SUMMARY
Honokiol, a compound extracted from Magnolia officinalis, has antitumor and antiangiogenic properties in several tumor models in vivo. Among the downstream pathways inhibited by honokiol is nuclear factor kappa beta (NFkB). A prime physiologic stimulus of NFkB is reactive oxygen species. The chemical structure of honokiol suggests that it may be an effective scavenger of reactive oxygen species. In this work we have studied the reactions of honokiol with superoxide and peroxyl radicals in cell-free and cellular systems using electron spin resonance (ESR) and HPLC techniques. Honokiol efficiently scavenged superoxide radicals in xanthine oxidase and cytochrome P-450 cell free systems with the rate constant 3.2×105 M−1s−1, which is similar to reactivity of ascorbic acid but 20-times higher than reactivity of vitamin E analog trolox. Honokiol potently scavenged intracellular superoxide within melanoma cells. In addition, honokiol scavenged peroxyl radicals generated by 2,2'-azo-bis(2-amidinopropane hydrochloride) (AAPH). The rate constant of the reaction of honokiol with peroxyl radicals (1.4×106 M−1s−1) was calculated from the competition with spin trap 5-(ethoxycarbonyl)-5-methyl-1-pyrroline N-oxide (EMPO), and was found close to reactivity of trolox (2.5×106 M−1s−1). Therefore, honokiol is an effective scavenger of both superoxide and peroxyl radicals, which may be important for physiological activity of honokiol.
1. Introduction
Honokiol is a small molecule that has been demonstrated to have antiangiogenic and antitumor properties in diverse tumors, including sarcomas, multiple myeloma, and chronic lymphocytic leukemia [1]. In these previous studies, honokiol was found to sensitize cells to tumor necrosis factor alpha apoptosis inducing ligand (TRAIL), TNF alpha, doxorubicin, and cladribine [2, 3]. TRAIL and TNF alpha are known to be pro-apoptotic under certain stimuli, but can also induce resistance to apoptosis due to induction of NFkB, a well known downstream effect of TRAIL and TNF alpha [4]. Indeed, inflammatory lesions that express TNF alpha are also known to express NFkB. One known stimulus of NFkB is reactive oxygen species, such as superoxide and peroxyl radicals [5–8]. Honokiol has been demonstrated to inhibit NFkB activation. Therefore, scavenging of reactive oxygen species may be a mechanism through which honokiol inhibits NFkB activation.
Honokiol molecule contains two phenolic groups (Figure 1) which can exhibit antioxidant properties similar to vitamin E [9] or polyphenols such as flavonoids [10]. However, scavenging of free radicals by honokiol has not been documented. Meanwhile, it has been reported that honokiol inhibited free radical induced lipid peroxidation [11] and prevented oxidative modification of LDL [12], reducing the oxLDL-induced cytotoxicity, apoptotic features and expression of adhesion molecules. These data suggest that attenuation of redox cell signaling [12] and inhibition of lipid peroxidation [11] may be important for physiological activity of honokiol. In this work we have investigated scavenging of superoxide and peroxyl radicals by honokiol and compared these reactions with well known antioxidants ascorbate and vitamin E analog trolox (Figure 1).
Figure 1.
Chemical structures of ascorbic acid, honokiol and trolox (vitamin E analog).
Previously, reactions of antioxidant with free radicals have been studied by competition with spin traps and spin probes [13]. For example, rate constant of antioxidant with superoxide radical can be calculated from inhibition of superoxide detection at various concentrations of an antioxidant using electron spin resonance (ESR) and spin probe 1-hydroxy-3-methoxycarbonyl-2,2,5,5-tetramethylpyrrolidine (CMH) [14]. CMH allows quantitative superoxide detection with high rate constant (1.2×104 M−1s−1) producing stable nitroxide radical 3-methoxycarbonyl-proxyl (CM) [14]. So, the rate constant of superoxide with honokiol can be calculated from inhibition of nitroxide ESR signal at increasing concentrations of honokiol.
An alternative method for detection of intracellular superoxide is based on dihydroethidium [15]. Recently, HPLC detection of intracellular superoxide by fluorescent product of dihydroethidium has been described [16]. In this work we have studied reactions of honokiol with superoxide in cell-free and in WM35PKB cells, a highly tumorigenic human melanoma cell line that overexpresses Akt, using HPLC detection of 2-hydroxyethidium [17].
Radical initiator 2,2'-azo-bis(2-amidinopropane hydrochloride) (AAPH) is commonly used to study lipid peroxidation. Peroxyl radicals generated by AAPH (reaction 1) can be detected by spin traps such as EMPO [18], which produces stable EMPO/•OR radical adduct (reaction 2) [19]. Meanwhile, scavenging of peroxyl radicals by honokiol will inhibit formation of the EMPO radical adduct (reaction 3).
| Reaction 1 |
| Reaction 2 |
| Reaction 3 |
In this work we report that honokiol is a potent scavenger of both superoxide and peroxyl radicals. This activity may underlie in part honokiol’s antitumor activity and suggests that honokiol may be useful in photoaging as well.
2. Materials and methods
2.1. Reagents
Honokiol was obtained from Wako Chemical Company (Tokyo, Japan). Diethylenetriaminepentaacetic acid (DTPA), menadione, xanthine, polyethylene glycol conjugated superoxide dismutase (PEG-SOD), and xanthine were obtained from Sigma–Aldrich (St. Louis, MO). Xanthine oxidase was purchased from Roche Molecular Biochemicals (Indianapolis, IN). The spin probe 1-hydroxy-3-methoxycarbonyl-2,2,5,5-tetramethyl-pyrrolidine (CMH), spin trap 5-ethoxycarbonyl-5-methyl-1-pyrroline N-oxide (EMPO) and trolox were purchased from Alexis Corporation (San Diego, USA). NADPH 2,2'-azo-bis(2-amidinopropane hydrochloride) (AAPH) was purchased from Cayman Chemical Co (Ann Arbor, MI). NADPH and cytochrome P-450 reductase were obtained from Calbiochem (San Diego, CA). Dihydroethidium was purchased from Molecular Probes (Eugene, OR) and dissolved in nitrogen-purged DMSO. The modified Krebs-HEPES buffer for cell studies was composed of (in mM) 99.01 NaCl, 4.69 KCl, 2.50 CaCl2, 1.20 MgSO4, 25 NaHCO3, 1.03 K2HPO4, 20 Na-HEPES, and 5.6 D-glucose, pH 7.35.
2.2. ESR experiments
All ESR samples were placed in a 10 mm flat cell (Wilmad, New Jersey). ESR experiments were carried out in 50 mM sodium phosphate buffer (pH 7.4) with 0.9% NaCl. To inhibit iron-catalyzed reactions, DTPA (100 µM) was added to all samples. ESR spectra were recorded at room temperature using an EMX ESR spectrometer (Bruker BioSpin, Massachusetts) and an SHQ microwave cavity. For EMPO samples, five consecutive ESR spectra were recorded, where each spectrum was scanned with the following settings: field sweep, 70 G; microwave frequency, 9.78 GHz; microwave power, 40 mW; modulation amplitude, 0.7 G; conversion time, 40.96 ms; time constant, 40.96 ms; receiver gain, 1×105; number of points, 1024; number of scans, 8. Superoxide detection by CMH was performed by following the low-field peak of the nitroxide ESR spectra using time scans with the following ESR settings: microwave frequency, 9.78 GHz; modulation amplitude, 2 G; microwave power, 20 mW; conversion time, 1.3 s; time constant, 5.2 s; receiver gain, 1×105; and number of points, 1024. ESR experiments were repeated at least three times.
2.3. Superoxide radical generation
The xanthine oxidase superoxide generating system [20] contained xanthine oxidase, xanthine (50 µM), and DTPA (0.1 mM) in 50 mM sodium phosphate buffer (pH 7.4) with 0.9% NaCl. Cytochrome P-450 reductase superoxide generating system [21] consisted of NADPH (0.1mM), menadione (10 µM), and 1µU cytochrome P-450 reductase.
2.4. Simulation of ESR spectra
Computer simulation of experimental ESR spectra was used for the calculation of hyperfine coupling constants. Programs for the simulation of ESR spectra and the spin-trap database are readily available to the public through the Internet (http://epr.niehs.nih.gov/). The details of this computer simulation program have been described elsewhere [22]. Hyperfine coupling constants are expressed as an average of ESR parameters obtained from computer simulations using at least three experimental spectra, which provides precision of not less than 0.05 G.
2.5. Preparation of spin probe stock solutions
Stock solutions of 1-hydroxy-3-methoxycarbonyl-2,2,5,5-tetramethylpyrrolidine (CMH) (10 mM), dissolved in 0.9% NaCl containing 0.1 mM DTPA and purged with argon, were prepared daily and kept under argon on ice. DTPA was used to decrease auto-oxidation of CMH catalyzed by trace amount of transition metals. CMH was used in a final concentration of 1 mM.
2.6. Cell culture
WM35pKB cells (gift of Joyce Slingerland, University of Miami) were plated in 100 mm dishes and grown to confluence. Cells were maintained at 37° Celsius, 5% CO2, and Dulbecco’s modified Eagle’s medium supplemented with 5% fetal calf serum [23].
2.7. Detection of intracellular superoxide with high-performance liquid chromatography
To evaluate intracellular production of superoxide, we measured the formation of 2-hydroxyethidium from dihydroethidium (DHE) using high-performance liquid chromatography (HPLC) analysis as recently reported [17]. Medium was removed from cells platted on 100 mm dish and replaced with 10 µM DHE in fresh Krebs-HEPES buffer. After 20 minute incubation at 37 °C buffer was aspirated, cells were collected into 300 µL methanol, and cell homogenate was filtered via 0.2 µm filter. Separation of ethidium, 2-hydroxyethidium, and dihydroethidium was performed with the use of an acetonitrile gradient and a C-18 reverse-phase column (Nucleosil 250-4.5 mm) on a Beckman HPLC system with a fluorescence detector Jasco FP-2020 (Easton, MD) using an emission wavelength of 580 nm and an excitation of 480 nm. 2-Hydroxyethidium was expressed per milligram of protein. In some samples, PEG-SOD (25 U/mL) or honokiol (10 µM) was added 20 hours prior to addition of dihydroethidium. PEG-SOD inhibited the 2-hydroxyethidium signal by 90%.
Of note, HPLC analysis (DHE protocol) did not show significant cellular metabolism of honokiol, while cellular levels of honokiol after 30-minutes and 20-hour incubation were identical. Acute treatments with honokiol led to similar decrease in superoxide detection as 20-hour incubation. However, 20-hour incubation was selected for comparison with PEG-SOD which required significant time for intracellular accumulation.
2.8. Statistical analysis
Data are presented as mean ± standard error. For comparison of two groups, a two-tailed t-test was employed using Excel software. Statistical significance was assumed when p < 0.05.
3. Results
3.1. Measurement of the rate constant of superoxide reaction with honokiol
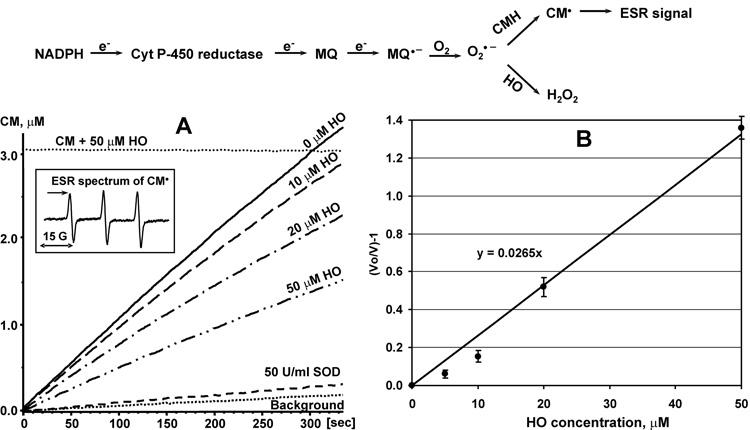
Previously, we have measured the rate constant of scavenging reactions by competition with spin probes such as CMH [24]. In this work redox-cycling agent menadione and NADPH dependent cytochrome P-450 reductase were used as superoxide generating system [21]. The rate constant of superoxide reaction with honokiol was determined by competition with CMH (Figure 2). Honokiol inhibited accumulation of CM-nitroxide in concentration dependent manner (Figure 2A). Linear regression of honokiol dependent inhibition provided the ratio of the rate constants for honokiol/CMH as high as 26.5±0.6 (Figure 2B), which gives an estimate for honokiol rate constant as 3.2×105 M−1s−1 (CMH rate constant is 1.2×104 M−1s−1). It is interesting that the rate constant of superoxide reaction with honokiol is very similar to one of the most efficient superoxide scavenger ascorbic acid (3.3×105 M−1s−1) [25] and 20-times higher than the rate constant of vitamin E analog trolox (1.7×104 M−1s−1) [26]. Meanwhile, honokiol is much more lipophilic than ascorbate. Therefore, honokiol may enhance superoxide scavenging in lipid and hydrophobic environment.
Figure 2.
Measurement of the rate constant of superoxide reaction with honokiol by competition with spin probe CMH (1mM) in cytochrome P-450 reductase system (10 µM menadione, 0.1mM NADPH, 1µU cytochrome P-450 reductase). (A) Accumulation of CM-nitroxide in superoxide generating system in the presence of various concentrations of honokiol. CM accumulation was followed by an increase in intensity of low field component of ESR spectrum indicated by arrow (insert). (B) Inhibition of CM accumulation by honokiol. ESR settings were as described in Materials and Methods.
3.2. Scavenging of superoxide in cell-free and cellular systems
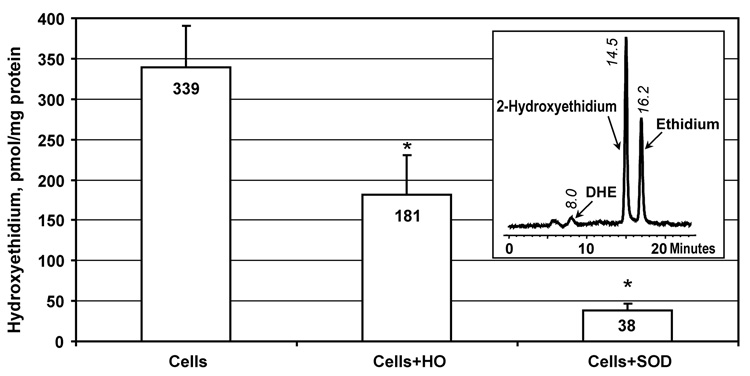
Dihydroethidium is a well known probe for intracellular superoxide [15]. It has been recently reported that the reaction of superoxide with dihydroethidium produces specific product 2-hydroxyethidium, which can be quantified by fluorescent detection with HPLC [16, 17]. We have studied the effect of honokiol on intracellular superoxide using dihydroethidium and HPLC. Superoxide production was compared in WM35pKB cells, cells treated with 10 µM honokiol or cells treated with cell permeable PEG-SOD. It was found that honokiol inhibited the formation of 2-hydroxyethidium by 47%, while PEG-SOD almost completely blocked superoxide detection (Figure 3).
Figure 3.
Detection of intracellular superoxide using dihydroethidium and HPLC in WM35pKB cells following 20-hour treatment with 10 µM honokiol or cell-permeable PEG-SOD (25 U/mL). Insert shows typical HPLC tracing of WM35pKB cells incubated with 10 µM DHE.
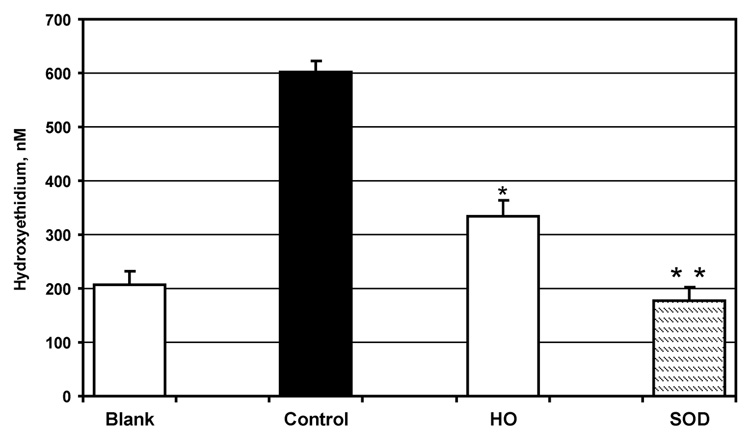
Effect of honokiol on intracellular superoxide was compared to inhibition of 2-hydroxyethidium formation in cell-free xanthine oxidase system. It was found that honokiol inhibited superoxide detection by 67% (Figure 4). Therefore, our cell-free data support that an inhibition of intracellular 2-hydroxyethidium is due to the scavenging of superoxide.
Figure 4.
Scavenging of superoxide by honokiol (10 µM) measured by dihydroethidium and HPLC in xanthine (50 µM) plus xanthine oxidase (1 mU/ml) superoxide generating cell-free system.
3.3. Spin trapping study of honokiol reaction with AAPH-derive peroxyl radical
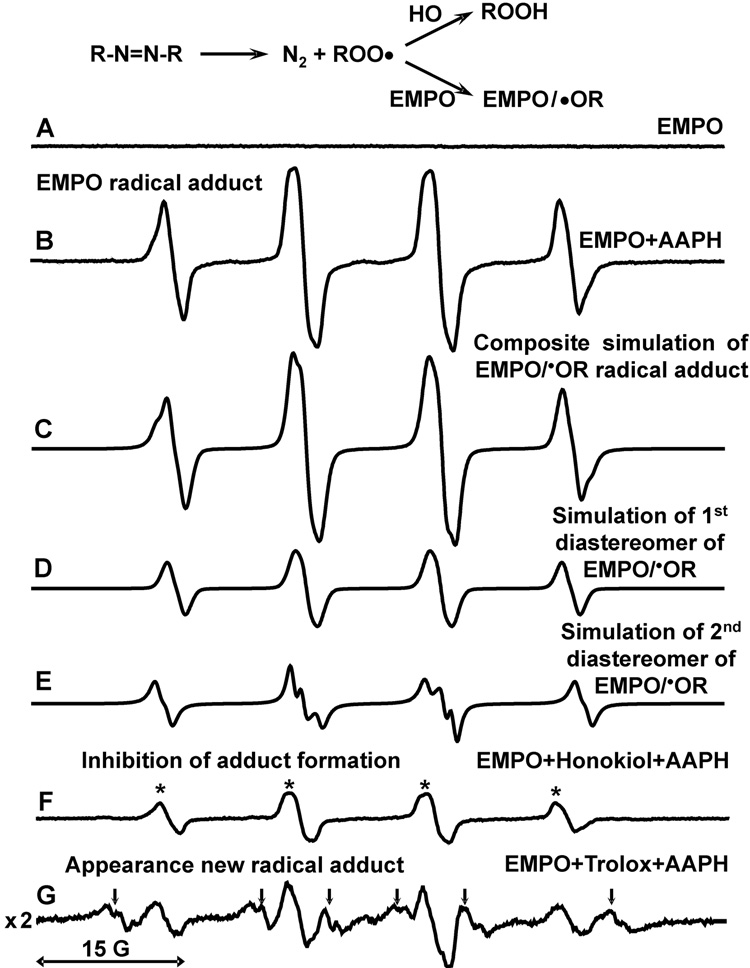
Reactions of peroxyl radicals were studied by spin trapping technique with EMPO [18]. It has been previously reported that spin trapping of peroxyl radicals with nitrones such as EMPO produces alkoxyl radical adduct [27]. Indeed, analysis of peroxyl radical generator AAPH with spin trap EMPO showed typical 4-line ESR spectrum of tertiary alkoxyl radical adduct (Figure 5B) [19]. Computer simulation (Figure 5C) revealed the presence of two diastereomers of the radical adduct (Figure 5 D,E) because of the presence of 2-chiral centers on the position 2 and 5 of the radical adduct [20]. Interestingly, addition of honokiol (0.2 mM) to EMPO (60 mM) strongly inhibited the formation of EMPO radical adduct (Figure 5F). It is important to note that honokiol inhibited the formation of EMPO radical adduct only when it was added before EMPO reaction with peroxyl radicals, while honokiol added 5-minutes after incubation of EMPO with AAPH did not affect ESR amplitude of EMPO radical adduct (Figure 5B), which shows no direct reduction of EMPO radical adduct by honokiol. Furthermore, addition of honokiol resulted only in decrease of peroxyl derived radical adduct (Figure 5, arrows) without the formation of new honokiol derived radical adducts.
Figure 5.
Scavenging of peroxyl radicals by honokiol. (A) ESR specrum of 60 mM EMPO; (B) specrum of EMPO plus 10 mM AAPH; (C) Composite computer simulation of (B); (D) First component of composite simulation with hyperfine coupling constants aN =13.6 G, aHβ=12.7 G, aHγ=0.9 G; (E) Second component of composite simulation with hyperfine coupling constants aN =13.6 G, aHβ=15.2 G, aHγ=0.9 G; (F) Addition of honokiol (0.2 mM) inhibited formation of the EMPO radical adduct due to scavenging of AAPH derived peroxyl radical by honokiol. (G) Addition of trolox (0.2mM) led to 6-fold decrease of EMPO/•OR amplitude and appearance of new two trolox derived carbon-centered radical adducts indicated by arrows (hyperfine coupling constants aN =14.6 G, aHβ=23.1 G and aN =14.5 G, aHβ=20.6 G). ESR settings were as described in Materials and Methods.
Vitamin E and its analog trolox (Figure 1) are well known scavengers of the peroxyl radicals. So, we have studied scavenging of peroxyl radical by trolox using spin trap EMPO and compared an inhibition of EMPO radical adduct formation with honokiol. Addition of 0.2 mM trolox led to 6-fold decrease of EMPO/•OR amplitude and appearance of new trolox derived carbon-centered radical adduct (Figure 5G, arrows). Comparison of the effects of honokiol and trolox showed that trolox had slightly higher efficiency of scavenging of peroxyl radicals. However, EMPO has detected secondary carbon-centerd radicals derived from trolox. These trolox-derived secondary radicals may be involved in propagation of free radical damage and, therefore, attenuate antioxidant activity of trolox.
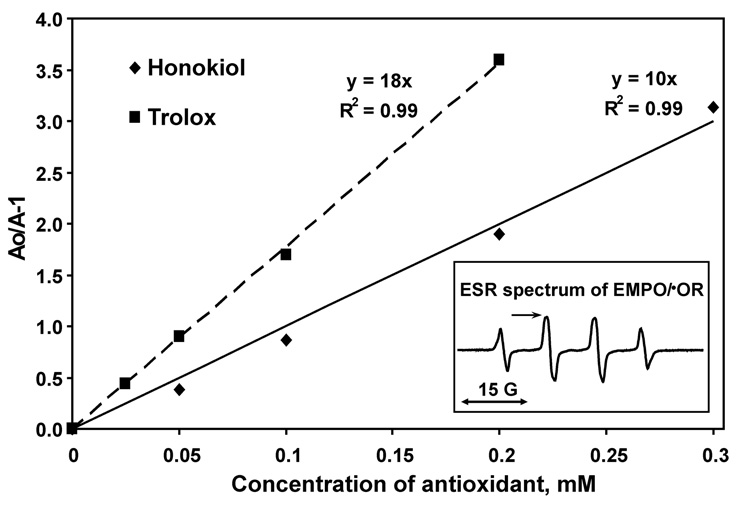
3.4. Measurement of the rate constant of honokiol reaction with peroxyl radical
Honokiol inhibited the formation of EMPO radical adduct in concentration dependent manner (Figure 6). Therefore, we calculated the rate constant of honokiol reaction with AAPH derived peroxyl radical by competition with spin trap EMPO and comparison with trolox (Figure 6). The ratio of the rate constants of trolox to EMPO was 1.8-fold higher than one of honokiol. The rate constant of trolox was previously determined as 2.5×106 M−1s−1 [28]. So, the rate constant of peroxyl radical reaction with honokiol is estimated as 1.4×106 M−1s−1. It is important to note that reactivity of honokiol with peroxyl radical is very high and it is close to one of vitamin E and its analog trolox. This may suggest that scavenging of peroxyl radicals by honokiol may contribute to antioxidant activity of honokiol.
Figure 6.
Calculation of the rate constant of peroxyl radical reaction with honokiol determined by competition with spin trap EMPO and comparison with vitamin E analog trolox. Insert shows typical ESR spectrum of EMPO/•OR in the presence of 60 mM EMPO and 10 mM AAPH. Accumulation of EMPO/•OR was followed by an increase in intensity of low field component of ESR spectrum indicated by arrow (insert). Ao is ESR amplitude of EMPO/•OR radical adduct in the absence of antioxidants, A is ESR amplitude of EMPO/•OR radical adduct in the presence of antioxidants. The slope of linear regression is equal to the ratio of the rate constants of scavenging reactions of antioxidants to the one of spin trap EMPO. The rate constant of trolox was previously determined as 2.5×106 M−1s−1. Thus, honokiol rate constant with peroxyl radicals is 1.4×106 M−1s−1.
4. Discussion
Various reactive oxygen species, including superoxide and peroxyl radicals, are generated as a consequence of cellular metabolism, and are accentuated by pathophysiologic processes. Dysregulation of reactive oxygen species has been implicated in numerous human disorders, including neoplasia, inflammation, degenerative diseases, and environmental exposures, especially ultraviolet light [29–34]. One of the cardinal features of reactive oxygen mediated disease is that cells undergo maladaptation to the initial insult which results in perpetuation of reactive oxygen generation. Examples of this include mitochondrial DNA mutations in photoaged skin as well as cancer, and the generation of reactive oxygen species in neurodegenerative disorders including Alzheimers and Parkinson’s disease [35, 36]. This persistent generation of reactive oxygen species serves as a therapeutic target for treatment. In the present study, we demonstrated that honokiol is an effective scavenger of both superoxide and peroxyl radicals.
By examining the competition between honokiol and spin probe CMH for we were able to calculate the rate constant of superoxide reaction with honokiol as 3.2×105 M−1s−1. The rate constant of the reaction of honokiol with peroxyl radicals was calculated from the competition of honokiol with spin trap EMPO and it was 1.4×106 M−1s−1. It is interesting that scavenging of superoxide radical by honokiol was similar to vitamin C but was much more effective than by vitamin E analog trolox. Meanwhile, scavenging of peroxyl radical by honokiol was similar to vitamin E. Our data have shown that honokiol reduced intracellular superoxide by 2-fold, which was similar to its effect in cell free system. Therefore, free radical scavenging may be an important for physiological activity of honokiol.
Previously, it has been reported that honokiol inhibited copper-induced oxidative modification of LDL [12] and iron-stimulated lipid peroxidation [11]. This inhibition may result from metal chelation. However, addition of ferric and cupric ions to honokiol did not affect optical absorption and honokiol did not inhibited metal-catalyzed oxidation of ascorbate (data not shown), which does not support chelation of transition metals by honokiol.
Meanwhile, it is known that overproduction stimulates lipid peroxidation. This may occur by the release of redox active iron from protein iron-sulfur clusters, such as aconitase, and reduction of metals feeding Fenton-like reactions. Our data showed that honokiol decreased the level of cellular by 47% which might also inhibit initiation of lipid peroxidation. Furthermore, by scavenging of lipid peroxyl radicals honokiol will inhibit propagation of lipid peroxidation. Therefore, honokiol may inhibit lipid peroxidation by blocking both initiation and propagation of lipid peroxidation which can be accounted for much higher than vitamin E antioxidant activity of honokiol in the liver and heart mitochondria [11, 37].
It has been reported that honokiol can induced cell apoptosis (IC50=49 µM) and the malignant cells are more susceptible [3]. However, 10 µM honokiol was not cytotoxic and it did not stimulate apoptosis [3]. Therefore, decrease of superoxide in honokiol treated cells in our experiments is not associated with apoptosis.
It was reported that honokiol diminished the activity of assembled-NADPH oxidase, a major reactive oxygen species producing enzyme in neutrophils by 40% without interfering with protein kinase C (PKC)-dependent assembly [38]. Our data suggest that honokiol is likely to act as a scavenger of superoxide rather than direct inhibitor of NADPH oxidase.
It is clear that scavenging of free radicals may directly or indirectly affect cell signaling. First, decrease in superoxide may reduce redox and metal dependent expression of E-selectin and VCAM-1 [39]. Second, decrease in lipid peroxidation may also affect cell signaling [40, 41]. Finally, free radicals directly react with nitric oxide leading to its inactivation [42]. Therefore, decrease in superoxide and lipid oxidation should lead to an increase in bioavalable nitric oxide, which will stimulate cGMP-dependent pathways [43].
In this work we showed that honokiol (10 µM) reduced cellular by almost 50% which may contribute to inhibition of redox-dependent activation of macrophages by honokiol (IC50=6.4 µM) [44]. Interestingly, other phenolic antioxidants such as flavonoids are also known to suppress cellular production (IC50=8.4 µM) and 3-day flavonoid treatment reduced redox-dependent expression of p47phox subunit of NADPH oxidase [45]. Regardless mechanisms of physiological activity (free radical scavenging, NADPH oxidase inhibition, increase in nitric oxide [46]) it is clear that effect of honokiol on cellular redox regulations is very important.
Attempts to use reactive oxygen scavenging in human disease have shown mixed results. Treatments with vitamins C and E have been complicated by issues of delivery and bioavailability [47]. Recent data suggests that high concentrations of vitamin C and E may have pro-oxidant activities [48, 49]. Clinical trials of antioxidants in cancer prevention may have been unsuccessful for this reason. On the other hand, disulfiram, a potent inhibitor of TNF-alpha-induced reactive oxygen species [50], has been effective in the treatment of melanoma in mice and humans [51, 52]. Thus, the choice and mechanism of antioxidant may be critical for optimal clinical benefits. Meanwhile, not all effects of honokiol may be explained on the basis of its free radical scavenging.
Honokiol has already been shown to be efficient in multiple tumor xenograft models in mice, including sarcoma, and hormone independent breast, and prostate cancers [1]. Honokiol downregulates NFkB activity [6], which may be due to free radical scavenging, but also inhibits phosphoinositol-3 kinase/akt signaling [1], which may be independent of reactive oxygen signaling. Honokiol may thus impact on several pathways, including reactive oxygen. Having more than one mechanism of action has been shown to be beneficial for drugs, as there is an increased incidence of tumor resistance to the newer specific targeted therapies.
Acknowledgments
This research was supported by National Institutes of Health grants PO-1 HL058000 and PO-1 HL075209 to S.D., by NIAMS Grants RO1AR 47901 and RO1 AR 050727 to J.L.A, Emory Skin Disease Research Core Center P30 AR 42687.
List of abbreviations
- AAPH
2,2'-azo-bis(2-amidinopropane hydrochloride
- CM
3-methoxycarbonyl-proxyl
- CMH
1-hydroxy-3-methoxycarbonyl-2,2,5,5-tetramethyl-pyrrolidine
- DHE
dihydroethidium
- DTPA
diethylenetriaminepentaacetic acid
- EMPO
5-ethoxycarbonyl-5-methyl-1-pyrroline N-oxide
- ESR
electron spin resonance
- HO
honokiol
- HPLC
high-performance liquid chromatography
- MQ
menadione
- NFkB
nuclear factor kappa beta
- PKC
protein kinase C
- PEG-SOD
polyethylene glycol conjugated superoxide dismutase.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Bai X, Cerimele F, Ushio-Fukai M, Waqas M, Campbell PM, Govindarajan B, Der CJ, Battle T, Frank DA, Ye K, Murad E, Dubiel W, Soff G, Arbiser JL. Honokiol, a small molecular weight natural product, inhibits angiogenesis in vitro and tumor growth in vivo. J Biol Chem. 2003;278:35501–35507. doi: 10.1074/jbc.M302967200. [DOI] [PubMed] [Google Scholar]
- 2.Ishitsuka K, Hideshima T, Hamasaki M, Raje N, Kumar S, Hideshima H, Shiraishi N, Yasui H, Roccaro AM, Richardson P, Podar K, Le Gouill S, Chauhan D, Tamura K, Arbiser J, Anderson KC. Honokiol overcomes conventional drug resistance in human multiple myeloma by induction of caspase-dependent and -independent apoptosis. Blood. 2005;106:1794–1800. doi: 10.1182/blood-2005-01-0346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Battle TE, Arbiser J, Frank DA. The natural product honokiol induces caspase-dependent apoptosis in B-cell chronic lymphocytic leukemia (B-CLL) cells. Blood. 2005;106:690–697. doi: 10.1182/blood-2004-11-4273. [DOI] [PubMed] [Google Scholar]
- 4.Chawla-Sarkar M, Bauer JA, Lupica JA, Morrison BH, Tang Z, Oates RK, Almasan A, DiDonato JA, Borden EC, Lindner DJ. Suppression of NF-kappa B survival signaling by nitrosylcobalamin sensitizes neoplasms to the anti-tumor effects of Apo2L/TRAIL. J Biol Chem. 2003;278:39461–39469. doi: 10.1074/jbc.M306111200. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 5.Oberley LW, Buettner GR. Role of superoxide dismutase in cancer: a review. Cancer Res. 1979;39:1141–1149. [PubMed] [Google Scholar]
- 6.Ahn KS, Sethi G, Shishodia S, Sung B, Arbiser JL, Aggarwal BB. Honokiol potentiates apoptosis, suppresses osteoclastogenesis, and inhibits invasion through modulation of nuclear factor-kappaB activation pathway. Mol Cancer Res. 2006;4:621–633. doi: 10.1158/1541-7786.MCR-06-0076. [DOI] [PubMed] [Google Scholar]
- 7.Schreck R, Rieber P, Baeuerle PA. Reactive oxygen intermediates as apparently widely used messengers in the activation of the NF-kappa B transcription factor and HIV-1. Embo J. 1991;10:2247–2258. doi: 10.1002/j.1460-2075.1991.tb07761.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Samlowski WE, Petersen R, Cuzzocrea S, Macarthur H, Burton D, McGregor JR, Salvemini D. A nonpeptidyl mimic of superoxide dismutase, M40403, inhibits dose-limiting hypotension associated with interleukin-2 and increases its antitumor effects. Nat Med. 2003;9:750–755. doi: 10.1038/nm874. [DOI] [PubMed] [Google Scholar]
- 9.Traber MG, Atkinson J. Vitamin E, antioxidant and nothing more. Free Radic Biol Med. 2007;43:4–15. doi: 10.1016/j.freeradbiomed.2007.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cao G, Sofic E, Prior RL. Antioxidant and prooxidant behavior of flavonoids: structure-activity relationships. Free Radic Biol Med. 1997;22:749–760. doi: 10.1016/s0891-5849(96)00351-6. [DOI] [PubMed] [Google Scholar]
- 11.Chiu JH, Ho CT, Wei YH, Lui WY, Hong CY. In vitro and in vivo protective effect of honokiol on rat liver from peroxidative injury. Life Sci. 1997;61:1961–1971. doi: 10.1016/s0024-3205(97)00836-9. [DOI] [PubMed] [Google Scholar]
- 12.Ou HC, Chou FP, Lin TM, Yang CH, Sheu WH. Protective effects of honokiol against oxidized LDL-induced cytotoxicity and adhesion molecule expression in endothelial cells. Chem Biol Interact. 2006;161:1–13. doi: 10.1016/j.cbi.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 13.Oettl K, Dikalov S, Freisleben HJ, Mlekusch W, Reibnegger G. Spin trapping study of antioxidant properties of neopterin and 7,8-dihydroneopterin. Biochem Biophys Res Commun. 1997;234:774–778. doi: 10.1006/bbrc.1997.6712. [DOI] [PubMed] [Google Scholar]
- 14.Kuzkaya N, Weissmann N, Harrison DG, Dikalo vS. Interactions of peroxynitrite, tetrahydrobiopterin, ascorbic acid, and thiols: implications for uncoupling endothelial nitric-oxide synthase. J Biol Chem. 2003;278:22546–22554. doi: 10.1074/jbc.M302227200. [DOI] [PubMed] [Google Scholar]
- 15.Benov L, Sztejnberg L, Fridovich I. Critical evaluation of the use of hydroethidine as a measure of superoxide anion radical. Free Radic Biol Med. 1998;25:826–831. doi: 10.1016/s0891-5849(98)00163-4. [DOI] [PubMed] [Google Scholar]
- 16.Zhao H, Joseph J, Fales HM, Sokoloski EA, Levine RL, Vasquez-Vivar J, Kalyanaraman B. Detection and characterization of the product of hydroethidine and intracellular superoxide by HPLC and limitations of fluorescence. Proc Natl Acad Sci U S A. 2005;102:5727–5732. doi: 10.1073/pnas.0501719102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dikalov S, Griendling KK, Harrison DG. Measurement of reactive oxygen species in cardiovascular studies. Hypertension. 2007;49:717–727. doi: 10.1161/01.HYP.0000258594.87211.6b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang H, Joseph J, Vasquez-Vivar J, Karoui H, Nsanzumuhire C, Martasek P, Tordo P, Kalyanaraman B. Detection of superoxide anion using an isotopically labeled nitrone spin trap: potential biological applications. FEBS Lett. 2000;473:58–62. doi: 10.1016/s0014-5793(00)01498-8. [DOI] [PubMed] [Google Scholar]
- 19.Dikalov SI, Mason RP. Reassignment of organic peroxyl radical adducts. Free Radic Biol Med. 1999;27:864–872. doi: 10.1016/s0891-5849(99)00134-3. [DOI] [PubMed] [Google Scholar]
- 20.Dikalov S, Jiang J, Mason RP. Characterization of the high-resolution ESR spectra of superoxide radical adducts of 5-(diethoxyphosphoryl)-5-methyl-1-pyrroline N-oxide (DEPMPO) and 5,5-dimethyl-1-pyrroline N-oxide (DMPO). Analysis of conformational exchange. Free Radic Res. 2005;39:825–836. doi: 10.1080/10715760500155688. [DOI] [PubMed] [Google Scholar]
- 21.Dikalov S, Landmesser U, Harrison DG. Geldanamycin leads to superoxide formation by enzymatic and non-enzymatic redox cycling. Implications for studies of Hsp90 and endothelial cell nitric-oxide synthase. J Biol Chem. 2002;277:25480–25485. doi: 10.1074/jbc.M203271200. [DOI] [PubMed] [Google Scholar]
- 22.Duling DR. Simulation of multiple isotropic spin-trap EPR spectra. J Magn Reson B. 1994;104:105–110. doi: 10.1006/jmrb.1994.1062. [DOI] [PubMed] [Google Scholar]
- 23.Liang J, Zubovitz J, Petrocelli T, Kotchetkov R, Connor MK, Han K, Lee JH, Ciarallo S, Catzavelos C, Beniston R, Franssen E, Slingerland JM. PKB/Akt phosphorylates p27, impairs nuclear import of p27 and opposes p27-mediated G1 arrest. Nat Med. 2002;8:1153–1160. doi: 10.1038/nm761. [DOI] [PubMed] [Google Scholar]
- 24.Kuzkaya N, Weissmann N, Harrison DG, Dikalov S. Interactions of peroxynitrite with uric acid in the presence of ascorbate and thiols: implications for uncoupling endothelial nitric oxide synthase. Biochem Pharmacol. 2005;70:343–354. doi: 10.1016/j.bcp.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 25.Mitsuta K, Mizuta Y, Kohno M, Hiramatsu M, Mori A. Reaction of superoxide with ascorbate. Bull Chem Soc Jpn. 1990;63:187–191. [Google Scholar]
- 26.Taubert D, Breitenbach T, Lazar A, Censarek P, Harlfinger S, Berkels R, Klaus W, Roesen R. Reaction rate constants of superoxide scavenging by plant antioxidants. Free Radic Biol Med. 2003;35:1599–1607. doi: 10.1016/j.freeradbiomed.2003.09.005. [DOI] [PubMed] [Google Scholar]
- 27.Dikalov SI, Mason RP. Spin trapping of polyunsaturated fatty acid-derived peroxyl radicals: reassignment to alkoxyl radical adducts. Free Radic Biol Med. 2001;30:187–197. doi: 10.1016/s0891-5849(00)00456-1. [DOI] [PubMed] [Google Scholar]
- 28.Simic MG. Peroxyl radical from oleic acid. Autoxid Food Biol Syst. 1980:17–26. [Google Scholar]
- 29.Bickers DR, Athar M. Oxidative stress in the pathogenesis of skin disease. J Invest Dermatol. 2006;126:2565–2575. doi: 10.1038/sj.jid.5700340. [DOI] [PubMed] [Google Scholar]
- 30.Arbiser JL, Petros J, Klafter R, Govindajaran B, McLaughlin ER, Brown LF, Cohen C, Moses M, Kilroy S, Arnold RS, Lambeth JD. Reactive oxygen generated by Nox1 triggers the angiogenic switch. Proc Natl Acad Sci U S A. 2002;99:715–720. doi: 10.1073/pnas.022630199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Petros JA, Baumann AK, Ruiz-Pesini E, Amin MB, Sun CQ, Hall J, Lim S, Issa MM, Flanders WD, Hosseini SH, Marshall FF, Wallace DC. mtDNA mutations increase tumorigenicity in prostate cancer. Proc Natl Acad Sci U S A. 2005;102:719–724. doi: 10.1073/pnas.0408894102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Podda M, Zollner TM, Grundmann-Kollmann M, Thiele JJ, Packer L, Kaufmann R. Activity of alpha-lipoic acid in the protection against oxidative stress in skin. Curr Probl Dermatol. 2001;29:43–51. doi: 10.1159/000060652. [DOI] [PubMed] [Google Scholar]
- 33.Khan N, Afaq F, Saleem M, Ahmad N, Mukhtar H. Targeting multiple signaling pathways by green tea polyphenol (−)-epigallocatechin-3-gallate. Cancer Res. 2006;66:2500–2505. doi: 10.1158/0008-5472.CAN-05-3636. [DOI] [PubMed] [Google Scholar]
- 34.Mantena SK, Meeran SM, Elmets CA, Katiyar SK. Orally administered green tea polyphenols prevent ultraviolet radiation-induced skin cancer in mice through activation of cytotoxic T cells and inhibition of angiogenesis in tumors. J Nutr. 2005;135:2871–2877. doi: 10.1093/jn/135.12.2871. [DOI] [PubMed] [Google Scholar]
- 35.Wallace DC. A mitochondrial paradigm of metabolic and degenerative diseases, aging, and cancer: a dawn for evolutionary medicine. Annu Rev Genet. 2005;39:359–407. doi: 10.1146/annurev.genet.39.110304.095751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Eshaghian A, Vleugels RA, Canter JA, McDonald MA, Stasko T, Sligh JE. Mitochondrial DNA deletions serve as biomarkers of aging in the skin, but are typically absent in nonmelanoma skin cancers. J Invest Dermatol. 2006;126:336–344. doi: 10.1038/sj.jid.5700088. [DOI] [PubMed] [Google Scholar]
- 37.Tsai SK, Huang SS, Hong CY. Myocardial protective effect of honokiol: an active component in Magnolia officinalis. Planta Med. 1996;62:503–506. doi: 10.1055/s-2006-957957. [DOI] [PubMed] [Google Scholar]
- 38.Liou KT, Shen YC, Chen CF, Tsao CM, Tsai SK. The anti-inflammatory effect of honokiol on neutrophils: mechanisms in the inhibition of reactive oxygen species production. Eur J Pharmacol. 2003;475:19–27. doi: 10.1016/s0014-2999(03)02121-6. [DOI] [PubMed] [Google Scholar]
- 39.Zhang WJ, Frei B. Intracellular metal ion chelators inhibit TNFalpha-induced SP-1 activation and adhesion molecule expression in human aortic endothelial cells. Free Radic Biol Med. 2003;34:674–682. doi: 10.1016/s0891-5849(02)01375-8. [DOI] [PubMed] [Google Scholar]
- 40.Ramana KV, Bhatnagar A, Srivastava S, Yadav UC, Awasthi S, Awasthi YC, Srivastava SK. Mitogenic responses of vascular smooth muscle cells to lipid peroxidation-derived aldehyde 4-hydroxy-trans-2-nonenal (HNE): role of aldose reductase-catalyzed reduction of the HNE-glutathione conjugates in regulating cell growth. J Biol Chem. 2006;281:17652–17660. doi: 10.1074/jbc.M600270200. [DOI] [PubMed] [Google Scholar]
- 41.Watanabe N, Zmijewski JW, Takabe W, Umezu-Goto M, Le Goffe C, Sekine A, Landar A, Watanabe A, Aoki J, Arai H, Kodama T, Murphy MP, Kalyanaraman R, Darley-Usmar VM, Noguchi N. Activation of mitogen-activated protein kinases by lysophosphatidylcholine-induced mitochondrial reactive oxygen species generation in endothelial cells. Am J Pathol. 2006;168:1737–1748. doi: 10.2353/ajpath.2006.050648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Huie RE, Padmaja S. The reaction of NO with superoxide. Free Radic Res Commun. 1993;18:195–199. doi: 10.3109/10715769309145868. [DOI] [PubMed] [Google Scholar]
- 43.Thomas DD, Ridnour LA, Espey MG, Donzelli S, Ambs S, Hussain SP, Harris CC, Degraff W, Roberts DD, Mitchell JB, Wink DA. Superoxide fluxes limit nitric oxide-induced signaling. J Biol Chem. 2006 doi: 10.1074/jbc.M602242200. [DOI] [PubMed] [Google Scholar]
- 44.Son HJ, Lee HJ, Yun-Choi HS, Ryu JH. Inhibitors of nitric oxide synthesis and TNF-alpha expression from Magnolia obovata in activated macrophages. Planta Med. 2000;66:469–471. doi: 10.1055/s-2000-8592. [DOI] [PubMed] [Google Scholar]
- 45.Jiang F, Guo N, Dusting GJ. Modulation of nicotinamide adenine dinucleotide phosphate oxidase expression and function by 3',4'-dihydroxyflavonol in phagocytic and vascular cells. J Pharmacol Exp Ther. 2008;324:261–269. doi: 10.1124/jpet.107.131433. [DOI] [PubMed] [Google Scholar]
- 46.Steffen Y, Gruber C, Schewe T, Sies H. Mono-O-methylated flavanols and other flavonoids as inhibitors of endothelial NADPH oxidase. Arch Biochem Biophys. 2008;469:209–219. doi: 10.1016/j.abb.2007.10.012. [DOI] [PubMed] [Google Scholar]
- 47.Alberts DS, Goldman R, Xu MJ, Dorr RT, Quinn J, Welch K, Guillen-Rodriguez J, Aickin M, Peng YM, Loescher L, Gensler H. Disposition and metabolism of topically administered alpha-tocopherol acetate: a common ingredient of commercially available sunscreens and cosmetics. Nutr Cancer. 1996;26:193–201. doi: 10.1080/01635589609514475. [DOI] [PubMed] [Google Scholar]
- 48.Mehlhorn RJ, Sumida S, Packer L. Tocopheroxyl radical persistence and tocopherol consumption in liposomes and in vitamin E-enriched rat liver mitochondria and microsomes. J Biol Chem. 1989;264:13448–13452. [PubMed] [Google Scholar]
- 49.Kagan VE, Freisleben HJ, Tsuchiya M, Forte T, Packer L. Generation of probucol radicals and their reduction by ascorbate and dihydrolipoic acid in human low density lipoproteins. Free Radic Res Commun. 1991;15:265–276. doi: 10.3109/10715769109105222. [DOI] [PubMed] [Google Scholar]
- 50.Zhao A, Wu ZQ, Pollack M, Rollwagen FM, Hirszel P, Zhou X. Disulfiram inhibits TNF-alpha-induced cell death. Cytokine. 2000;12:1356–1367. doi: 10.1006/cyto.2000.0725. [DOI] [PubMed] [Google Scholar]
- 51.Meyskens FL, Jr, McNulty SE, Buckmeier JA, Tohidian NB, Spillane TJ, Kahlon RS, Gonzalez RI. Aberrant redox regulation in human metastatic melanoma cells compared to normal melanocytes. Free Radic Biol Med. 2001;31:799–808. doi: 10.1016/s0891-5849(01)00650-5. [DOI] [PubMed] [Google Scholar]
- 52.Brar SS, Grigg C, Wilson KS, Holder WD, Jr, Dreau D, Austin C, Foster M, Ghio AJ, Whorton AR, Stowell GW, Whittall LB, Whittle RR, White DP, Kennedy TP. Disulfiram inhibits activating transcription factor/cyclic AMP-responsive element binding protein and human melanoma growth in a metal-dependent manner in vitro, in mice and in a patient with metastatic disease. Mol Cancer Ther. 2004;3:1049–1060. [PubMed] [Google Scholar]