Abstract
Dynamic protein localization is an integral component of the regulatory circuit that drives the Caulobacter cell cycle. The ClpXP protease is localized to the Caulobacter cell pole, where it catalyzes the degradation of the CtrA master regulator at specific times in the cell cycle. Clearance of active CtrA at the G1/S transition allows the initiation of DNA replication and cell-cycle progression. The polar localization of ClpXP is dependent on the polar positioning of the CpdR single-domain response regulator. Only the unphosphorylated form of CpdR localizes and activates ClpXP. We demonstrate that another single domain response regulator, DivK, promotes the polar accumulation of unphosphorylated CpdR and that CpdR is subsequently degraded at the cell pole by the localized ClpXP protease. Thus, CpdR function is regulated by a feedback loop that incorporates its differential phosphorylation, the transient polar localization and activity of the ClpXP protease, and the clearance of the CpdR by polar ClpXP that, in turn, releases ClpXP from the pole relieving the degradation of CtrA. CtrA∼P then accumulates and activates the transcription of cpdR, completing the regulatory loop, establishing an integrated network that controls a robust cell-cycle transition.
Keywords: Caulobacter, ClpXP, feedback, cell-cycle, CtrA
The Caulobacter crescentus cell cycle is temporally and spatially regulated by a control network that includes signal transduction elements, transcriptional controls, and regulated proteolytic pathways that operate in positive and negative feedback loops (1). These processes drive and are driven by protein complexes that are localized to specific subcellular locations at defined times in the cell cycle (2). Caulobacter cell division is asymmetric, yielding morphologically distinct cells with different cell fates: a flagellated swarmer cell and a sessile stalked cell (Fig. 1B). The differentiation of the swarmer cell into a stalked cell coincides with the initiation of DNA replication, an event equivalent to the eukaryotic G1-to-S transition.
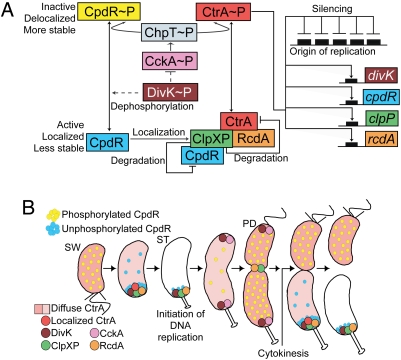
Fig. 1.
Model for CpdR and CtrA regulation (A) and cell cycle progression (B). (A) Continuous and dashed lines indicate direct or possible indirect effects, respectively. The black boxes represent CtrA binding sites. (B) The differential spatial distribution of multiple regulatory elements that drive cell-cycle progression are shown. PD, predivisional cell, ST, stalked cell; SW, swarmer cell.
CtrA, a central regulatory protein in the Caulobacter cell-cycle circuit, directly controls the transcription of ≈95 cell cycle-regulated genes (3), as well as binding to and silencing the origin of replication (4). CtrA is a member of the response regulator family of proteins, and is activated by phosphorylation to perform these functions (5, 6). CtrA∼P must be cleared from the cell during the swarmer-to-stalked cell transition to allow the initiation of DNA replication (see Fig. 1B) (5). In vitro, CtrA is degraded by ClpXP in the absence of any protein cofactor (7). Because ClpXP is present throughout the cell cycle (8), some other mechanism must signal the temporal control of CtrA proteolysis. The transient polar localization of ClpXP and the RcdA-dependent recruitment of the CtrA substrate to the polar protease (9–11) contributes to the degradation of CtrA at critical points in the cell cycle. The polar localization and consequently the activity of ClpXP is controlled by the CpdR single-domain response regulator (9). In its unphosphorylated state, CpdR localizes to the cell pole, promoting the polar positioning of the protease complex and enabling its proteolytic activity (9). When CpdR is phosphorylated (CpdR∼P), it does not localize to the cell pole, consequently ClpXP does not accumulate at the pole and CtrA is not degraded. The temporally controlled phosphorylation of CpdR is mediated by the same phospho-signaling pathway that phosphorylates and activates CtrA (9, 12) (Fig. 1A). The hybrid histidine kinase CckA phosphorylates the histidine phosphotransferase ChpT, which in turn phosphorylates both CtrA and CpdR (12). Thus, the CckA phospho-signaling pathway controls both the activity and stability of CtrA, providing robust control of CtrA function during the cell cycle. The DivK response regulator is another factor that contributes to CtrA degradation (13) by inhibiting the CckA-ChpT pathway in a unknown way (12).
The dynamic polar positioning and release of the CpdR localization factor is a critical lynch-pin in the cell cycle control of the CtrA master regulator. Here, we show that CpdR function is regulated by a feedback loop that incorporates its differential phosphorylation state mediated by DivK. The release of ClpXP from the pole, after the clearance of CtrA, is facilitated by the degradation of CpdR by ClpXP. CtrA∼P then accumulates and activates the transcription of cpdR, completing the regulatory loop and allowing cell-cycle progression.
Results
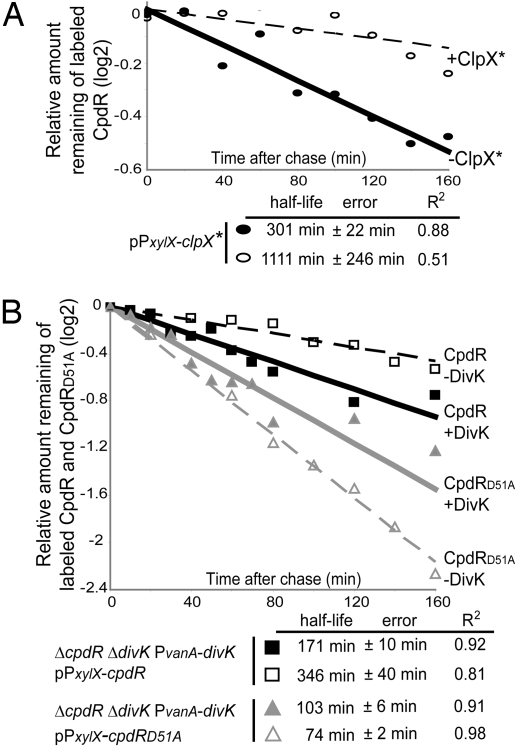
CpdR Is Degraded in a ClpX-Dependent Manner.
The accumulation of CpdR is cell cycle-regulated [supporting information (SI) Fig. S1] (9) with a peak in late predivisional cells and a minimum at the stalked cells stage just after CtrA is cleared from the cell and DNA replication is initiated. The CpdR-C terminus has a hydrophobic sequence, similar to that found at the C-terminal sequence of CtrA (11). We asked if the ClpX chaperone targets CpdR for proteolysis. Accordingly, we measured the half-life of CpdR in a WT strain containing a low-copy number plasmid bearing a dominant negative allele of clpX, clpX* (14). The clpX* allele, which has a loss of function mutation in the ATP binding site of ClpX, is under the control of the inducible PxylX promoter (15). Thus, when grown in the presence of xylose, the mutated allele clpX* is highly expressed, inhibiting the degradation of ClpXP substrates (14). The half-life of CpdR after 4 h of xylose induction is ≈3.7-fold longer than in the absence of xylose (Fig. 2A) (see Note About the Half-Life of CpdR in SI Results), indicating that CpdR is degraded in a ClpX-dependent manner. This requirement is likely to be direct because we have shown previously, by coimmunoprecipitation assays, that CpdR interacts with both ClpX and ClpP (9). Thus, CpdR activates ClpXP by positioning it at the cell pole, ClpXP proteolyzes CtrA, and then CpdR is degraded in a ClpX-dependent manner. We propose that the degradation of CpdR promotes the observed polar detachment of ClpXP (10), thereby preventing the degradation of newly synthesized CtrA later in the cell cycle.
Fig. 2.
The ClpX-mediated degradation of CpdR is dependent on its phosphorylation state and on DivK. (A) WT cells harboring the plasmid pPxylX-clpX* (14) were grown in M2G medium in the absence of xylose. Culture samples were incubated for 4 h either in the absence (solid line) or in the presence of xylose to allow clpX* induction (dashed line). (B) Pulse–chase experiments showing the half-lives of CpdR (black lines) and CpdRD51A (gray lines) in the presence (solid lines) or absence (dashed lines) of DivK. Each strain was incubated in the presence of xylose to induce cpdR or cpdRD51A and in the presence or absence of vanillate (presence or absence of DivK, respectively). R2 is the regression coefficient.
The Stability of CpdR Depends on DivK Regulation of the CpdR Phosphorylation State.
The unphosphorylated form of CpdR is localized to the cell pole where it actively localizes ClpXP, whereas the phosphorylated form of CpdR is delocalized and unable to localize ClpXP to the pole (9). The CckA-ChpT phospho-signaling cascade promotes the accumulation of phosphorylated CpdR (see Fig. 1A) (9, 12). Because the DivK response regulator has been proposed to be an inhibitor of CckA activity (12) and is required for the degradation of ClpXP's polar substrates, such as CtrA and the McpA chemoreceptor (9, 13), DivK is a possible mediator of CpdR function. We thus asked if the proteolysis of CpdR is related to its phosphorylation state and if it is mediated by DivK. We generated an isogenic DivK-depletion strain (LS4400) bearing a divK deletion with a single chromosomal divK gene under the control of the vanillate inducible PvanA promoter (16) (Fig. 3A Lower and Fig. S2). DivK depletion strains were then generated that contained a chromosomal cpdR deletion, complemented by a high-copy number plasmid bearing either a PxylX-inducible cpdR (LS4401) or cpdRD51A (LS4402). CpdRD51A is a mutated form of CpdR that cannot be phosphorylated (9). We have observed that CpdRD51A is degraded in a ClpX-dependent manner (data not shown). Expression of WT cpdR in the presence of vanillate (presence of DivK) yields both phosphorylated and nonphosphorylated CpdR. However, the expression of cpdRD51A yields only nonphosphorylatable protein. We observed that the half-life of the unphosphorylatable CpdRD51A (see Fig. 2B, solid gray line) was ≈1.7-fold shorter than the half-life of the mixture of phosphorylated and non-phosphorylated CpdR present in the WT strain (see Fig. 2B, solid black line). Thus, the unphosphorylated form of CpdR is less stable than CpdR∼P.
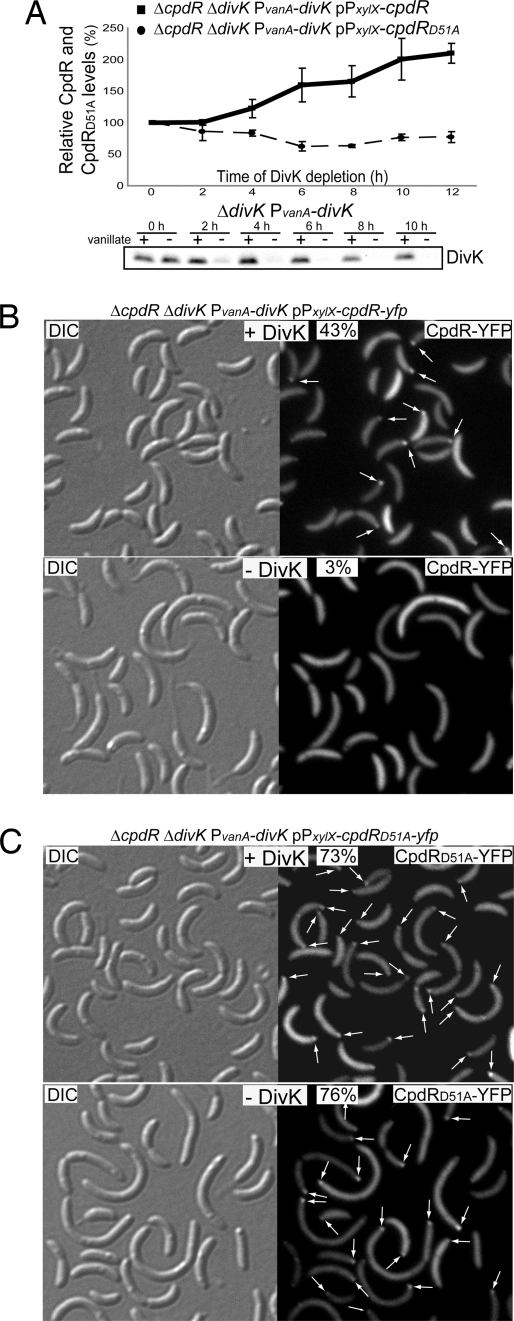
Fig. 3.
(A) CpdR (solid line), but not CpdRD51A (dashed line) accumulates in the absence of DivK. Indicated strains were incubated in the absence of vanillate (absence of DivK), but in the presence of xylose (presence of CpdR or CpdRD51A). Samples were taken every 2 h for Western blot analysis using anti-CpdR antibodies, and the amounts of CpdR or CpdRD51A were determined. Immunoblot using anti-DivK antibodies showing the levels of DivK accumulation after DivK depletion by vanillate removal (Bottom). Cultures of strain ΔdivK PvanA-divK were grown in M2G media in presence of vanillate (presence of DivK). After vanillate removal, two aliquots of the same culture were grown in the presence or absence of vanillate. Western blot analysis was performed on samples collected from each culture every 2 h over the duration of the experiment. DivK was significantly decreased after 2 h of incubation in the absence of vanillate, and was undetectable after 6 h. (B) DivK controls the polar localization of CpdR. Differential interference contrast (DIC) and fluorescence images of CpdR-YFP or CpdRD51A-YFP in the presence or absence of DivK. Cultures from the indicated strains were grown in M2G media with vanillate (presence of DivK). After vanillate removal, culture samples were incubated in either the presence or absence of vanillate. After 8 h of DivK depletion, cells were induced with xylose (for cpdR-yfp or cpdRD51A-yfp expression) for 1 h. The proportion of cells showing at least one polar YFP fluorescence focus (white arrows) and number of cells observed are indicated.
If DivK stimulates CpdR's ability to localize and activate ClpXP, the absence of DivK should result in an increase in CpdR stability, a decrease in CpdR localization, and a decrease in the ability of CpdR to localize and thereby activate ClpXP, all of which are characteristic properties of the phosphorylated form of CpdR. To determine if DivK affects the accumulation of CpdR, we depleted DivK from the strain LS4401 and observed the levels of CpdR. After vanillate removal, the levels of CpdR increased in proportion to the time of DivK depletion (see Fig. 3A). This was also observed with CpdR-YFP (data not shown). Because CpdR stability depends on its phosphorylation state (see Fig. 2B), we used the strain expressing cpdRD51A instead of the WT cpdR to ask if the stability of CpdR protein after DivK depletion could be explained by the phosphorylation state of CpdR. If unphosphorylatable CpdRD51A accumulation increased during depletion of DivK, it would indicate that DivK controls the degradation of CpdR independently of its phosphorylation state. If not, it would suggest that DivK controls the degradation of CpdR by increasing the relative amount of unphosphorylated CpdR, which is less stable. Fig. 3A shows that the levels of CpdRD51A remained fairly constant upon depletion of DivK, suggesting that DivK increases the relative accumulation of unphosphorylated CpdR. To assess the relative stability of the two forms of CpdR, we compared the half-life of CpdR and CpdRD51A after 8 h of DivK depletion. As shown in Fig. 2B, CpdR was less stable in the presence of DivK (solid black line) than in its absence (dashed black line), demonstrating that DivK promotes the degradation of CpdR. However, the absence of DivK did not have a similar stabilizing effect on CpdRD51A (see Fig. 2B, dashed gray line) (see Note About the Half-Life of CpdRD51A in the Absence of DivK in SI Results), showing that in the absence of DivK, only the WT CpdR, which is able to be phosphorylated, was stabilized, and not the unphosphorylatable CpdRD51A. We therefore propose that DivK controls the degradation of CpdR by promoting the presence of the less-stable unphosphorylated form of CpdR.
DivK Regulates the Localization of CpdR.
The dynamic localization and function of CpdR at the stalked pole depends on its phosphorylation state: CpdR is able to localize at the cell pole while the CpdR∼P is not (9). To test if DivK affects the localization of CpdR, we determined the proportion of cells with a CpdR-YFP polar fluorescent focus in the presence and in the absence of DivK (Fig. 3B). We constructed strain LS4403 bearing in frame chromosomal deletions of cpdR and divK, a chromosomal copy of divK under the control of a vanillate-inducible promoter, and cpdR-yfp under the control of the xylose-inducible promoter on a high-copy number plasmid. CpdR-YFP fully complements a deletion of cpdR (9). In the presence of DivK, polar CpdR-YFP was found in 43% of the cells observed (see Fig. 3B). However, in the absence of DivK, only 3% of the cells had a CpdR-YFP focus, indicating that DivK regulates the localization of CpdR. To determine whether the decrease in polar CpdR-YFP foci in the absence of DivK is dependent on the phosphorylation state of CpdR rather than a failure of CpdR polar recruitment because of the physical absence of DivK, we repeated the experiment exchanging CpdR-YFP for CpdRD51A-YFP (see Fig. 3C), which is not regulated by phosphorylation (9). We observed that the number of cells having at least one polar CpdRD51A-YFP focus was the same in the presence (73%) or absence (76%) of DivK. Thus, we propose that DivK controls the localization of CpdR by promoting the accumulation of unphosphorylated CpdR.
DivK Regulates the Localization of Both ClpX and CtrA.
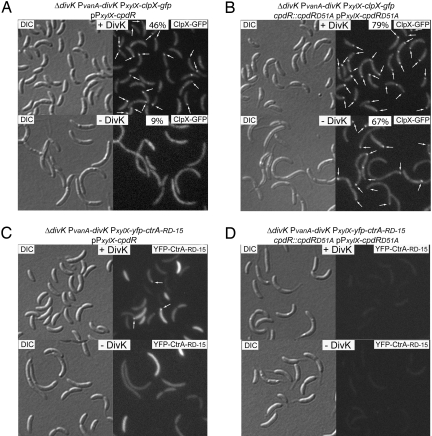
Because the phosphorylation state and localization of CpdR regulates the polar positioning and activity of the ClpXP protease (9), and because DivK appears to promote polar CpdR localization by increasing the level of unphosphorylated CpdR, we predict that ClpX would be delocalized in the absence of DivK. To test this, we used a DivK-depletion strain with a xylose-inducible chromosomal clpX-gfp and a high-copy number plasmid bearing PxylX-cpdR (strain LS4405), and observed that in the presence of DivK, polar ClpX-GFP foci were found in 46% of the cells (Fig. 4A). However, in the absence of DivK, the number of cells with a polar ClpX-GFP focus was reduced to 9%. To determine if this decrease in ClpX-GFP localization was also dependent on the phosphorylation state of CpdR, we repeated these experiments exchanging all copies of cpdR for cpdRD51A (Fig. 4B). The number of cells with at least one polar ClpX-GFP focus was 79% and 67% in the presence or absence of DivK, respectively. The increase in the number of ClpX-GFP foci observed in the presence of CpdRD51A versus in the presence of CpdR was reported previously (9), and is consistent with the role of unphosphorylated CpdR in recruiting ClpXP to the cell pole. Thus, we propose that DivK controls the polar localization of ClpX by modulating the phosphorylation state of CpdR.
Fig. 4.
DivK controls the polar localization of ClpX and CtrA. (A and B) DIC and fluorescence images of ClpX-GFP in the presence of CpdR or CpdRD51A, and in the presence or absence of DivK. Cultures from the indicated strains were grown in M2G medium with vanillate, to induce divK expression, and aliquots were incubated in either the presence or absence of vanillate. After 8 h of DivK depletion, cells were induced with xylose (for clpX-gfp and cpdR or cpdRD51A expression) for 2 h. Proportion of cells showing at least one polar ClpX-GFP fluorescence focus (white arrows) and number of cells observed are indicated. (C and D) DIC and fluorescence images of YFP-CtrA-RD-15 in the presence of CpdR or CpdRD51A, and in the presence or absence of DivK. After 8 h of DivK depletion, cells from the indicated strains were induced with xylose (for yfp-ctrA-RD-15 and cpdR or cpdRD51A expression) for 2 h. Polar YFP-CtrA-RD-15 fluorescence foci are indicated (white arrows).
The pathway for the polar localization of CpdR and ClpX has a crucial final objective: the clearance of the CtrA∼P master regulator, a direct inhibitor of the initiation of DNA replication (4). As stated above, the degradation of CtrA requires its polar localization (9–11). We have demonstrated here that CpdR and ClpX fail to localize in the absence of DivK (see Figs. 3B and 4A), and we reported previously that the polar localization and degradation of CtrA requires the polar placement of ClpXP (9). Therefore, we predict that in the absence of DivK, CtrA would fail to form polar foci and would remain dispersed in the cytoplasm. To test this possibility, we used the LS4407 DivK-depletion strain, with only one copy of divK under the control of the vanillate-inducible promoter, bearing the plasmid pPxylX-cpdR and the low-copy number plasmid pPxylX-yfp-ctrA-RD-15. The presence of xylose induces the accumulation of YFP-CtrA-RD-15 protein, which contains all of the elements necessary for its transient localization and degradation (11). In the presence of DivK, a portion of the cells exhibited polar YFP-CtrA-RD-15 foci, indicating that CtrA was being properly degraded (Fig. 4C). When the cells were depleted of DivK, no polar foci were detected in any of the cells observed. To determine if the effect of DivK on CtrA localization is dependent on the phosphorylation state of CpdR, we observed YFP-CtrA-RD-15 in strain LS4408, bearing only cpdRD51A instead of WT cpdR (Fig. 4D). Based on our observations that CpdRD51A and ClpX are more often localized in this strain, we would expect to see an increased removal of CtrA. Indeed, irrespectively of the presence or absence of DivK, we were unable to detect even cytoplasmic YFP-CtrA-RD-15 fluorescence, indicating that DivK promotes the degradation of CtrA in a CpdR phosphorylation state-dependent manner.
DivK Is Not Required for Viability upon Constitutive Presence of Unphosphorylated CpdR.
It has been proposed that DivK is not only involved in CtrA degradation but also in CtrA dephosphorylation, because cells are arrested in G1 phase in a cold-sensitive divK mutant (12, 13). We have shown here that in the absence of DivK, CpdR is stable, delocalized, and unable to localize ClpXP. The inability to localize the ClpXP protease and its CtrA substrate results in the failure to degrade CtrA. We have also shown that these phenotypes can be reversed if CpdR is modified so that it is independent of phosphorylation-dephosphorylation regulation. Therefore, a strain containing the unphosphorylatable CpdRD51A should bypass the cell lethality caused by the absence of DivK. To test this hypothesis, we used the strains LS4401 and LS4402, in which divK and cpdR or cpdRD51A are under the control of the vanillate- or xylose-inducible promoter, respectively (see Fig. 5 for strain descriptions). We determined the viability of these strains on PYE plates with or without vanillate or xylose. As a control, we showed that in the absence of DivK (no vanillate) and the absence of either CpdR or CpdRD51A (with glucose), the viability of both strains dropped more than a thousand fold compared with strains with a native copy of divK (see Fig. 5A Right). In the presence of DivK (with vanillate), both strains behaved equally, independent of whether CpdR or CpdRD51A is present (data not shown). The low viability in the absence of DivK (no vanillate) was rescued only in the presence CpdRD51A (with xylose), but not CpdR (see Fig. 5A Left). Thus, DivK is dispensable for viability if CpdR is kept in the unphosphorylated state, because the possibility of degrading CtrA is restored.
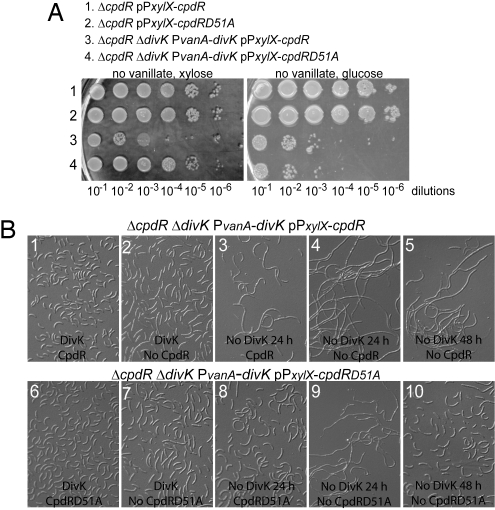
Fig. 5.
The presence of the unphosphorylatable CpdRD51A bypasses the requirement of DivK for cell survival. (A) Four indicated strains were grown in PYE media in the presence of vanillate (divK expression) and xylose (cpdR or cpdRD51A expression) to an O.D.660 of 0.6. The cultures were serially diluted, and 5 μl of each sample was spotted onto PYE plates containing no vanillate but xylose (Left), and no vanillate but glucose (Right). (B) Cells from the strains ΔcpdR ΔdivK PvanA-divK pPxylX-cpdR (i–v) and ΔcpdR ΔdivK PvanA-divK pPxylX-cpdRD51A (vi–x) were grown in M2G media in the presence of vanillate (presence of DivK) and xylose (presence of CpdR or CpdRD51A). Culture samples were washed and incubated in M2G media in the presence or absence of vanillate or xylose before microscopic observation. All cultures were kept in exponential phase growth for the entire experiment.
When DivK and either CpdR or CpdRD51A were present, the cell morphology was normal (see Fig. 5 Bi and Bvi). In presence of DivK, but in the absence of either CpdR or CpdRD51A, the cells were slightly bigger and straighter than wild-type, as described previously (see Fig. 5 Bii and Bvii) (9). In the absence of either CpdR or CpdRD51A and after 24 h of DivK depletion, cells became filamentous (see Fig. 5 Biv and Bix). The abnormal phenotype of cells lacking DivK was suppressed only by the presence of CpdRD51A, and not by CpdR (see Fig. 5 Biii and Bviii), even after 48 h of DivK depletion (see Fig. 5 Bv and Bx). These results confirm that the essential regulator DivK can be dispensable for viability if the CtrA degradation mechanism is made functional by replacing CpdR with the unphosphorylatable CpdRD51A. We have shown here that the function of CpdRD51A is independent of DivK, indicating that WT CpdR needs the action of DivK for its localization, its ability to localize and activate ClpXP, and its own degradation. Because CpdR localization, function, and stability are intrinsically dependent on its phosphorylation state, and the observation that DivK promotes CpdR localization, function, and degradation (see Figs. 2B, 3, and 4), we propose that DivK promotes the accumulation of unphosphorylated CpdR.
Discussion
The bacterial cell cycle is driven by irreversible transitions that are triggered by transient signals. The chemical irreversibility of proteolysis provides directionality at critical steps, regulating the timing of cell-cycle transitions. For example, the proteolysis of CtrA during the differentiation of Caulobacter swarmer cells into stalked cells, which coincides with the G1/S transition, allows the initiation of DNA replication (4, 5), and the degradation of a cyclin-cyclin-dependent kinase inhibitor in eukaryotic cells promotes the transition of G1-to-S phase (17). Here, we present evidence for a unique feedback mechanism that integrates transient polar localization and controlled proteolysis of regulatory proteins. This feedback loop controls the function of a key cell-cycle regulator, the CpdR single-domain response regulator. At the transition of a swarmer cell into a stalked cell, CpdR localizes to the stalked pole where it functions to position ClpXP at the cell pole, thereby enabling proteolysis of the CtrA master regulator (see Fig. 1B) (9). The temporally controlled clearance of CtrA via CpdR is central to cell-cycle progression. Once CpdR function is complete, a critical event is the loss of CpdR from the pole, thereby releasing the ClpXP protease and thus halting the degradation of CtrA. CpdR is cleared from the pole by the ClpXP protease that is localized and activated by CpdR.
CpdR polar localization, activation of ClpXP polar positioning and activity, and CpdR degradation are all determined by the CpdR phosphorylation state. The essential CckA histidine kinase transfers phosphate to both CtrA and CpdR through the ChpT phosphotransferase (see Fig. 1A) (12). CpdR is phosphorylated and inactive in the predivisional cell, and it remains phosphorylated in the progeny swarmer cell until the swarmer-to-stalked cell transition (9), when the dephosphorylated form accumulates at the cell pole to localize and activate the ClpXP protease (see Fig. 1B). Here, we demonstrate that once unphosphorylated CpdR localizes to the pole, it enables not only the degradation of CtrA, but also its own proteolysis. The osmotic stress response in Saccharomyces cerevisiae is regulated in a similar manner by the unphosphorylated Ssk1p response regulator, which is selectively degraded when in its unphosphorylated state (18).
The essential response regulator, DivK, has been shown to be required for the clearance of active CtrA from the cell, thus allowing the initiation of DNA replication (13). To explore the role of DivK in CtrA degradation, we have constructed a DivK-depletion strain and shown that the loss of DivK results in increased CpdR stability, decreased CpdR polar localization, and concomitant delocalization of the ClpXP protease, and thus the inability to degrade CtrA. DivK is dispensable for viability if CpdR is forced to be artificially kept in the unphosphorylated state, leading to an increase in CtrA degradation. In support of the argument that the essential role of DivK is to inactivate CtrA, CtrA bearing a point mutation that may partially affect DNA-protein binding and a CtrA mutation that exhibits defects in cell division, are both able to suppress lethality caused by the absence of DivK (19, 20). Although we demonstrate that DivK controls CpdR polar localization and degradation, the biochemical mechanism underlying these outcomes is unknown. By taking advantage of the unphosphorylatable form of CpdR, CpdRD51A, we have provided evidence that DivK contributes to CpdR function by promoting the accumulation of unphosphorylated CpdR. However, we were unable to directly demonstrate a differential increase in phosphorylated CpdR because we observed an increase in both the amount of total CpdR protein and CpdR∼P in the absence of DivK (Fig. S3).
All proteins necessary for CtrA degradation, such as DivK, RcdA, ClpP, ClpX, and CpdR, are present but not active in swarmer cells (8–10, 21) until CpdR is localized to the cell pole at the swarmer-to-stalked cell transition. We propose that it is the DivK-stimulated accumulation of unphosphorylated and polar CpdR that promotes the localization of the ClpXP-RcdA-CtrA complex needed for the degradation of CtrA at the swarmer-to-stalked cell transition (see Fig. 1). It has been proposed that DivK inhibits CckA activity in the stalked cells (12), thus blocking phosphate transfer from ChpT to CpdR, resulting in the accumulation of unphosphorylated CpdR (see Fig. 1A). At this time in the cell cycle, DivK, is in its phosphorylated state and is positioned at the cell pole (see Fig. 1B) (21). DivK could also contribute to an increase in the accumulation of unphosphorylated CpdR by promoting its dephosphorylation.
After the polar activation of ClpXP by CpdR, and upon CtrA clearance, CpdR is degraded in a ClpX-dependent manner, leading to the polar detachment of ClpXP. These processes provide a feedback loop for CpdR and ClpXP regulation that is dependent on both positional information and controlled proteolysis (see Fig. 1). After the initiation of DNA replication and the release of ClpXP from the cell pole, there is new synthesis of CtrA. At this time, the CckA histidine kinase in its phosphorylated state localizes to the cell pole (6, 22), leading to the phosphorylation of both CtrA and CpdR via the CckA-ChpT pathway (12) (see Fig. 1). This temporally regulated phosphorylation yields active CtrA∼P and prevents CpdR-mediated CtrA degradation, resulting in the activation of genes that encode proteins required for CtrA proteolysis, such as CpdR (Fig. S4), DivK, ClpP, and RcdA (see Fig. 1A) (3, 13). Thus, feedback loops that incorporate a spatial dimension function to coordinate and integrate the regulatory system that drives the cell cycle progression.
Materials and Methods
Bacterial Strains, Plasmids, and Growth Conditions.
Escherichia coli TOP10 (Invitrogen) were used for general cloning purposes. All Caulobacter strains used in this study were derived from the synchronizable WT CB15N. Unless stated otherwise, Caulobacter was grown at 28°C in PYE rich media, M2G minimal media, or M5G low-phosphate media supplemented with glutamate (1 mM) (21). Where necessary, media were supplemented with 0.3% xylose, 0.2% glucose, or 0.5-mM vanillate. When appropriate, media were supplemented with antibiotics at the following concentrations (liquid/solid media for Caulobacter; liquid/solid media for E. coli; in μg/ml): kanamycin (5/25; 30/50), gentamycin (0.6/5; 15/20), oxytetracycline (1/2; 12/12), chloramphenicol (1/1; 20/30). Cultures were maintained in log phase during all DivK-depletion experiments. Plasmids and strains used in this study, and their construction are described in Tables S1 and S2, respectively.
Microscopy.
All strains were analyzed in exponential growth phase. Cells were immobilized on 1% agarose-M2G. Microscopy was performed on a Nikon Eclipse E800 microscope equipped with a Nikon Plan Apo 100×/1.40 DIC objective and a 5-MHz MicroMAX cooled CCD camera (Princeton Instruments). Fluorescein (Chroma 96170M) and YFP (Chroma 41028) filter sets were used for GFP and YFP fluorescence, respectively. Exposure times were 50 ms for DIC images and 2 to 3 s for fluorescence images. Images were processed with Metamorph 4.5 (Universal Imaging Group) and Adobe Photoshop 6.0 (Adobe Systems).
Supplementary Material
Acknowledgments.
We thank H. McAdams, P. McGrath, and the members of the Shapiro laboratory for critical review of the manuscript. This work was supported by National Institutes of Health Grant GM32506 and Department of Energy Grants DE-FG03ER632219-A001 and DE-FG02ER632219.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0808807105/DCSupplemental.
References
- 1.Goley ED, Iniesta AA, Shapiro L. Cell cycle regulation in Caulobacter: Location, location, location. J Cell Sci. 2007;120:3501–3507. doi: 10.1242/jcs.005967. [DOI] [PubMed] [Google Scholar]
- 2.McAdams HH, Shapiro L. A bacterial cell-cycle regulatory network operating in time and space. Science. 2003;301:1874–1877. doi: 10.1126/science.1087694. [DOI] [PubMed] [Google Scholar]
- 3.Laub MT, Chen SL, Shapiro L, McAdams HH. Genes directly controlled by CtrA, a master regulator of the Caulobacter cell cycle. Proc Natl Acad Sci USA. 2002;99:4632–4637. doi: 10.1073/pnas.062065699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Quon KC, Yang B, Domian IJ, Shapiro L, Marczynski GT. Negative control of bacterial DNA replication by a cell cycle regulatory protein that binds at the chromosome origin. Proc Natl Acad Sci USA. 1998;95:120–125. doi: 10.1073/pnas.95.1.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Domian IJ, Quon KC, Shapiro L. Cell type specific phosphorylation and proteolysis of a transcriptional regulator controls the G1-to-S transition in a bacterial cell cycle. Cell. 1997;90:415–424. doi: 10.1016/s0092-8674(00)80502-4. [DOI] [PubMed] [Google Scholar]
- 6.Jacobs C, Ausmees N, Cordwell SJ, Shapiro L, Laub MT. Functions of the CckA histidine kinase in Caulobacter cell cycle control. Mol Microbiol. 2003;47:1279–1290. doi: 10.1046/j.1365-2958.2003.03379.x. [DOI] [PubMed] [Google Scholar]
- 7.Chien P, Perchuk BS, Laub MT, Sauer RT, Baker TA. Direct and adaptor-mediated substrate recognition by an essential AAA+ protease. Proc Natl Acad Sci USA. 2007;104:6590–6595. doi: 10.1073/pnas.0701776104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jenal U, Fuchs T. An essential protease involved in bacterial cell-cycle control. EMBO J. 1998;17:5658–5669. doi: 10.1093/emboj/17.19.5658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Iniesta AA, McGrath PT, Reisenauer A, McAdams HH, Shapiro L. A phospho-signaling pathway controls the localization and activity of a protease complex critical for bacterial cell cycle progression. Proc Natl Acad Sci USA. 2006;103:10935–10940. doi: 10.1073/pnas.0604554103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McGrath PT, Iniesta AA, Ryan KR, Shapiro L, McAdams HH. A dynamically localized protease complex and a polar specificity factor control a cell cycle master regulator. Cell. 2006;124:535–547. doi: 10.1016/j.cell.2005.12.033. [DOI] [PubMed] [Google Scholar]
- 11.Ryan KR, Huntwork S, Shapiro L. Recruitment of a cytoplasmic response regulator to the cell pole is linked to its cell cycle-regulated proteolysis. Proc Natl Acad Sci USA. 2004;101:7415–7420. doi: 10.1073/pnas.0402153101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Biondi EG, et al. Regulation of the bacterial cell cycle by an integrated genetic circuit. Nature. 2006;444:899–904. doi: 10.1038/nature05321. [DOI] [PubMed] [Google Scholar]
- 13.Hung DY, Shapiro L. A signal transduction protein cues proteolytic events critical to Caulobacter cell cycle progression. Proc Natl Acad Sci USA. 2002;99:13160–13165. doi: 10.1073/pnas.202495099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Potocka I, Thein M, ØSterås M, Jenal U, Alley MR. Degradation of a Caulobacter soluble cytoplasmic chemoreceptor is ClpX dependent. J Bacteriol. 2002;184:6635–6641. doi: 10.1128/JB.184.23.6635-6641.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Meisenzahl AC, Shapiro L, Jenal U. Isolation and characterization of a xylose-dependent promoter from Caulobacter crescentus. J Bacteriol. 1997;179:592–600. doi: 10.1128/jb.179.3.592-600.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thanbichler M, Iniesta AA, Shapiro L. A comprehensive set of plasmids for vanillate- and xylose-inducible gene expression in Caulobacter crescentus. Nucleic Acids Res. 2007;35:e137. doi: 10.1093/nar/gkm818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.King RW, Deshaies RJ, Peters JM, Kirschner MW. How proteolysis drives the cell cycle. Science. 1996;274:1652–1659. doi: 10.1126/science.274.5293.1652. [DOI] [PubMed] [Google Scholar]
- 18.Sato N, Kawahara H, Toh-e A, Maeda T. Phosphorelay-regulated degradation of the yeast Ssk1p response regulator by the ubiquitin-proteasome system. Mol Cell Biol. 2003;23:6662–6671. doi: 10.1128/MCB.23.18.6662-6671.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Paul R, et al. Allosteric regulation of histidine kinases by their cognate response regulator determines cell fate. Cell. 2008;133:452–461. doi: 10.1016/j.cell.2008.02.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wu J, Ohta N, Newton A. An essential, multicomponent signal transduction pathway required for cell cycle regulation in Caulobacter. Proc Natl Acad Sci USA. 1998;95:1443–1448. doi: 10.1073/pnas.95.4.1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jacobs C, Hung D, Shapiro L. Dynamic localization of a cytoplasmic signal transduction response regulator controls morphogenesis during the Caulobacter cell cycle. Proc Natl Acad Sci USA. 2001;98:4095–4100. doi: 10.1073/pnas.051609998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jacobs C, Domian IJ, Maddock JR, Shapiro L. Cell cycle-dependent polar localization of an essential bacterial histidine kinase that controls DNA replication and cell division. Cell. 1999;97:111–120. doi: 10.1016/s0092-8674(00)80719-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.