Abstract
In Euplotes crassus, most of the micronuclear genome is eliminated during formation of a transcriptionally active macronucleus. To understand how this is mediated throughout the genome, we have examined the chromatin structure of the macronucleus-destined sequences and Tec transposons, which are dispersed in 15,000 copies in the micronuclear genome and completely eliminated during formation of the macronuclear genome. Whereas the macronucleus-destined sequences show a typical pattern of nucleosomal repeats in micrococcal nuclease digests, the Tec element chromatin structure digests to a nucleosome-like repeat pattern that is not typical: the minimum digestion products are ∼300–600 base pairs, or “subnucleosomal,” in size. In addition, the excised, circular forms of the Tec elements are exceedingly resistant to nucleases. Nevertheless, an underlying nucleosomal structure of the Tec elements can be demonstrated from the size differences between repeats in partial micrococcal nuclease digests and by trypsin treatment of nuclei, which results in mononucleosome-sized products. Characterization of the most micrococcal nuclease–resistant DNA indicates that micronuclear telomeres are organized into a chromatin structure with digestion properties identical to those of the Tec elements in the developing macronucleus. Thus, these major repetitive sequence components of the micronuclear genome differ in their chromatin structure from the macronuclear-destined sequences during DNA elimination. The potential role of developmental stage–specific histone variants in this chromatin differentiation is discussed.
INTRODUCTION
Like other ciliated protozoa, Euplotes crassus forms a transcriptionally active macronucleus from a micronucleus during the sexual phase of its life cycle (reviewed by Klobutcher and Jahn, 1991; Prescott, 1994). In the “hypotrichous” ciliates, such as Euplotes, Stylonychia, and Oxytricha, as much as 95% of the genome is eliminated during the formation of a macronucleus and the chromosomes are fragmented into gene-sized pieces of DNA. Studies of genome organization and macronuclear development in E. crassus indicate that DNA elimination occurs in multiple phases and via multiple mechanisms (Klobutcher and Jahn, 1991). In the micronuclear genome, the sequences that are destined to become the macronuclear linear DNA molecules (henceforth referred to as mac-destined sequences) are clustered together (Figure 1). Within these clusters are short stretches of eliminated DNA that either (a) are internal to the mac-destined sequences (referred to as IES for “internal eliminated sequences”) or (b) reside between two chromosome fragmentation sites (referred to as “junction regions”). These sequences are eliminated by a precise deletion process (IES excision) and chromosome fragmentation, respectively (Figure 1). We estimate that ∼20% of the E. crassus micronuclear genome is destined for the macronucleus and that IES and junction regions account for ∼10% of the DNA, which means that as much as 70% of the eliminated DNA exists outside of these mac-destined sequence clusters (Jahn et al., 1988). The majority of these eliminated sequences are single-copy sequences.
Figure 1.
DNA replication and processing during macronuclear development in E. crassus. On the left, the two types of DNA-processing events that produce the macronuclear linear DNA molecules are diagrammed. Precursors to macronuclear sequences are designated as open boxes, where each box corresponds to a gene that will become a linear DNA molecule (0.5–20 kb in size) bearing telomeric repeats. IES are designated by black boxes for the small, unique sequence IES (SU-IES) and double arrowheads for the Tec elements (Tec IES). Junction regions lying between two mac-destined sequences and other eliminated sequences are shown as heavy black lines. The excision of Tec elements that interrupt mac-destined sequences as IES is diagrammed on the left. The extrachromosomal circular forms are designated EC. This contrasts with non-IES Tec elements, shown on the right, in which the element is juxtaposed with other eliminated sequences. These sequences replicate late and become degraded around the time of chromosome fragmentation and telomere addition. At a given chromosomal locus, which is eightfold replicated during the first S phase, not all of the Tec elements are excised, and copies with and without the element are further amplified during the second S phase. Approximately 50% of the Tec element IES are excised in this first round. During the second excision, both Tec elements and SU-IES are removed, and variability in the extent of excision exists from locus to locus. For simplicity, this variability was not included in the figure.
We have characterized two families of transposable elements, called Tec1 and Tec2, that are highly dispersed in the E. crassus micronuclear genome and completely eliminated during macronuclear development (Jahn et al., 1988, 1989, 1993; Baird et al., 1989; Krikau and Jahn, 1991). Approximately one-third of the 7500 elements per haploid genome reside within the mac-destined sequence clusters and interrupt genes as IES (Figure 1, Tec IES), whereas the remainder are interspersed with the eliminated unique sequences (Figure 1, non-IES Tec) (Jahn et al., 1988). By determining the copy number as a function of time of macronuclear development for a large number of sequences that flank Tec elements, we demonstrated that mac-destined sequence clusters are differentially replicated compared with the bulk of the eliminated DNA during the early stage of macronuclear development, when micronuclear chromosomes are polytenized (Frels et al., 1996). Mac-destined sequences replicate during the two S phases of the polytene stage, whereas the eliminated sequences that are not associated with the mac-destined sequence clusters replicate during the second of the two S phases (Frels and Jahn, 1995; Frels et al., 1996). Our studies also demonstrated that the Tec elements within the mac-destined sequence clusters (Tec IES) undergo precise excision during both S phases of the polytene stage (Figure 1). In contrast, the Tec elements within the late replicating regions (non-IES Tecs) are eliminated along with surrounding sequences at the time of chromosome fragmentation and may or may not be specifically excised (Figure 1). Thus, the Tec elements are eliminated at different times, and possibly by different mechanisms, depending on their sequence environs. Because the primary difference in environs for the Tec elements has to do with whether or not they reside within genes (the mac-destined sequences), it seemed possible that chromatin structural differences involved in transcriptional activation of genes might be a controlling factor for excision. Thus, the goal of the work described here was to determine whether differences in chromatin structure could be defined for eliminated versus retained (mac-destined) sequences or for Tec elements within different environs.
We have investigated the chromatin structure of Tec elements and of mac-destined sequences with the use of nuclease digestion of chromatin. The enzymes micrococcal nuclease (MNase) and DNase I have differing activities with respect to the nucleosomal structure of chromatin, but both can be used to define differences in the accessibility of DNA within the chromatin fiber (Igo-Kemenes et al., 1982). Hypersensitivity and increased sensitivity to DNase I have been widely correlated with transcriptional activity or with a chromatin structure that is accessible to transcriptional machinery (Gross and Garrard, 1988). Heterochromatic sequences, such as genes located on an inactive X chromosome or silenced in a given tissue type, are not as readily digested. Although MNase has not been as widely used for comparisons of active and inactive genes, heterochromatic sequences such as telomeres can have nonnucleosomal structures that alter their MNase digestion properties (Gottschling and Cech, 1984; Price, 1990; Wright et al., 1992). We demonstrate here that the Tec elements are packaged differently than the mac-destined sequences and become highly resistant to nuclease digestion in conjunction with their excision.
MATERIALS AND METHODS
Cell Culture, Nuclear Isolations, and Nuclease Treatments
E. crassus strains X1 and X2 were cultured and harvested as described previously (Roth et al., 1985; Krikau and Jahn, 1991). Nuclei were prepared by suspending the cells in 10 mM Tris-HCl, 1 mM EDTA, pH 8, with protease inhibitors (defined below) followed by the addition of Triton X-100 to 0.5% and homogenization in a Dounce homogenizer (vegetative or mated cells at time points up to 30 h) or by sonication (preparations of developing macronuclei at 30–60 h). Protease inhibitors (Sigma, St. Louis, MO) were as follows: N-tosyl-l-phenylalanine chloromethyl ketone, 100 μg/ml; Nα-p-tosyl-l-lysine chloromethyl ketone, 50 μg/ml; leupeptin, 2 μg/ml; aprotinin, 1 μg/ml. Mixtures of macronuclei and anlagen (the developing macronuclei) were prepared by centrifugation at 1000 × g for 5 min, which does not pellet the micronuclei. When necessary, anlagen were further purified by progressive filtration through Nitex mesh (Tetko, Elmsford, NY) with 30-, 25-, and 20-μm pores and concentrated on 10-μm mesh followed by centrifugation as described above. A preparation of “old macronuclei and micronuclei” from 45-h mated cells was prepared by progressive filtration of a homogenized sample through Nitex (anlagen were retained at 30 μm, and the other nuclei passed through). Nuclei were suspended in digestion buffer (0.25 M sucrose, 50 mM Tris-HCl, pH 7.5, 15 mM NaCl, 3 mM MgCl2, 0.5 mM CaCl2) at a concentration of 106-107 nuclei/ml. MNase or DNase I (Sigma) was added at concentrations ranging between 0.1 and 5 U/ml. Digestions were carried out at 37°C for varying periods of time, typically 1, 3, 5, 10, 15, and 30 min. The most extensive digestions were for 45 min. Digests were stopped by the addition of EDTA to 10 mM and placement on ice. NaCl and SDS were added directly to the EDTA-treated samples at 0.5 M and 0.5%, respectively, followed by proteinase K (GIBCO-BRL, Gaithersburg, MD) digestion (200 μg/ml) at 65°C for 2–5 h. DNA was prepared by phenol-chloroform extraction.
Trypsin digestion of anlagen was carried out before the MNase treatment by incubating the nuclei (106/ml) in PBS containing trypsin (Sigma) at 10-fold dilutions ranging from 10 ng/ml to 10 μg/ml for 10 min at 37°C. Reactions were stopped by the addition of hen egg white trypsin inhibitor (Boehringer Mannheim, Indianapolis, IN) at 1 mg/ml, Nα-p-tosyl-l-lysine chloromethyl ketone at 50 μg/ml, and N-tosyl-l-phenylalanine chloromethyl ketone at 100 μg/ml, followed by pelleting and washing in the presence of the same inhibitors. Nuclei were then suspended in digestion buffer for MNase treatment (0.5 U/ml) as described above. Under these conditions, gel electrophoresis of the histones indicated that the H2A and H2B histones were digested but that the H3 and H4 histones were intact.
Agarose Gels, Southern Blotting, Hybridizations, and Slot Blot Quantitation
DNA samples (0.1–1 μg of DNA) were prepared in TAE buffer (0.04 M Tris, 0.02 M sodium acetate, 0.002 M EDTA, pH 7.0, with acetic acid) and 10% Ficoll without loading dyes because the bromphenol blue was found to interfere with blotting. Gels were typically 1.5% agarose or 1.5 or 2% low-melting-point agarose (GIBCO-BRL) in TAE buffer and were run at low voltage (25–30 V) overnight. Blotting was carried out for 6–20 h with the use of 0.4 N NaOH, 1 M NaCl, and ZetaBind (Bio-Rad, Richmond, CA). DNA was cross-linked to the membrane with a Stratagene (La Jolla, CA) UV cross-linker. Hybridization probes consisted of DNA fragments in low-melting-point agarose labeled by the random hexamer procedure (Feinberg and Vogelstein, 1983). Gel lanes containing the marker were blotted alongside the samples and then cut off and hybridized with radiolabeled marker. The macronuclear sequence probe was prepared by Bal31 treatment of 50 μg of total E. crassus DNA with 1 U of Bal31 for 1 min (Jahn, 1988), which resulted in no size alteration of the DNA, as judged by agarose gel electrophoresis. Hybridization and washing conditions were as described previously (Krikau and Jahn, 1991). Blots hybridized with the smallest fragments used as probes (100–200 base pairs [bp]) were washed only with 6× SSC (1× SSC is 0.15 M NaCl and 0.015 M Na citrate), 0.5% SDS at 65°C. Restriction enzyme digestions were carried out with the use of the buffers recommended by the manufacturer of the enzyme (Life Technologies, Gaithersburg, MD; New England Biolabs, Beverly, MA).
RESULTS
Developmental Changes in Nuclease Resistance of the Tec Element Chromatin Structure
We have previously demonstrated that the spacing of nucleosomes in the developing macronucleus (referred to as anlagen) differs from that of its micronuclear precursor and that the change in spacing correlates with the first round of replication in the newly formed anlagen (Jahn et al., 1997). Comparison of MNase digestion products over a range of MNase concentrations and times of digestion indicated that additional changes in the Tec element chromatin structure were evident. To determine how the Tec element chromatin structure in the anlagen related to micronuclear and macronuclear chromatin structure and to the mac-destined sequences, we used three types of hybridization probes in consecutive hybridizations of the same Southern blots of DNA purified from MNase- or DNase I–digested nuclei.
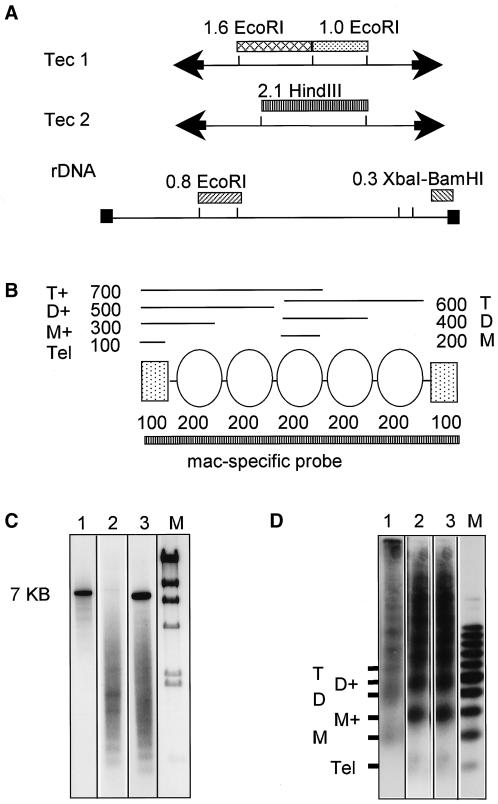
The hybridization probes are illustrated in Figure 2. The Tec element probes consisted of internal restriction fragments (i.e., not including the inverted repeats) from representatives of the Tec1 and Tec2 families (Figure 2A). Each probe recognizes all of the ∼7500 Tec1 or Tec2 family members; thus, we detected the overall nucleosomal or higher-order packing of these highly dispersed, repeated sequences. The “mac-specific” probe consists of size-fractionated, Bal31-treated macronuclear DNA molecules (<4 kilobase [kb] in size). By size selection, we have avoided inclusion of the macronuclear rDNA, which is present at 100-fold higher copy number than the other macronuclear genes. Because the average size of the macronuclear linear DNA molecules is ∼2 kb, this size-selected probe is representative of a large number of different genes (∼104). In the selected size range, most of the molecules are similar in copy number in the macronucleus; thus, we reasoned that it would be an ideal probe to determine the overall chromatin organization of mac-destined sequences in the developing macronucleus.
Figure 2.
Hybridization probes. (A) Consensus restriction maps of the Tec1 and Tec2 elements (representative of most family members) with the restriction fragments that were isolated from cloned elements for use as Tec-specific probes. The map of the macronuclear rDNA molecule shows the internal (0.8-kb EcoRI) and terminal (0.3-kb XbaI-BamHI) fragments used for comparison with the mac-specific probe in C and D. Black boxes symbolize the telomeres. (B) Scheme of the nucleosomal structure of macronuclear linear DNA molecules. The terminal C4A4 repeats are organized in a nonnucleosomal complex that protects ∼100 bp (rectangles). Nucleosomes are shown as ovals. Hybridization probes that detect the telomeric repeats will hybridize to a pattern of DNA fragments produced by partial MNase digestion of chromatin that corresponds to a nucleosomal multimeric repeat pattern that is shifted by 100 bp because it includes the 100-bp telomeric complex. (C) Hybridization of the mac-specific probe (lane 2) to Southern-blotted native macronuclear DNA analyzed on a 1% agarose gel compared with the hybridization of internal (0.8-kb EcoRI) and terminal (0.3-kb XbaI-BamHI) fragments from the rDNA molecule (lanes 1 and 3, respectively). The same three probes were hybridized consecutively to a Southern blot of DNA from MNase-digested macronuclei shown in D. The markers shown in C and D are a phage λ HindIII digest and a 100-bp ladder, respectively. The 100-bp telomeric complex is designated Tel, with the corresponding nucleosome-plus-telomere complex products at 300 and 500 bp designated M+ (for monomer plus telomere) and D+. The nucleosomal repeats detected by the internal rDNA fragment, corresponding to multimers of 200 bp, are labeled M, D, and T because they represent a typical monomer, dimer, trimer nucleosomal pattern.
Hybridization of this mac-specific probe to DNA from MNase digests of vegetative macronuclei indicates that even though we treated the DNA with Bal31 to remove telomeres, it detects a pattern of nucleosomes that resembles the pattern that would be detected by a telomeric sequence (Figure 2, B and D). The macronuclear telomeric sequences in E. crassus are associated with a telomere-binding protein that generates a structure that protects the first 100 bp of each macronuclear linear DNA molecule from digestion with MNase (Gottschling and Cech, 1984; Price, 1990). Internal to this 100-bp complex, the molecules are associated with nucleosomes. Thus, when DNA from MNase digests of macronuclei is hybridized with a telomeric probe (i.e., C4A4 repeats), a pattern of multimeric fragments corresponding to the telomere complex plus each multimer of a nucleosome is seen and each fragment size is 100 bp larger than the expected size of each nucleosomal multimer (Gottschling and Cech, 1984). In Figure 2, C and D, we compare the hybridization of three probes (the mac-specific probe and two probes derived from the cloned macronuclear rDNA) with native macronuclear DNA, which ranges in size from 500 bp to 20 kb, and with DNA prepared from MNase-digested macronuclei. The internal 0.8-kb EcoRI fragment from the rDNA hybridizes to the rDNA molecule, 7 kb in size, in the native macronuclear DNA and detects a nucleosomal repeat pattern of ∼200-, 400-, and 600-bp fragments in the MNase digests. In contrast, the 0.3-kb XbaI-BamHI fragment from the terminus of the rDNA, which contains the C4A4 telomeric repeat sequences, detects both the 7-kb rDNA molecule and a smear of hybridization to other macronuclear sequences. The mac-specific probe also hybridizes to a smear of macronuclear molecules, which would be expected regardless of whether it detected the telomeric repeat sequences. Because the probe primarily detects a size range of molecules corresponding to what was size selected and does not hybridize to the macronuclear rDNA molecule, it clearly detects sequences internal to the telomeric C4A4 repeats and not just the C4A4 repeats. However, both the 0.3-kb terminal rDNA probe and the mac-specific probe detect the alternative spacing of nucleosomes at ∼300, 500, and 700 bp that results from the 100-bp complex at macronuclear telomeres (Figure 2B).
We interpret the hybridization of the mac-specific probe as follows. In the size fraction that we are detecting, the chromatin fragments produced with telomeric ends are more abundant than the fragments without telomeric ends; thus, the predominant pattern seen is that of the telomeric complex. This situation could arise if the accessibility to nuclease digestion decreases in the vicinity of telomeres such that the ends of the molecules (including the first three or four nucleosomes) digest to the “telomere-plus-nucleosome” products while the middle of the macronuclear molecules digest to the nucleosomal multimers. We have noted that the sizes of MNase digestion products that are visible by ethidium bromide staining (rather than hybridization) change dramatically during digestion. The multimeric repeats that we detect with the 0.8-kb EcoRI fragment from the macronuclear rDNA are visible by ethidium bromide staining only at early time points of digestion. Later in the digestion, the ethidium bromide–stained DNA is only slightly larger than what we see with the mac-specific hybridization probe. Similar size changes are apparent in MNase digests of E. crassus macronuclear chromatin described previously (Price, 1990). Thus, we suspect that nucleosomal spacing and accessibility differ across a macronuclear DNA molecule and that the “telomere effect” seen with the mac-specific probe may involve chromatin structure differences arising from both the telomeric complex and a more MNase-resistant nucleosomal structure in the telomere-adjacent DNA.
As seen below, when the mac-specific probe is used with MNase-digested anlagen samples before the time of fragmentation and telomere addition, it shows a typical multimeric nucleosomal repeat pattern of ∼200, 400, and 600 bp. After telomere addition (52 h), it shows the telomere-like pattern of ∼300-, 500-, and 700-bp repeats. Thus, for the purpose of detecting the overall chromatin organization of mac-destined sequences, the probe behaves as if it detects a wide range of macronuclear sequences: when telomeres are present, it detects the “telomeric pattern” of sequences adjacent to telomeres, and when they are not present, it detects a more typical nucleosomal repeat pattern.
The differences between the Tec element chromatin structure of the anlagen and that in the micronucleus are illustrated in Figure 3. Total nuclei (macronuclei and micronuclei) from vegetative cells and the combined sample of developing macronuclei and “old” macronuclei from mated cells at 43 h of development were digested under identical conditions with MNase. (Macronuclear development is referred to in hours after mixing of two different mating types; macronuclear development begins at ∼16 h). At 43 h of development, the two phases of DNA replication to form polytene chromosomes are completed, but fragmentation has not started. In the nuclease-digested samples from cells at 43 h, the digestion pattern of the Tec elements present in the anlagen can be compared with the digestion pattern of the sequences in the old macronuclei that are coisolated and nuclease treated together with the developing macronucleus. Because the copy number of mac-destined sequences in the anlagen is a maximum of 64 times their micronuclear copy number at this stage, and the macronuclear sequences in the old macronucleus are ∼1000-fold amplified, the mac-specific probe predominantly detects sequences from the old macronucleus.
Figure 3.
Comparison of Tec element chromatin in the micronucleus with that of the anlagen. Total nuclei from vegetative cells (micronuclei and macronuclei) and the combination of developing macronuclei (anlagen) and old macronuclei from cells at 43 h of development were treated at 37°C with MNase at several concentrations of enzyme (0.05, 0.25, or 1.25 U/ml) and for varying times (10, 30, or 45 min), as shown above each lane. The DNA was isolated, electrophoresed in 1.5% agarose, Southern blotted, and then probed consecutively with a Tec element probe (the 1-kb EcoRI fragment from Tec1, shown in Figure 2), the mac-specific probe, and the 0.8-kb EcoRI fragment from the macronuclear rDNA molecule (only the vegetative samples are shown for the rDNA probe). Samples from nuclei incubated for 45 min without the addition of MNase, to control for endogenous nuclease activity, are shown for comparison (lanes E). The marker lanes shown are 100- and 123-bp ladders.
These MNase digests showed two unusual results with the Tec element probes. First is the change in the spacing of nucleosomal repeats from 150 bp average to 185 bp average, as described previously (Jahn et al., 1997). Second, the smallest size seen for the Tec element chromatin digestion products in the anlagen is 300–600 bp, which is larger than the digestion product expected for a sequence protected by a nucleosome. Other Tec1 hybridization probes and the Tec2 probe (see Figure 2) show the same change in structure; thus, the alterations observed are generalizable to both families of elements (our unpublished data). Comparison of the blots for the vegetative sample and the 43-h sample by hybridization to the mac-specific probe and the rDNA probe shows that the time course of digestion was similar for these two samples. These hybridizations show that the digestion kinetics for the macronuclear sequences are much more rapid than those seen for the Tec elements in either the micronucleus or the anlagen. This might be expected for a comparison between a transcriptionally active nucleus (the macronucleus) and a nucleus or chromatin structure that is silent. The difference between the “mac sequence” probe and the rDNA probe further demonstrates the different behavior of the “telomere-associated” chromatin in the macronucleus. The internal sequences of the macronuclear rDNA that are detected with the 0.8-kb EcoRI fragment are rapidly digested to a dimer and “core monomer” size (i.e., 146 bp) under the conditions we used to generate the partial digestion products of micronuclear or micronuclear-limited sequences. Under these conditions, the mac-specific probe detects primarily the 250- to 300-bp “telomeric” complex, which must be more stable than the monomer- and dimer-sized products produced at internal sites.
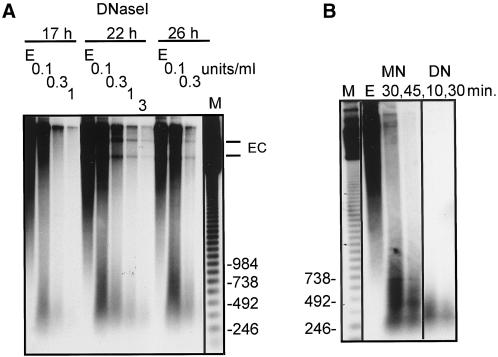
We also digested nuclei with DNase I to determine if the differences in chromatin structure would be apparent. In general, DNase I has a greater accessibility to DNA in chromatin and digests DNA in a nucleosomal core to a greater extent than MNase (Igo-Kemenes et al., 1982). Digests of mixtures of developing and old macronuclei at 17, 22, and 26 h of development are shown in Figure 4. The DNase I digests did not produce a “nucleosome-like” pattern of repeats. However, as observed for the MNase digests, the smallest product of DNase digestion is 300–600 bp in size. These three time points encompass the first period of Tec element excision, and the circular products of excision can be seen. These excised circular forms are especially resistant to DNase I digestion. In numerous experiments, we have digested nuclei isolated over a wide range of developmental time points with MNase and DNase I. At all time points later than 20 h of development, and in many cases as early as 17 h, the minimal digestion products seen with the Tec element probes are larger than a typical mononucleosome fraction. Given that there is some variability in the synchrony of mating, such that the overall synchrony of developmental events can differ by 2–4 h for a mass mating, the detection of this altered chromatin structure at 17–20 h indicates that it is assembled during the first S phase of DNA replication to form the polytene chromosomes (Figure 1). With the E. crassus strains we used, the excision of Tec elements occurs at 20–22 h. In all of the MNase and DNase I digests, whenever Tec element extrachromosomal circular excision products are present, they are highly resistant to digestion by either nuclease.
Figure 4.
Pancreatic DNase I digestion of Tec element sequences in chromatin early during macronuclear development and the size of the minimal digestion products seen with Tec element probes. (A) Nuclei (a mixture of anlagen and macronuclei) from cells at 17, 22, and 26 h of development were digested with DNase I at 0, 0.1, 0.3, 1, and 3 U/ml DNase I for 10 min, and the DNA was isolated and analyzed on a 1.5% agarose gel as described for Figure 3. Extrachromosomal circular forms of Tec elements are indicated (—EC). (B) “Limit” digests of anlagen prepared from cells at 45 h of development were carried out by extensive treatment with MNase and DNase I. Lane E corresponds to nuclei incubated for 45 min at 37°C to control for endogenous nuclease activity. MNase was added at 5 U/ml for 30 and 45 min, and DNase I was added at 50 U/ml for 10 and 30 min. The marker (M) lane for both blots is a 123-bp ladder with sizes in base pairs indicated.
To determine whether the slow rate of digestion of Tec elements in the micronucleus and anlagen relative to macronuclear sequences meant that neither DNase I nor MNase had reached an extent of digestion that would give rise to a true “mononucleosome,” we incubated nuclei with high concentrations of the enzymes for 30 or 45 min (Figure 4B). Both enzymes predominantly showed the same pattern of fragments > 300 bp in size. Thus, this size range of DNA must correspond to the most nuclease-resistant structure in the Tec element chromatin.
Comparison of the Chromatin Structure of Mac-destined Sequences and Tec Elements at the Late Polytene Stage
In the experiments described above, we compared the Tec element chromatin with the macronuclear chromatin, i.e., two different types of nuclei that we coisolated. To compare the chromatin structure of the mac-destined sequences with the Tec element chromatin in the same nucleus, we isolated anlagen at the end of the polytene stage (40–45 h). We used sonication to break open the cells and disrupt the old macronuclei; this yielded anlagen that were completely free of macronuclear contamination, as judged by Southern blotting of the DNA and hybridization with the macronuclear rDNA gene. Comparison of the Tec element chromatin structure seen with sonicated nuclei prepared from the same cells as a “homogenized” sample (which contains old macronuclei) demonstrated that the sonication did not disrupt the Tec element chromatin structure, because the MNase digestion pattern was unaltered.
When DNA samples from MNase-digested, sonicated, and purified anlagen are hybridized consecutively with the Tec element and mac-specific probes, differences in the pattern of digestion of the two sequence classes are apparent. As seen in Figure 5A, at 45 h, which is immediately before chromosome fragmentation, the pattern of products for Tec element chromatin is shifted toward a higher molecular weight than that for the mac-destined sequences. These anlagen MNase digestion samples were electrophoresed side by side with MNase digestion products from a mixture of old macronuclei and micronuclei prepared from the same cells. This demonstrates that the mac sequence probe detects the mac-destined sequences in the anlagen without contamination by old macronuclear sequences. In addition, it demonstrates that the multimeric repeats for the Tec element chromatin in the anlagen are more similar in size to the “telomere” repeat pattern of the old macronucleus than to the multimeric products of the mac-destined sequences in the anlagen. This is again apparent in the blots of the 42-h samples shown in Figure 5B and in the densitometric scans of the 42-h samples shown in Figure 5C, where the peaks observed with the Tec probe do not align with the peaks seen with the mac-specific probe even when different extents of digestion are compared. Thus, these digests clearly show that the multimeric repeat sizes and the size of the minimal digestion product differ for the Tec element chromatin.
Figure 5.
Micrococcal nuclease digests of purified anlagen from the late polytene stage of macronuclear development. (A) DNA isolated from anlagen purified by sonication from cells at 45 h and digested with MNase at 0.5 U/ml for 2, 5, or 10 min was analyzed on the same gel as samples from old (degenerating) macronuclei and micronuclei isolated (by homogenization) from the same mated cells and digested under identical conditions. The DNA samples were electrophoresed in 2% low-melting-point agarose. Comparison of the hybridization demonstrates that no contamination of the anlagen sample with old macronuclei is apparent and that the mac-specific probe detects an ∼200-bp nucleosomal multimer pattern in the anlagen sample instead of the nucleosome-plus-telomere complex pattern seen for the old macronucleus. The pattern of Tec element digestion in the micronucleus at 45 h (Tec hybridization to the old macronuclei-plus-micronuclei sample) resembles the vegetative micronucleus. (B) Anlagen were purified (by sonication) from cells at 42 or 52 h of development and incubated at 37°C with MNase at 0.5 U/ml for 2, 5, 10, and 15 min of digestion. Lanes E show DNA from nuclei incubated for 30 min without MNase to control for endogenous nuclease activity. The DNA was isolated, electrophoresed in 1.5% agarose, Southern blotted, and hybridized with a Tec probe (Tec). Each blot was stripped and hybridized with the mac-specific probe (Mac). Samples from 42 and 52 h were analyzed on the same gel, so the blots correspond directly to each other. Asterisks identify the regions of subnucleosome-sized fragments detected with the Tec probe. The marker lane is a 123-bp ladder. (C) Densitometric scans of the 2-, 5-, and 10-min digestion samples from the autoradiograms shown for the 42-h samples in B for the Tec element and mac-specific probes for direct comparison. M, D, T, M+, D+, T+, Tel, and EC are as defined in previous figures.
In the 42- and 45-h samples, telomeres are not present on the sequences detected by the mac-specific probe, and the pattern of nucleosomes is typical of a repeat of 180- to 200-bp nucleosomal units. We have previously shown that chromosome fragmentation in the E. crassus strains used in these experiments occurs at 46–48 h of development (Frels and Jahn, 1995; Frels et al., 1996). Thus, in the 52-h anlagen (Figure 5B), chromosome fragmentation and telomere addition to the mac-destined sequences has occurred. In these samples, the mac-specific probe once again shows a nucleosomal repeat pattern typical of the telomere complex repeat pattern, indicating that telomere addition has occurred.
The pattern of digestion of the mac-destined sequences more closely resembles a “typical” MNase digestion of nuclei from other organisms than the pattern from the Tec elements. In a typical digestion, multimers of nucleosomal repeats are reduced to a monomer size (∼150–200 bp), the monomer size reduces to a core size (146 bp), and then smaller fragments are produced (Noll, 1974; Axel, 1975; Sollner-Webb and Felsenfeld, 1975). The monomer and core represent “pauses” in the digestion process. For the mac-destined sequences (Figure 5), the monomer size is the primary pause in the digestion process and becomes a broad smear from 150 to 200 bp. As a result of our methods of blotting and hybridization, the hybridization to small fragment sizes (<150 bp) is greatly reduced and we do not see the accumulation of fragments that are smaller than the core monomer size unless we alter the stringency of hybridization (see below). In contrast to findings with the mac-destined sequences, we never see accumulation of the monomer sizes (150–200 bp) in the hybridizations with Tec element probes. All of the accumulation occurs at the 300- to 600-bp size range, with a minority of the hybridization occurring at 150 bp and smaller sizes (see densitometric scans in Figure 5C). Thus, for these sequences, the structure that is most protected from digestion corresponds to a structure larger than a single nucleosome and most likely related to a dimer of nucleosomes (see DISCUSSION). Presumably, these sequences are converted from this more stable structure to the smallest fragment sizes, which are not as readily detected by hybridization (see experiments described below). Prolonged digestion with either MNase or DNase I at high enzyme concentrations does not result in the accumulation of a monomer-sized product for the Tec element sequences in the sonicated nuclear samples. In contrast, under a wide range of enzyme concentrations and digestion times and with varying extents of sonication of the nuclei, we have always seen a typical monomer, dimer, trimer relationship for the mac-destined sequences before fragmentation and telomere addition.
We have previously shown that Tec element excision occurs in two discrete periods: the first at 20–22 h and the second at 40–42 h (Frels and Jahn, 1995; Frels et al., 1996). Thus, at 42 h, a second group of Tec elements excises as extrachromosomal circles. As observed at the earlier time points, the Tec element circular forms produced at 42 h are highly resistant to MNase (Figure 5B). At 52 h, circular forms of the Tec elements are not as abundant.
Tec Elements Are Organized into Nucleosomes in the Developing Macronucleus
Our finding that the majority of the digestion products for the Tec elements are larger than the size of a nucleosomal monomer suggests that some nonnucleosomal structure may be forming. Nevertheless, several other results indicate that this higher-order structure of the Tec elements forms from a typical nucleosomal substructure. For instance, throughout development, the Tec elements in the anlagen can be detected in a nucleosome-like repeat spacing that is distinct from the spacing seen in the micronucleus (Jahn et al., 1997) (Figures 3 and 5), even when monomer-sized fragments are not produced. Calculation of the spacing between multimers from the scans shown in Figure 5C indicates that the spacing of repeats in the Tec elements is 190.2 (mean) ± 28.9 (SD) bp, which is close to what we measured as the spacing visible at the beginning of macronuclear development, when a change in spacing (relative to the micronucleus) is first visible (187 ± 27 bp) (Jahn et al., 1997). The spacing measured from the same gels of the 42-h samples for the mac-destined sequences is 191.5 ± 25.2 bp, which is somewhat larger than the size measured for vegetative macronuclear sequences with the use of the rDNA as a hybridization probe (175 ± 27 and 181 ± 29 bp) (Jahn et al., 1997). Thus, even though the sizes of the multimers released for the Tec element sequences do not correspond to the sizes of the multimers released for the mac-destined sequences in the 42-h samples (i.e., the peaks do not align in the densitometric scans shown in Figure 5C), the spacing of nucleosomes appears the same. This suggests that the preferred cutting sites for digestion to release the multimers differ between these two types of sequences.
Second, we have considered the possibility that the pattern of repeats we detected for the Tec element chromatin is related to the telomeric complex pattern seen when the mac-specific probe is hybridized to MNase digests of macronuclei. That is, the pattern would arise from an unusual structure at one site within the element. This seemed unlikely given the fact that two different probes encompassing 1.0 and 1.6 kb of the element behaved identically. Nevertheless, to test this possibility, we hybridized 11 different small probes (80–250 bp) from sites scattered throughout the Tec1 element to MNase digestion products (Figure 6). The most extensive digestions were chosen for analysis to determine what size range of products was most abundant. Because we used small probes, our hybridization conditions were of low stringency to optimize hybridization with small fragments. All of these probes hybridized to the unusual minimum digestion size of ∼300 bp and higher multimers, i.e., all of these probes show a pattern of hybridization that is identical to the larger Tec element probes. None of the probes hybridize to a nucleosome monomer-sized product. Under the lower hybridization stringencies used, most of these small probes hybridized to fragments that were “submonomer” in size, in addition to the unusual minimum digest products. The smallest fragments detected by these probes migrate between the 100- and 123-bp bands in the marker lanes, and the largest size is equivalent to a core monomer (146 bp). As discussed below, this suggests that the Tec element chromatin digests directly from a structure that is larger than a single nucleosome to something that is equivalent to or smaller than a core monomer. Together, these hybridizations with numerous small probes demonstrate that the unusual minimum digestion products and the unusual multimeric repeats arise throughout the element and thus are not due to a “terminal” complex. Furthermore, because we cannot identify any region within the element that digests to a typical mononucleosomal product, the chromatin structure of the Tec elements must be uniform and cannot be a mixture of two types of nucleosomal structures.
Figure 6.
Demonstration that the Tec element chromatin structure is uniform across the element and that the chromatin is organized in nucleosomes. Four sets of three MNase samples from total nuclei at 43 h of development (corresponding to 0.25 U/ml MNase at 30 and 45 min and 1.25 U/ml MNase at 10 min, as in Figure 3) were electrophoresed in 1.5% agarose, Southern blotted, and hybridized consecutively with 11 different Tec1 probes ranging in size from 80 to 250 bp. The two types of hybridization patterns obtained are shown for one blot probed with two different probes. (B) The Tec1 element is shown, with the black boxes indicating the positions of the restriction fragments used as small probes. The four fragments with stars above them gave the hybridization pattern seen with probe 1, in which little detection of submonomer fragment sizes is seen. The rest of the fragments gave a pattern identical to that seen with probe 2, in which both the ∼300-bp unusual “dimer” size and smaller fragments are observed. (C) Anlagen purified from cells at 45 h of development were treated with varying amounts (10-fold differences) of trypsin before digestion with MNase (see MATERIALS AND METHODS). All five samples designated +MNase were digested identically with MNase. The −MNase lane contains DNA isolated from untreated nuclei. Samples were electrophoresed on 1.5% agarose gels, Southern blotted, and hybridized with a Tec element probe. The marker lane is a 100-bp ladder with sizes indicated in base pairs.
To further elucidate whether the subunit structure of the Tec element chromatin is nucleosomal, we treated purified anlagen with increasing amounts of trypsin followed by MNase digestion. Trypsin is known to digest histone termini without disrupting the core nucleosome structure (Allan et al., 1982; Ausio et al., 1989; Hayes et al., 1991); thus, it should increase the accessibility of spacer regions between nucleosomes to MNase. As seen in Figure 6C, the trypsin treatment converts the structure of the Tec element chromatin from the unusual minimum digestion product and higher-order repeats to a nucleosomal monomer-sized protected fragment. The size range of this “monomer” fraction is 150–250 bp. The nucleosomal spacing determined from the trypsin-treated samples is 185 ± 25 bp; thus, the overall chromatin structure does not appear to be disrupted by the treatment. Therefore, it appears that the unusual behavior of the Tec element sequences arises from an underlying nucleosomal structure.
Are the Tec Elements Representative of Other Eliminated Sequences?
We have not tested cloned eliminated or retained unique sequences as hybridization probes because the amounts of the MNase or DNase digestions that we subjected to Southern blotting are not sufficient to detect single-copy sequences. However, we carried out several experiments to define (a) what sequences are most resistant to MNase and (b) whether these sequences behave like the Tec elements with respect to MNase digestion. We found that when MNase-digested nuclei (45-h anlagen) are treated with EDTA and then centrifuged, nucleosomal monomers and smaller submonomer fragments are released from the nuclei and larger nucleosomal multimers are pelleted. This is illustrated in Figure 7A, in which the monomers that hybridize to the mac-specific probe are shown to fractionate into the supernatant after treatment of MNase-digested nuclei with EDTA; the Tec hybridization indicates that none of the Tec element chromatin is within the size range to be released. By carrying out this fractionation after extensive MNase digestion, we derived a fraction that contains very few remaining Tec elements or mac-destined sequences (equivalent to the highest extent of digestion shown in Figure 4B). Even though 50% of the starting DNA remains in this nuclear pellet fraction after EDTA addition (as determined from the UV absorbance of the pellet samples), the hybridization of the Tec element probes or the macronuclear sequence probe to this fraction was reduced 100-fold relative to the starting DNA. Thus, we reasoned that the DNA remaining in the pellet fraction must be composed primarily of other classes of eliminated sequences. To determine whether this fraction showed a chromatin organization resembling that of the Tec elements, we radiolabeled the pellet fraction and hybridized it to the samples from MNase-digested anlagen. As seen in Figure 7B, this pellet fraction behaves like the Tec element probes. Thus, the most MNase-resistant fraction of the DNA shows a digestion pattern that is identical to that of the Tec elements.
Figure 7.
Hybridization of non-Tec–eliminated sequences to MNase digests. (A) The fractionation of chromatin into pellet and supernatant after the addition of EDTA to a MNase digest of purified 45-h anlagen. The DNA was extracted from the total, unfractionated MNase digest (T) and compared with the pellet (P) and supernatant (S) fractions. The blot was hybridized with the mac-specific probe and then stripped and hybridized with a Tec probe. M and D indicate the monomer and dimer sizes in the mac-specific probe hybridization. (B) The pellet fraction from a more extensive digestion than that shown in A, which showed very little remaining hybridization with Tec or macronuclear sequences in Southern blots or slot blots, was radiolabeled and used as a hybridization probe to a Southern blot of the 42-h MNase digests (identical to the 2-, 5-, and 10-min lanes in Figure 5, B and C), which were electrophoresed on a 2% low-melting-point agarose gel. The blot was stripped and hybridized with a Tec element probe. The marker lane (M) shown is a 100-bp ladder. (C) Micronuclear DNA, either undigested (U) or digested with EcoRI, HindIII, or XbaI (E, H, and X, respectively), was electrophoresed in 1% agarose, Southern blotted, and hybridized consecutively with the pellet probe (as in B) or a Tec1 or Tec2 probe. The hybridization to the pellet probe is either at the loading well or >20 kb in size. Positions and sizes (in kilobases) of a λ HindIII digest run in an adjacent lane are shown to the left. (D) Additional digests of micronuclear DNA with EcoRV, HhaI, HincII, and MseI (lanes RV, Hh, H2, and M, respectively) were electrophoresed in 1% agarose, Southern blotted, and hybridized with the pellet probe. Again, most of the hybridizing DNA was >20 kb in size. (E) The cloned macronuclear rDNA (7.5 kb in the pBS vector) was digested with EcoRI, HindIII, or XbaI, electrophoresed in 1% agarose, Southern blotted, and hybridized to the pellet probe. The fragment sizes detected by the probe correspond to those of fragments that contain the telomeric repeats from the rDNA molecule (Erbeznik et al., 1999). (F) The locations of the hybridizing fragments are shown below the restriction map of the rDNA. Fragments designated with arrows included adjacent plasmid sequences and thus are >3 kb in size in the autoradiogram shown in E. The positions of the telomeric repeats are shown as small boxes labeled “tel.”
We proceeded to characterize the sequence content of this MNase-resistant pellet fraction. We reasoned that the pellet fraction consisted primarily of non-Tec element–eliminated sequences; therefore, when used as a hybridization probe to genomic or cloned DNA, it might behave either as a repetitive sequence probe that would identify non-Tec element repetitive sequences or as a “bulk” unique sequence probe for eliminated unique sequence DNA (similar to our mac-sequence probe). Because of the high sequence representation of this fraction (50% of the starting nuclear DNA), these hybridizations are unlikely to detect single-copy sequences. Initial experiments demonstrated that the probe hybridized only to high-molecular-weight (>20 kb) DNA in total vegetative DNA samples (macronuclear plus micronuclear), indicating that no hybridization to macronuclear DNA (linear molecules < 20 kb in size) was detectable, thus verifying that the probe was specific for only micronucleus-limited sequences (i.e., eliminated DNA).
To determine whether the micronucleus-specific nature of this probe could be due to a class of repeated DNA that we have not previously characterized, we used the “pellet” fraction as a hybridization probe to Southern-blotted, restriction enzyme–digested micronuclear DNA (Figure 7C). Whereas the bulk of the eliminated unique sequence DNA should be detectable as a smear of fragment sizes, repetitive sequences can give rise to discrete bands because of the occurrence of the identical restriction enzyme cleavage sequences in multiple copies of the repeats. The blot of the genomic digests was stripped and hybridized with probes from the Tec1 and Tec2 elements to determine to what extent the pellet probe hybridized to known consensus fragment sizes for these two element families (Figure 7C). As expected from our quantitation of hybridization of Tec elements to this fraction, the hybridization of the pellet fraction to the genomic digests demonstrated only weak hybridization to Tec element sequences, in that it did not detect the major EcoRI, HindIII, or XbaI fragments that arise from the conserved sites within these two families of elements (Figure 7C). Surprisingly, the DNA that is most readily detected with the pellet probe did not digest with EcoRI, HindIII, or XbaI. We performed similar hybridizations to micronuclear DNA digested with enzymes that cut more frequently (EcoRV, HhaI, HincII, and MseI) and again observed no digestion of the sequences (Figure 7D); however, hybridization of the same blots with Tec element probes indicated that the digestions were complete. This lack of restriction enzyme digestion is similar to findings with micronuclear telomeric sequences that have been characterized in other hypotrichous ciliates (Dawson and Herrick, 1984; Jahn, 1988). The micronuclear telomeres are composed of long stretches of C4A4 repeats that are resistant to restriction enzyme digestion because of their sequence composition.
To determine whether the pellet probe was detecting telomeric sequences, we hybridized it to digests of the cloned macronuclear rDNA molecule (Figure 7E). This demonstrated that the probe hybridized only to fragments known to contain the telomeric repeats (Figure 7F). The smallest of these fragments is 90 bp and contains 42 bp of C4A4 repeats and 30 bp of the vector polylinker. Similar results were obtained with blotted digests of two other cloned macronuclear DNA molecules. Thus, we conclude that the “pellet” fraction detects the micronuclear telomeric sequences and that the chromatin structure of these sequences in the anlagen behaves similar to the Tec element sequences.
DISCUSSION
The results described above have documented several alterations of chromatin structure in the E. crassus anlagen. We have demonstrated a dramatic difference between the Tec element chromatin structure and the chromatin structure of the mac-destined sequences. Furthermore, we have presented evidence that the micronuclear telomeres have a chromatin structure in the anlagen that behaves like the Tec elements. Analysis of the MNase and DNase I digestion products indicates that there may be two types of altered chromatin structure associated with Tec elements. The circular excision products are highly resistant to both MNase and DNase I, whereas the rest of the Tec element sequences are organized into a structure that is more accessible but does not accumulate as typical nucleosomal digestion products. This contrasts with the mac-destined sequences in the anlagen, which readily digest and accumulate as a nucleosomal monomer and higher multimer fragments before chromosome fragmentation and telomere addition. Thus, we appear to be detecting three types of chromatin structure: (1) a typical nucleosome structure of the mac-destined sequences, (2) an unusual nucleosomal structure of Tec elements (in their nonexcised state) and the micronuclear telomeres that we believe involves some degree of inaccessibility of DNA between two nucleosomes, and (3) a highly compacted structure for the excised Tec element circular forms. The nuclease insensitivity of the circular forms and the unusual nucleosomal products are more dramatic alterations of chromatin structure than the nuclease digestion patterns obtained for sequences subject to transcriptional repression by heterochromatin at position effect–variegating loci in Drosophila (Wallrath and Elgin, 1995) or the silent mating type loci in yeast (Nasmyth, 1982). The extreme resistance of the extrachromosomal circular Tec elements resembles the lack of digestibility seen for male-specific sequences in mealybugs, in which the paternal chromosome set is heterochromatic (Khosla et al., 1996). Thus, we believe that the Tec elements form a unique type of heterochromatin structure.
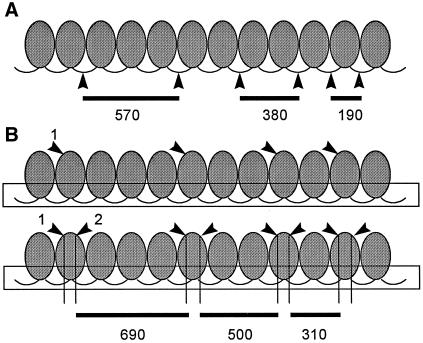
We have provided evidence that the unusual multimeric MNase products seen for Tec element chromatin arise from an underlying nucleosomal repeat. The fact that we can detect “subnucleosomal” fragments and the unusual minimum digestion products without any typical monomer products, even though a nucleosomal repeat is present, suggests that we are detecting a chromatin structure that does not allow digestion within the “linker” or “spacer” regions. Precedence for an alternative cutting pattern for a nucleosomal array comes from studies of DNase II digestion of chromatin in which a 100-bp ladder was produced by cutting both within the spacer region and at sites within the core-associated DNA (Horz et al., 1980; Horz and Zachau, 1980). The 100-bp alternative was produced by cutting at sites that were 100 bp apart within the 147-bp core-associated DNA and was sensitive to ionic conditions that altered higher-order chromatin structure. Based on this pattern, we suggest the following scenario (Figure 8). If the MNase digestion pattern of Tec elements were altered such that cutting within the spacer region was completely suppressed, multimers could arise via digestion at multiple sites within the nucleosomal core–associated DNA and the digestion products would go directly from a size corresponding to two partial nucleosomes with one intact nucleosome and two spacers (i.e., the unusual minimal digestion product) to the submonomer sizes, essentially as we observe. Depending on the spacing of the preferred cutting sites within the core DNA, the predicted sizes of nucleosomal multimers could resemble the telomeric pattern seen for E. crassus telomere-associated mac sequences (as observed for the Tec element in the anlagen) and would be displaced by ∼100 bp from the typical nucleosomal pattern (see Figure 8). Furthermore, the difference in size between these multimers would be the same as the difference in size between multimers seen by cutting only in the spacer region, i.e., the apparent nucleosomal spacing would be the same (Figure 8, compare A and B).
Figure 8.
A model to explain the unusual multimeric sizes of the Tec element chromatin MNase digestion products. (A) The normal pattern of digestion of a nucleosomal array by MNase along with fragment sizes predicted for nucleosomes that show a repeat spacing of 190 bp. (B) An alternative pattern of cutting that could give rise to the unusual multimers seen for the Tec elements. Instead of preferential digestion in the spacer regions, we propose a pattern of cutting within the nucleosomal core–associated DNA. We assume that a higher-order chromatin structure (shown as a box surrounding the spacer regions) prevents accessibility of MNase in the spacer region. We propose that asymmetry in the cut sites that give rise to the multimers arises because multiple cuts occur within a core, leaving behind portions of nucleosomal core–associated DNA. This could happen in a stepwise manner as shown, in which one site within the core is preferred and then additional sites within the core become sensitive after the first site is attacked. If the amount of DNA removed by cutting within the core-associated DNA is approximately one loop, or 78–79 bp, the multimers would be ∼110 bp larger than those produced from the pattern shown in A. Note that both patterns of cutting give rise to multimers that differ by 190 bp, as seen for both the Tec element and mac-destined sequences.
At present, we have not found a means of releasing the multimers or minimal digestion products of the Tec element chromatin from the anlagen. This has hindered our ability to identify the proteins responsible for the chromatin structural change. The unusually resistant structure of the anlagen (alluded to in RESULTS) prevents dissociation of the Tec element chromatin under any conditions that leave the nucleosomes intact (Jahn and Sharp, unpublished observations). This behavior is similar to that of sea urchin sperm nuclei, in which histone H1, H2B, and H2A variants have been characterized (Simpson and Bergman, 1980). The sperm nuclei were resistant to any form of homogenization or sonication, as well as to EDTA or β-mercaptoethanol, that would normally release nucleosomes and their multimers after MNase treatment.
As in the sea urchin sperm nuclei, the complement of histones that are found in the E. crassus developing macronucleus could play a role in forming the unusual Tec element chromatin structure. We have previously described a histone H3 variant that is specific to the polytene chromatin stage of macronuclear development (Jahn et al., 1997). In addition, we have recently sequenced cDNA clones for an H2A variant that is transcribed only in conjunction with polytene chromosome DNA replications (C. Jahn, unpublished observations). The H3(P) variant is 15 amino acids longer at its amino terminus (Jahn et al., 1997), and the H2A histone is longer at its carboxyl terminus (Jahn, unpublished observations). The x-ray crystallographic structure of a nucleosome indicates that the histone H3 amino terminus and the histone H2A carboxyl terminus could interact with DNA in the spacer region between nucleosomes and with each other (Luger et al., 1997). Thus, the anlagen-specific core histone variants in E. crassus may alter the accessibility of DNA in the spacer region or provide an interaction between two nucleosomes that help generate a higher-order structure. Because these histone variants are abundant and appear to be incorporated into the chromatin structure of both eliminated and retained sequences, it seems unlikely that they are the sole determinants of this altered chromatin structure. However, it is also possible that some type of differential modification of core histones occurs within the eliminated versus the retained sequence classes. The fact that the retained macronuclear chromosomes are essentially individual transcription units with very little noncoding DNA suggests that their chromatin structure might be differentially modified during macronuclear development as the transcription machinery is put into place.
We would expect that histone H1 would play a role in determining the accessibility of the linker region, but the E. crassus anlagen does not appear to have a typical linker histone. Characterization of histone H1 proteins and genes in E. crassus has identified two genes. One has been shown to be the major histone H1 present in the vegetative macronucleus (Ray et al., 1999). The other gene most likely corresponds to a second perchloric acid–soluble histone that is in much lower abundance in vegetative cells. Neither of these proteins (or any other smaller histone H1–like protein) can be isolated from the anlagen (Jahn, unpublished observations). Thus, histone H1 most likely does not play a role in this chromatin structure differentiation. However, we have recently found an abundant, lysine-rich, perchloric acid–soluble (and sulfuric acid–soluble) protein that migrates at 85 kDa in SDS-PAGE and thus is much larger than a histone (Sharp and Jahn, unpublished observations). Antibodies to this protein indicate that it is specific to the anlagen and is not uniformly distributed in chromatin. Thus, this protein may be a “specialized” linker histone that has a specific subchromosomal distribution. We are in the process of determining whether this protein is associated with the Tec elements, micronuclear telomeres, or other eliminated DNA.
Despite the large size of the Tec element repetitive sequence families (7500 copies per haploid genome in each family), the elements appear to be uniform in their chromatin organization. The results of MNase and DNase I digestion of nuclei during the first replication and excision period (17–22 h), when the replication is restricted to regions of the genome where Tec elements are primarily in the mac-destined sequence environs (i.e., as IES), suggest that nuclease resistance develops before excision. In this period, preferential replication of the mac-destined sequence clusters would lead to a higher copy number of the Tec IES, and they would represent approximately one-half to two-thirds of the sequences we would detect with our probes. Thus, the Tec IES most likely become organized into a structure that differs from the mac-destined sequences before their excision. The only evidence for two distinct classes of elements (in which the Tec IES would behave differently from the non-IES Tecs) is the increased resistance of the Tec IES after their excision. It seems likely that the excision mechanism, which produces circular molecules that are supercoiled, may increase the compaction of the chromatin in the process of forming the circles.
Although we do not detect a difference in Tec element chromatin structure that can be attributed to a difference in environs for the IES and non-IES elements, we do detect a difference between the mac-destined sequence chromatin and the Tec element chromatin before the time of fragmentation. Thus, the environs of the Tec IES differ from that of the Tec element. This suggests that Tec elements constitute a discrete chromatin domain even when they interrupt the mac-destined sequences as IES. The ability of the DNA-processing machinery to excise the Tec IES may depend on the juxtaposition of the more typical nucleosomal structure of the mac-destined sequences with the unusual chromatin structure of the Tec elements. We suspect that most of the eliminated DNA may be organized into a chromatin structure resembling that of the Tec element. Thus, the environs of a non-IES Tec may not differ from that of the Tec element; therefore, DNA-processing machinery would not specifically recognize the boundaries of the non-IES Tec element.
The chromatin structure of the Tec elements and the mac-destined sequences we have defined by nuclease treatment of the E. crassus anlagen may be directly related to two fiber types seen by electron microscopy of chromatin in the Stylonychia polytene anlagen (Meyer and Lipps, 1980, 1981). These studies revealed large loops of 30-nm-wide fibers with strands of 12-nm fibers in between (Meyer and Lipps, 1980, 1981). During elimination, the loops were released as dense chromatin circles that disappeared from the nucleus, leaving behind the 12-nm fibers that became the short linear molecules organized as nucleosomes. To date, the proteins involved in organizing these structures and the specific distribution of DNA sequences relative to the structures have not been determined. The nuclease digestion properties of the E. crassus mac-destined sequences parallel the finding that the sequences organized as 12-nm fibers were retained in the late-stage (postelimination) developing macronucleus and were recognizably nucleosomal fibers. The unusual nucleosomal organization we see for E. crassus Tec elements could represent the organization of nucleosomes into a higher-order structure corresponding to the loops of 30-nm fibers seen by Meyer and Lipps (1980, 1981), although the distribution of loop sizes seen in Stylonychia was substantially larger than that of Tec elements. Because Stylonychia does not have Tec elements and these two organisms are evolutionarily quite distant (despite similarities in their macronuclear development), it is difficult to determine what types of eliminated sequences were represented in the heterochromatic loops seen in Stylonychia. However, it will be interesting to determine if Stylonychia has histone variants related to those found in E. crassus.
Although it is somewhat counterintuitive that DNA that is going to be degraded would become packaged in a nuclease-resistant chromatin structure, this actually appears to be a common theme in DNA elimination. Heterochromatization and compaction of chromatin is associated with elimination of chromosomes in Sciara (Rieffel and Crouse, 1966), with elimination of interstitial blocks of DNA in nematodes (Muller et al., 1996), and with pyknosis and elimination of nuclei during erythropoiesis in mammals and during apoptosis in many organisms (Kerr et al., 1972; Papayannopoulou and Abkowitz, 1991). In addition to the “heterochromatic” fibers seen in Stylonychia and referred to above, recent studies of the Pdd1 protein in Tetrahymena thermophila have generated another link between heterochromatin formation and DNA elimination in ciliates (Madireddi et al., 1994, 1996). Pdd1p is a chromodomain protein, i.e., it shares an amino acid sequence domain with proteins that are associated with heterochromatin in Drosophila, yeast, and mammals (Paro and Hogness, 1991; Koonin et al., 1995). Pdd1p forms heterochromatic “vesicle” structures resembling nucleoli that colocalize with eliminated DNA; hence, the Pdd1 protein and heterochromatin formation are assumed to be involved in the process of DNA elimination or degradation in Tetrahymena (Madireddi et al., 1996). To date, it is unclear whether the formation of heterochromatin during elimination is due to a repression of transcription that accompanies sequence elimination or whether condensation or compaction has other effects. The Tec elements do not appear to be forming the type of vesicle structure seen with the Pdd1 protein in Tetrahymena. In E. crassus, a distinct chromosomal architecture is apparent in the developing anlagen throughout the stages we examined here, and in situ hybridization with Tec elements does not indicate any coalescence of the eliminated DNA (Sharp and Jahn, unpublished observations). The mechanisms of heterochromatin formation in eliminated DNA may be quite different for different ciliated protozoans, but the end result could be similar. Condensation of the chromatin could facilitate its recognition by machinery that either partitions the sequences to an intranuclear compartment that degrades the DNA or allows transport of the sequences out of the nucleus to be degraded. Further studies of the histone variants and the unusual lysine-rich, 85-kDa protein from E. crassus may elucidate the role of chromatin differentiation in DNA elimination.
ACKNOWLEDGMENTS
This work was supported in part by National Science Foundation grant MCB-9319009 and by Northwestern University.
REFERENCES
- Allan J, Harborne N, Rau DC, Gould H. Participation of core histone “tails” in the stabilization of the chromatin solenoid. J Cell Biol. 1982;93:285–297. doi: 10.1083/jcb.93.2.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ausio J, Dong F, van Holde KE. Use of selectively trypsinized nucleosome core particles to analyze the role of the histone “tails” in the stabilization of the nucleosome. J Mol Biol. 1989;206:451–463. doi: 10.1016/0022-2836(89)90493-2. [DOI] [PubMed] [Google Scholar]
- Axel R. Cleavage of DNA in nuclei and chromatin with staphylococcal nuclease. Biochemistry. 1975;14:2921–2925. doi: 10.1021/bi00684a020. [DOI] [PubMed] [Google Scholar]
- Baird SE, Fino GM, Tausta SL, Klobutcher LA. Micronuclear genome organization in Euplotes crassus: a transposon-like element is removed during macronuclear development. Mol Cell Biol. 1989;9:3793–3807. doi: 10.1128/mcb.9.9.3793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson D, Herrick G. Telomeric properties of C4A4-homologous sequences in micronuclear DNA of Oxytricha fallax. Cell. 1984;36:171–177. doi: 10.1016/0092-8674(84)90086-2. [DOI] [PubMed] [Google Scholar]
- Erbeznik M, Yao MC, Jahn CL. Characterization of the Euplotes crassus macronuclear rDNA and its potential as a DNA transformation vehicle. J Eukaryot Microbiol. 1999;46:206–216. doi: 10.1111/j.1550-7408.1999.tb04605.x. [DOI] [PubMed] [Google Scholar]
- Feinberg AP, Vogelstein B. A technique for radiolabeling DNA fragments to high specific activity. Anal Biochem. 1983;132:6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Frels JS, Jahn CL. DNA rearrangements in Euplotes crassus coincide with discrete periods of DNA replication during the polytene chromosome stage of macronuclear development. Mol Cell Biol. 1995;15:6488–6495. doi: 10.1128/mcb.15.12.6488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frels JS, Tebeau CM, Doktor SZ, Jahn CL. Differential replication and DNA elimination in the polytene chromosomes of Euplotes crassus. Mol Biol Cell. 1996;7:755–768. doi: 10.1091/mbc.7.5.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottschling DE, Cech TR. Chromatin structure of the molecular ends of Oxytricha macronuclear DNA: phased nucleosomes and a telomeric complex. Cell. 1984;38:501–510. doi: 10.1016/0092-8674(84)90505-1. [DOI] [PubMed] [Google Scholar]
- Gross DS, Garrard WT. Nuclease hypersensitive sites in chromatin. Annu Rev Biochem. 1988;57:159–197. doi: 10.1146/annurev.bi.57.070188.001111. [DOI] [PubMed] [Google Scholar]
- Hayes JJ, Clark DJ, Wolffe AP. Histone contributions to the structure of DNA in the nucleosome. Proc Natl Acad Sci USA. 1991;88:6829–6833. doi: 10.1073/pnas.88.15.6829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horz W, Miller F, Klobeck G, Zachau HG. Deoxyribonuclease II as a probe for chromatin structure. II. Mode of cleavage. J Mol Biol. 1980;144:329–351. doi: 10.1016/0022-2836(80)90094-7. [DOI] [PubMed] [Google Scholar]
- Horz W, Zachau HG. Deoxyribonuclease II as a probe for chromatin structure. I. Location of cleavage sites. J Mol Biol. 1980;144:305–327. doi: 10.1016/0022-2836(80)90093-5. [DOI] [PubMed] [Google Scholar]
- Igo-Kemenes T, Horz W, Zachau HG. Chromatin. Annu Rev Biochem. 1982;51:89–121. doi: 10.1146/annurev.bi.51.070182.000513. [DOI] [PubMed] [Google Scholar]
- Jahn CL. Bal 31 sensitivity of micronuclear sequences homologous to C4A4/G4T4 repeats in Oxytricha nova. Exp Cell Res. 1988;177:162–175. doi: 10.1016/0014-4827(88)90034-1. [DOI] [PubMed] [Google Scholar]
- Jahn CL, Doktor SZ, Frels JS, Jaraczewski JW, Krikau MF. Structure of the Euplotes crassus Tec1 and Tec2 elements: identification of putative transposase coding regions. Gene. 1993;133:71–78. doi: 10.1016/0378-1119(93)90226-s. [DOI] [PubMed] [Google Scholar]
- Jahn CL, Krikau MF, Shyman S. Developmentally coordinated en masse excision of a highly repetitive element in E. crassus. Cell. 1989;59:1009–1018. doi: 10.1016/0092-8674(89)90757-5. [DOI] [PubMed] [Google Scholar]
- Jahn CL, Ling Z, Tebeau CM, Klobutcher LA. An unusual histone H3 specific for early macronuclear development in Euplotes crassus. Proc Natl Acad Sci USA. 1997;94:1332–1337. doi: 10.1073/pnas.94.4.1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahn CL, Nilles LA, Krikau MF. Organization of the Euplotes crassus micronuclear genome. J Protozool. 1988;35:590–601. doi: 10.1111/j.1550-7408.1988.tb04157.x. [DOI] [PubMed] [Google Scholar]
- Kerr JFR, Wyllie AH, Currie AR. Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer. 1972;26:239–257. doi: 10.1038/bjc.1972.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khosla S, Kantheti P, Brachmachari V, Chandra HS. A male-specific nuclease-resistant chromatin fraction in the mealybug Planococcus lilacinus. Chromosoma. 1996;104:386–392. doi: 10.1007/BF00337228. [DOI] [PubMed] [Google Scholar]
- Klobutcher LA, Jahn CL. Developmentally controlled genomic rearrangements in ciliated protozoa. Curr Opin Gen Dev. 1991;1:397–403. doi: 10.1016/s0959-437x(05)80306-5. [DOI] [PubMed] [Google Scholar]
- Koonin EV, Zhou S, Lucchesi JC. The chromo superfamily: new members, duplication of the chromodomain and possible role in delivering transcriptional regulators to chromatin. Nucleic Acids Res. 1995;23:4229–4233. doi: 10.1093/nar/23.21.4229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krikau MF, Jahn CL. Tec2: a second transposon-like element demonstrating developmentally coordinated excision in Euplotes crassus. Mol Cell Biol. 1991;11:4751–4759. doi: 10.1128/mcb.11.9.4751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luger K, Mader AW, Richmond RK, Sargent DF, Richmond TJ. Crystal structure of the nucleosome core particle at 2.8 A resolution. Nature. 1997;389:251–260. doi: 10.1038/38444. [DOI] [PubMed] [Google Scholar]
- Madireddi MT, Coyne RS, Smothers JF, Mickey KM, Yao M-C, Allis CD. Pdd1p, a novel chromodomain-containing protein, links heterochromatin assembly and DNA elimination in Tetrahymena. Cell. 1996;87:75–84. doi: 10.1016/s0092-8674(00)81324-0. [DOI] [PubMed] [Google Scholar]
- Madireddi MT, Davis MC, Allis CD. Identification of a novel polypeptide involved in the formation of DNA-containing vesicles during macronuclear development in Tetrahymena. Dev Biol. 1994;165:418–431. doi: 10.1006/dbio.1994.1264. [DOI] [PubMed] [Google Scholar]
- Meyer GF, Lipps HJ. Chromatin elimination in the hypotrichous ciliate Stylonychia mytilus. Chromosoma. 1980;77:285–297. doi: 10.1007/BF00286054. [DOI] [PubMed] [Google Scholar]
- Meyer GF, Lipps HJ. The formation of polytene chromosomes during macronuclear development of the hypotrichous ciliate Stylonychia mytilus. Chromosoma. 1981;82:309–314. doi: 10.1007/BF00286113. [DOI] [PubMed] [Google Scholar]
- Muller F, Bernard V, Tobler H. Chromatin diminution in nematodes. Bioessays. 1996;18:133–138. doi: 10.1002/bies.950180209. [DOI] [PubMed] [Google Scholar]
- Nasmyth KA. The regulation of yeast mating-type chromatin structure by SIR: an action at a distance affecting both transcription and transposition. Cell. 1982;30:567–578. doi: 10.1016/0092-8674(82)90253-7. [DOI] [PubMed] [Google Scholar]
- Noll M. Subunit structure of chromatin. Nature. 1974;251:249–251. doi: 10.1038/251249a0. [DOI] [PubMed] [Google Scholar]
- Papayannopoulou T, Abkowitz J. Biology of erythropoiesis, erythroid differentiation and maturation. In: Hoffman R, Bentz EJJ, Shattil SJ, Furie B, Cohen H J, editors. Hematology: Basic Principles and Practice. New York: Churchill Livingstone; 1991. pp. 252–257. [Google Scholar]
- Paro R, Hogness D. The Polycomb protein shares a homologous domain with a heterochromatin associated protein of Drosophila. Proc Natl Acad Sci USA. 1991;88:262–267. doi: 10.1073/pnas.88.1.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prescott DM. The DNA of ciliated protozoa. Microbiol Rev. 1994;58:233–267. doi: 10.1128/mr.58.2.233-267.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price CM. Telomere structure in Euplotes crassus: characterization of DNA-protein interactions and isolation of a telomere-binding protein. Mol Cell Biol. 1990;10:3421–3431. doi: 10.1128/mcb.10.7.3421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray S, Jahn CL, Tebeau C, Larson M, Price CM. Differential expression of linker histone variants in Euplotes crassus. Gene. 1999;231:15–20. doi: 10.1016/s0378-1119(99)00107-9. [DOI] [PubMed] [Google Scholar]
- Rieffel SM, Crouse HV. The elimination and differentiation of chromosomes in the germ line of sciara. Chromosoma. 1966;19:231–276. doi: 10.1007/BF00326917. [DOI] [PubMed] [Google Scholar]
- Roth MR, Lin M-Y, Prescott DM. Large scale synchronous mating and the study of macronuclear development in Euplotes crassus. J Cell Biol. 1985;101:79–84. doi: 10.1083/jcb.101.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson RT, Bergman LW. Structure of sea urchin sperm chromatin core particle. J Biol Chem. 1980;255:10702–10709. [PubMed] [Google Scholar]
- Sollner-Webb B, Felsenfeld G. A comparison of digestion of nuclei and chromatin by staphylococcal nuclease. Biochemistry. 1975;14:2915–2920. doi: 10.1021/bi00684a019. [DOI] [PubMed] [Google Scholar]
- Wallrath L, Elgin SC. Position effect variegation in Drosophila is associated with an altered chromatin structure. Genes Dev. 1995;9:1263–1277. doi: 10.1101/gad.9.10.1263. [DOI] [PubMed] [Google Scholar]
- Wright JH, Gottschling DE, Zakian VA. Saccharomyces telomeres assume a nonnucleosomal chromatin structure. Genes Dev. 1992;6:197–210. doi: 10.1101/gad.6.2.197. [DOI] [PubMed] [Google Scholar]