005; Weisel 2005; Lord 2007). This short review will briefly summarize the aspects of fibrin structure most closely related to its mechanical properties, examine the unique mechanical properties of fibrin that appear to be advantageous for its clinical application and report some recent examples of its potential for both tissue engineering and therapy.
2. Structural features of fibrinogen and its polymerization
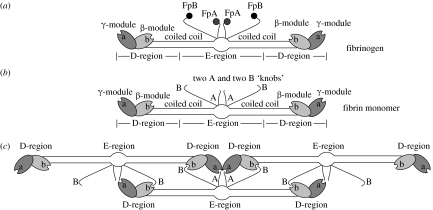
Fibrinogen is a glycoprotein 45 nm in length and made up of three pairs of polypeptide chains, (AαBβγ)2, including the fibrinopeptides A and B, held together by 29 disulphide bonds (Weisel 2005). The amino termini of all six chains are joined in the central region, which is connected to the end domains by α-helical coiled coils. The central region also contains the two pairs of fibrinopeptides (figure 2a). The distal end regions comprise the C-terminal γ-chains, while the proximal end regions comprise the C-terminal Bβ-chains. The Aα-chains fold back to comprise a fourth strand of the coiled coil and connect to the αC-domains, which bind to the central region. Fibrinogen has three high-affinity binding sites for calcium ions and several lower affinity sites.
Figure 2.
(a–c) Schematic of the structure of fibrin monomers and their assembly into protofibrils.
Fibrin polymerization is initiated by the action of the proteolytic enzyme, thrombin, to cleave the fibrinopeptides from the middle of fibrinogen to produce fibrin monomer (Weisel 2005). Thrombin, which is generated by the clotting cascade from prothrombin, cleaves at specific Arg-Gly residues, with the A fibrinopeptides being cleaved more rapidly than the B fibrinopeptides. Cleavage of the A fibrinopeptides exposes knobs ‘A’ that are complementary to holes ‘a’ that are always exposed near the ends of the molecule in the γC-module, as shown schematically in figure 2b. There are also knobs ‘B’ exposed by the removal of the B fibrinopeptides that are complementary to holes ‘b’ in the βC-module. Specific interactions between knobs ‘A’ and holes ‘a’ yield protofibrils in which the fibrin monomers are half-staggered, since the central region of one molecule binds to the end of an adjacent molecule (figure 2c). B fibrinopeptide cleavage occurs primarily from fibrin oligomers and enhances lateral aggregation, such that the clots made up of thicker fibres are produced with the cleavage of both fibrinopeptides rather than from the removal of the A peptides only. The molecular mechanisms underlying the effects of B fibrinopeptide removal are not well understood, but may include B–b interactions, release of the αC-domains and binding interactions of the B peptides (Weisel 2007b).
These initial oligomers lengthen with the addition of more monomers to make two-stranded protofibrils, which aggregate into fibres having a repeat of 22.5 nm, half the molecular length of fibrin(ogen). Protofibril assembly occurs by highly specific protein–protein interactions, and micrometre-long single, semiflexible protofibrils of approximately 10 nm diameter are stable at high pH (8.5) and [NaCl] (0.45 M) (Janmey & Ferry 1986). Under physiological ionic conditions, protofibrils assemble into fibres by lateral interactions that involve both specific, relatively weak protein bonds and interactions with chloride ions (Di Stasio et al. 1998). Gels can be formed by either protofibrils or fibres, to make so-called fine and coarse clots, respectively (Ferry & Morrison 1947). Lateral growth of fibres appears to be limited because protofibrils are twisted and the interactions in lateral aggregation are specific, so that, as the fibre diameter is increased, they must be stretched to traverse an increasing path length (Weisel et al. 1987). With this model, fibre growth ceases when the energy to stretch an added protofibril exceeds the energy of bonding.
Fibres branch to make a three-dimensional network, and most branch points are trifunctional, in other words consisting of the junction of three fibres (Ryan et al. 1999). Branching is generally the opposite of lateral aggregation, in that the conditions that favour lateral aggregation yield clots made of thick fibres with few branch points, whereas the conditions that hinder lateral aggregation yield clots with thin fibres and many branch points. Branching is essential for fibrin to make a space-filling gel.
The properties of clots vary greatly depending on the conditions of polymerization (Weisel 2007a). The thickness of fibres, the extent of branching and the pore size of clots can vary to a very large extent, depending on a whole host of factors. For example, the thrombin concentration can have dramatic effects on clot structure, with low thrombin yielding clots with thick fibres, few branch points and large pores. Similarly, salt concentration, pH and many plasma proteins affect clot structure and properties.
The blood clot is stabilized by the formation of covalent bonds introduced by the plasma transglutaminase, factor XIIIa, which makes it more stable mechanically and more resistant to proteolytic digestion (Ariëns et al. 2002; Lorand & Graham 2003). The active enzyme, factor XIIIa, is generated from its precursor, factor XIII, by the action of thrombin in the presence of calcium ions and fibrin. Specific glutamine and lysine side chains are joined, first those near the C-terminal end of the γ-chains, yielding primarily γ-dimers, followed by others near the C-terminal ends of the α-chains, yielding a whole series of α-polymers.
3. Rheological features of fibrin gels
Fibrin is a viscoelastic polymer, with both elastic and viscous properties. Its viscoelastic properties can vary greatly, depending on clot structure and biochemical properties (Weisel 2004). During polymerization, finite clot stiffness can first be detected when the network is established, at the gel point, and the stiffness increases with time. For small strains over short times, the clots are highly elastic and there is little inelastic component over a wide range of frequencies or rate of application of strain. At higher frequencies of strain, inelastic deformation becomes apparent and increases in magnitude with the rate of change. Creep in fibrin occurs over very long times and substantial deformation can take place. However, surprisingly for fine clots the recovery of the original stiffness after removal of the initial stress is nearly complete. In other words, apparently there are no net changes in structure during the rearrangements responsible for the creep.
4. Nonlinear elasticity of fibrin gels
The elongated, but still compliant, nature of the fibrin strands and the inherent branches (cross links) built into the polymerization mechanism produce protein networks with viscoelastic properties that differ in many respects from those of synthetic hydrogels. One of the most interesting rheological properties of fibrin is its nonlinear elasticity. The elastic modulus of fibrin, measured in either shear or elongational strain, strongly increases the more the material is deformed (strained), as shown in figure 3. The strain-stiffening nature of blood clots and purified fibrin gels has been experimentally evident for decades (Janmey et al. 1983), but theoretical models to explain this effect have only recently been developed. Several different models can account for nonlinear elasticity of elongated fibre networks (MacKintosh et al. 1995; Didonna & Lubensky 2005; Onck et al. 2005; Storm et al. 2005; Heussinger et al. 2007; Huisman et al. 2007), and which one applies depends on how flexible the filaments are, compared with the mesh size of the networks. More precisely, if the persistence length of the filament is similar to the mesh size of the network, as it is for fine clots, then strain stiffening emerges naturally from an entropic model that considers how the thermal fluctuations of semiflexible polymer are constrained as the end-to-end distance of the filament between cross-linking points changes when the sample is deformed (MacKintosh et al. 1995; Storm et al. 2005). This entropic model, which assumes no changes in the protein structure until the filament is pulled nearly straight, accounts very well for the elasticity of networks containing fibrin protofibrils (fine clots), as shown schematically in figure 4. Under more physiological conditions, fibrin forms thicker bundles containing dozens or hundreds of protofibrils, which appear too straight in light micrographs to exhibit any significant thermal fluctuations. Coarse fibrin clots, as well as plasma clots, still strain stiffen, and for this case, alternative mainly enthalpic models, based on the orientation and stretching of the fibres are proposed to account for the nonlinear elasticity observed in the experiment (Didonna & Lubensky 2005; Onck et al. 2005; Heussinger et al. 2007; Huisman et al. 2007). The basic idea of these models, shown schematically in figure 4, is that stiff fibres are easier to bend than to stretch, and therefore as strain increases and the filaments align more in the strain direction, a transition occurs from bending to stretching modes, which leads to increased stiffness at increased strains. This latter model is essentially enthalpic and predicts that at strains where the samples stiffen, changes in the protein structure can be expected, and indeed a change from α-helical to β-structures is observed for fibrin fibres at large deformation. These structural changes presumably involve the helical coils connecting the E- and D-regions (Brown et al. 2007).
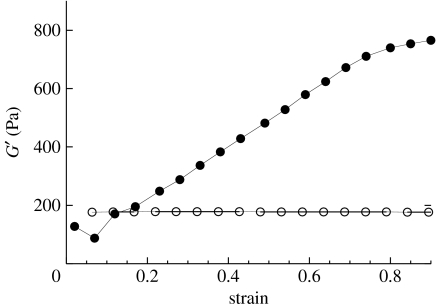
Figure 3.
Strain stiffening of fibrin gels under shear deformation. Dynamic shear storage moduli measured with a strain-controlled rheometer at different maximal strain amplitudes are shown for 6 mg ml−1 human fibrinogen polymerized under coarse clotting conditions (filled circles), and for 5% polyacrylamide polymerized with ammonium persulphate and TEMED (open circles) by standard methods. Detailed methods are given in Storm et al. (2005).
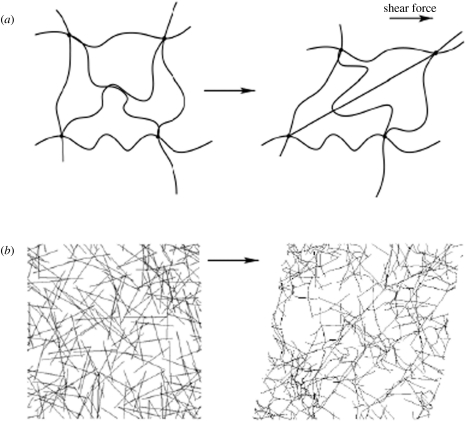
Figure 4.
Schematics for two different mechanisms of strain stiffening. (a) Semiflexible polymers linked at ends in network junctions lose configurational entropy as their end-to-end distances are increased or decreased from their resting lengths during shear deformation. Filaments with intrinsically nonlinear force elongation relationships resist more strongly when they are stretched more to the limit at which the end-to-end distance equals their contour length. Adapted from MacKintosh et al. (1995). (b) Stiff filaments deform initially by bending at small strains and then by stretching at larger strains when their end-to-end vectors align in the shear field. In this mode, fibres with linear force–extension relationships produce strain stiffening in networks owing to the geometrical changes as they align in shear. Adapted from Onck et al. (2005).
Both fine and coarse clots also exhibit negative normal stress under simple shear deformation (Janmey et al. 2007). This property, which is not observed in conventional synthetic gels, implies that at time scales when water can diffuse out of a clot, a shear deformation leads to a downward stress that tends to compress the clot. Such an effect might have relevance to the ways the clots deform under stresses in vivo. The entropic and enthalpic models for nonlinear elasticity predict quantitatively different dependencies of elastic modulus and normal stress on mesh size and strain magnitude, and further studies of fibrin and other biopolymer gels will allow testing of these theories.
5. Relationship between filament flexibility, bundling and macroscopic elastic modulus
A striking mechanical feature of fibrin filaments is that, compared with other filamentous biopolymers, they are very soft. For example, a direct measurement of the stiffness of a 200 nm thick fibrin fibre, consisting of the order of 100 fibrin protofibrils, was made by using an optical trap to pull on a latex bead directly attached to a strand within a fibrin network (Collet et al. 2005). The force required to deform a filament of measured length spanning two branch points to a measured degree of deflection was used to calculate the bending modulus of the filament, k. Young's modulus of the fibrin strand, an intensive variable defining the material properties of the strand, can be calculated from the filament's bending modulus from the relationship EI=κ, where I is the moment of inertia of the cylindrical filaments (=πa4/4, where a is the radius of the fibre). This measurement yields Young's modulus for fibrin of 1.7 MPa, if the filament is not cross-linked by FXIIIa, and of 14.5 MPa, if the fibre is covalently linked by γ–γ- and α–α-bonds (Collet et al. 2005). Deformation of fibrin fibres has also been measured by atomic force microscopy, using an elegant system that allows lateral force to be applied to a filament suspended over a nanofabricated channel. These studies show that an unligated fibrin fibre can be strained 120 per cent without damage, and a ligated fibre can withstand strains of more than 400 per cent without breaking (Liu et al. 2006). The remarkable softness and resistance to breakage distinguish fibrin fibres from all other characterized biopolymers (Guthold et al. 2007), and direct comparisons of biopolymer mechanical properties are shown in table 1.
Table 1.
Comparison of Young's moduli of various filamentous biopolymers.
| filament type | Young's modulus | method | reference |
|---|---|---|---|
| fibrin protofibril | 4 MPa | persistence length | Storm et al. (2005) |
| fibrin fibre−XIIIa | 1.7 MPa | bending | Collet et al. (2005) |
| fibrin fibre+XIIIa | 14 MPa | bending | Collet et al. (2005) |
| F-actin | 100 MPa to 1 GPa | bending | Gittes et al. (1993) |
| microtubule | >1 GPa | bending | Gittes et al. (1993) |
| intermediate filaments | 1.6–8 MPa | various | Wagner et al. (2007) |
Young's modulus of a single fibrin protofibril has not yet been directly measured by force–deflection studies but can be inferred from its measured persistence length λp, a geometric quantity, derived from electron micrographs of fibrin protofibrils (Janmey & Ferry 1986), that quantifies the distance over which the protofibril's contour is approximately straight. More precisely, persistence length is defined by the expression
| (5.1) |
where 〈cos θ(s)〉 is an ensemble average of the angle θ formed by two tangents drawn at distances s along the contour.
The persistence length is related to the elastic bending constant of the filament κ by the expression
| (5.2) |
where kBT is the thermal energy. The persistence length of fibrin protofibrils is measured to be 500 nm (Storm et al. 2005), corresponding to Young's modulus of 4 MPa taking the diameter of a protofibril to be 10 nm.
The similarity of Young's moduli of fibrin protofibrils and fibres implies that the bonds holding protofibrils together within a fibre are strong enough to prevent protofibril slippage as a fibre is bent, and suggests that the compliance of the fibre comes from longitudinal stretching due to the flexibility of the superhelical coils formed by the three strands in the regions connecting the central and end domains. Recent studies by atomic force microscopy have shown that stretching and limited unfolding of fibrin monomers occur at lower forces (Brown et al. 2007) than those required to detach a fibrin monomer from the lateral bonds holding it within the protofibril (Litvinov et al. 2005; Brown et al. 2007; Chtcheglova et al. 2008).
The softness of the fibrin strand (1.5 to 10 MPa) is evident by comparison of its Young's modulus with that of actin filaments (44 MPa; Blange et al. 1997) and microtubules (100–1000 MPa; Mizushima-Sugano et al. 1983; Gittes et al. 1993). In terms of persistence length, fibrin protofibrils are similar to intermediate filaments (λp=0.2–1 μm) but much more flexible than either F-actin (λp=17 μm) or microtubules (λp=>1 mM) (Wagner et al. 2007).
6. Current clinical and bioengineering uses
6.1 Haemostatic glue and wound repair
Fibrin glue is currently being used clinically as an adjunct therapy to stem bleeding and to replace sutures in certain applications (Weisel & Cederholm-Williams 1997; Albala & Lawson 2006). Since the blood comes from a common pool, there is a risk of disease or prion transmission, even though the solutions are carefully processed and screened. To avoid this risk, researchers have developed systems to concentrate autologous fibrin glue from a patient's serum. Two major limitations of these systems are that the sealant solutions can take several hours to prepare and the patient cannot suffer from any clotting disorders.
To ensure that clotting occurs quickly and effectively, fibrin glues contain very high amounts of fibrinogen and thrombin, up to 60 mg ml−1 fibrinogen and 300 IU ml−1 thrombin after mixing (Buchta et al. 2004, 2005). Current clinical uses include mesh fixation for inguinal hernia repair, severed sciatic nerve reattachment, stabilization of microsurgical anastomoses and skin graft adhesion (Martins et al. 2005; Mittermayr et al. 2006; Andree et al. 2008). Using fibrin glue instead of sutures or staples enhances healing, minimizes scarring, eases application and in the case of hernia repair lowers the risk of nerve injury and postoperative neuralgia (Santoro et al. 2007). Fibrin glue has also been shown to be effective in helping to anchor a deep brain stimulation electrode for the treatment of Parkinson's disease by acting as a protective layer between the dura and the methyl methacrylate bioglue that anchors the upper portion of the electrode to a stabilized titanium plate (Bjarkam et al. 2007).
6.2 Drug delivery
To provide injury repair, it is often desirable to locally deliver tissue-specific growth factors in a controlled manner. Fibrin is an appealing drug delivery vehicle because it can be injected where it gels in situ, it is degraded naturally and it stimulates the body's own wound healing response. By modifying the interaction between the growth factor and the fibrin scaffold, it is possible to vary the release profile from hours to weeks. The release rate is dependent upon the growth factor's initial concentration, diffusion rate and interaction with the matrix. Increasing the fibrinogen concentration would reduce the matrix pore size and thus retard the permeation of large solutes; however, increasing the concentration also retards endothelial cell migration and capillary formation (Nehls & Herrmann 1996; Nehls et al. 1998; Bhang et al. 2007).
Most strategies to regulate growth factor release from fibrin involve modulating the interaction between the gel and the signalling protein. Some proteins such as basic fibroblast growth factor, vascular endothelial growth factor, connective tissue growth factor and heparin reversibly bind fibrin with a known KD (Odrljin et al. 1996; Sahni et al. 1998; Sahni & Francis 2000; Yoshida & Munakata 2007). Heparin can act as a linker between the fibrin network and the growth factors that do not interact with fibrin but do bind heparin such as nerve growth factor and neurotrophin-3 (Lobb et al. 1986; Taylor & Sakiyama-Elbert 2006).
To achieve more controlled release, one group used a phage display assay to identify peptides with a high binding coefficient for nerve growth factor and linked them to a transglutaminase substrate domain, which factor XIIIa then covalently incorporated into the polymerizing fibrin (Willerth et al. 2007). To synchronize bone morphogenic protein-2 (BMP-2) release with gel degradation, Schmoekel et al. designed a fusion protein of BMP-2, a plasmin cleavage domain and a transglutaminase domain (Schmoekel et al. 2005). Since growth factor release is now dependent upon the presence of plasmin and surface availability of the fibres, the initial burst release seen in diffusion-limited release is damped. In an interesting reversal, one study used fibrin glue as a perfusion barrier to protect the spinal canal and nerve root from bone ingrowth stimulated by the application of recombinant human BMP-2 to facilitate spinal disc fusion (Patel et al. 2006). These methods allow researchers to design a release profile that fits the particular need rather than rely on nature's limitations.
6.3 Cell delivery
Growth factor delivery is sufficient only if there are cells in the injury area capable of responding to the signals. When defects exceed a critical size, the local cell population is not sufficient to repair the injury and additional cells must be introduced. This is another application where fibrin has proven useful. Keratinocytes suspended in fibrin were effective in reconstituting full thickness wounds in both mouse and human subjects, while skin fibroblast-coated fibrin microbeads decreased the time for granulation tissue formation (Horch et al. 1998, 2001; Gorodetsky et al. 1999). By layering a film of keratinocytes suspended in plasma on top of a layer of suspended fibroblasts, a fibrin-based bilayer skin equivalent was developed, which when grafted onto a mouse skin defect resulted in successful integration with the surrounding skin and a mature dermal–epidermal junction (Mazlyzam et al. 2007).
In plastic surgery, fat tissue transplantation is often required to create the desired look but this process can result in donor site deformity and also a gradual resorption of the transplanted tissue. Preadipocyte-seeded fibrin has been investigated as a possible alternative to autologous tissue transplantation, and in mice has resulted in the formation of stable adipose tissue (Torio-Padron et al. 2007). An experiment of particular interest showed that injecting skeletal myoblasts in a fibrin scaffold into infarct regions of a live rat heart that had been occluded for 17 min before 7 days improved cell survival and reduced the infarct scar (Christman et al. 2004). In this setting, the elastic modulus of the scaffold may be of particular significance since myocyte development does not proceed normally on materials with abnormally high stiffness (Engler et al. 2004).
6.4 Cell differentiation and tissue engineering
Unlike a synthetic hydrogel, fibrin is not just a passive cell delivery matrix, but it binds specifically many growth factors as well as clot components, such as fibronectin, hyaluronic acid and von Willebrand factor (Weisel 2005). Fibrin has two pairs of RGD sites and a pair of AGDV sites through which it can interact with integrins and has several sites that interact with the leucocyte integrin αmβ2 (Cheresh et al. 1989; Altieri et al. 1990; Lishko et al. 2002; Chernousov & Carey 2003). Once fibrinopeptide B has been cleaved by thrombin to reveal β15–42, endothelial cells can also bind to the network through VE cadherins (Bach et al. 1998).
This bioactivity makes fibrin an attractive matrix for stem cell differentiation and tissue engineering. By modulating the mechanical and chemical properties of a fibrin-based matrix, human mesenchymal stem cells have been differentiated into osteoblasts and mouse embryonic stem cells have been coaxed down neural and astroglial lineages (Catelas et al. 2006; Willerth et al. 2006). Osteogenic differentiation required very high concentrations of fibrinogen and Ca2+, whereas neurogenic differentiation required more physiological fibrinogen and Ca2+ concentrations and preculturing of the embryoid bodies in retinoic acid-containing media.
Though fibrin has been effective in vitro at promoting osteogenesis, it lacks the mechanical strength required for bone tissue engineering and in vivo studies have been disappointing even when a stiff scaffold encases the fibrin (Kneser et al. 2005; Arkudas et al. 2007). Fibrin has been more effective as a scaffold for cartilage, cardiovascular and nervous tissue engineering. In cartilage tissue engineering, fibrin promotes glycosaminoglycan and collagen II while inhibiting collagen I deposition by primary or stem cell-derived chondrocytes (Dragoo et al. 2007; Eyrich et al. 2007). This trend is further enhanced by adding hyaluronic acid to the gel (Park et al. 2005). To enhance the strength of the fibrin gel, a combination strategy can be employed whereby a highly porous synthetic polymer scaffold with appropriate mechanical properties is filled with chondrocytes suspended in a fibrin gel and this whole construct is implanted at the desired junction (Eyrich et al. 2007).
Severed peripheral nerve axons have some capacity to repair themselves but only if the defect is below a certain critical length. Fibrin sealant has been used for promoting nerve repair for a long time (Young & Medawar 1940). To promote regeneration over longer lengths, channels filled with stimulatory signals are used to bridge the severed ends. Synthetic polymer channels filled with fibrin improve nerve repair and this is further enhanced if Schwann cells, which bind fibrin through αvβ8 integrins and are required for healthy axon development, are suspended in the fibrin (Chernousov & Carey 2003; Galla et al. 2004; Tsai et al. 2006).
In addition to its central role in haemostasis, the fibrin clot also serves as a scaffold for angiogenesis. This makes fibrin uniquely suited for cardiovascular tissue engineering. As with cartilage and nerve regeneration, the best results are achieved when a synthetic scaffold with the desired mechanical properties is combined with a biologically tuned fibrin gel (Mol et al. 2005; Sreerekha & Krishnan 2006). One of the most advanced applications of a fibrin gel in cardiovascular engineering is the development of a moulding system to produce tricuspid heart valve replacements from fibrin, fibroblasts and smooth muscle myocytes (Jockenhoevel et al. 2001). Again mechanical properties need to be controlled, and in this case the engineered valves were cultured in a pulsatile flow bioreactor, which resulted in less shrinkage as well as improved alignment of the cells and the ECM components, strengthening the final valve (Flanagan et al. 2007).
6.5 Patterning
Most of the techniques described above create chemically and structurally uniform gels, but many tissues are patterned. Growth factor patterning has been achieved in two dimensions by using ink-jet printing to control application of bFGF onto fibrin films (Campbell et al. 2005). Cell adhesion was higher in areas where bFGF was applied, and this difference was maintained over at least 5 days (Miller et al. 2006). Extension of this printing method to three dimensions would be useful for the production of fibrin bandages or skin equivalents.
An increasingly common method to pattern tissue equivalents is by culturing them in a bioreactor. The bioreactors are individually designed to apply physiologically relevant forces to precondition the implants for their in vivo application. When a constant 25 per cent or greater tensile strain is applied to a 3 mg ml−1 fibrin gel, the fibril bundles and myoblasts or endothelial cells cultured within this gel align and divide along the bundles, forming lines of cells (Matsumoto et al. 2007). This could prove useful in creating linearly organized tissues but the authors do not describe what happens to the cells when the tensile force is released.
Lastly, the grafts can be patterned by orienting the fibrin fibrils as they are polymerizing, which results in an anisotropic structure independent of applied force. This can be done by controlling the initiation sites, polymerizing fibrin under flow or in a high magnetic field or by electrospinning the protein on to a rotating target (Dubey et al. 2001; McManus et al. 2006, 2007). Aligned fibrin has been shown to enhance neurite alignment and could be a useful filler for nerve guidance channels (Dubey et al. 2001). Electrospinning has the added advantage of being able to design more advanced patterns, such as cross-hatching, by manipulating the electric field (Teo et al. 2006). Control of solution conditions can also control fibre thickness and therefore permeability through the matrix (Sell et al. 2008).
7. Conclusions
Although it is one of the most abundant and well-characterized polymers in nature, fibrin continues to reveal fascinating features of self-assembly and soft elasticity, and presents many potential uses both as a haemostatic agent in clinical applications and as a scaffold for tissue engineering. Unlike nearly all native cellular matrices and even many synthetic scaffolds, fibrin is designed to assemble rapidly under physiological conditions by the activation of the soluble fibrinogen monomer by the proteolytic enzyme thrombin. From a practical perspective, methods to purify gram quantities of highly purified fibrinogen, and correspondingly large amounts of thrombin, from both mammalian and non-mammalian blood plasma have enabled many tests of its potential application in biological settings. The fact that both fibrinogen and thrombin retain activity after lyophilization and prolonged storage at ambient temperatures, sterilization by heat and gamma irradiation, and processing by methods such as electrospinning is a significant advantage over other protein polymers. Potential disadvantages for its use in vivo include the possibility of immune response or infectious disease transmission when using heterologous proteins. Improved methods to inactivate bacteria and viruses and the potential to produce both fibrinogen and thrombin as recombinant proteins secreted into culture by mammalian cell lines may significantly improve the safety and effectiveness of fibrin-based materials.
As an interface for cells grown on two-dimensional or within three-dimensional matrices, fibrin has unique properties that are not matched by other materials. The fibrin fibre is reported to be the most highly extensible of any filamentous biopolymer, and can resist stretching to more than five times its resting length without breakage. Even elongational strains greater than 100 per cent are completely recoverable when the stress is released. The high degree of deformation tolerated by the fibrin fibre is accommodated in part by very loose packing of monomers and high water content in the fibre and by partially reversible secondary structural transitions within the monomers. The softness and large compliance of the fibrin gel appear to be essential for its efficiency as a matrix for cells such as neurons that normally reside in very soft tissues such as brain. Continued study of the combined effects of fibrin network mechanics and the biochemical features of fibrin is likely to lead to many new applications of this venerable biomaterial.
References
- Albala D.M, Lawson J.H. Recent clinical and investigational applications of fibrin sealant in selected surgical specialties. J. Am. College Surg. 2006;202:685–697. doi: 10.1016/j.jamcollsurg.2005.11.027. [DOI] [PubMed] [Google Scholar]
- Altieri D.C, Agbanyo F.R, Plescia J, Ginsberg M.H, Edgington T.S, Plow E.F. A unique recognition site mediates the interaction of fibrinogen with the leukocyte integrin Mac-1 (CD11b/CD18) J. Biol. Chem. 1990;265:12 119–12 122. [PubMed] [Google Scholar]
- Andree, C. et al 2008 Improved safety of autologous breast reconstruction surgery by stabilisation of microsurgical vessel anastomoses using fibrin sealant in 349 free DIEP or fascia-muscle-sparing (fms)-TRAM flaps: a two-centre study. Breast 17, 492–498. ( 10.1016/j.breast.2008.03.010) [DOI] [PubMed]
- Ariëns R.A.S, Lai T.-S, Weisel J.W, Greenberg C.S, Grant P.J. Role of factor XIII in fibrin clot formation and effects of genetic polymorphisms. Blood. 2002;100:743–754. doi: 10.1182/blood.V100.3.743. [DOI] [PubMed] [Google Scholar]
- Arkudas A, Tjiawi J, Bleiziffer O, Grabinger L, Polykandriotis E, Beier J.P, Sturzl M, Horch R.E, Kneser U. Fibrin gel-immobilized VEGF and bFGF efficiently stimulate angiogenesis in the AV loop model. Mol. Med. 2007;13:480–487. doi: 10.2119/2007-00057.Arkudas. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bach T.L, Barsigian C, Chalupowicz D.G, Busler D, Yaen C.H, Grant D.S, Martinez J. VE-Cadherin mediates endothelial cell capillary tube formation in fibrin and collagen gels. Exp. Cell Res. 1998;238:324–334. doi: 10.1006/excr.1997.3844. [DOI] [PubMed] [Google Scholar]
- Bhang S.H, Jeon O, Choi C.Y, Kwon Y.H, Kim B.S. Controlled release of nerve growth factor from fibrin gel. J. Biomed. Mater. Res. A. 2007;80:998–1002. doi: 10.1002/jbm.a.31050. [DOI] [PubMed] [Google Scholar]
- Bjarkam C.R, Jorgensen R.L, Jensen K.N, Sunde N.A, Sørensen J.C. Deep brain stimulation electrode anchoring using BioGlue®, a protective electrode covering, and a titanium microplate. J. Neurosci. Methods. 2007;168:151–155. doi: 10.1016/j.jneumeth.2007.09.008. [DOI] [PubMed] [Google Scholar]
- Blange T, van der Heide U.A, Treijtel B.W, de Beer E.L. The effect of actin filament compliance on the interpretation of the elastic properties of skeletal muscle fibres. J. Muscle Res. Cell Motil. 1997;18:125–131. doi: 10.1023/A:1018649420778. [DOI] [PubMed] [Google Scholar]
- Brown L.F, Lanir N, McDonagh J, Tognazzi K, Dvorak A.M, Dvorak H.F. Fibroblast migration in fibrin gel matrices. Am. J. Pathol. 1993;142:273–283. [PMC free article] [PubMed] [Google Scholar]
- Brown A.E, Litvinov R.I, Discher D.E, Weisel J.W. Forced unfolding of coiled-coils in fibrinogen by single-molecule AFM. Biophys. J. 2007;92:L39–L41. doi: 10.1529/biophysj.106.101261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchta C, Dettke M, Funovics P.T, Hirschl A.M, Macher M, Worel N, Hocker P. Impact of manufacturing, irradiation and filtration steps to bacterial contamination of autologous fibrin sealant. Biologicals. 2004;32:165–169. doi: 10.1016/j.biologicals.2004.03.004. [DOI] [PubMed] [Google Scholar]
- Buchta C, Hedrich H.C, Macher M, Hocker P, Redl H. Biochemical characterization of autologous fibrin sealants produced by CryoSeal and Vivostat in comparison to the homologous fibrin sealant product Tissucol/Tisseel. Biomaterials. 2005;26:6233–6241. doi: 10.1016/j.biomaterials.2005.04.014. [DOI] [PubMed] [Google Scholar]
- Campbell P.G, Miller E.D, Fisher G.W, Walker L.M, Weiss L.E. Engineered spatial patterns of FGF-2 immobilized on fibrin direct cell organization. Biomaterials. 2005;26:6762–6770. doi: 10.1016/j.biomaterials.2005.04.032. [DOI] [PubMed] [Google Scholar]
- Catelas I, Sese N, Wu B.M, Dunn J.C, Helgerson S, Tawil B. Human mesenchymal stem cell proliferation and osteogenic differentiation in fibrin gels in vitro. Tissue Eng. 2006;12:2385–2396. doi: 10.1089/ten.2006.12.2385. [DOI] [PubMed] [Google Scholar]
- Cheresh D.A, Berliner S.A, Vicente V, Ruggeri Z.M. Recognition of distinct adhesive sites on fibrinogen by related integrins on platelets and endothelial cells. Cell. 1989;58:945–953. doi: 10.1016/0092-8674(89)90946-X. [DOI] [PubMed] [Google Scholar]
- Chernousov M.A, Carey D.J. αVβ8 integrin is a Schwann cell receptor for fibrin. Exp. Cell Res. 2003;291:514–524. doi: 10.1016/S0014-4827(03)00409-9. [DOI] [PubMed] [Google Scholar]
- Christman K.L, Vardanian A.J, Fang Q, Sievers R.E, Fok H.H, Lee R.J. Injectable fibrin scaffold improves cell transplant survival, reduces infarct expansion, and induces neovasculature formation in ischemic myocardium. J. Am. Coll. Cardiol. 2004;44:654–660. doi: 10.1016/j.jacc.2004.04.040. [DOI] [PubMed] [Google Scholar]
- Chtcheglova L.A, Haeberli A, Dietler G. Force spectroscopy of the fibrin(ogen)–fibrinogen interaction. Biopolymers. 2008;89:292–301. doi: 10.1002/bip.20910. [DOI] [PubMed] [Google Scholar]
- Ciano P.S, Colvin R.B, Dvorak A.M, McDonagh J, Dvorak H.F. Macrophage migration in fibrin gel matrices. Lab. Invest. 1986;54:62–70. [PubMed] [Google Scholar]
- Cohen I, Gabbay J, Glaser T, Oplatka A. Fibrin-blood platelet interaction in a contracting clot. Br. J. Haematol. 1975;31:45–50. doi: 10.1111/j.1365-2141.1975.tb00830.x. [DOI] [PubMed] [Google Scholar]
- Collet J.P, Shuman H, Ledger R.E, Lee S, Weisel J.W. The elasticity of an individual fibrin fiber in a clot. Proc. Natl Acad. Sci. USA. 2005;102:9133–9137. doi: 10.1073/pnas.0504120102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Didonna B, Lubensky T. Nonaffinity and nonlinearity in random elastic networks. Phys. Rev. E. 2005;72:066 619. doi: 10.1103/PhysRevE.72.066619. [DOI] [PubMed] [Google Scholar]
- Di Stasio E, Nagaswami C, Weisel J.W, Di Cera E. Cl− regulates the structure of the fibrin clot. Biophys. J. 1998;75:1973–1979. doi: 10.1016/S0006-3495(98)77638-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dragoo J.L, Carlson G, McCormick F, Khan-Farooqi H, Zhu M, Zuk P.A, Benhaim P. Healing full-thickness cartilage defects using adipose-derived stem cells. Tissue Eng. 2007;13:1615–1621. doi: 10.1089/ten.2006.0249. [DOI] [PubMed] [Google Scholar]
- Dubey N, Letourneau P.C, Tranquillo R.T. Neuronal contact guidance in magnetically aligned fibrin gels: effect of variation in gel mechano-structural properties. Biomaterials. 2001;22:1065–1075. doi: 10.1016/S0142-9612(00)00341-0. [DOI] [PubMed] [Google Scholar]
- Engler A.J, Griffin M.A, Sen S, Bonnemann C.G, Sweeney H.L, Discher D.E. Myotubes differentiate optimally on substrates with tissue-like stiffness: pathological implications for soft or stiff microenvironments. J. Cell Biol. 2004;166:877–887. doi: 10.1083/jcb.200405004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eyrich D, Brandl F, Appel B, Wiese H, Maier G, Wenzel M, Staudenmaier R, Goepferich A, Blunk T. Long-term stable fibrin gels for cartilage engineering. Biomaterials. 2007;28:55–65. doi: 10.1016/j.biomaterials.2006.08.027. [DOI] [PubMed] [Google Scholar]
- Ferry J.D, Morrison P.R. Preparation and properties of serum and plasma proteins. VIII. The conversion of human fibrinogen to fibrin under various conditions. J. Am. Chem Soc. 1947;69:388–400. doi: 10.1021/ja01194a066. [DOI] [PubMed] [Google Scholar]
- Flanagan T.C, Cornelissen C, Koch S, Tschoeke B, Sachweh J.S, Schmitz-Rode T, Jockenhoevel S. The in vitro development of autologous fibrin-based tissue-engineered heart valves through optimised dynamic conditioning. Biomaterials. 2007;28:3388–3397. doi: 10.1016/j.biomaterials.2007.04.012. [DOI] [PubMed] [Google Scholar]
- Galla T.J, Vedecnik S.V, Halbgewachs J, Steinmann S, Friedrich C, Stark G.B. Fibrin/Schwann cell matrix in poly-epsilon-caprolactone conduits enhances guided nerve regeneration. Int. J. Artif. Organs. 2004;27:127–136. doi: 10.1177/039139880402700208. [DOI] [PubMed] [Google Scholar]
- Gittes F, Mickey B, Nettleton J, Howard J. Flexural rigidity of microtubules and actin filaments measured from thermal fluctuations in shape. J. Cell Biol. 1993;120:923–934. doi: 10.1083/jcb.120.4.923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorodetsky R, Clark R.A, An J, Gailit J, Levdansky L, Vexler A, Berman E, Marx G. Fibrin microbeads (FMB) as biodegradable carriers for culturing cells and for accelerating wound healing. J. Invest. Dermatol. 1999;112:866–872. doi: 10.1046/j.1523-1747.1999.00600.x. [DOI] [PubMed] [Google Scholar]
- Guthold M, Liu W, Sparks E.A, Jawerth L.M, Peng L, Falvo M, Superfine R, Hantgan R.R, Lord S.T. A comparison of the mechanical and structural properties of fibrin fibers with other protein fibers. Cell Biochem. Biophys. 2007;49:165–181. doi: 10.1007/s12013-007-9001-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heussinger C, Schaefer B, Frey E. Nonaffine rubber elasticity for stiff polymer networks. Phys. Rev. E Stat. Nonlin. Soft Matter Phys. 2007;76:031 906. doi: 10.1103/PhysRevE.76.031906. [DOI] [PubMed] [Google Scholar]
- Horch R.E, Bannasch H, Kopp J, Andree C, Stark G.B. Single-cell suspensions of cultured human keratinocytes in fibrin-glue reconstitute the epidermis. Cell Transplant. 1998;7:309–317. doi: 10.1016/S0963-6897(98)00005-0. [DOI] [PubMed] [Google Scholar]
- Horch R.E, Bannasch H, Stark G.B. Transplantation of cultured autologous keratinocytes in fibrin sealant biomatrix to resurface chronic wounds. Transplant. Proc. 2001;33:642–644. doi: 10.1016/S0041-1345(00)02181-3. [DOI] [PubMed] [Google Scholar]
- Huisman E.M, van Dillen T, Onck P.R, Van der Giessen E. Three-dimensional cross-linked F-actin networks: relation between network architecture and mechanical behavior. Phys. Rev. Lett. 2007;99:208 103. doi: 10.1103/PhysRevLett.99.208103. [DOI] [PubMed] [Google Scholar]
- Janmey P.A. Kinetics of formation of fibrin oligomers. I. Theory. Biopolymers. 1982;21:2253–2264. doi: 10.1002/bip.360211112. [DOI] [PubMed] [Google Scholar]
- Janmey P.A, Ferry J.D. Gel formation by fibrin oligomers without addition of monomers. Biopolymers. 1986;25:1337–1344. doi: 10.1002/bip.360250712. [DOI] [PubMed] [Google Scholar]
- Janmey P.A, Amis E, Ferry J. Rheology of fibrin clots. VI. Stress relaxation, creep and differential dynamic modulus of fine clots in large shearing deformations. J. Rheol. 1983;27:135–153. doi: 10.1122/1.549722. [DOI] [Google Scholar]
- Janmey P.A, McCormick M.E, Rammensee S, Leight J.L, Georges P.C, Mackintosh F.C. Negative normal stress in semiflexible biopolymer gels. Nat. Mater. 2007;6:48–51. doi: 10.1038/nmat1810. [DOI] [PubMed] [Google Scholar]
- Jockenhoevel S, Zund G, Hoerstrup S.P, Chalabi K, Sachweh J.S, Demircan L, Messmer B.J, Turina M. Fibrin gel—advantages of a new scaffold in cardiovascular tissue engineering. Eur. J. Cardiothorac. Surg. 2001;19:424–430. doi: 10.1016/S1010-7940(01)00624-8. [DOI] [PubMed] [Google Scholar]
- Kneser U, Voogd A, Ohnolz J, Buettner O, Stangenberg L, Zhang Y.H, Stark G.B, Schaefer D.J. Fibrin gel-immobilized primary osteoblasts in calcium phosphate bone cement: in vivo evaluation with regard to application as injectable biological bone substitute. Cells Tissues Organs. 2005;179:158–169. doi: 10.1159/000085951. [DOI] [PubMed] [Google Scholar]
- Kuntamukkula M.S, McIntire L.V, Moake J.L, Peterson D.M, Thompson W.J. Rheological studies of the contractile force within platelet-fibrin clots: effects of prostaglandin E1, dibutyryl-cAMP and dibutyryl-cGMP. Thromb. Res. 1978;13:957–969. doi: 10.1016/0049-3848(78)90225-6. [DOI] [PubMed] [Google Scholar]
- Laurens N, Koolwijk P, de Maat M.P. Fibrin structure and wound healing. J. Thromb. Haemost. 2006;4:932–939. doi: 10.1111/j.1538-7836.2006.01861.x. [DOI] [PubMed] [Google Scholar]
- Lishko V.K, Kudryk B, Yakubenko V.P, Yee V.C, Ugarova T.P. Regulated unmasking of the cryptic binding site for integrin αM β2 in the γ C-domain of fibrinogen. Biochemistry. 2002;41:12 942–12 951. doi: 10.1021/bi026324c. [DOI] [PubMed] [Google Scholar]
- Litvinov R.I, Gorkun O.V, Owen S.F, Shuman H, Weisel J.W. Polymerization of fibrin: specificity, strength, and stability of knob–hole interactions studied at the single-molecule level. Blood. 2005;106:2944–2951. doi: 10.1182/blood-2005-05-2039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W, Jawerth L.M, Sparks E.A, Falvo M.R, Hantgan R.R, Superfine R, Lord S.T, Guthold M. Fibrin fibers have extraordinary extensibility and elasticity. Science. 2006;313:634. doi: 10.1126/science.1127317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobb R, Sasse J, Sullivan R, Shing Y, D'Amore P, Jacobs J, Klagsbrun M. Purification and characterization of heparin-binding endothelial cell growth factors. J. Biol. Chem. 1986;261:1924–1928. [PubMed] [Google Scholar]
- Lorand L, Graham R. Transglutaminases: crosslinking enzymes with pleiotropic functions. Nat. Rev. Mol. Cell. Biol. 2003;4:140–156. doi: 10.1038/nrm1014. [DOI] [PubMed] [Google Scholar]
- Lord S.T. Fibrinogen and fibrin: scaffold proteins in hemostasis. Curr. Opin. Hematol. 2007;14:236–241. doi: 10.1097/MOH.0b013e3280dce58c. [DOI] [PubMed] [Google Scholar]
- MacKintosh F.C, Kas J, Janmey P.A. Elasticity of semiflexible biopolymer networks. Phys. Rev. Lett. 1995;75:4425–4428. doi: 10.1103/PhysRevLett.75.4425. [DOI] [PubMed] [Google Scholar]
- Martins R.S, Siqueira M.G, Da Silva C.F, Plese J.P. Overall assessment of regeneration in peripheral nerve lesion repair using fibrin glue, suture, or a combination of the 2 techniques in a rat model. Which is the ideal choice? Surg. Neurol. 2005;64(Suppl. 1):10–16. doi: 10.1016/j.surneu.2005.04.022. [DOI] [PubMed] [Google Scholar]
- Matsumoto T, Sasaki J, Alsberg E, Egusa H, Yatani H, Sohmura T. Three-dimensional cell and tissue patterning in a strained fibrin gel system. PLoS ONE. 2007;2:e1211. doi: 10.1371/journal.pone.0001211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazlyzam A.L, Aminuddin B.S, Fuzina N.H, Norhayati M.M, Fauziah O, Isa M.R, Saim L, Ruszymah B.H. Reconstruction of living bilayer human skin equivalent utilizing human fibrin as a scaffold. Burns. 2007;33:355–363. doi: 10.1016/j.burns.2006.08.022. [DOI] [PubMed] [Google Scholar]
- McManus M.C, Boland E.D, Koo H.P, Barnes C.P, Pawlowski K.J, Wnek G.E, Simpson D.G, Bowlin G.L. Mechanical properties of electrospun fibrinogen structures. Acta Biomater. 2006;2:19–28. doi: 10.1016/j.actbio.2005.09.008. [DOI] [PubMed] [Google Scholar]
- McManus M.C, Boland E.D, Simpson D.G, Barnes C.P, Bowlin G.L. Electrospun fibrinogen: feasibility as a tissue engineering scaffold in a rat cell culture model. J. Biomed. Mater. Res. A. 2007;81:299–309. doi: 10.1002/jbm.a.30989. [DOI] [PubMed] [Google Scholar]
- Miller E.D, Fisher G.W, Weiss L.E, Walker L.M, Campbell P.G. Dose-dependent cell growth in response to concentration modulated patterns of FGF-2 printed on fibrin. Biomaterials. 2006;27:2213–2221. doi: 10.1016/j.biomaterials.2005.10.021. [DOI] [PubMed] [Google Scholar]
- Mittermayr R, Wassermann E, Thurnher M, Simunek M, Redl H. Skin graft fixation by slow clotting fibrin sealant applied as a thin layer. Burns. 2006;32:305–311. doi: 10.1016/j.burns.2005.10.010. [DOI] [PubMed] [Google Scholar]
- Mizushima-Sugano J, Maeda T, Miki-Noumura T. Flexural rigidity of singlet microtubules estimated from statistical analysis of their contour lengths and end-to-end distances. Biochim. Biophys. Acta. 1983;755:257–262. doi: 10.1016/0304-4165(83)90212-x. [DOI] [PubMed] [Google Scholar]
- Mol A, van Lieshout M.I, Dam-de Veen C.G, Neuenschwander S, Hoerstrup S.P, Baaijens F.P, Bouten C.V. Fibrin as a cell carrier in cardiovascular tissue engineering applications. Biomaterials. 2005;26:3113–3121. doi: 10.1016/j.biomaterials.2004.08.007. [DOI] [PubMed] [Google Scholar]
- Mosesson M.W. Fibrinogen and fibrin structure and functions. J. Thromb. Haemost. 2005;3:1894–1904. doi: 10.1111/j.1538-7836.2005.01365.x. [DOI] [PubMed] [Google Scholar]
- Nehls V, Herrmann R. The configuration of fibrin clots determines capillary morphogenesis and endothelial cell migration. Microvasc. Res. 1996;51:347–364. doi: 10.1006/mvre.1996.0032. [DOI] [PubMed] [Google Scholar]
- Nehls V, Herrmann R, Huhnken M. Guided migration as a novel mechanism of capillary network remodeling is regulated by basic fibroblast growth factor. Histochem. Cell Biol. 1998;109:319–329. doi: 10.1007/s004180050232. [DOI] [PubMed] [Google Scholar]
- Odrljin T.M, Francis C.W, Sporn L.A, Bunce L.A, Marder V.J, Simpson-Haidaris P.J. Heparin-binding domain of fibrin mediates its binding to endothelial cells. Arterioscler. Thromb. Vasc. Biol. 1996;16:1544–1551. doi: 10.1161/01.atv.16.12.1544. [DOI] [PubMed] [Google Scholar]
- Onck P.R, Koeman T, van Dillen T, van der Giessen E. Alternative explanation of stiffening in cross-linked semiflexible networks. Phys. Rev. Lett. 2005;95:178 102. doi: 10.1103/PhysRevLett.95.178102. [DOI] [PubMed] [Google Scholar]
- Park S.H, Park S.R, Chung S.I, Pai K.S, Min B.H. Tissue-engineered cartilage using fibrin/hyaluronan composite gel and its in vivo implantation. Artif. Organs. 2005;29:838–845. doi: 10.1111/j.1525-1594.2005.00137.x. [DOI] [PubMed] [Google Scholar]
- Patel V.V, Zhao L, Wong P, Kanim L, Bae H.W, Pradhan B.B, Delamarter R.B. Controlling bone morphogenetic protein diffusion and bone morphogenetic protein-stimulated bone growth using fibrin glue. Spine. 2006;31:1201–1206. doi: 10.1097/01.brs.0000217650.90861.99. [DOI] [PubMed] [Google Scholar]
- Ryan E.A, Mockros L.F, Weisel J.W, Lorand L. Structural origins of fibrin clot rheology. Biophys. J. 1999;77:2813–2826. doi: 10.1016/S0006-3495(99)77113-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahni A, Francis C.W. Vascular endothelial growth factor binds to fibrinogen and fibrin and stimulates endothelial cell proliferation. Blood. 2000;96:3772–3778. [PubMed] [Google Scholar]
- Sahni A, Odrljin T, Francis C.W. Binding of basic fibroblast growth factor to fibrinogen and fibrin. J. Biol. Chem. 1998;273:7554–7559. doi: 10.1074/jbc.273.13.7554. [DOI] [PubMed] [Google Scholar]
- Santoro E, Agresta F, Buscaglia F, Mulieri G, Mazzarolo G, Bedin N, Mulieri M. Preliminary experience using fibrin glue for mesh fixation in 250 patients undergoing minilaparoscopic transabdominal preperitoneal hernia repair. J. Laparoendosc. Adv. Surg. Tech. A. 2007;17:12–15. doi: 10.1089/lap.2006.0107. [DOI] [PubMed] [Google Scholar]
- Schmoekel H.G, Weber F.E, Schense J.C, Gratz K.W, Schawalder P, Hubbell J.A. Bone repair with a form of BMP-2 engineered for incorporation into fibrin cell ingrowth matrices. Biotechnol. Bioeng. 2005;89:253–262. doi: 10.1002/bit.20168. [DOI] [PubMed] [Google Scholar]
- Sell S, Barnes C, Simpson D, Bowlin G. Scaffold permeability as a means to determine fiber diameter and pore size of electrospun fibrinogen. J. Biomed. Mater. Res. A. 2008;85:115–126. doi: 10.1002/jbm.a.31556. [DOI] [PubMed] [Google Scholar]
- Shah J, Janmey P. Strain hardening of fibrin gels and plasma clots. Rheol. Acta. 1997;36:262–268. doi: 10.1007/s003970050044. [DOI] [Google Scholar]
- Sreerekha P.R, Krishnan L.K. Cultivation of endothelial progenitor cells on fibrin matrix and layering on dacron/polytetrafluoroethylene vascular grafts. Artif. Organs. 2006;30:242–249. doi: 10.1111/j.1525-1594.2006.00211.x. [DOI] [PubMed] [Google Scholar]
- Storm C, Pastore J.J, MacKintosh F.C, Lubensky T.C, Janmey P.A. Nonlinear elasticity in biological gels. Nature. 2005;435:191–194. doi: 10.1038/nature03521. [DOI] [PubMed] [Google Scholar]
- Taylor S.J, Sakiyama-Elbert S.E. Effect of controlled delivery of neurotrophin-3 from fibrin on spinal cord injury in a long term model. J. Control. Rel. 2006;116:204–210. doi: 10.1016/j.jconrel.2006.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teo W.E, He W, Ramakrishna S. Electrospun scaffold tailored for tissue-specific extracellular matrix. Biotechnol. J. 2006;1:918–929. doi: 10.1002/biot.200600044. [DOI] [PubMed] [Google Scholar]
- Torio-Padron N, Baerlecken N, Momeni A, Stark G.B, Borges J. Engineering of adipose tissue by injection of human preadipocytes in fibrin. Aesthetic Plast. Surg. 2007;31:285–293. doi: 10.1007/s00266-006-0221-6. [DOI] [PubMed] [Google Scholar]
- Tsai E.C, Dalton P.D, Shoichet M.S, Tator C.H. Matrix inclusion within synthetic hydrogel guidance channels improves specific supraspinal and local axonal regeneration after complete spinal cord transection. Biomaterials. 2006;27:519–533. doi: 10.1016/j.biomaterials.2005.07.025. [DOI] [PubMed] [Google Scholar]
- Wagner O.I, Rammensee S, Korde N, Wen Q, Leterrier J.F, Janmey P.A. Softness, strength and self-repair in intermediate filament networks. Exp. Cell Res. 2007;313:2228–2235. doi: 10.1016/j.yexcr.2007.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisel J.W. The mechanical properties of fibrin for basic scientists and clinicians. Biophys.Chem. 2004;112:267–276. doi: 10.1016/j.bpc.2004.07.029. [DOI] [PubMed] [Google Scholar]
- Weisel J.W. Fibrinogen and fibrin. Adv. Protein Chem. 2005;70:247–299. doi: 10.1016/S0065-3233(05)70008-5. [DOI] [PubMed] [Google Scholar]
- Weisel J.W. Structure of fibrin: impact on clot stability. J. Thromb. Haemost. 2007a;5(Suppl. 1):116–124. doi: 10.1111/j.1538-7836.2007.02504.x. [DOI] [PubMed] [Google Scholar]
- Weisel J.W. Which knobs fit into which holes in fibrin polymerization. J. Thromb. Haemost. 2007b;5:2340–2343. doi: 10.1111/j.1538-7836.2007.02794.x. [DOI] [PubMed] [Google Scholar]
- Weisel J.W, Cederholm-Williams S.A. Fibrinogen and fibrin: characterization, processing and medical applications. In: Domb A.J, Kost J, Wiseman D.M, editors. Handbook of biodegradable polymers. Harwood; Amsterdam, The Netherlands: 1997. pp. 347–365. [Google Scholar]
- Weisel J.W, Nagaswami C, Makowski L. Twisting of fibrin fibers limits their radial growth. Proc. Natl Acad. Sci. USA. 1987;84:8991–8995. doi: 10.1073/pnas.84.24.8991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willerth S.M, Arendas K.J, Gottlieb D.I, Sakiyama-Elbert S.E. Optimization of fibrin scaffolds for differentiation of murine embryonic stem cells into neural lineage cells. Biomaterials. 2006;27:5990–6003. doi: 10.1016/j.biomaterials.2006.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willerth S.M, Johnson P.J, Maxwell D.J, Parsons S.R, Doukas M.E, Sakiyama-Elbert S.E. Rationally designed peptides for controlled release of nerve growth factor from fibrin matrices. J. Biomed. Mater. Res. A. 2007;80:13–23. doi: 10.1002/jbm.a.30844. [DOI] [PubMed] [Google Scholar]
- Yoshida K, Munakata H. Connective tissue growth factor binds to fibronectin through the type I repeat modules and enhances the affinity of fibronectin to fibrin. Biochim. Biophys. Acta. 2007;1770:672–680. doi: 10.1016/j.bbagen.2006.11.010. [DOI] [PubMed] [Google Scholar]
- Young J.Z, Medawar P.B. Fibrin suture of peripheral nerves. Lancet. 1940;236:126–132. doi: 10.1016/S0140-6736(01)07978-8. [DOI] [Google Scholar]