Abstract
The effect of incorporating α,α’-diethylglycine and α-aminocyclopentane carboxylic acid at the P2 position of inhibitors on μ-calpain inhibition was studied. Compound 3 with α,α’-diethylglycine was over 20-fold more potent than 2 with α-aminocyclopentane carboxylic acid. Additionally, 3 was over 35-fold selective for μ-calpain compared to cathepsin B, while 2 was 3-fold selective for cathepsin B compared to μ-calpain. Thus, the conformation induced by the P2 residue influenced the activities of the compounds versus the closely related cysteine proteases and suggests an approach to the discovery of selective μ-calpain inhibitors.
Calpain is a papain-like cytosolic cysteine protease that requires calcium ions for activation.1 Several calpain isoforms have been reported of which μ–calpain and μ-calpain are the most abundantly distributed in mammalian cells and have been termed ubiquitous calpains.1–3 Calpain is a promiscuous enzyme that catalyzes the limited proteolysis of a broad range of substrates in vivo.2,3 The physiological role of the enzyme is evolving and it has been shown to participate in signal transduction pathways.4–7 Calpain has attracted considerable interest due in part to implication of the enzyme in a variety of pathological conditions including neurological disorders (e.g., stroke and Alzheimer’s disease), cataract, and cancer.8–13 This has led to the search for selective calpain inhibitors as potential therapeutic agents and as biochemical probes. Several compounds are known to inhibit the ubiquitous calpains. However, most of the inhibitors are not specific for the calpains because they also inhibit the closely related cysteine cathepsins (e.g., cathepsin B).14,15 Hence there is a continuing need for new calpain inhibitors with improved selectivity for the enzyme.
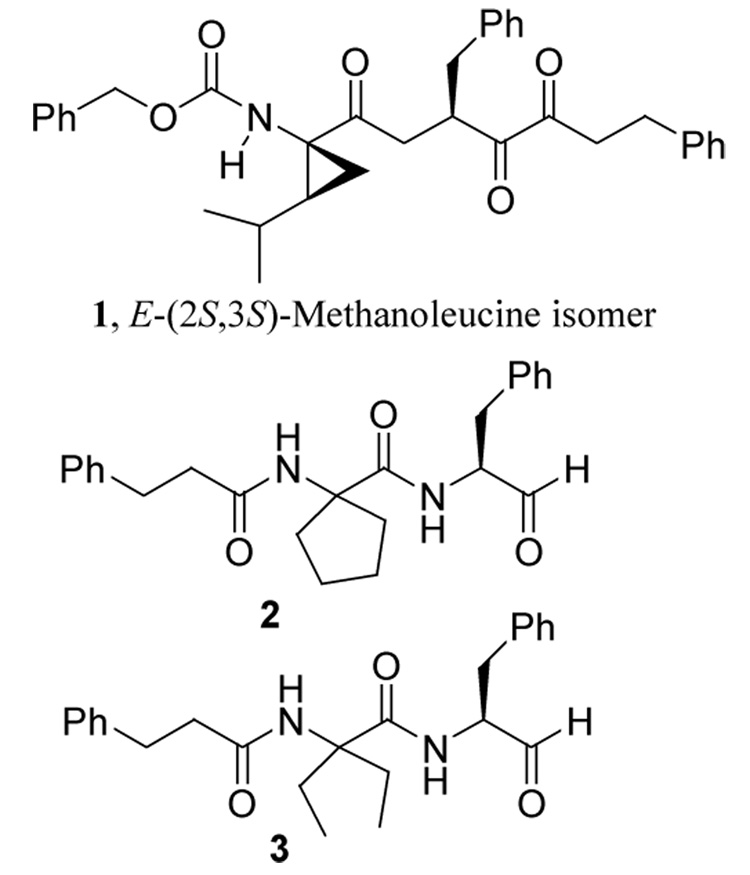
Incorporation of constrained amino acids such as α,α’-dialkylglycines and α-aminocycloalkane carboxylic acids into a peptide restricts the conformational freedom of the peptide in the vicinity of the constrained amino acid.16 This allows one to study the effect of local conformational constraints on bioactivity because peptides containing α,α’-dialkylglycine residues generally adopt fully extended conformation while those with α-aminocycloalkane carboxylic acids prefer a folded conformation.16 We demonstrated in a previous study that incorporation of 2,3-methanoleucine stereoisomers at the P2 position of peptidyl inhibitors influences μ-calpain inhibition. Compound 1 with a 2,3-methanoleucine stereoisomer of E-(2S,3S) configuration at the P2 position (Figure 1) was the most potent and selective member of the series albeit modest selectivity for μ–calpain versus cathepsin B.17 This study showed that the S2-subsite of μ–calpain is more stereosensitive than that of cathepsin B, which suggested that compounds that adopt different conformations would exhibit differential affinities for μ–calpain and cathepsin B. To further probe the active sites of these closely related cysteine proteases with the goal of generating selective μ–calpain inhibitors we studied the effect of incorporating α-aminocyclopentane carboxylic acid (as in 2, Figure 1) and α,α’-diethylglycine (as in 3) at the P2 position on inhibition of μ–calpain and cathepsin B.
Figure 1.
Structures of calpain inhibitors with constrained amino acids
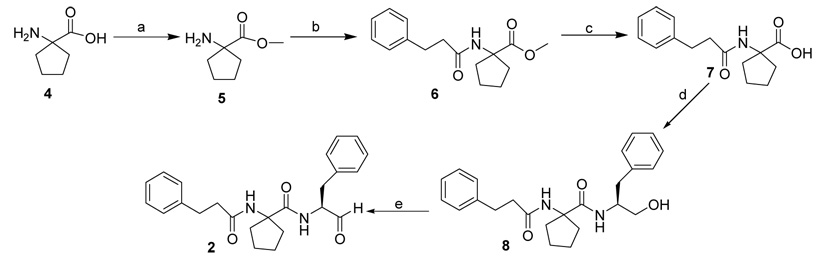
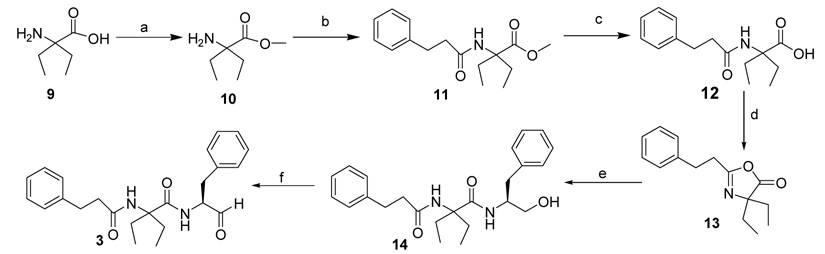
Compounds 2 and 3 were synthesized using general coupling procedures as outlined in Scheme 1 and Scheme 2. 1-Amino-1-cyclopentane carboxylic acid 4 (Scheme 1) or diethylglycine 9 (Scheme 2) were refluxed in SOCl2 and MeOH for 24 h to give esters 5 and 10, respectively. The esters were coupled with 3-phenyl propionic acid, using EDC, NMM, and HOBT, to give the dipeptide esters 6 and 11, which were hydrolysed using 2N NaOH to give the corresponding dipeptide acids 7 and 12. Compound 7 was coupled with l-phenylalaninol in the presence of EDC, NMM, and HOBT to give tripeptide alcohol 8. Compound 12 (Scheme 2) formed the cyclic intermediate 13 in the presence of EDC. This was coupled with l-phenylalaninol in the presence of EDC, NMM, and HOBT to give the tripeptide alcohol 14. The tripeptide alcohols 8 and 14 were oxidized using Dess-Martin periodinane reagent to afford the target compounds 2 (Scheme 1) and 3 (Scheme 2), respectively. The final products were purified by flash column chromatography and/or recrystallization from hexanes/CH2Cl2 (1:1).18 The compounds were evaluated19 as inhibitors of porcine erythrocyte μ–calpain (Calbiochem) and human liver cathepsin B (Calbiochem) using ALLN as positive control. Table 1 shows the results of the study. The nature of the P2 residue influenced the inhibitory activity of the compounds. Compound 3 with α,α’-diethylglycine at the P2 position inhibited μ–calpain with Ki of 0.08 µM. It was over 20-fold a better inhibitor of μ–calpain compared to 2 with α-aminocyclopentane carboxylic acid as the P2 residue. Furthermore, 3 was over 35-fold selective for μ–calpain versus cathepsin B. Compound 2 on the contrary, inhibited cathepsin B 3-fold better than 3. Clearly, our data suggest that compounds that adopt extended conformation are better inhibitors of μ–calpain compared to those with folded conformation. This is consistent with recent X-ray crystallographic studies of the complexes of μ–calpain with bound inhibitors, which showed that the inhibitors occupy the active site pocket of the enzyme in extended conformation.20–22 Thus, unlike μ–calpain, cathepsin B did not display significant preference for any of the two compounds suggesting that the enzyme is not very sensitive to the conformational differences induced by the constrained amino acids at the P2 position of the inhibitors. This is consistent with our previous finding using compounds with 2,3-methanoleucine stereoisomers as the P2 residue, which suggested that the S2-subsite of cathepsin B is not as stereosensitive as that of μ–calpain.17
Scheme 1.
Reagents: (a) SOCl2, MeOH; (b) 3-Phenylpropionic acid, EDC, NMM, HOBT; (c) 2N NaOH, MeOH; (d) L-Phenylalaninol, EDC, NMM, HOBT; (e) Dess-Martin reagent
Scheme 2.
Reagents: (a) SOCl2, MeOH; (b) 3-Phenylpropionic acid, EDC, NMM, HOBT; (c) 2N NaOH, MeOH; (d) EDC, DMF, (e) L-Phenylalaninol, EDC, NMM, HOBT; (f) Dess-Martin reagent
Table 1.
Inhibition of porcine erythrocyte μ–calpain and human liver cathepsin B by compounds 2 and 3.
| Compound | μ-Calpain aKi (µM) | Cathepsin B Ki (µM) | bSR |
|---|---|---|---|
| 2 | 1.94 ± 0.81 | 0.88 ± 0.01 | 0.45 |
| 3 | 0.08 ± 0.01 | 2.91 ± 0.62 | 36.37 |
| cALLN | 0.19 ± 0.02 | 0.15 ± 0.01 | 0.79 |
Ki values are means of triplicate determinations obtained by Dixon plots with correlation coefficient of ≥ 0.95.
SR is selectivity ratio, which was determined by dividing the Ki value for cathepsin B inhibition by that for calpain inhibition.
ALLN was purchased from Calbiochem.
In summary, we have demonstrated using constrained amino acids as the P2 residue that peptidomimetic compounds that adopt extended conformation are potentially potent and selective inhibitors of μ–calpain versus cathepsin B compared to related analogues that adopt folded conformation.
Acknowledgements
This work was supported by NIH grant R15 HL083968-01 to I.O.D.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and notes
- 1.Croall DE, Demartino GN. Physiol. Rev. 1991;71:813. doi: 10.1152/physrev.1991.71.3.813. [DOI] [PubMed] [Google Scholar]
- 2.Suzuki K, Sorimachi H, Yoshizawa T, Kinbara K, Ishiura S. Biol Chem. 1995;376:523. doi: 10.1515/bchm3.1995.376.9.523. [DOI] [PubMed] [Google Scholar]
- 3.Sorimachi H, Ishiura S, Suzuki K. Biochem. J. 1997;328:721. doi: 10.1042/bj3280721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bano D, Young KW, Guerin CJ, Lefeuvre R, Rothwell NJ, Naldini L, Rizzuto R, Carafoli E, Nicotera P. Cell. 2005;120:275. doi: 10.1016/j.cell.2004.11.049. [DOI] [PubMed] [Google Scholar]
- 5.Atencio IA, Ramachandra M, Shabram P, Demers GW. Cell Growth & Differentiation. 2000;11:247. [PubMed] [Google Scholar]
- 6.Dietrich C, Bartsch T, Schanz F, Oesch F, Wieser RJ. Proc. Natl. Acad. Sci. USA. 1996;93:10815. doi: 10.1073/pnas.93.20.10815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Choi YH, Lee SJ, Nguyen P, Jang JS, Lee J, Wu ML, Takano E, Maki M, Henkart PA, Trepel JB. J. Biol. Chem. 1997;272:28479. doi: 10.1074/jbc.272.45.28479. [DOI] [PubMed] [Google Scholar]
- 8.Zatz A, Starling A. N Engl J Med. 2005;352:2413. doi: 10.1056/NEJMra043361. [DOI] [PubMed] [Google Scholar]
- 9.Saido T, Sorimachi H, Suzuki K. FASEB J. 1994;8:814. [PubMed] [Google Scholar]
- 10.Nixon RA. Ann NY Acad. Sci. 1989;568:198. doi: 10.1111/j.1749-6632.1989.tb12509.x. [DOI] [PubMed] [Google Scholar]
- 11.Wang KKW, Yuen P-W. Trends Pharm. Sci. 1994;15:412. doi: 10.1016/0165-6147(94)90090-6. [DOI] [PubMed] [Google Scholar]
- 12.Wang KKW, Yuen P-W. Adv. Pharm. 1997;37:117. doi: 10.1016/s1054-3589(08)60949-7. [DOI] [PubMed] [Google Scholar]
- 13.Bartus RT, Chen EY, Lynch G, Kordower JH. Exp. Neurol. 1999;155:315. doi: 10.1006/exnr.1998.7001. [DOI] [PubMed] [Google Scholar]
- 14.Donkor IO. Curr Med Chem. 2000;12:1171. doi: 10.2174/0929867003374129. [DOI] [PubMed] [Google Scholar]
- 15.Neffe AT, Abell AD. Curr Opin Drug Discov Devel. 2005;6:684. [PubMed] [Google Scholar]
- 16.Wolff ME, editor. Burger’s Medicinal Chemistry and Drug Discovery, Vol. 1: Principles and Practice. 5th Ed. New York, NY: John Wiley and Sons, Inc.; pp. 817–181. [Google Scholar]
- 17.Donkor IO, Zheng X, Miller DD. Bioorg. Med. Lett. 2000;10:2497. doi: 10.1016/s0960-894x(00)00518-7. [DOI] [PubMed] [Google Scholar]
- 18.General coupling method. A solution of the carboxylic acid (1 eq) in CH2Cl2 was cooled in an ice-bath and EDC (1.05 eq), HOBT (1.05 eq), NMM (3 eq) and the appropriate amine (1 eq), were added consecutively. The reaction mixture was stirred overnight at room temperature. Water was added to the reaction mixture and extracted with CH2Cl2. The combined organic extracts were washed successively with saturated NaHCO3 solution (30 mL), 0.5 N HCl (30 mL), and then water (30 mL) and dried over Na2SO4. Evaporation of the solvent followed by column chromatographic purification afforded the desired amide.General oxidation method. Dess Martin periodinane reagent (1.05 eq) was added to a solution of the appropriate alcohol (1 eq) in CH2Cl2 (20 mL), and the reaction mixture was stirred for 2 h at room temperature. Na2S2O3.5H2O (14–28 eq) in saturated NaHCO3 solution was added and stirred for additional 10 min. The mixture was extracted with CH2Cl2 (3 × 30 mL). The combined CH2Cl2 extracts were washed with 0.5 N HCl (30 mL), water (30 mL), and dried over Na2SO4. The solvent was evaporated and crude product was purified by column chromatography.1-(3-Phenyl-propionylamino)-cyclopentane carboxylic acid (1-benzyl-2-oxo-ethyl)-amide (2). Compound 8 was transformed to 2 as described under the general method for oxidation. The product was obtained as a white crystalline solid (0.5 g, 77%). mp 148–150 °C. 1H NMR (CDCl3, 300 MHz): δ 9.57 (s, 1H), 7.29 (m, 11H), 5.46 (s, 1H), 4.55 (q, J = 9 Hz, 1H), 3.10 (m, 2H), 2.93 (t, J = 9 Hz, 2H), 2.63 (t, J = 7.5 Hz, 2H), 2.16 (m, 2H), 1.79 (m, 2H), 1.66 (m, 2H), 1.53 (m, 2H). Anal. (C24H28N2O3), C, H, N. Calculated: C 73.44, H 7.19, N 7.14. Found: C 73.21, H 7.24, N 7.04. ESI MS: m/z 447 (M + Na + CH3OH)+.N-(1-Benzyl-2-oxo-ethyl)-2-ethyl-2-(3-phenyl-propionylamino)-butyramide (3). Compound 14 was transformed to 3 as described under the general method for oxidation. The product was obtained as a white crystalline solid (0.3 mg, 98.3%). mp 98–100 °C. 1H NMR (CDCl3, 300 MHz): δ 9.62 (s, 1H), 7.26 (m, 10H), 6.48 (s, 1H), 6.25 (d, J = 6.6 Hz, 1H), 4.74 (q, J = 6.9 Hz, 1H), 3.12 (d, J = 7.2 Hz, 2H), 2.94 (t, J = 7.5 Hz, 2H), 2.48 (m, 4H), 1.48 (m, 1H), 1.38 (m, 1H), 0.644 (t, J = 7.2 Hz, 3H), 0.54 (t, J = 7.2 Hz, 3H). Anal. (C24H30N2O3), C, H, N. Calculated: C 73.07, H 7.66, N 7.10. Found: C 72.99, H 7.75, N 7.10. ESI MS: m/z 449 (M + Na + CH3OH)+.1-Amino-cyclopentane carboxylic acid methyl ester (5). SOCl2 (1 mL) was added drop wise to a solution of 1-amino-1-cyclopentane carboxylic acid (0.4 g, 3.1 mmol) in methanol (10 mL) at 0 °C and the mixture was refluxed for 3 h. It was cooled and evaporated in vacuo to give the product as a white solid (0.52 g, 93.5%). mp 203–205 °C. 1H NMR (CDCl3, 300 MHz): δ 8.98 (s, 2H), 3.84 (s, 3H), 2.29 (m, 4H), 2.13 (m, 2H), 1.89 (m, 2H).1-(3-Phenyl-propionylamino)-cyclopentane carboxylic acid methyl ester (6). Compound 6 was obtained from 5 and commercially available 3-phenyl propionic acid using the general coupling procedure. The product was obtained as a white solid (0.76 g, 99.4%). mp 65–67 °C 1H NMR (CDCl3, 300 MHz): δ 7.25 (m, 5H), 5.86 (s, 1H), 3.70 (s, 3H), 2.93 (t, J = 9 Hz, 2H), 2.49 (t, J = 9 Hz, 2H), 2.20 (m, 2H), 1.87 (m, 2H), 1.72 (m, 4H).1-(3-Phenyl-propionylamino)-cyclopentane carboxylic acid (7). 2N NaOH (15 mL) was added to a solution of 6 (0.66 g, 3.67 mmol) in MeOH (15 mL) and the mixture was stirred for 6 h at room temperature. The reaction mixture was cooled to 0 °C, ethyl acetate (50 mL) was added and it was acidified with 5% HCl (pH = 4.5). The mixture was extracted with EtOAc (2 × 30 mL). The combined organic extracts were washed with brine (25 mL), dried with Na2SO4 and evaporated to give 7 as a white solid (0.53 g, 55.3%). mp 170–172 °C. 1H NMR (CDCl3, 300 MHz): δ 8.18 (s, 1H), 7.21 (m, 5H), 2.89 (t, J = 8 Hz, 2H), 2.47 (t, J = 7.2 Hz, 2H), 2.13 (m, 2H), 1.88 (m, 2H), 1.67 (m, 4H).1-(3-Phenyl-propionylamino)-cyclopentane carboxylic acid (1-benzyl-2-hydroxy-ethyl)-amide (8). Compound 8 was obtained from 7 and commercially available L-phenylalaninol using the general coupling procedure. The product was obtained as a white solid (0.69 g, 91.3%). mp 50–52 °C. 1H NMR (CDCl3, 300 MHz): δ 7.24 (m, 10H), 6.64 (s, 1H), 5.84 (s, 1H), 3.63 (m, 1H), 3.45 (m, 1H), 3.05 (m, 1H), 2.96 (m, 3H), 2.83 (m, 2H), 2.5 (m, 2H), 2.25 (m, 1H), 1.94 (m, 1H), 1.71 (m, 4H), 1.55 (m, 2H).2-Amino-2-ethylbutyric acid methyl ester (10). SOCl2 (10 mL) was added dropwise to a solution of diethylglycine (0.7g, 5.3 mmol) in methanol (30 mL) at 0 °C and the mixture was refluxed for 24 h followed by solvent removal in vacuo. Further rinsing with MeOH/CH2Cl2 (2 × 30 mL) and evaporation afforded 10 as a semi-viscous yellowish green oil (0.92 g, 95.6%). 1H NMR (CDCl3, 300 MHz): δ 8.5 (s, 2H), 3.77 (s, 3H), 1.83 (q, J = 6 Hz, 4H), 0.877 (t, J = 7.2 Hz, 6H).2-Ethyl-2-(3-phenyl-propionylamino)-butyric acid methyl ester (11). Compound 11 was obtained from 10 and commercially available 3-phenyl propionic acid using the general coupling procedure. The product was obtained as viscous oil (0.17 mg, 94.4%). 1H NMR (CDCl3, 500 MHz): δ 7.25 (m, 5H), 6.32 (s, 1H), 3.74 (s, 3H), 2.96 (t, J = 6.2 Hz, 2H), 2.54 (t, J = 6.2 Hz, 2H), 2.45 (m, 2H), 1.75 (m, 2H), 0.64 (t, J = 7 Hz, 6H).2-Ethyl-2-(3-phenyl-propionylamino)-butyric acid (12). 2N NaOH (50 mL) was added to a solution of 11 (0.8g, 2.88 mmol) in MeOH (35 mL) and the mixture was stirred for 36 h at room temperature. EtOAc (50 mL) was added to the reaction mixture at 0 °C and then acidified with 5% HCl (pH = 4.5). The mixture was extracted with EtOAc (2 × 50 mL) and the combined organic extracts were washed with brine, dried with Na2SO4 and evaporated to give 12 as a white powdery solid (0.66 g, 87%). mp 200–202 °C. 1H NMR (DMSO, 300 MHz): δ 7.49 (s, 1H), 7.21 (m, 5H), 2.78 (t, J = 9 Hz, 2H), 2.43 (t, J = 9 Hz, 2H), 1.80 (s, 1H), 1.78 (m, 4H), 0.62 (t, J = 6 Hz, 6H).4,4-Diethyl-2-phenethyl-4H-oxazol-5-one (13). EDC (0.087g, 0.46 mmol) was added to a solution of 12 (0.8g, 2.88 mmol) in CH2Cl2/DMF (10:2) at 0 °C and the mixture was stirred for 24 h at room temperature. The reaction mixture was diluted with CH2Cl2 (60 mL), washed with saturated NaHCO3, 0.5N HCl and water successively and dried with Na2SO4. Evaporation of the solvent gave 13 as colorless oil (0.92 g, 98.69%). mp 180–182 °C. 1H NMR (CDCl3, 300 MHz): δ 7.27 (m, 5H), 3.05 (m, 2H), 2.87 (m, 2H), 1.76 (q, J = 6Hz, 4H), 0.71 (t, J = 6 Hz, 6H).N-(1-Benzyl-2-hydroxy-ethyl)-2-ethyl-2-(3-phenyl-propionylamino)-butyramide (14). Compound 14 was obtained from 13 and commercially available L-phenylalaninol using the general coupling procedure. The product was obtained as a white crystalline solid (0.6 mg, 94.6%). 1H NMR (CDCl3, 300 MHz): δ 7.28 (m, 10H), 6.06 (s, 1H), 6.09 (d, J = 7.2 Hz, 1H), 4.25 (m, 1H), 3.65 (m, 2H), 3.15 (s, 1H), 2.95 (m, 3H), 2.81 (m, 1H), 2.53 (t, J = 6 Hz, 2H), 2.33 (m, 2H), 1.53 (m, 1H), 1.38 (m, 1H), 0.63 (t, J = 6 Hz, 3H), 0.45 (t, J = 6 Hz, 3H).
- 19.Calpain inhibition assay. The Ki values for inhibition of porcine erythrocyte μ-calpain (Calbiochem) activity was monitored as previously reported23 in a buffer consisting of 50 mM Tris HCl (pH 7.4), 50 mM NaCl, 10 mM dithiothreitol, 1 mM EDTA, 1 mM EGTA, 0.2 mM or 1.0 mM Suc-Leu-Tyr-AMC (Calbiochem), 2 µg of calpain from porcine erythrocyte (Calbiochem), varying concentrations of the inhibitor in DMSO (2% total concentration) and 5 mM CaCl2 in a final volume of 250 µL in a microtiter plate.Cathepsin B inhibition assay. The Ki values for inhibition of human liver cathepsin B (Calbiochem) activity was determined as described for calpain using a reaction mixture containing 1 nM human liver cathepsin B (Calbiochem), 50 mM NaOAc (pH 6.0), 1 mM EDTA, 0.5 mM DTT, 50 µM or 250 µM of substrate (Z-Arg-Arg-AMC), and varying concentrations of inhibitor in DMSO (2% total concentration) in a final volume of 250 µL.
- 20.Cuerrier D, Moldoveanu T, Inoue J, Davies PL, Campbell RL. Biochemistry. 2006;45:7446. doi: 10.1021/bi060425j. [DOI] [PubMed] [Google Scholar]
- 21.Li Q, Hanzlik RP, Weaver RF, Schonbrunn E. Biochem. 2006;45:701. doi: 10.1021/bi052077b. [DOI] [PubMed] [Google Scholar]
- 22.Moldoveanu T, Campbell RL, Cuerrier D, Davies PL. J. Mol. Biol. 2004;343:1313. doi: 10.1016/j.jmb.2004.09.016. [DOI] [PubMed] [Google Scholar]
- 23.Donkor IO, Korukonda R, Huang TL, LeCour L., Jr Bioorg. Med. Chem. Lett. 2003;13:783. doi: 10.1016/s0960-894x(03)00021-0. [DOI] [PubMed] [Google Scholar]