Abstract
β-Catenin is a central mediator of Wnt signaling pathway, components of which have been implicated in B cell development and function. B cell progenitors and bone marrow stromal cells express Wnt ligands, Frizzled receptors and Wnt antagonists, suggesting fine tuned regulation of this pathway in B cell development. In particular, deletion of Frizzled 9 gene results in developmental defects at the pre-B stage of development and an accumulation of plasma cells. Furthermore, Wnt signals regulate B cell proliferation through lymphocyte enhancer-binding factor-1. However, it is not known whether Wnt signaling in B cell development is mediated by β-catenin and whether β-catenin plays a role in mature B cell function. In this report, we show that mice bearing B cell-specific deletion of β-catenin have normal B cell development in bone marrow and periphery. A modest defect in plasma cell generation in vitro was documented, which correlated with a defective expression of IRF-4 and Blimp-1. However, B cell response to T-dependent and T-independent Ags in vivo was found to be normal. Thus, β-catenin expression was found to be dispensable for normal B cell development and function.
B cell development from stem cells is a complex, multi-stage process regulated by cell intrinsic signals and cues provided by the bone marrow (BM)2 environment (1). The external signals that regulate stage specific gene expression during B cell development include growth factors and cell-cell contact with the stromal cells. Of particular interest are transcription factors that execute cell fate decisions by regulating pattern of gene expression. Transcription factors that are important for early B lineage commitment and B cell development include PAX5, E2A, EBF, and PU.1. In periphery, B cells undergo further development to become long-lived circulating follicular B cells (FO B), noncirculating marginal zone B cells (MZ B), or B-1 cells which reside primarily in peritoneal cavity (1). Strength of BCR signals determine the differentiation into different lineages, while signal through BAFF-receptor (BAFF-R) is essential for the development of both FO and MZ B cells (2, 3). Transcription factors required for the differentiation of these lineages include c-Myb for FO B cells, Notch-2, and Aiolos for MZ B cells as well as Aiolos and c-Myb for B-1 cells (2). Upon encounter foreign Ags, MZ B cells and FO B cells proliferate and rapidly give rise to short lived plasma cells (PC). When they encounter T cell-dependent Ags, FO B cells form a germinal center where they undergo proliferation accompanied by affinity maturation and class-switch recombination (CSR) of Ig, and subsequently give rise to long lived PCs and memory B cells. Transcription factors Blimp-1, Xbp-1, and IRF-4 are involved in the differentiation of PCs, with Blimp-1 being the master regulator for this process (4–6).
Lymphocyte enhancer factor (LEF)-1, a member of the LEF-1/T cell factor (TCF) family of transcription factors, is implicated in early B cell development (7). LEF-1 was originally identified in lymphocytes, and later, LEF-1/TCF family of transcription factors were shown to be the major effectors of the canonical Wnt signaling pathway with β-catenin as a cofactor. Wnts are secreted growth factors that mediated cell to cell communication by interacting with Frizzled receptors in a complex with the low-density lipoprotein receptor-related protein 5 and 6. Engagement of Frizzled receptors by Wnt ligand results in the accumulation of β-catenin protein. In the absence of Wnt signal, β-catenin is found in a complex with scaffolding protein Axin, the tumor suppressor ad-enomatous polyposis coli, GSK3β, and β-TrCP. In this complex, β-catenin is phosphorylated by GSK-3β and tagged for ubiquitination and degradation. When Wnt binds Frizzled receptors, Dishevelled blocks GSK-3β-mediated phosphorylation and rescues it from subsequent degradation. Unphosphorylated β-catenin accumulates for the duration of the Wnt signal and translocates to the nucleus to function as a cofactor for the LEF/TCF family of transcription factors.
Evidence that Wnt signaling pathway is important for B cells comes from observation that several components of the Wnt signaling cascade, including Wnt proteins, Frizzled receptors, and LEF are expressed in B cell progenitors. Wnt proteins induce proliferation of B cells and deletion of LEF-1 results in increased apoptosis of Pro-B cells and reduced proliferation of developing B cells (7). Frizzled 9 deficient (Fzd9−/−) mice show pronounced reduction in pre-B cells and an accumulation of plasma cells (8). More recently, gain of function studies have demonstrated that constitutively activated β-catenin in hematopoietic stem cells blocks multilineage differentiation, including B cell differentiation at early stages, suggesting the importance of fine tuning of the Wnt-β-catenin signaling pathway for normal B cell development (9–11). Furthermore, BCR signals induce the accumulation of β-catenin, suggesting a possible role of β-catenin in both B cell development and function (12).
The current work was undertaken to investigate the role of β-catenin, a central mediator of the Wnt signaling pathway, in B cell development and function. We demonstrate that in CAT-KO mice bearing B cell-specific deletion of β-catenin, B cells developed normally but showed a statistically significant reduction in plasma cell generation and increase in class switching to IgG1 in vitro. However, β-catenin deficient B cells showed normal T-dependent and T-independent B cell responses in vivo. These data suggest that β-catenin expression was dispensable for B cell development and function in vivo.
Materials and Methods
Mice
CD19-Cre mice (13) and CATlox/lox (14) mice were purchased from The Jackson Laboratory. Both mice are on C57BL/6 background. CD19-Cre mice were bred with CATlox/lox mice to generate Cre−CATlox/lox, Cre+CATlox/wt, and Cre+CATlox/lox (CAT-KO) mice. The mice were maintained in animal facility at Gerontology Research Center, National Institute on Aging, according to National Institutes of Health regulations.
Flow cytometry
BM or splenic cells were harvested, stained and analyzed on a FACSCalibur (BD Biosciences). The data were analyzed by FlowJo software (Tree Star) and dead cells were excluded by forward scatter and propidium iodide gating. Abs with the following specificities were used for staining: APC-B220, PerCP-Cy5.5-IgM, PerCP-Cy5.5-CD19, FITC-CD43, PE-AA4.1, PE-IgD, FITC-CD23, PE-CD21, PE-CD11b, FITC-CD5, FITC-IgG1, FITC-IgG3, and PE-Syndecan-1 (all from BD Pharmingen). Where indicated, Pro-B (AA4+CD19+CD43+B220+) cells and CD43−B220+ cells were electronically sorted from BM cells and B220+ cells were sorted from spleen on a DakoCytomation Moflo.
For analyzing 4-hydroxy-3-nitrophenylacetyl (NP)-binding B cells after in vivo immunization, the following Abs were used: fixable aqua vital dye (Invitrogen), PE-Cy5-CD4 and CD8, biotin-CD138, streptavidin-PerCP-Cy5.5 (BD Biosciences), PE-Cy5-Gr-1 and F4/80, PE-Cy7-IgM, APC-Alexa750-B220, Alexa700-CD19 (eBioscience), biotin-IgD, PE-λ, FITC-Igβ and κ (Southern Biotechnology Associates). APC-NP was conjugated in the Cancro Laboratory. Data were collected (at least 2,000,000 events) on a BD LSR II flow cytometer and analyzed with FlowJo software (Tree Star). Significance levels were determined by Student’s t test.
Cell culture
Splenic B cells were purified by negative selection with anti-CD43 beads (Miltenyi Biotec) to >98% purity. The cells were cultured at 1 × 106/ml for 3 days with the following stimuli: LPS 1 μg/ml (Sigma-Aldrich), IL-4 50 ng/ml (R&D Systems), anti-CD40 1 μg/ml (BD Pharmingen), and anti-IgM 10 μg/ml (Jackson ImmunoResearch Laboratories). The cells were then harvested, counted for viable cells by trypan blue exclusion and stained with Abs as indicated. Significance levels were determined by Student’s t test.
Immunizations
For T-dependent immune response, mice were immunized i.p. with 50 μg NP16-CGG in alum. Six weeks later, some mice were boosted with same dose of NP16-CGG. Seven days after the boost, mice were sacrificed and analyzed for memory and plasma B cells. For T-independent response, mice were immunized i.p. with 50 μg NP-Ficoll in PBS. Seven days after, mice were sacrificed and analyzed for memory and plasma B cells. Significance levels were determined by Student’s t test.
Anti-NP Ab ELISAs
Costar E.I.A./R.I.A. plates were coated overnight at 4°C with 10 μg/ml NP30BSA in 100 mM sodium bicarbonate buffer. Plates were blocked with 2% BSA in PBS for at least 1 h and diluted serum was incubated for at least 1 h. IgG1 standard was a gift of G. Kelsoe (Duke University, Durham, NC). HRP-conjugated goat anti-mouse IgM and IgG1 (Southern Biotechnology Associates) were used for detection and the enzymatic reaction carried using a TMB substrate kit (BD Biosciences). All washes were performed using PBS + 0.1% Tween 20. Plates were run on an Emax Precision microplate reader and data collected using Softmax Pro software (Molecular Devices).
In vitro BrdU labeling
BrdU was added into cell culture at 10 μM, and incubated at 37°C for 1 h. The cells were then harvested and intracellular staining for BrdU was performed with FITC-BrdU Flow Kit according to the manufacturer’s protocol (BD Pharmingen).
Biochemical assays
For Quantitative (real-time) RT-PCR, total RNA from cells was reverse transcribed using poly:(dT) and Superscript III reverse transcriptase (Invitrogen). The cDNA was subjected to RT-PCR amplification (Applied Biosystems) for 40 cycles with annealing and extension temperature at 60°C. Primer sequences available upon request. For Western blot analysis, cells were lysed in Nonidet P-40 lysis buffer and the lysates were resolved on 4–12% SDS-PAGE (Invitrogen) and then transferred to nitrocellulose membrane. Blots were incubated with monoclonal anti-β-catenin Ab (BD Pharmingen) or anti-PKCμ Ab (Santa Cruz Biotechnology), followed by HRP-conjugated anti-mouse-IgG or anti-rabbit-IgG. Reactivity was revealed by ECL.
Results
Deletion of β-catenin allele during B cell development
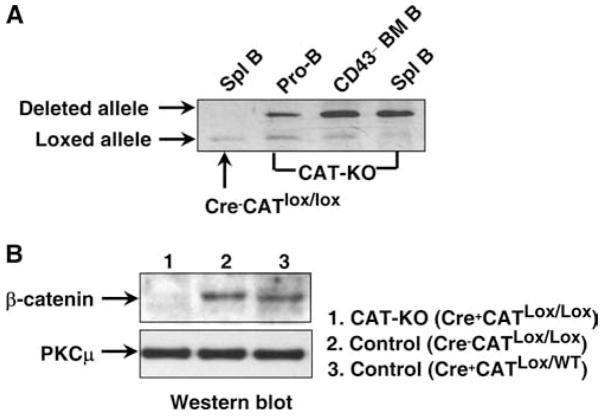
To delete β-catenin in B lineage cells, CD19-Cre mice (13) were bred to mice bearing the floxed β-catenin allele (CATlox/lox) (14) to generate CD19-Cre+CATlox/lox (CAT-KO) mice. To assess the deletion of β-catenin allele in these mice, we performed DNA PCR with primers that amplify DNA sequences surrounding β-catenin allele and give rise to different lengths of PCR products from loxed allele and deleted allele (14). Significant deletion was observed in sorted Pro-B cells (AA4+CD19+CD43+B220+) from BM of CAT-KO mice and the degree of deletion increased progressively in CD43−B cells (which contained fractions D, E and F) from BM and in splenic B cells (Fig. 1A). Splenic B cells from Cre−CATlox/lox were used as control in which no deletion was observed (Fig. 1A). Consistent with the above data, the mRNA level for β-catenin was markedly reduced in CAT-KO Pro-B cells and the subsequent populations (data not shown). We next examined β-catenin protein level in CAT-KO splenic B cells by Western blot, with the expression level of β-catenin being normalized to PKCμ. Protein was extracted from freshly isolated CAT-KO, Cre−CATlox/lox, and Cre+CATlox/wt control B cells and then subjected to Western blot analysis. CAT-KO B cells showed very little β-catenin protein compared with both types of control B cells (Fig. 1B). We conclude that deletion of β-catenin gene results in the absence of β-catenin protein in CAT-KO B cells.
Figure 1.
Deletion of β-catenin gene in B lineage cells. A, Genomic DNA was extracted from electronically sorted pro-B cells and CD43−B cells from BM and B cells from spleen. The DNA was subjected to PCR to detect deleted β-catenin loci (upper band) or loxed β-catenin loci (lower band). B, Western blot was performed on cell lysates from freshly isolated Cre−CATlox/lox (wild type) Cre+CATlox/wt (control) and Cre+CATlox/lox (KO) splenic B cells for expression of β-catenin and PKCμ.
Normal B cell development in BM from CAT-KO mice
We first examined B cell development in BM from CAT-KO mice. We used both Cre−CATlox/lox and Cre+CATlox/wt mice as controls for CAT-KO mice for two reasons: First, to control for the level of CD19 expression in Cre+CATlox/wt and CAT-KO mice compared with Cre−CATlox/lox mice, as insertion of Cre in the CD19 promoter disrupts CD19 transcription and thus B cells in both Cre+CATlox/wt and CAT-KO mice have a slightly lower level of CD19 expression compared with Cre−CATlox/lox mice (data not shown; Ref. 15); second, to control for the expression of Cre protein. Because B cells from Cre+CATlox/wt and Cre−CATlox/lox mice showed comparable results in all assays performed, data from the two control strains is shown only for the BM analysis in Fig. 2. In subsequent figures data from the two control strains are merged and shown as “control”.
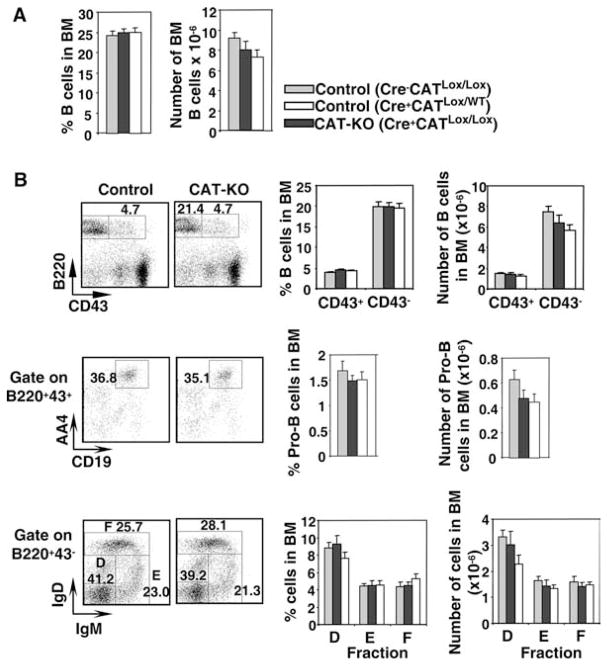
Figure 2.
B cell development in BM from CAT-KO mice. A, BM cells were isolated from femur of control or CAT-KO mice and stained for multiple markers. Left panel, Percentage of B220+ cells in BM; Right panel, Absolute number of B220+ in BM (n = 6). There were no statistically significant differences between either of the two controls and CAT-KO mice. B, Left top panel, CD43+B220+ cells and CD43−B220− cells in BM; Left middle panel, Pro-B cells defined as CD43+B220+ AA4.1+CD19+; Left bottom panel, Fractions D, E, and F in the CD43−B220+ population defined by expression of surface IgD and IgM. Graphs show percentage and number of the above populations in BM (n = 6). There were no statistically significant differences between either of the two controls and CAT-KO mice.
Both the percentage and absolute number of B220+ cells were comparable between two types of control mice and CAT-KO mice (Fig. 2A). The percentage and number of relatively immature CD43+ B cells and the relatively mature CD43− B cells were also comparable between control and CAT-KO mice (Fig. 2B, top panels). Further characterization of developmental subsets showed that CAT-KO mice had normal percentage of Pro-B cells in BM, defined as AA4+CD19+CD43+B220+ cells (16) (Fig. 2B, middle panels). The number of Pro-B cells showed a slight reduction in Cre+CATlox/wt and CAT-KO mice compared with Cre−CATlox/lox mice, but this was not statistically significant (Fig. 2B, middle panels). The fractions D, E, and F, defined by expression of B220, CD43, IgD, and IgM, were also similar between control and CAT-KO mice (Fig. 2B, bottom panels). Thus, β-catenin deficiency does not significantly affect B cell development in the BM.
Subtle abnormalities in B cell maturation in the spleen in CAT-KO mice
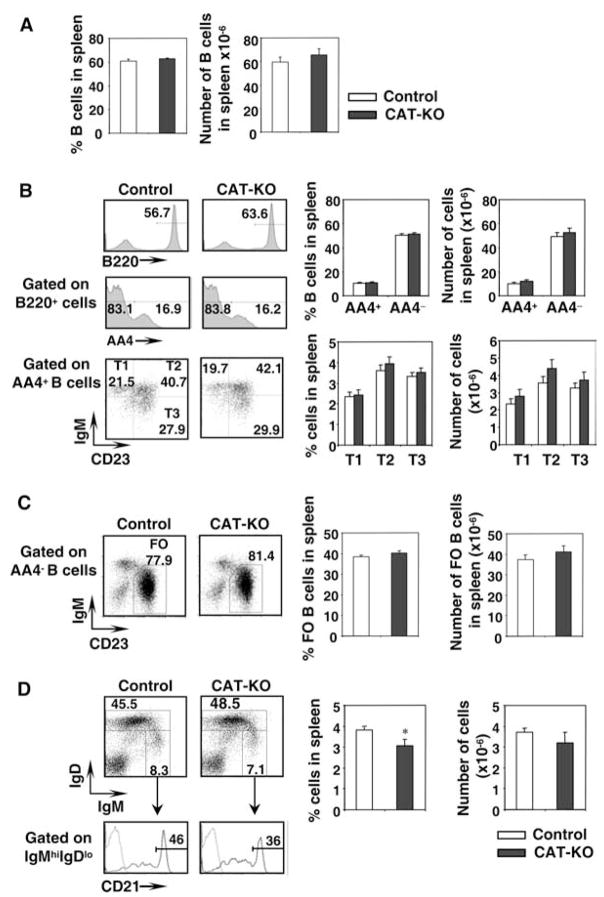
We next examined splenic B cell development in CAT-KO mice. We found nearly identical phenotype in Cre−CATlox/lox and Cre+CATlox/wt mice and thus include data from both types of mice in the control group. The percentage and absolute number of spleen B220+ cells were similar between control and CAT-KO mice (Fig. 3A). The percentage and absolute number of the AA4+ (transitional) B cells and the mature AA4− B cells were also comparable between control and CAT-KO mice (Fig. 3B). Further characterization of transitional subsets among AA4+B cells showed that the amount of all the three subsets, T1, T2, and T3 (17), was similar between control and CAT-KO mice (Fig. 3B). The percentage and absolute number of mature follicular B cells, defined as IgMlowIgD+AA4− B cells (17), was also normal in CAT-KO mice (Fig. 3C). Fraction of marginal zone B cells, defined as CD21highIgMhighIgDlow cells (18), showed a small but statistically significant decrease in CAT-KO mice, while the absolute number of cells for this population remained comparable to control (Fig. 3D). Thus, B cell development in the spleen is grossly normal, with a slight decrease in the fraction of marginal zone B cells.
Figure 3.
B cell maturation in spleen from CAT-KO mice. A, Percent and number of B cells in spleen. Spleen cells from control mice (include both Cre−CATlox/lox and Cre+CATlox/wt mice) or CAT-KO mice were stained for multiple markers. Percentage of B220+ cells in spleen (left panel); Absolute number of B220+ in spleen (right panel). Control mice, n = 15; CAT-KO mice, n = 8. B, Transitional and mature B cells. Left top panels, B220+ cells in spleen; Left middle panels, AA4.1+ and AA4.1− cells among B220+ cells; Left bottom panels, T1, T2, and T3 populations among AA4.1+B220+ cells defined by surface IgM and CD23. Histograms on the right show the percentage and number of the above populations in spleen. Control mice, n = 15; CAT-KO mice, n = 8. C, Follicular B cells. Left panels, Follicular B cells among AA4.1−B220+ population, defined as IgMlowCD23+ cells; Right panels, Percentage and number of follicular B cells in spleen. Control mice, n = 15; CAT-KO mice, n = 8. D, Marginal zone B cells. Left panels, Cytometric profiles of marginal zone cells defined as IgMhighIgDlowCD21high cells; Right panels, Percentage and number of marginal zone cells in spleen. Control mice, n = 15; CAT-KO mice, n = 8. *, Statistically significant difference (p < 0.05).
Normal development of B1 cells in CAT-KO mice
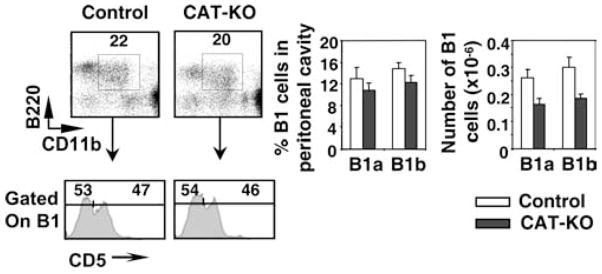
We next examined B1a and B1b cells in peritoneal cavity of CAT-KO mice, based on the expression of B220, CD11b and CD5. The percentage and absolute number of both B1a and B1b cells showed a small reduction in CAT-KO mice, which was not statistically significant (Fig. 4). We conclude that development of B1 cells is grossly normal in CAT-KO mice.
Figure 4.
Development of B1 cells in CAT-KO mice. Cells from peritoneal cavity were collected and stained for B220, CD11b, IgM, and CD5. Left upper panel, B1 cells defined as B220+CD11b+ cells; Left lower panel, B1a cells, defined as CD5+B1 cells and B1b cells, defined as CD5—B1 cells. Graphs show percentage and number of the above populations (n = 3).
Enhanced class switch to IgG3 and IgG1 and decreased plasma cell formation in CAT-KO B cells in vitro
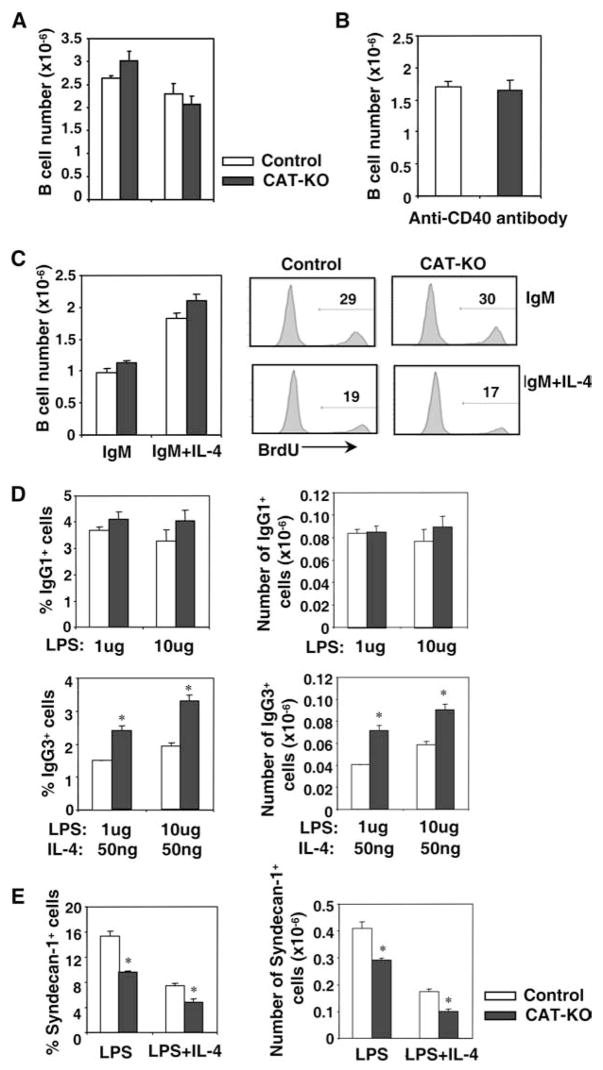
We next investigated CAT-KO B cells for their ability to proliferate, undergo CSR and form plasma cells upon activation in vitro. Splenic B cells were stimulated with LPS or LPS plus IL-4 for 3 days and viable cell numbers were counted as described in Materials and Methods. CAT-KO B cells expanded normally compared with control B cells (Fig. 5A). CAT-KO B cells also expanded normally in response to anti-CD40 plus IL-4 stimulation, which mimics activation by T cell dependent Ags (Fig. 5B). Furthermore, CAT-KO B cells proliferated normally to anti-IgM, in the presence or absence of IL-4, as assessed by both cell numbers and BrdU incorporation assays (Fig. 5C). We conclude that B cell proliferation does not require β-catenin expression.
Figure 5.
In vitro proliferation, class switch and plasma cell formation of CAT-KO B cells. A, Proliferation of B cells to LPS. One × 106 purified splenic B cells were stimulated with LPS or LPS plus IL-4 for 3 days and viable cell number was shown (n = 4). B, Proliferation of B cells to anti-CD40. One × 106 purified splenic B cells were stimulated with anti-CD40 Ab plus IL-4 for 3 days and viable cell number was shown (n = 6). C, Proliferation of B cells to anti-IgM. One × 106 purified splenic B cells were stimulated with anti-IgM or anti-IgM plus IL-4 for 3 days. Left panel, Viable cell number (n = 3); Right panels, cells were incubated with BrdU on day 3 of culture and BrdU incorporation was assessed by Ab staining. D, Class switch. B cells stimulated with LPS plus IL-4 or LPS for 3 days were stained for surface IgG1 or IgG3, respectively. Percentage and absolute number of IgG1+or IgG3+ cells are shown (n = 4). *, Statistically significant difference (p < 0.05). E, Plasma cell formation. B cells stimulated as above for 3 days were stained for syndecan-1. Percentage and absolute number of sydecan-1+ cells are shown (n = 4). *, Statistically significant difference (p < 0.05).
To assess CSR, we stained B cells stimulated with LPS plus IL-4 or LPS for surface IgG1 and IgG3, respectively. CAT-KO B cells showed a moderate but statistically significant increase in CSR to IgG3 and a small increase in CSR to IgG1 compared with control B cells (Fig. 5D). To assess generation of plasma cells, we stained B cells stimulated with LPS or LPS plus IL-4 for surface expression of syndecan-1 (CD138). CAT-KO B cells showed a statistically significant reduction in plasma cell generation compared with control B cells, in response both to LPS and to LPS plus IL-4 (Fig. 5E). We conclude that CAT-KO B cells demonstrate a small but statistically significant increase in CSR to IgG3 and IgG1 and decreased ability to differentiate into plasma cells upon stimulation in vitro.
Impaired Blimp-1 up-regulation in stimulated CAT-KO B cells
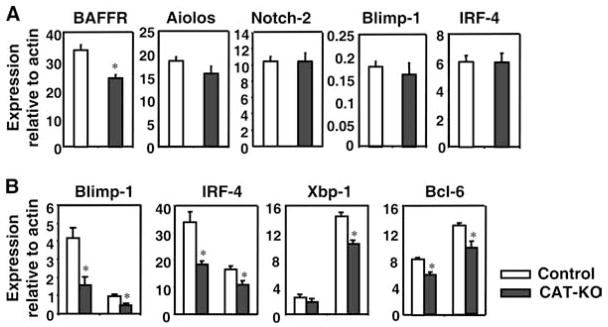
To investigate the molecular basis for the slight decrease in marginal zone B cells and the small reduction in plasma cell formation, we analyzed expression of transcriptional regulators of MZ B cells and PCs. RT-PCR analysis showed that BAFF-R expression was decreased in freshly isolated CAT-KO B cells, suggesting a putative mechanism for the decrease in marginal zone B cells in CAT-KO mice (Fig. 6A). The other two molecules implicated in marginal zone B cell development, Aiolos and Notch-2, were expressed at normal level (Fig. 6A). Both Blimp-1 and IRF-4, regulators of plasma cell generation, were expressed at normal, low level in fresh CAT-KO B cells (Fig. 6A). However, after stimulation with LPS plus IL-4 for 1 day, CAT-KO B cells showed significantly lower level of both Blimp-1 and IRF-4 expression compared with control B cells (Fig. 6B), which may underlie the defective plasma cell generation in CAT-KO cells. Xbp-1, a target of Blimp-1, was also expressed at lower levels in stimulated CAT-KO B cells (Fig. 6B). The decrease in Blimp-1 was not due to enhanced expression of Bcl-6, a repressor of Blimp-1 expression (Fig. 6B). In fact, Bcl-6 expression showed a small but statistically significant decrease in CAT-KO B cells (Fig. 6B). In stimulated CAT-KO B cells, BAFF-R and B cell-specific factors BSAP and Pax5 were expressed at levels comparable to control B cells (data not shown), despite the fact that BAFF-R was expressed at lower level in freshly isolated CAT-KO B cells. Thus, expression of BAFF-R is decreased in freshly isolated CAT-KO B cells, which may underlie the decrease in marginal zone B cells. Expression of both Blimp-1 and IRF-4 is decreased in stimulated CAT-KO B cells, which explains the decrease in plasma cell formation. We conclude that the modest changes in the CAT-KO B cells may reflect that fine tuning of the immune response may be regulated by β-catenin.
Figure 6.
Gene expression in freshly isolated and stimulated B cells. A, Gene expression in ex vivo B cells. RNA from freshly isolated splenic B cells was reverse transcribed and subjected to RT-PCR. Expression of different genes relative to β-actin is shown (n = 5). *, Statistically significant difference (p < 0.05). B, Gene expression in stimulated B cells. RNA from splenic B cells that have been stimulated with LPS+IL-4 for 1 day was subjected to real-time RT-PCR. Expression of different genes relative to β-actin is shown (n = 4). Star indicates statistically significant difference (p < 0.05).
Normal T-dependent and -independent immune responses in CAT-KO mice in vivo
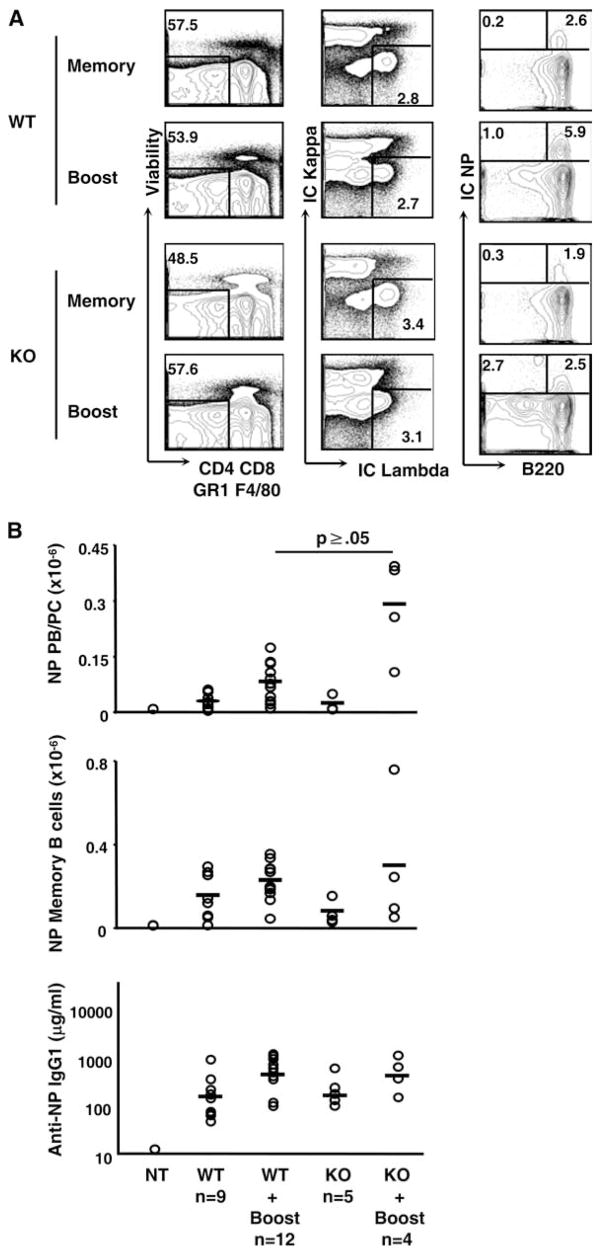
To test the effects of B cell-specific β-catenin deletion on T-dependent immune responses in vivo, we immunized mice with NP-CGG and tracked primary Ab titers, memory, and secondary responses (Fig. 7). Control and CAT-KO mice both responded to immunization with similar kinetics and magnitudes of IgG1 NP-specific serum Ab on days 7 and day 14 of the primary response (data not shown). To test the persistence of memory and the magnitude of recall responses, mice were immunized with NP-CGG, rested for 6 wk (19), and then injected again with NP-CGG (Fig. 7). C57BL/6 B cells reactive to NP bear λ L chains, therefore we gated live, single cells by both intra and extra-cellular NP binding and λ L chain usage, excluding cells bearing κ L chains, CD4, CD8, GR-1, and expressing F4/80. Memory B cells were further delineated by their high expression of B220, while plasma cells and plasma blasts express lower levels or no B220 (Fig. 7A) (20, 21). Standing memory and long-lived plasma cells in the bone marrow and the spleen were no different (Fig. 7B and data not shown). Although no differences were seen in the number of either resting, long-lived plasma cells or memory B cells, we consistently observed a slight increase in the number of plasma cells generated upon secondary immunization (Fig. 7B). Nevertheless, there were no differences in Ab titers between control and CAT-KO cells (Fig. 7B), suggesting that CAT-KO Ab secreting cells (ASCs) may generate less Ab on a per cell basis.
Figure 7.
T-dependent immune responses in CAT-KO mice in vivo. A, CAT-KO and control mice were immunized with 50 μg NP-CGG in alum, rested for 6 wk, boosted with 50 μg NP-CGG in alum, and analyzed at day 7. Live lymphocytes were gated for single cells and size using forward scatter and side scatter profile and CD4, CD8, GR-1, and F4/80 expressing cells excluded. Cells were further gated for expression of intracellular Igλ and NP-binding, then for B220− (plasma cell/plasma blast) or B220+ (memory/GC B cells). B, The number of NP-binding B220− plasma cells/plasma blasts and memory/GC B cells were calculated from the frequency of NP+ cells shown in A and the total number of live splenocytes harvested. Levels of anti-NP IgG1 Ab following NP-CGG boost were determined by ELISA. Each point represents one mouse with the mean indicated by horizontal bars. Graph represents combined results from two experiments. Significance levels are indicated and were determined by Student’s t test.
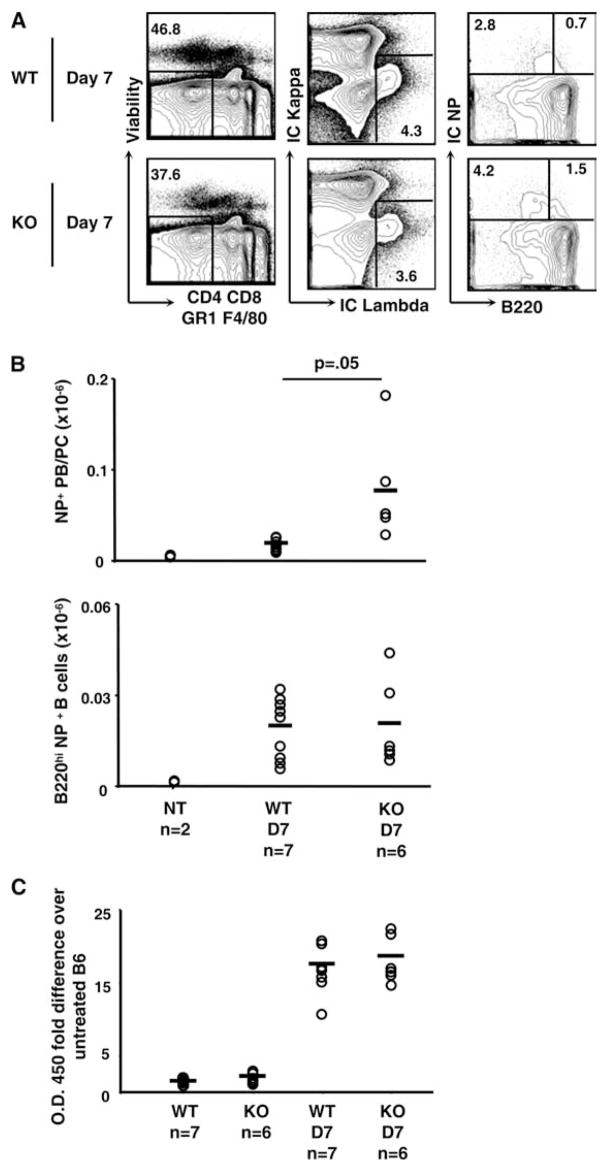
We next tested T-independent immune responses using NP-Ficoll. Mice were injected i.p. with 50 μl NP-Ficoll and NP-reactive populations were assessed. We gated live, single cells by both intra and extra-cellular NP binding and λ L chain usage; excluding cells bearing κ chains, CD4, CD8, GR-1, and expressing F4/80. Activated B cells were further delineated by their high expression of B220, while plasma cells and plasma blasts express lower levels or no B220 (Fig. 8A). Again, no difference in B220 high cells was documented, but expression of plasma cell markers were increased (Fig. 8B). Despite an increase in plasma cells, NP-specific Ab titers were similar between groups, consistent with the results seen in T-dependent responses (Fig. 7C). Taken together, CAT-KO mice can elicit grossly normal in vivo T-dependent and T-independent immune responses, despite a slight augmentation in plasma cell populations. We conclude that β-catenin expression was dispensable for B cell function.
Figure 8.
T-independent immune responses in CAT-KO mice in vivo. CAT-KO and control mice were immunized with 50 μg NP-Ficoll in PBS and analyzed at day 7. A, Single, live lymphocytes were gated by using forward scatter and side scatter and CD4, CD8, GR-1, and F4/80 expressing cells excluded. Cells were gated for expression of intracellular Igλ and NP-binding, then further gated for B220− (plasma cell/plasma blast) or B220+ (activated B cells). B, Number of NP-binding B220− plasma cells/plasma blasts and activated B cells following NP-Ficoll treatment were calculated from the frequency of NP+ cells shown in A and the total number of live splenocytes. Each point represents one mouse with the mean indicated by horizontal bars. Graph represents combined results from two experiments. C, Anti-NP IgM Ab titers were determined by ELISA and fold increase calculated over untreated B6 mouse. Each point represents one mouse with the mean indicated by horizontal bars. Graph represents combined results from two separate experiments. Significance levels are indicated and were determined by Student’s t test.
Discussion
Defective B cell development in mice deficient for LEF-1 or Frizzled-9, both players in Wnt-β-catenin-TCF/LEF signaling pathways, suggests a role for β-catenin in B cells. In this report, using mice with B cell-specific deletion of β-catenin, we show that β-catenin is dispensable for B cell development in the BM and the spleen. This is unlike the development of T cells where T cell-specific deletion of β-catenin leads to impaired thymocyte development at the β-selection transition (22). In in vitro stimulation culture, mature CAT-KO B cells show enhanced CSR to IgG3, and to a lesser extent, to IgG1. They also show impaired generation of plasma cells, due to failure to adequately induce the expression of Blimp-1 and IRF-4. However, CAT-KO mice can elicit grossly normal T-dependent and T-independent immune responses in vivo, with a slight increase in the number of plasma cells, which may have a lower ability to produce Abs.
β-catenin deficient BM HSCs develop normally into both B and T cells upon transfer into host mice (23). To investigate the physiological function of β-catenin in B cell development and function, we generated B cell specific β-catenin deficient mice in which β-catenin deletion starts at Pro-B stage. Our data confirm that β-catenin is dispensable for B cell development both in the BM and in the spleen. Thus, the function of LEF-1 and Frizzle-9 in early B cell development is independent of the presence of β-catenin. Together with the report that β-catenin and γ-catenin double deficient HSCs can also undergo normal B cell development in host mice (23, 24), we suggest that non-canonical Wnt signaling pathways that are independent of β-catenin and γ-catenin mediate functions of Frizzled-9 and LEF in B cell development. Thus, this work provides important genetic evidence that β-catenin is dispensable for B cell development using lineage-specific gene deletion approach.
Next, we investigated the role of β-catenin in mature B cell function. We demonstrate that β-catenin contributes to the optimal generation of PCs in vitro. The effect of β-catenin in PC formation, albeit small, is highly consistent and statistically significant. Correlating with the defective PC formation, the expression of Blimp-1 is significantly lower in CAT-KO cells than control cells early after LPS/LPS plus IL-4 stimulation. The other two relevant transcription factors, Xbp-1 and IRF-4, also showed a decrease. Because the decrease in all these transcription factors occurred very early after LPS stimulation, it is likely to be the molecular mechanism for the consequent defective plasma cell differentiation. Furthermore, because Blimp-1 promotes PC formation and simultaneously suppresses CSR (25), the defective Blimp-1 expression in CAT-KO B cells may also underlie the slightly enhanced CSR to IgG3 and IgG1. The mechanism by which β-catenin affects Blimp-1 and IRF-4 expression remain to be investigated, although it’s clear that β-catenin does not promote Blimp-1 by enhancing Bcl-6 expression. The regulatory regions of Prdm-1 (the gene encoding Blimp-1) and IRF-4 contain TCF-1 binding sites, thus β-catenin and TCF has the potential to directly regulate the transcription of Blimp-1 and IRF-4.
Despite the cell intrinsic effect of β-catenin on plasma cell differentiation and class switch of IgG observed in the in vitro, T-dependent and T-independent B cell responses in CAT-KO mice are grossly normal in vivo. The number of activated B cells and memory B cells generated serum IgG levels documented in primary response and secondary response, and long-lived plasma cells were comparable in CAT-KO and control mice. There was a slight increase in number of plasma cells in the T-dependent secondary response as well as T-independent response in CAT-KO mice. The increased number of plasma cells combined with normal Ab titers suggests the CAT-KO plasma cells secrete less Ab on a per cell basis. β-Catenin deficiency renders plasma cell generation in vitro slightly defective, in contrast a slight increase in plasma cell generation is noted in vivo. These two observations are not necessarily at odds. Blimp-1 plays an integral role in the development of plasma cells and reduced expression may contribute to the less rapid plasma cell differentiation in vitro, while a reduction in Blimp-1 expression in vivo may lead to increased survival of ASCs due to increased cell death of cells expressing Blimp-1 (26). These plasma cell differences may also represent unknown factors in the in vivo milieu or a difference in syndecan-1 expression in short-term in vitro cultures. Additionally, while in vitro cultures were conducted for 3 days, in vivo immune responses were assessed after day 7. This window could allow for increased proliferation and/or survival of ASCs in CAT-KO mice that could not be distinguished due to limitations of in vitro culture systems.
In conclusion, our data demonstrate that despite the requirement of Wnt and LEF-1, β-catenin expression is dispensable for B cell development. β-Catenin contributes to the optimal generation of plasma cells after in vitro stimulation and may impair the optimal generation of plasma cells in vivo. Together these data suggest that β-catenin expression may be largely dispensable for B cell development and responses in vivo.
Acknowledgments
We thank Dr. Robert Wersto, Francis J. Chrest, and Cuong Nguyen for expert cell sorting of B cell subpopulations; Donna Tignor, Dawn Phillips, Dawn Nines, Anna Butler, Crystal Gifford, and Ernest Dabney for maintaining animals; Dr. Shengyuan Luo and his team for genotyping animals; P. J. O’Neill and M. Z. Hossain for technical help; and members of our laboratories for support throughout the project. This research was supported by the Intramural Research Program of the National Institute on Aging at the National Institutes of Health and in part by an appointment to the Oak Ridge Institute for Science and Education’s Research Associates Program at the National Institutes of Health.
Footnotes
Abbreviations used in this paper: BM, bone marrow; FO B, follicular B cell; MZ B, marginal zone B cell; BAFF-R, BAFF-receptor; PC, plasma cell; CSR, class-switch recombination; LEF, lymphocyte enhancer factor; TCF, T cell factor; RT, real time; ASC, Ab secreting cell; NP, 4-hydroxy-3-nitrophenylacetyl.
Disclosures
The authors have no financial conflict of interest.
References
- 1.Hardy RR, Hayakawa K. B cell development pathways. Annu Rev Immunol. 2001;19:595–621. doi: 10.1146/annurev.immunol.19.1.595. [DOI] [PubMed] [Google Scholar]
- 2.Casola S. Control of peripheral B-cell development. Curr Opin Immunol. 2007;19:143–149. doi: 10.1016/j.coi.2007.02.010. [DOI] [PubMed] [Google Scholar]
- 3.Lopes-Carvalho T, Foote J, Kearney JF. Marginal zone B cells in lymphocyte activation and regulation. Curr Opin Immunol. 2005;17:244–250. doi: 10.1016/j.coi.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 4.Shapiro-Shelef M, Lin KI, McHeyzer-Williams LJ, Liao J, McHeyzer-Williams MG, Calame K. Blimp-1 is required for the formation of immunoglobulin secreting plasma cells and pre-plasma memory B cells. Immunity. 2003;19:607–620. doi: 10.1016/s1074-7613(03)00267-x. [DOI] [PubMed] [Google Scholar]
- 5.Johnson K, Shapiro-Shelef M, Tunyaplin C, Calame K. Regulatory events in early and late B-cell differentiation. Mol Immunol. 2005;42:749–761. doi: 10.1016/j.molimm.2004.06.039. [DOI] [PubMed] [Google Scholar]
- 6.Shapiro-Shelef M, Calame K. Regulation of plasma-cell development. Nat Rev Immunol. 2005;5:230–242. doi: 10.1038/nri1572. [DOI] [PubMed] [Google Scholar]
- 7.Reya T, Okamura R, Grosschedl R. Control of lymphocyte differentiation by the LEF-1/TCF family of transcription factors. Cold Spring Harbor Symp Quant Biol. 1999;64:133–140. doi: 10.1101/sqb.1999.64.133. [DOI] [PubMed] [Google Scholar]
- 8.Ranheim EA, Kwan HC, Reya T, Wang YK, Weissman IL, Francke U. Frizzled 9 knock-out mice have abnormal B-cell development. Blood. 2005;105:2487–2494. doi: 10.1182/blood-2004-06-2334. [DOI] [PubMed] [Google Scholar]
- 9.Scheller M, Huelsken J, Rosenbauer F, Taketo MM, Birchmeier W, Tenen DG, Leutz A. Hematopoietic stem cell and multilineage defects generated by constitutive β-catenin activation. Nat Immunol. 2006;7:1037–1047. doi: 10.1038/ni1387. [DOI] [PubMed] [Google Scholar]
- 10.Baba Y, Garrett KP, Kincade PW. Constitutively active β-catenin confers multilineage differentiation potential on lymphoid and myeloid progenitors. Immunity. 2005;23:599–609. doi: 10.1016/j.immuni.2005.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baba Y, Yokota T, Spits H, Garrett KP, Hayashi S, Kincade PW. Constitutively active β-catenin promotes expansion of multipotent hematopoietic progenitors in culture. J Immunol. 2006;177:2294–2303. doi: 10.4049/jimmunol.177.4.2294. [DOI] [PubMed] [Google Scholar]
- 12.Christian SL, Sims PV, Gold MR. The B cell antigen receptor regulates the transcriptional activator β-catenin via protein kinase C-mediated inhibition of glycogen synthase kinase-3. J Immunol. 2002;169:758–769. doi: 10.4049/jimmunol.169.2.758. [DOI] [PubMed] [Google Scholar]
- 13.Rickert RC, Roes J, Rajewsky K. B lymphocyte-specific, Cre-mediated mutagenesis in mice. Nucleic Acids Res. 1997;25:1317–1318. doi: 10.1093/nar/25.6.1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brault V, Moore R, Kutsch S, Ishibashi M, Rowitch DH, McMahon AP, Sommer L, Boussadia O, Kemler R. Inactivation of the β-catenin gene by Wnt1-Cre-mediated deletion results in dramatic brain malformation and failure of craniofacial development. Development. 2001;128:1253–1264. doi: 10.1242/dev.128.8.1253. [DOI] [PubMed] [Google Scholar]
- 15.Rickert RC, Rajewsky K, Roes J. Impairment of T-cell-dependent B-cell responses and B-1 cell development in CD19-deficient mice. Nature. 1995;376:352–355. doi: 10.1038/376352a0. [DOI] [PubMed] [Google Scholar]
- 16.Miller JP, Allman D. The decline in B lymphopoiesis in aged mice reflects loss of very early B-lineage precursors. J Immunol. 2003;171:2326–2330. doi: 10.4049/jimmunol.171.5.2326. [DOI] [PubMed] [Google Scholar]
- 17.Allman D, Lindsley RC, DeMuth W, Rudd K, Shinton SA, Hardy RR. Resolution of three nonproliferative immature splenic B cell subsets reveals multiple selection points during peripheral B cell maturation. J Immunol. 2001;167:6834–6840. doi: 10.4049/jimmunol.167.12.6834. [DOI] [PubMed] [Google Scholar]
- 18.Cariappa A, Liou HC, Horwitz BH, Pillai S. Nuclear factor kappa B is required for the development of marginal zone B lymphocytes. J Exp Med. 2000;192:1175–1182. doi: 10.1084/jem.192.8.1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.DiLillo DJ, Hamaguchi Y, Ueda Y, Yang K, Uchida J, Haas KM, Kelsoe G, Tedder TF. Maintenance of long-lived plasma cells and serological memory despite mature and memory B cell depletion during CD20 immunotherapy in mice. J Immunol. 2008;180:361–371. doi: 10.4049/jimmunol.180.1.361. [DOI] [PubMed] [Google Scholar]
- 20.Takahashi Y, Dutta PR, Cerasoli DM, Kelsoe G. In situ studies of the primary immune response to (4-hydroxy-3-nitrophenyl)acetyl: V. Affinity maturation develops in two stages of clonal selection. J Exp Med. 1998;187:885–895. doi: 10.1084/jem.187.6.885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kallies A, Hasbold J, Tarlinton DM, Dietrich W, Corcoran LM, Hodgkin PD, Nutt SL. Plasma cell ontogeny defined by quantitative changes in blimp-1 expression. J Exp Med. 2004;200:967–977. doi: 10.1084/jem.20040973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xu Y, Banerjee D, Huelsken J, Birchmeier W, Sen JM. Deletion of β-catenin impairs T cell development. Nat Immunol. 2003;4:1177–1182. doi: 10.1038/ni1008. [DOI] [PubMed] [Google Scholar]
- 23.Cobas M, Wilson A, Ernst B, Mancini SJ, MacDonald HR, Kemler R, Radtke F. β-catenin is dispensable for hematopoiesis and lymphopoiesis. J Exp Med. 2004;199:221–229. doi: 10.1084/jem.20031615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Koch U, Wilson A, Cobas M, Kemler R, Macdonald HR, Radtke F. Simultaneous loss of β- and γ-catenin does not perturb hematopoiesis or lymphopoiesis. Blood. 2008;111:160–164. doi: 10.1182/blood-2007-07-099754. [DOI] [PubMed] [Google Scholar]
- 25.Omori SA, Cato MH, Anzelon-Mills A, Puri KD, Shapiro-Shelef M, Calame K, Rickert RC. Regulation of class-switch recombination and plasma cell differentiation by phosphatidylinositol 3-kinase signaling. Immunity. 2006;25:545–557. doi: 10.1016/j.immuni.2006.08.015. [DOI] [PubMed] [Google Scholar]
- 26.Messika EJ, Lu PS, Sung YJ, Yao T, Chi JT, Chien YH, Davis MM. Differential effect of B lymphocyte-induced maturation protein (Blimp-1) expression on cell fate during B cell development. J Exp Med. 1998;188:515–525. doi: 10.1084/jem.188.3.515. [DOI] [PMC free article] [PubMed] [Google Scholar]