Abstract
The discriminative stimulus properties of ethanol are functionally regulated by ionotropic GABAA and NMDA receptors in specific limbic brain regions including the nucleus accumbens, amygdala, and hippocampus, as determined by microinjection studies. The purpose of the present work was to further investigate potential neural substrates of ethanol’s discriminative stimulus effects by examining if ethanol discrimination learning produces changes in brain regional response to ethanol. To accomplish this goal, immunohistochemistry was used to assess the effects of ethanol (2 g/kg) on c-Fos immunoreactivity (Fos-IR). Comparisons in ethanol-induced Fos-IR were made between a group of rats that was trained to discriminate the stimulus properties of ethanol (2 g/kg, IG) from water (IG) and a drug/behavior-matched control group that did not receive differential reinforcement for lever selection, which precluded acquisition of discriminative stimulus control by ethanol. In some brain regions discrimination training had no effect on ethanol-induced Fos-IR changes (caudate putamen, bed nucleus of the stria terminalis, and CA1 region of the hippocampus). In contrast, discrimination training altered the pattern of ethanol-induced Fos-IR in the nucleus accumbens (core), medial septum, and the hippocampus (dentate and CA3). These results indicate that having behavior under the stimulus control of ethanol can change ethanol-induced Fos-IR in some brain regions. This suggests that learning about the subjective properties of ethanol produces adaptive changes in how the brain responds to acute ethanol exposure.
Keywords: ethanol, c-Fos, drug discrimination, discriminative stimulus, immunohistochemistry, learning
1. Introduction
Drug discrimination procedures are commonly used to assess the subjective, or interoceptive, properties of drugs of abuse (Colpaert, 1987). The discriminative stimulus effects of ethanol are mediated in part by inhibitory γ-aminobutyric acid type-A (GABAA) and excitatory N-methyl-D-aspartate (NMDA) receptors. For example, in a variety of species systemic administration of GABAA positive modulators such as benzodiazepines, barbiturates, and neurosteroids substitute fully for the stimulus properties of ethanol (Ator et al., 1993; Bienkowski et al., 1997; Grant et al., 2000; Jarbe and McMillan, 1983; Shelton and Grant, 2002). Similarly, systemic administration of noncompetitive NMDA antagonists, such as PCP and MK-801 also produce ethanol-like stimulus effects (Hundt et al., 1998; Schechter et al., 1993; Shelton and Grant, 2002; Vivian et al., 2002). Given the ubiquitous expression of these and other receptor systems that regulate ethanol discrimination, systemic administration studies do not provide information regarding involvement of specific brain regions or neural pathways.
Microinjection procedures, in which a biologically active compound is infused into a brain region(s), have been widely used to determine functional involvement of specific nuclei or sub-nuclei in the behavioral effects of drugs. We have used this strategy to determine the involvement of specific limbic brain regions in ethanol’s discriminative stimulus effects. For example, direct activation of GABAA receptors by muscimol in the nucleus accumbens, or the amygdala, fully substitutes for systemic ethanol (1 g/kg; Besheer et al., 2003; Hodge and Aiken, 1996; Hodge and Cox, 1998), and partially substitutes for ethanol after administration into the prelimbic cortex (Hodge and Cox, 1998). Other GABAA positive modulators such as pentobarbital and allopregnanolone also substitute for systemic ethanol when administered into the nucleus accumbens, and produce partial substitution for ethanol when administered into the hippocampus (Hodge et al., 2001). Antagonism of NMDA receptors by MK-801 in the hippocampus or the nucleus accumbens produces full substitution for ethanol, and partial substitution when administered into the prelimbic cortex (Hodge and Cox, 1998). Further, interaction of GABAA and NMDA receptors within brain regions and interactions between GABAA receptors across brain regions have also been reported (Besheer et al., 2003; Hodge and Cox, 1998). Together these studies show that the discriminative stimulus effects of ethanol are mediated by GABAA and NMDA receptors in specific mesocorticolimbic brain regions.
Studies have also measured expression of immediate early genes, such as c-fos, to identify neurons within specific brain nuclei that are activated by neuroactive drugs or specific stimuli (Dragunow and Faull, 1989). Along with several other drugs of abuse, ethanol administration alters Fos immunoreactivity in a manner that varies by brain region, ethanol dose, strain of animal, and previous ethanol exposure (Canales, 2004; Eisenman et al., 2002; Herring et al., 2004; Hitzemann and Hitzemann, 1997; Knapp et al., 2001; Ryabinin et al., 1997). Fos has also been used to identify brain regions that are activated by environmental stimuli and learning situations. For example, Fos activation has been reported in specific prefrontal and limbic brain regions when animals are exposed to an environment that had previously been paired with nicotine or chocolate (Schroeder et al., 2001), indicating that learning about reward-associated cues can induce a Fos response. Fos in the amygdala has been reported to vary during different stages of odor discrimination learning (Hess et al., 1997), demonstrating that different anatomical regions are recruited during different stages of learning. Fos has also been used to identify anatomical structures that mediate the discriminative stimulus properties of methamphetamine (Nakajima et al., 2004).
The purpose of this study was to determine if ethanol discrimination learning produces changes in brain regional response to ethanol. To accomplish this goal, the effects of ethanol on brain regional patterns of Fos immunoreactivity (Fos-IR) were examined in rats trained to discriminate the stimulus properties of ethanol (2 g/kg, IG) from water (IG). In order to assess the contribution of discrimination learning to ethanol-induced Fos-IR, comparisons were made between a group of rats trained to discriminate ethanol (2 g/kg) from water and an ethanol/behavior–matched control group for which ethanol did not serve as a discriminative stimulus. This control group received identical exposure to ethanol as the discrimination trained group and was required to lever press on the same schedule of reinforcement (FR10) as the discrimination-trained group in order to receive sucrose reinforcement; however, both levers were active during the training sessions such that completion of an FR10 on either lever resulted in reinforcement. Therefore, this group received no differential reinforcement for lever selection and ethanol did not serve as a discriminative stimulus. Comparisons were made to the corresponding water-treated group to determine whether Fos-IR induced by ethanol (2 g/kg), the stimulus properties of which served as the discriminative stimulus for the discrimination trained animals, resulted in a unique neural activation pattern.
2. Results
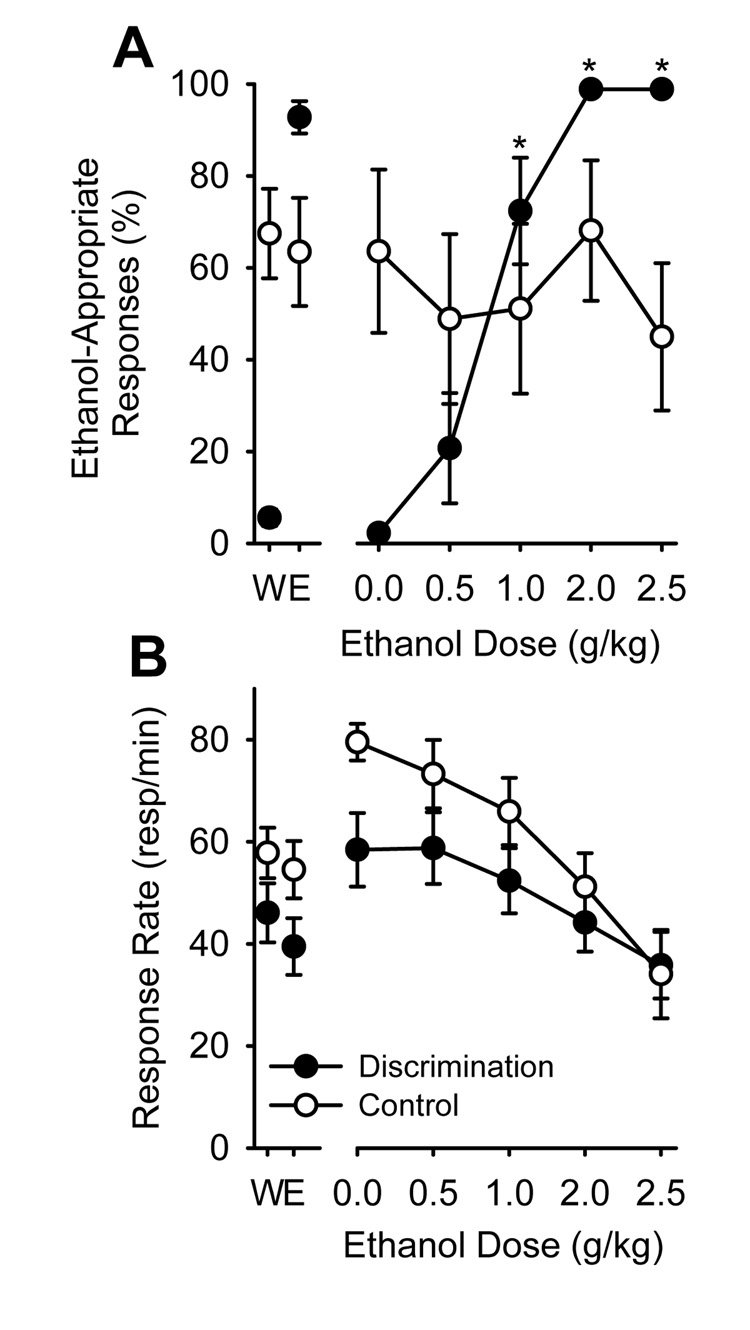
Figure 1A shows the percentage of ethanol-appropriate lever responding on completion of the first FR 10 during testing of different ethanol doses in the discrimination trained and control groups. The left-most panel of the graph shows the average ethanol-appropriate responses for the 10 days of training preceding the beginning of the testing procedures. The two-way ANOVA found a significant main effect of ethanol dose [F(4,56)=8.79, p<0.001], and a significant interaction [F(4,56)=6.47, p<0.001]. In the discrimination-trained group, ethanol-appropriate responding increased as a function of ethanol test dose, with the 1, 2, and 2.5 g/kg doses producing greater ethanol-appropriate responding than water administration (ps<0.001). Further, full substitution (greater than 80% ethanol-appropriate responses) for the 2 g/kg ethanol training dose was observed at 2 and 2.5 g/kg ethanol. This data pattern indicates that the discrimination training procedure established reliable stimulus control by ethanol (2 g/kg). In contrast, in the control group, ethanol-appropriate responses did not vary depending on ethanol dose, and remained at chance levels (50%), indicating that ethanol did not serve as a discriminative stimulus and confirmed the establishment of effective control procedures.
Figure 1.
Panel A. Mean (± SEM) percentage of ethanol-appropriate responding upon completion of the first FR10 at each ethanol dose tested in the discrimination-trained (Discrimination) and ethanol/behavior-matched control (Control). Panel B. Mean (± SEM) test session response rate at each ethanol dose tested. Data plotted on the left side of the x-axis break represent mean performance during the last 10 training days. * Indicates significant difference from 0 (water) in the discrimination-trained group (p<0.05).
Figure 1B illustrates total session response rate for the discrimination-trained and control groups. The left most panel of the graph shows the average response rate for the 10 days of training preceding the beginning of the testing procedures. The two-way ANOVA on this data showed no significant differences, indicating that water and ethanol training sessions resulted in similar response rates and both groups showed similar response rates. The two-way ANOVA on response rate for the ethanol substitution test showed a significant main effect of ethanol dose [F(4,56)=18.64, p<0.001], with a significant reduction in response rate at the 2 and 2.5 g/kg ethanol doses relative to water (ps<0.01). The main effect of training condition and interaction were not significant.
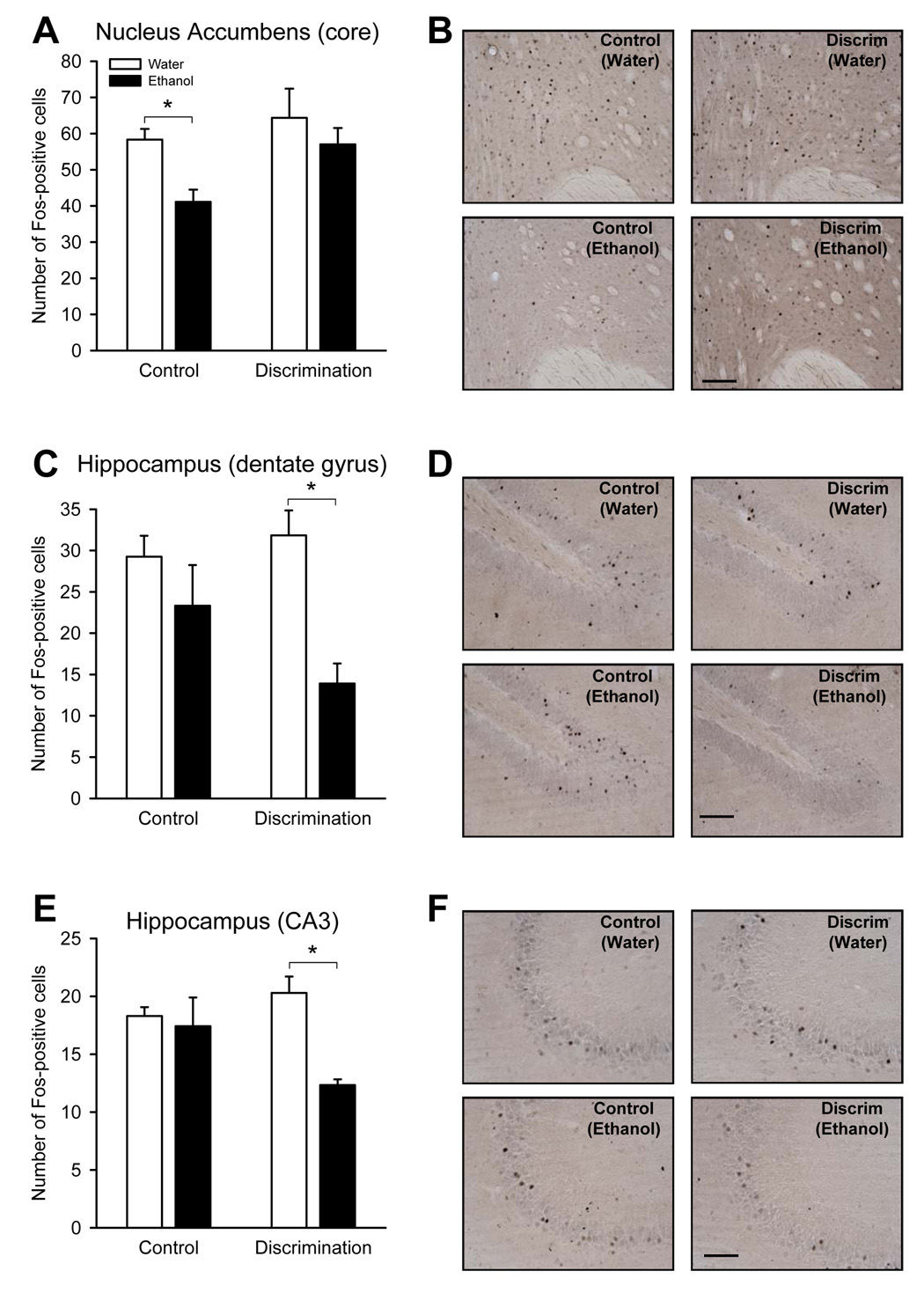
The number of Fos-positive cells for all the surveyed brain regions are shown in Table 1. In the caudate putamen, nucleus accumbens (core), bed nucleus of the stria terminalis, and the hippocampus (dentate, CA1 and CA3 regions) ethanol significantly reduced the number of Fos-positive cells as determined by significant main effect of treatment (caudate putamen [F(1,11)=22.93, p<0.001]; nucleus accumbens core: [F(1,11)=5.09, p=0.045]; bed nucleus of the stria terminalis (BNST): [F(1,12)=17.98, p=0.001], dentate gyrus: [F(1,12)=12.46, p=0.004], CA1: [F(1,12)=21.58, p<0.001]; CA3: [F(1,12)=8.68, p=0.01]). In the caudate putamen, CA1 region of the hippocampus and the BNST, the ethanol-induced reduction in the number of Fos-positive cells occurred regardless of training history (i.e., both the ethanol-injected control and discrimination-trained groups showed reduced Fos-IR; ps<0.03). In the nucleus accumbens (core; Figure 2A and B), ethanol significantly reduced the number of Fos-positive cells in the control group only, (p<0.01). This effect was prevented by discrimination training. In contrast, in the dentate (Figure 2C and D) and CA3 (Figure 2E–F) regions of the hippocampus, discrimination training resulted in significant ethanol-induced reductions in the number of Fos-positive cells, (ps<0.002); no change was observed in the control group. In contrast to the ethanol-induced reductions in the number of Fos-positive cells, ethanol produced a significant increase in the number of Fos-positive cells in the medial septum [F(1,12)=6.53, p=0.025]. This increase was observed in the control group (p=0.04; Table 1), and was not evident in the discrimination-trained group.
Table 1.
Mean number of Fos-positive cells (± S.E.M.) in specified brain regions in the ethanol/behaviormatched control (Control) and the discrimination-trained (Discrimination) groups.
| Control | Discrimination | |||
|---|---|---|---|---|
| Brain Region | Water | Ethanol (2 g/kg) | Water | Ethanol (2 g/kg) |
| Infralimbic cortex | 69.50 ± 5.87 | 68.83 ± 6.69 | 77.92 ± 5.15 | 60.58 ± 6.64 |
| Piriform cortex | 61.21 ± 5.85 | 53.21 ± 11.41 | 63.17 ± 5.56 | 50.25 ± 5.62 |
| Prelimbic cortex | 143.63 ± 17.61 | 136.33 ± 16.88 | 164.96 ± 8.76 | 120.25 ± 15.40 |
| Lateral septum | ||||
| Ventral portion | 56.73 ± 13.24 | 44.56 ± 5.15 | 45.48 ± 5.35 | 47.17 ± 5.55 |
| Intermediate portion | 46.73 ± 7.38 | 47.06 ± 8.20 | 48.31 ± 2.19 | 36.88 ± 5.54 |
| Medial septum | 30.63 ± 2.28 | 42.38 ± 3.96* | 30.88 ± 1.81 | 37.00 ± 4.98 |
| Indusium griseum | 21.25 ± 1.65 | 19.83 ± 2.73 | 18 ± 2.82 | 24.50 ± 4.29 |
| Caudate putamen | 109.00 ± 9.81 | 64.33 ± 1.26* | 103.5 ± 11.09 | 66.0 ± 5.40* |
| Nucleus accumbens | ||||
| Core | 58.33 ± 2.97 | 41.11 ± 3.38* | 64.33 ± 8.08 | 57.00 ± 4.52 |
| Shell | 63.92 ± 3.62 | 53.44 ± 1.28 | 75.58 ± 3.21† | 74.67 ± 5.29† |
| Bed nucleus of the stria terminalis | 22.08 ± 1.02 | 18.06 ± 0.58* | 23.77 ± 1.08 | 16.23 ± 2.21* |
| Amygdala | ||||
| Basolateral | 27.33 ± 1.39 | 28.50 ± 1.73 | 28.58 ± 2.43 | 29.42 ± 2.16 |
| Central nucleus | 46.83 ± 11.75 | 46.67 ± 3.09 | 33.79 ± 4.55† | 29.33 ± 3.34† |
| Hippocampus | ||||
| Dentate gyrus | 29.25 ± 2.54 | 23.33 ± 4.91 | 31.83 ± 3.03 | 13.92 ± 2.42* |
| CA1 | 28.67 ± 3.15 | 16.42 ± 2.91* | 27.50 ± 1.67 | 16.33 ± 2.05* |
| CA3 | 18.29 ± 0.77 | 17.42 ± 2.48 | 20.29 ± 1.42 | 12.33 ± 0.49* |
p ≤ 0.05 versus respective water treatment
p ≤ 0.05 versus Control group (i.e., represents a main effect of training condition)
Figure 2.
Mean (± SEM) of Fos-positive cells in the ethanol/behavior-matched control group (Control) and the discrimination-trained group (Discrimination) after water (open bars) and 2 g/kg ethanol (filled bars) in the nucleus accumbens (core; Panel A), hippocampus (dentate gyrus; Panel C), and the hippocampus (CA3; Panel E), respectively. *p <0.05. Panels B, D, and F show representative photomicrographs (20X) of Fos-IR in the corresponding brain regions for each of the groups. Scale bar = 100 microns.
The central nucleus of the amygdala and the nucleus accumbens (shell) showed a significant main effect of training condition. Discrimination training produced an overall increase in the number of Fos-positive cells in the nucleus accumbens (shell), [F(1,11)=17.53, p=0.002; Table 1] and a significant reduction in the central nucleus of the amygdala [F(1,12)=5.14, p=0.04; Table 1]. The number of Fos-positive cells for the other surveyed brain regions are also shown in Table 1 and did not show any changes in Fos-IR.
3. Discussion
The purpose of this study was to use immunohistochemistry to identify anatomical substrates associated with ethanol serving as a discriminative stimulus. Accordingly, comparisons in Fos-IR were made between a group of rats that was trained to discriminate the stimulus properties of ethanol (2 g/kg, IG) from water and a drug/behavior-matched control group that did not receive differential reinforcement for lever selection, which precluded acquisition of discriminative stimulus control by ethanol in this group. The results of this study suggest that having behavior under stimulus control of ethanol changes the effects of ethanol on Fos-IR in specific brain regions including the nucleus accumbens (core), medial septum, and the hippocampus (dentate gyrus and CA3).
Behavioral results of the present study showed that ethanol functioned as a discriminative stimulus only in the discrimination trained rats. That is, test doses of ethanol (0 – 2.5 g/kg) produced dose-dependent substitution for ethanol (2 g/kg) in the discrimination trained group, but lever-choice by the control group remained at chance levels (50%) regardless of the ethanol dose tested. This indicates that the procedures established reliable stimulus control by ethanol in the discrimination-trained group but not in the drug/behavior-matched control group. Importantly, these groups received an identical history of ethanol injections, exhibited the same response rate (during training and on the final test day), and received the same number of reinforcers in all phases of the experiment. This suggests that the groups differed only in whether they learned the ethanol discrimination and that any group differences in Fos-IR in response to ethanol was attributable to a learning-associated adaptation.
The overall results of this study suggest that having behavior under the stimulus control of ethanol changes the effects of ethanol on Fos-IR in specific brain regions. These results are in agreement with evidence indicating that expression of inducible transcription factors, such as c-Fos, is changed by learning (Gall et al., 1998; Kaczmarek, 1993; Robertson, 1992). In the present work, discrimination training prevented an ethanol-induced reduction of Fos-IR in the nucleus accumbens (core) and prevented an ethanol-induced increase in the medial septum. These findings suggest that ethanol discrimination learning is associated with adaptive changes in these brain regions that prevent, or inhibit, response to ethanol. In contrast, in the dentate gyrus and CA3 regions of the hippocampus discrimination training produced an ethanol-induced reduction in Fos-IR, that was not evident in ethanol-injected controls. Thus, discrimination learning may be associated with adaptive changes in these brain regions that enhance some effects of ethanol. Further, discrimination training was associated with a general reduction in Fos-IR in the central nucleus of the amygdala and a general increase in Fos-IR in the shell of the nucleus accumbens, which suggests that learning the ethanol discrimination results in a general inhibition or activation of these brain regions irrespective of ethanol treatment.
Prior research, utilizing microinjection techniques, has shown functional involvement of the nucleus accumbens and the amygdala in ethanol’s discriminative stimulus properties (Hodge and Aiken, 1996; Hodge and Cox, 1998; Hodge et al., 2001). At the present time, there are no published data regarding the potential functional role of the dentate gyrus and CA3 regions of the hippocampus or the medial septum. However, these structures are known to regulate aspects of learning and memory, and this pattern of results draws attention to these regions as potential substrates of ethanol’s discriminative stimulus effects.
The BNST, caudate putamen and the CA1 region of the hippocampus showed ethanol-induced reductions in Fos-IR regardless of training history (i.e., reductions in Fos-IR were observed in both training groups), which suggests that ethanol discrimination learning may not change how these brain regions respond to ethanol. Reductions in Fos-IR in several brain regions including the CA1 region of the hippocampus and the caudate putamen following ethanol administration have been reported (Ryabinin et al., 1997; Hitzemann and Hitzemann, 1997). It is interesting to note, however, that the CA1 region of the hippocampus has been shown to functionally regulate ethanol’s discriminative stimulus properties (Hodge and Cox, 1998). This suggests that specific brain regions, such as the CA1, may functionally regulate ethanol discrimination in the absence of adaptive changes in response to discrimination learning.
The results of this study, showing changes in Fos-IR in specific brain regions as a function of discrimination training are in general agreement with the only other published study that used Fos-IR to identify anatomical substrates of drug discrimination (Nakajima et al., 2004). In that study, the nucleus accumbens (core and shell) and the ventral tegmental area showed an adaptive response to discrimination learning in rats trained to discriminate methamphetamine (0.5 mg/kg) from saline. Specifically, relative to a naive control group and a control group that received operant training but no methamphetamine exposure or discrimination training, discrimination-trained rats showed significant methamphetamine- and saline-induced increases in Fos-IR in these brain regions. Together with the present findings, these data lend further support to the notion that discrimination learning produces adaptive changes in how specific brain regions respond to drugs of abuse.
An alternative explanation of the present findings is that the changes in brain regional response to ethanol represent attentional processes rather than learning. That is, animals for which ethanol served as a discriminative stimulus were trained to specifically attend to the ethanol cue. In contrast, for the controls, ethanol was likely not specifically attended to given that it did not serve as a discriminative stimulus in this particular situation. Thus, the differences in Fos-IR may be representative of brain regions involved in mediating attention to internal stimuli. However, given that areas shown to be involved in attentional processes (as measured by Fos) such as the frontal and cingulate cortices (Bucci and Macleod, 2007), as well as nuclei within the amygdala (for review see Knapska et al., 2007), did not show a selective ethanol-induced alteration in Fos-IR this explanation may be less likely. However, future studies can examine this possibility using a drug naive behavior-matched control group that is administered ethanol for the first time on the final session; thus, this group would serve as a control for attention to the internal stimulus produced by ethanol.
In conclusion, prior studies have shown that specific limbic brain regions (Hodge and Aiken, 1996; Hodge and Cox, 1998; Hodge et al., 2001) and neural circuits (Besheer et al., 2003) functionally regulate the discriminative stimulus effects of ethanol. The goal of this study was to use Fos-IR as an index of neuronal activity to identify anatomical substrates associated with ethanol serving as a discriminative stimulus. Results indicate that the effects of ethanol on Fos-IR are modified by ethanol discrimination training in mesolimbic brain regions including the nucleus accumbens and the hippocampus. These results indicate that having behavior under the stimulus control of ethanol can change ethanol-induced Fos-IR in some brain regions. This suggests that learning about the subjective properties of ethanol produces adaptive changes in how the brain responds to acute ethanol exposure.
4. Method
Animals
Sixteen male Long Evans rats (Harlan Sprague Dawley, Indianapolis, IN) were individually housed in Plexiglas cages. Long Evan rats were chosen based on previous work from this laboratory using this strain to examine ethanol’s discriminative stimulus properties (Besheer et al., 2003; Besheer and Hodge, 2005; Besheer et al., 2006; Hodge and Aiken, 1996; Hodge and Cox, 1998; Hodge et al., 2001). Body weights were maintained at approximately 325 g via caloric regulation and water was continuously available in the home cage. The colony room was maintained on a 12-h light/dark cycle and experiments were conducted during the light portion of the cycle. All procedures were carried out in accordance with the NIH Guide to Care and Use of Laboratory Animals (Publication No. 85–23, revised 1985) and institutional guidelines.
Procedure
Discrimination Training
Rats were assigned to the Discrimination (n=8) or the Drug/Behavior-Matched Control Group (n=8). A detailed description of lever press training and the chambers (Med Associates, Georgia, VT) used in this study are described in (Besheer and Hodge, 2005). For both groups, training sessions were conducted at approximately 9:00 am, 5 days per week (M–F) during which ethanol (2 g/kg) or water was administered IG prior to the start of the 15-min sessions. Immediately following ethanol or water administration the rats were placed in the chambers. After 10 min the house light was illuminated and both levers were introduced into the chamber signaling the beginning of the session. In the discrimination group, after ethanol administration, completion of 10 responses on the ethanol-appropriate lever resulted in the presentation of the sucrose (10% w/v) solution. Following water administration, completion of 10 responses on the water-appropriate lever resulted in sucrose delivery. During both ethanol and water sessions, responses on the inappropriate lever were recorded but produced no programmed consequences. The control group received the same exposure to ethanol and water, however responses were not differentially reinforced; both levers were active on an FR10 schedule during all sessions. That is, during ethanol and water sessions 10 responses on either lever resulted in presentation of the sucrose solution. For the discrimination group, the lever associated with ethanol or water administration was randomly assigned and counterbalanced across animals. For the control group, an “ethanol-appropriate lever” was randomly assigned for data analysis purposes. Water and ethanol administration varied on a double alternation schedule (W, W, E, E …). For rats in the discrimination group, training continued until the percentage of ethanol- and water-appropriate lever press responses emitted prior to the first reinforcer, and during the entire session equaled or exceeded 80% for ten consecutive days. Once these criteria were met, testing began. Once rats in the discrimination group began testing, rats from the control group were tested in parallel.
Testing Procedures
During the test sessions, which were 2 min in duration, completion of an FR10 on either lever resulted in sucrose delivery (for both groups). For the discrimination group, these sessions were interspersed with training sessions only if performance during the previous 5 training sessions met the accuracy criteria. If the criteria were not met, sessions continued until response accuracy was 80% or greater for 5 consecutive days. For the control group, animals had to maintain a response rate of 20 responses per min or greater for 5 consecutive days in order to be tested. In 5 different test sessions, various ethanol doses (0, 0.5, 1, 2, and 2.5 g/kg IG) were administered to determine an ethanol substitution curve. Rats received each ethanol dose in a random order.
Final training session
In order to preserve the daily routine for the animals, the final day of the experiment was a standard training session that occurred at approximately 9:00 am. For half of the rats in each group this session was a water session (n=4 per group), and for the other half, the session was an ethanol (2 g/kg) session (n=4 per group). Response rates were similar on this final training session as determined by a two-way ANOVA (no significant main effects or interactions). After the session, animals were returned to the home cage.
Immunohistochemistry
Approximately 2 h after ethanol or water administration, the animals were deeply anesthetized with pentobarbital (100 mg/kg IP) and perfused transcardially with 0.1 M phosphate buffered saline (PBS), pH 7.4, at 4°C followed by 4% formaldehyde in 0.2 M phosphate buffer, pH 7.4, at 4°C. The brains were removed from the skull and placed in the same fixative solution for 24 h before being washed with PBS and sliced coronally on a vibratome into 40 µm sections. Free-floating sections were blocked in 10% goat serum and 0.1% Triton X-100 in PBS, and then incubated in 3% goat serum, 0.1% Triton X-100 in PBS, and rabbit anti-c-Fos antibody (1:20,000 dilution; Oncogene Research Products/Calbiochem, USA) for 48 hours at 4° C with agitation. Sections were then washed in PBS and incubated for 1 h in a solution of biotinylated secondary anti-rabbit antibody, and then rinsed in PBS. Sections were next processed with avidin-biotin complex (Vector ABC kit, Vector Laboratories, USA), and Fos-IR was visualized using a diaminobenzidine solution containing 0.006% hydrogen peroxide, 0.005% cobalt, and 0.0075% nickel.
Quantification of Fos-IR
Sections were viewed under a light microscope, and the number of Fos-positive cells in an optical field were counted manually by an observer blinded to experimental treatment. Fos positive cells were identified based on black reaction product confined to the nucleus. The areas (mm2) of optical fields for each brain region are listed below. Cell counts were performed at 20X magnification except for the hippocampal counts which were performed at 10X magnification. The brain regions listed below were matched as closely as possible for each animal according to the atlas of Paxinos and Watson, 1998. When possible, the value of three separate counts including the left and right hemisphere of an individual brain region for each animal (e.g., 2 sections) was averaged and used as a single data point.
Brain regions examined, AP coordinates and area of quantified region
Prelimbic cortex (+2.7 mm; 0.25 mm2); infralimbic cortex (+2.7 mm; 0.125 mm2); piriform cortex (+2.7 mm; 0.025 mm2); indusium griseum (+1.6 mm; 0.0175 mm2); nucleus accumbens (core; +1.2 to 1.0 mm; 0.125 mm2); nucleus accumbens (shell; +1.2 to 1.0 mm; 0.1 mm2); caudate putamen (dorsal portion; +1.0 to 0.7 mm; 0.25 mm2); medial septum (+1.0 to 0.7 mm; 0.1 mm2); lateral septum (ventral portion; +0.2 mm; 0.125 mm2); lateral septum (intermediate portion; +0.2 mm; 0.175 mm2); bed nucleus of the stria terminalis (+0.2 mm; 0.07 mm2); amygdala (basolateral; −2.56 mm; 0.07 mm2); amygdala (central nucleus; −2.56 mm; 0.105 mm2); hippocampus (CA1; −3.3 mm; 0.1 mm2); hippocampus (CA3; −3.3 mm; 0.1 mm2); hippocampus (dentate; −3.3 mm; 0.1 mm2).
Data Analyses
The behavioral data (ethanol substitution curve) was analyzed using a two-way ANOVA with ethanol dose as a repeated measure and training condition as a between group measure. Tukey post hoc tests were used to examine differences when significant main effects and/or interactions were observed. For the control group, an “ethanol-appropriate lever” was designated for the purpose of analysis. Fos-IR quantification is presented as mean number of positive cells per optical field ± S.E.M. The number of Fos-positive cells were analyzed using a two-way ANOVA. Significant main effects of ethanol treatment were followed up with t-tests (i.e., comparing ethanol vs. water) to assess whether ethanol induced a similar pattern of Fos-IR in the control and discrimination groups. Statistical significance was declared at p≤0.05.
Acknowledgements
This work was supported by Grants AA016009 (JB) and AA011605 (CWH) from the National Institute on Alcohol Abuse and Alcoholism and by the Bowles Center for Alcohol Studies. We are grateful to Dr. Darin Knapp for his advice with immunohistochemistry and quantification techniques, and to Mary Beth Wilkie and Julie Grondin for technical assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ator NA, Grant KA, Purdy RH, Paul SM, Griffiths RR. Drug discrimination analysis of endogenous neuroactive steroids in rats. Eur J Pharmacol. 1993;241(2–3):237–243. doi: 10.1016/0014-2999(93)90208-y. [DOI] [PubMed] [Google Scholar]
- Besheer J, Cox AA, Hodge CW. Coregulation of ethanol discrimination by the nucleus accumbens and amygdala. Alcohol Clin Exp Res. 2003;27(3):450–456. doi: 10.1097/01.ALC.0000057036.64169.C1. [DOI] [PubMed] [Google Scholar]
- Besheer J, Hodge CW. Pharmacological and anatomical evidence for an interaction between mGluR5- and GABA(A) alpha1-containing receptors in the discriminative stimulus effects of ethanol. Neuropsychopharmacology. 2005;30(4):747–757. doi: 10.1038/sj.npp.1300616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besheer J, Stevenson RA, Hodge CW. mGlu5 receptors are involved in the discriminative stimulus effects of self-administered ethanol in rats. Eur J Pharmacol. 2006;551(1–3):71–75. doi: 10.1016/j.ejphar.2006.08.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bienkowski P, Iwinska K, Stefanski R, Kostowski W. Discriminative stimulus properties of ethanol in the rat: differential effects of selective and nonselective benzodiazepine receptor agonists. Pharmacol Biochem Behav. 1997;58(4):969–973. doi: 10.1016/s0091-3057(97)00034-8. [DOI] [PubMed] [Google Scholar]
- Bucci DJ, Macleod JE. Changes in neural activity associated with a surprising change in the predictive validity of a conditioned stimulus. Eur J Neurosci. 2007;26(9):2669–2676. doi: 10.1111/j.1460-9568.2007.05902.x. [DOI] [PubMed] [Google Scholar]
- Canales JJ. Catalase-independent early-gene expression in rat brain following acute ethanol exposure. Brain Res. 2004;1016(1):96–101. doi: 10.1016/j.brainres.2004.04.078. [DOI] [PubMed] [Google Scholar]
- Colpaert FC. Drug discrimination: methods of manipulation, measurement, and analysis. In: Bozarth MA, editor. Methods of assessing the reinforcing properties of abused drugs. New York: Springer-Verlag; 1987. pp. 341–372. [Google Scholar]
- Dragunow M, Faull R. The use of c-fos as a metabolic marker in neuronal pathway tracing. Journal of Neuroscience Methods. 1989;29(3):261–265. doi: 10.1016/0165-0270(89)90150-7. [DOI] [PubMed] [Google Scholar]
- Eisenman LM, Tran MH, Scott Donovan H. Acute ethanol administration produces specific patterns of localization of Fos-immunoreactivity in the cerebellum and inferior olive of two inbred strains of mice. Brain Res. 2002;952(1):135–141. doi: 10.1016/s0006-8993(02)03184-0. [DOI] [PubMed] [Google Scholar]
- Gall CM, Hess US, Lynch G. Mapping brain networks engaged by, and changed by, learning. Neurobiol Learn Mem. 1998;70(1–2):14–36. doi: 10.1006/nlme.1998.3835. [DOI] [PubMed] [Google Scholar]
- Grant KA, Waters CA, Green-Jordan K, Azarov A, Szeliga KT. Characterization of the discriminative stimulus effects of GABA(A) receptor ligands in Macaca fascicularis monkeys under different ethanol training conditions. Psychopharmacology (Berl) 2000;152(2):181–188. doi: 10.1007/s002130000510. [DOI] [PubMed] [Google Scholar]
- Herring BE, Mayfield RD, Camp MC, Alcantara AA. Ethanol-induced Fos immunoreactivity in the extended amygdala and hypothalamus of the rat brain: focus on cholinergic interneurons of the nucleus accumbens. Alcohol Clin Exp Res. 2004;28(4):588–597. doi: 10.1097/01.alc.0000122765.58324.6d. [DOI] [PubMed] [Google Scholar]
- Hess US, Gall CM, Granger R, Lynch G. Differential patterns of c-fos mRNA expression in amygdala during successive stages of odor discrimination learning. Learn Mem. 1997;4(3):262–283. doi: 10.1101/lm.4.3.262. [DOI] [PubMed] [Google Scholar]
- Hitzemann B, Hitzemann R. Genetics ethanol and the Fos response: a comparison of the C57BL/6J and DBA/2J inbred mouse strains. Alcohol Clin Exp Res. 1997;21(8):1497–1507. [PubMed] [Google Scholar]
- Hodge CW, Aiken AS. Discriminative stimulus function of ethanol: role of GABAA receptors in the nucleus accumbens. Alcoholism: Clinical & Experimental Research. 1996;20(7):1221–1228. doi: 10.1111/j.1530-0277.1996.tb01116.x. [DOI] [PubMed] [Google Scholar]
- Hodge CW, Cox AA. The discriminative stimulus effects of ethanol are mediated by NMDA and GABA(A) receptors in specific limbic brain regions. Psychopharmacology (Berl) 1998;139(1–2):95–107. doi: 10.1007/s002130050694. [DOI] [PubMed] [Google Scholar]
- Hodge CW, Nannini MA, Olive MF, Kelley SP, Mehmert KK. Allopregnanolone and pentobarbital infused into the nucleus accumbens substitute for the discriminative stimulus effects of ethanol. Alcoholism: Clinical & Experimental Research. 2001;25(10):1441–1447. doi: 10.1097/00000374-200110000-00006. [DOI] [PubMed] [Google Scholar]
- Hundt W, Danysz W, Hèolter SM, Spanagel R. Ethanol and N-methyl-D-aspartate receptor complex interactions: a detailed drug discrimination study in the rat. Psychopharmacology (Berl) 1998;135(1):44–51. doi: 10.1007/s002130050484. [DOI] [PubMed] [Google Scholar]
- Jarbe TU, McMillan DE. Interaction of the discriminative stimulus properties of diazepam and ethanol in pigeons. Pharmacol Biochem Behav. 1983;18(1):73–80. doi: 10.1016/0091-3057(83)90254-x. [DOI] [PubMed] [Google Scholar]
- Kaczmarek L. Molecular biology of vertebrate learning: is c-fos a new beginning? J Neurosci Res. 1993;34(4):377–381. doi: 10.1002/jnr.490340402. [DOI] [PubMed] [Google Scholar]
- Knapp DJ, Braun CJ, Duncan GE, Qian Y, Fernandes A, Crews FT, Breese GR. Regional specificity of ethanol and NMDA action in brain revealed with FOS-like immunohistochemistry and differential routes of drug administration. Alcohol Clin Exp Res. 2001;25(11):1662–1672. [PubMed] [Google Scholar]
- Knapska E, Radwanska K, Werka T, Kaczmarek L. Functional internal complexity of amygdala: focus on gene activity mapping after behavioral training and drugs of abuse. Physiol Rev. 2007;87(4):1113–1173. doi: 10.1152/physrev.00037.2006. [DOI] [PubMed] [Google Scholar]
- Nakajima A, Yamada K, He J, Zeng N, Nitta A, Nabeshima T. Anatomical substrates for the discriminative stimulus effects of methamphetamine in rats. J Neurochem. 2004;91(2):308–317. doi: 10.1111/j.1471-4159.2004.02705.x. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Fourth ed. Sydney: Academic Press; 1998. [Google Scholar]
- Robertson HA. Immediate-early genes, neuronal plasticity, and memory. Biochem Cell Biol. 1992;70(9):729–737. doi: 10.1139/o92-112. [DOI] [PubMed] [Google Scholar]
- Ryabinin AE, Criado JR, Henriksen SJ, Bloom FE, Wilson MC. Differential sensitivity of c-Fos expression in hippocampus and other brain regions to moderate and low doses of alcohol. Mol Psychiatry. 1997;2(1):32–43. doi: 10.1038/sj.mp.4000206. [DOI] [PubMed] [Google Scholar]
- Schechter MD, Meehan SM, Gordon TL, McBurney DM. The NMDA receptor antagonist MK-801 produces ethanol-like discrimination in the rat. Alcohol. 1993;10(3):197–201. doi: 10.1016/0741-8329(93)90035-m. [DOI] [PubMed] [Google Scholar]
- Schroeder BE, Binzak JM, Kelley AE. A common profile of prefrontal cortical activation following exposure to nicotine- or chocolate-associated contextual cues. Neuroscience. 2001;105(3):535–545. doi: 10.1016/s0306-4522(01)00221-4. [DOI] [PubMed] [Google Scholar]
- Shelton KL, Grant KA. Discriminative stimulus effects of ethanol in C57BL/6J and DBA/2J inbred mice. Alcohol Clin Exp Res. 2002;26(6):747–757. [PubMed] [Google Scholar]
- Vivian JA, Waters CA, Szeliga KT, Jordan K, Grant KA. Characterization of the discriminative stimulus effects of N-methyl- D-aspartate ligands under different ethanol training conditions in the cynomolgus monkey ( Macaca fascicularis) Psychopharmacology (Berl) 2002;162(3):273–281. doi: 10.1007/s00213-002-1086-2. [DOI] [PubMed] [Google Scholar]