Abstract
Cell–cell interactions organize lens fiber cells into highly ordered structures to maintain transparency. However, signals regulating such interactions have not been well characterized. We report here that ephrin-A5, a ligand of the Eph receptor tyrosine kinases, plays a key role in lens fiber cell shape and cell–cell interactions. Lens fiber cells in mice lacking ephrin-A5 function appear rounded and irregular in cross-section, in contrast to their normal hexagonal appearance in WT lenses. Cataracts eventually develop in 87% of ephrin-A5 KO mice. We further demonstrate that ephrin-A5 interacts with the EphA2 receptor to regulate the adherens junction complex by enhancing recruitment of β-catenin to N-cadherin. These results indicate that the Eph receptors and their ligands are critical regulators of lens development and maintenance.
Keywords: β-catenin, Eph receptor, N-cadherin
Cataract, or the opacification of the lens, is the leading cause of visual impairment and blindness worldwide (1). The molecular events underlying lens development and the processes by which the lens maintains transparency over a lifetime are unclear (2). In addition, the cellular and biochemical mechanisms underlying the pathological changes leading to cataract remain poorly understood.
The lens is composed of a single layer of epithelial cells on the anterior surface, which, over a lifetime, divide and differentiate into the underlying lens fiber cells that comprise the bulk of the lens (3, 4). Initially during lens development, primary lens fiber cells differentiate and elongate from the posterior pole. In later embryogenesis and throughout life, secondary lens fiber cells differentiate from lens epithelial cells located at the equator. In cross section, the lens fiber cells resemble flattened hexagons with two broad and four short sides (3). These cells are organized in a highly ordered and closely packed manner, and interact through extensive intercellular adhesion complexes including gap and adherens junctions (5). Fiber cell gap junctions are composed of connexins (Cx) 46 and 50 (6), inactivation of which leads to the degeneration of the inner fiber cells and the development of cataract in mice (7, 8). Mutations in human Cx genes have also been associated with cataractogenesis (9, 10). As the lens is completely enclosed by an acellular, avascular capsule, it is believed that these cell–cell junctions are critical for providing nutrient transport, removal of metabolic wastes, and maintenance of homeostasis (11, 12). In addition to gap junctions, widespread adherens junctions containing N-cadherin and its associated protein β-catenin exist between lens fiber cells (13–16), and may play important roles in lens development and function.
Although cell–cell interaction is critical for maintaining lens transparency, little is known about the molecular mechanisms underlying these interactions. We have identified an unexpected regulator of lens fiber cell–cell interaction, the axon guidance molecule ephrin-A5 (17–19), and have shown that the loss of its function leads to alterations of cell shape and severe cataracts in the adult mouse. Our studies identify a novel function of ephrin-A5 in lens development and suggest unique regulation of downstream signaling mechanisms. We show here that a disruption in EphA2–ephrin-A5 interaction leads to the internalization of N-cadherin and a disruption in the binding of N-cadherin with β-catenin.
Results and Discussion
Ephrin-A5−/− Mice Develop Cataracts.
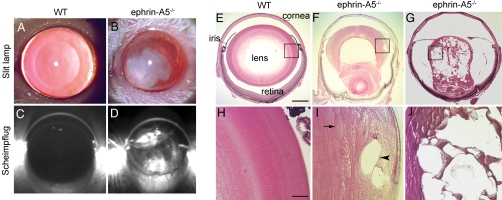
Examination of ephrin-A5−/− mutant mice using slit-lamp biomicroscopy and Scheimpflug imaging revealed large regions of opacification in the adult mutant lenses (Fig. 1 A–D). Such cataracts developed in 87% of mutant mice older than 6 months (n = 22), but not in any WT controls or heterozygous animals (n = 24). The overall size and morphology of the heterozygous lenses were indistinguishable from that of the WT lens. In the mutant lens, histological analysis revealed ruptures of the posterior lens capsule and lens disruptions with varying degrees of severity in the mutant mice (Fig. 1 F, G, I, and J). In the most severe cases, the lens completely degenerated, leaving tissue remnants impinging against the retina and sometimes the iris.
Fig. 1.
Development of cataracts in ephrin-A5−/− mice. (A and B) Slit-lamp images of adult WT (A) and ephrin-A5−/− (B) mouse lenses. (C and D) Scheimpflug images of adult WT (C) and ephrin-A5−/− (D) mouse lenses. (E–J) Sections (5 μm thick) of the WT (E and H) and mutant (F, G, I, and J) lens. (H, I, and J) Higher magnification images of the bow region of images shown in E, F, and G, respectively. Unusually large fiber cells (arrow) and vacuoles (arrowhead) were observed in mutant lenses. (Scale bar in E, 500 μm; in H, 100 μm.)
Loss of Cell Shape Control in Ephrin-A5−/− Lenses.
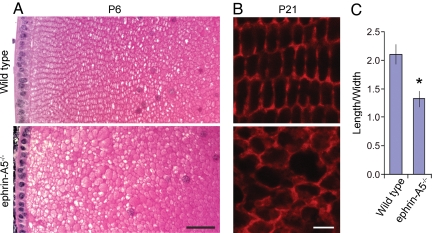
To examine the nature and timing of the initial defects, lenses from WT and ephrin-A5−/− mice were collected at various developmental stages (E14, E17, P0, P6, P21, P30, and P60), sectioned, and stained with H&E. The first clear morphological alterations occurred in a small proportion of mutant lenses at P6, in which tiny vacuoles appeared in the cortical region near the lens bow (not shown). Most noticeably, the lens fiber cells in the mutant animals did not display the columnar organization of the meridional rows, nor did they exhibit the closely packed hexagonal morphology typically found in WT lens fiber cells (Fig. 2 A and B). Fiber cell length-to-width ratio was reduced from 2.1:1 in the WT to a more cuboidal 1.3:1 in mutant lenses (Fig. 2C). Severe lens degeneration occurred at approximately 2 months of age. Changes in cell shape and organization in the mutant lens could be observed before the development of any gross morphological defects, suggesting that they are not caused by secondary disruptions.
Fig. 2.
Loss of cell shape control in ephrin-A5−/− lens. (A) Lens fiber cell morphology of P6 WT and ephrin-A5−/− lenses. The lens sections (5 μm thick) were stained with H&E. Notable differences in cell size, shape, and packing organization were observed in the mutant lens. (B) Lens fiber cell morphology of P21 WT and ephrin-A5−/− lenses. Cross-sections of WT and mutant lenses were prepared (10 μm thick) and stained with Alexa Fluor 546-phalloidin to delineate cell morphology. (C) Quantification of the length-to-width ratio in WT and ephrin-A5−/− lenses. *Significant difference at P < 0.05 (t test). (Scale bar in A, 40 μm; in B, 5 μm.)
To determine whether the normal complement of lens crystallins was present in mutant lenses and whether aberrant lens fiber cell differentiation might account for the morphological changes, sections of WT and ephrin-A5−/− lenses from adult mice were stained with antibodies (20, 21) against the differentiation markers α-crystallin, β-crystallin, γ-crystallin, and major intrinsic lens protein 26 (22–25). Mutant lenses were positive for all of these markers (data not shown), suggesting that the fiber cells had normal differentiation.
Abnormal Distribution of N-Cadherin in Ephrin-A5−/− Lenses.
To examine whether alterations in lens fiber cell shape were caused by changes in cell–cell interactions, P21 lenses were analyzed for changes in gap and adherens junctions. Gap junctions were detected using antibodies against either Cx46 or the Cx46 interacting protein ZO-1 (26). In P21 WT lenses, the majority of the gap junctions were located on the broad side of the fiber cell hexagonal profile as previously reported (15, 26). In contrast, Cx46 was randomly distributed in the mutant lens, possibly resulting from cell shape alterations [supporting information (SI) Fig. S1]. The distribution of ZO-1 is similarly disorganized but remains co-localized with Cx46 (Fig. 3 and Fig. S1), suggesting that the interaction between Cx46 and ZO-1 was not interrupted. Although ephrin-B ligands have been shown to inhibit gap junction functions (27, 28), whether they are affected in the ephrin-A5−/− lens is unclear.
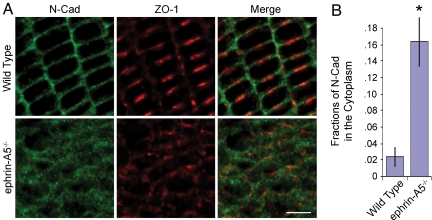
Fig. 3.
Change in N-cadherin localization in ephrin-A5−/− lens. (A) Altered patterns of expression of N-cadherin and the gap junction protein ZO-1 in ephrin-A5−/− lenses. P21 WT and ephrin-A5−/− lens cryosections were prepared (10 μm thick) and stained with anti–N-cadherin and anti–ZO-1 antibodies. (B) Fractions of N-cadherin signals detected in the cytoplasm in P21 WT and ephrin-A5−/− lenses. The fractions were obtained by dividing the fluorescent signals in the cytoplasm by the signals of the entire cell. Cell boundaries are defined by staining with Alexa Fluor 546-phalloidin. *Significant at P < 0.05 (t test). (Scale bar in A, 5 μm.)
In contrast, staining of P21 WT and mutant lenses with an anti–N-cadherin monoclonal antibody revealed striking differences in the distribution of the protein (Fig. 3). In the WT lens, N-cadherin is mostly localized to the short ends of the lens fiber cell hexagonal faces as previously reported (Fig. 3A) (15). In the mutant lens, N-cadherin became internalized from the cell membrane into the cytoplasm (Fig. 3A). Quantitative analysis of the change in relative signal intensity between the membrane and cytoplasm revealed an eightfold increase in the levels of intracellular N-cadherin (Fig. 3B), suggesting severe disruption of adherens junctions in the mutant lens. The antibody used to identify N-cadherin recognizes the extracellular domain (29), suggesting that the whole molecule, rather than the cytoplasmic domain only, was internalized. Previous studies showed that E-cadherin can also be internalized (30–32). Additionally, NMDA receptor activity increased N-cadherin turnover through endocytosis to modulate adhesion (33). Our observations here suggest that ephrin-A5 functions to promote N-cadherin membrane localization during lens development.
Decreased EphA2 Activation in Ephrin-A5−/− Lenses.
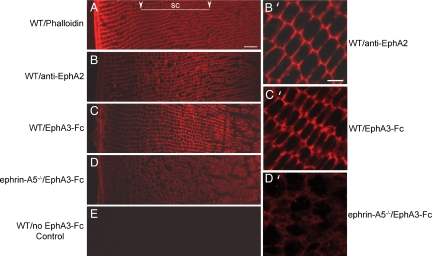
To identify which Eph receptors mediate ephrin-A5 function in lens development, we examined the expression of Eph receptors in WT lenses by PCR. Expression of EphA2, EphA3, EphA5, EphA7, EphA8, and all EphB receptors was detected (not shown). Examination of lenses from EphA3- (A. Brown, personal communication), EphA5-, and EphB1-null mice failed to detect any morphological defects. Therefore, we proceeded to examine the expression of EphA2 in the developing lens. To determine where EphA2 protein was expressed, we performed double immunofluorescence studies for subcellular localization of both EphA2 receptor and ephrin-A5 proteins in the P21 lens. EphA2 protein was detected with a goat anti-EphA2 antibody coupled with a Cy3-conjugated anti-goat secondary antibody. For analysis of ephrin-A5 expression, several commercially available anti–ephrin-A5 antibodies were first tested for the ligand detection in WT lenses, using ephrin-A5−/− lens sections as controls. None of the tested antibodies provided specific staining (data not shown). Thus, ephrin-A5 expression was analyzed with the EphA receptor body EphA3-Fc, which binds the ligand (34). As EphA3-Fc may bind to other ephrin-A ligands, ephrin-A5−/− lens sections were also stained in parallel to evaluate the contribution of ephrin-A5 to the staining signals. Both the anti-EphA2 antibody and EphA3-Fc detected strong signals in the subcortical regions of the lens (Fig. 4 B and C). The signals detected by EphA3-Fc were lost in the ephrin-A5−/− lens (Fig. 4D), indicating that signals detected by EphA3-Fc were primarily from ephrin-A5. Confocal imaging of the stained lens sections showed that both ephrin-A5 and EphA2 are expressed at the highest levels at the junction where neighboring cells make contact with each other, suggesting that the receptor–ligand pair interacts in vivo (Fig. 4 B′ and C′). Double immunostaining using anti-EphA2 antibody and EphA3-Fc showed that EphA2 and ephrin-A ligands co-localize (Fig. S2).
Fig. 4.
Both EphA2 and ephrin-A ligands are expressed at the cell junctions. (A) Phalloidin staining of WT lens shows lens fiber cell organization. (B and C) WT transverse sections of P21 lenses stained with anti-EphA2 and EphA3-Fc, respectively. Low-magnification images demonstrate that both EphA2 and A-ephrins are normally expressed at higher levels in the subcortical region. (D) EphA3-Fc staining on ephrin-A5−/− lens sections. Staining was mostly lost on mutant lenses indicating that the subcortical signals were a result of ephrin-A5 expression. (B′–D′) High-magnification confocal images of B–D. Note that WT EphA2 receptor (B′) and ephrin-A5 (C′) expression is the highest at the cell–cell junctions. (E) WT control without primary antibody. Images were collected with equal exposure times. Arrows in A denote the subcortical (sc) lens fiber region for A–E. (Scale bar in top left, 20 μm; top right, 5 μm.)
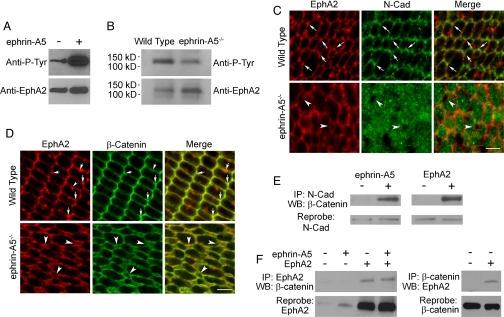
To determine whether ephrin-A5 may serve as a ligand for EphA2, we examined the ability of ephrin-A5 to activate EphA2. EphA2 cDNA was expressed in 293T cells, which normally do not express this receptor at high levels. The transfected cells were then stimulated with 2 μg/ml of ephrin-A5 and lysed, followed by EphA2 immunoprecipitation. The immunoprecipitates were then analyzed by Western blotting using an anti-phosphotyrosine antibody. This analysis showed that EphA2 was readily activated by ephrin-A5 in the transfected cells (Fig. 5A). To test whether loss of the ligand led to a reduction of EphA2 activation in the mutant lens, P6 lenses from ephrin-A5−/− and WT animals were collected, lysed, and immunoprecipitated for the EphA2 receptor. Western blot analysis for tyrosine phosphorylation revealed a remarkable downregulation in the levels of EphA2 tyrosine phosphorylation in the mutant lens (Fig. 5B), supporting the conclusion that ephrin-A5 is a physiological ligand of EphA2 in the lens.
Fig. 5.
Activation of EphA2 promotes recruitment of β-catenin to N-cadherin. (A) Ephrin-A5 activates the EphA2 receptor in 293T cells. (B) Reduction of EphA2 phosphorylation in ephrin-A5−/− P6 lenses. (C) EphA2 and N-cadherin co-localize on the P6 WT lens fiber cell membrane. Note the increased cytoplasmic N-cadherin staining in the mutant lens. Arrows indicate vertices where EphA2 and N-cadherin show strong co-localization. Arrowheads denote extracellular space between the fiber cells in the mutant lens, which are devoid of cytoplasmic N-cadherin staining. (D) Double immunofluorescence staining of P21 lens fiber cells with anti-EphA2 and anti–β-catenin antibodies. Arrows in the upper panels indicate where strong EphA2 and β-catenin co-localization was observed in the WT lenses. Arrowhead in the upper left panel indicates some of the EphA2 expression is not co-localized with beta-catenin. Arrowheads in the lower panels show spaces between the lens fiber cells. (E) Ephrin-A5 stimulation and EphA2 transfection promote the recruitment of β-catenin to N-cadherin. (F) EphA2 co-immunoprecipitates with β-catenin. (Scale bars in C and D, 5 μm.)
Regulation of N-Cadherin and β-Catenin Interaction by Ephrin-A5.
As N-cadherin distribution was altered in ephrin-A5−/− lens fiber cells, we determined the subcellular localization of EphA2 protein in relation to N-cadherin and β-catenin, which is associated with adherens junctions, in double immunofluorescence studies. These analyses revealed that EphA2 and N-cadherin were both localized primarily at the short ends of the lens fiber cell hexagons (Fig. 5C). The co-localization is especially prominent at the vertices of the hexagons (arrows in Fig. 5C). In ephrin-A5–null lenses, a considerable amount of N-cadherin was found in the cytoplasm (Fig. 5C). In addition, neighboring fiber cells appeared to pull away from each other, leaving small extracellular vacuoles between the cells, suggesting weakened cell–cell interaction (Fig. 5C).
Association with β-catenin is indicative of the adhesive function of N-cadherin (35, 36). We therefore examined whether EphA2 co-localized with β-catenin. Double immunofluorescence experiments showed that both EphA2 and β-catenin were localized on the short ends of the fiber cell hexagons as well, particularly at the vertices in the WT lens (Fig. 5D). In the mutant lens, β-catenin is still localized on the cell membranes (Fig. 5D), indicating that its membrane-proximal localization is not dependent on ephrin-A5 signaling. Consistent with this finding, we showed that EphA2 and β-catenin interact in 293T cells regardless of ephrin-A5 stimulation (Fig. 5F), indicating that EphA2 may serve as a membrane anchoring mechanism for β-catenin. In addition, small vacuoles were also frequently observed at these cell junctions using anti–β-catenin antibody staining, supporting a loss of cell–cell adhesion (Fig. 5D). To test the possibility that ephrin-A5 functions to increase interaction between β-catenin and N-cadherin, we analyzed whether ephrin-A5 promotes the association of β-catenin to N-cadherin. Stimulation of 293T cells with ephrin-A5 led to a strong increase in the amount of β-catenin bound to N-cadherin (Fig. 5E). Although 293T cells do not express EphA2 at high levels, they do express the EphA7 receptor (not shown). Thus, ephrin-A5 may stimulate β-catenin binding to N-cadherin through activation of endogenous EphA receptors. To examine whether EphA2 could also promote β-catenin–N-cadherin interaction, we transfected EphA2 into 293T cells and examined the effects of over-expression. Transfection of EphA2 alone led to a sharp increase in the amount of β-catenin binding to N-cadherin (Fig. 5E). Treatment of the transfected cells with ephrin-A5 did not lead to further increases in binding, possibly because of spontaneous activation of the transfected receptors when expressed at high levels (37). These observations indicate that loss of ephrin-A5 signals does not alter the membrane localization of β-catenin, but rather leads to the loss of N-cadherin binding to β-catenin and the recruitment to the membrane anchored adherens junction complex. Consistent with our studies, conditional loss of β-catenin in mice led to a disruption in lens cell adhesion and malformation of the lens (38–40). These observations together support a critical role for the β-catenin complex in lens cell–cell interactions.
Previous studies also demonstrated that activation of EphB receptors by ephrin-B1 or ephrin-B2 results in the activation of E-cadherin adhesion (41, 42). These studies are consistent with our observations here that the loss of ephrin-A5 leads to the disruption of N-cadherin–β-catenin interactions, resulting in a loss of cell–cell adhesion. Intriguingly, the regulation may work both ways. There is evidence that the integrity of E/VE-cadherin–based adhesion between cells aids in the aggregation of EphA2 receptors along the cell membrane, enhancing receptor tyrosine phosphorylation (43–45).
Lens fiber cells interact with each other to form a tightly packed, well organized stereotypical cytoarchitecture to achieve and maintain transparency. This investigation has identified a novel function of ephrin-A5 as a key regulator of lens cell organization and provided evidence that this function may be mediated through an interaction with the N-cadherin cell adhesion complex. Our report establishes that ephrin-A5 activates EphA2, leading to the increased recruitment of β-catenin to N-cadherin. Loss of ephrin-A5 results in the loss of N-cadherin from the cytoplasmic membrane and likely the loss of lens cell–cell adhesion evident in the eventual degeneration of the fiber cells and the subsequent formation of cataract. In addition to regulation of cadherin functions, ephrin-A5 may also affect the organization of gap junctions, as the gap junction complexes were also disorganized in the ephrin-A5−/− lens, although it is not known at present whether the effects are direct or indirect. Although other Eph receptors may still play a role in regulating lens development, our study provides strong evidence that the β-catenin–N-cadherin interaction is regulated by the ephrin-A5–EphA2 ligand–receptor pair for proper lens fiber cell adhesion.
Materials and Methods
Animal Care.
Mice were bred and maintained under standard conditions and treated in strict accordance to the Guidelines for the Care and Use of Laboratory Animals of Rutgers University.
Cataract Detection and Gross Morphology.
Mouse cataracts and gross lens morphology were assessed using slit-lamp examinations and H&E histologic analysis, as described in the SI text.
Immunohistochemistry.
Expression and cellular localization of various lens proteins were detected using colorimetric or fluorescence immunohistochemistry. Detailed methods are described in the SI text.
Western Blot Analysis and Co-Immunoprecipitation.
EphA2 activation was assessed using Western blot analysis with an anti-phosphotyrosine antibody and interactions among EphA2, β-catenin, and N-cadherin were assayed with co-immunoprecipitation experiments (additional details presented in the SI Text).
Supplementary Material
Acknowledgments.
We thank J. Frisen (Karolinska Institute, Stockholm, Sweden) for generously providing ephrin-A5−/− mice; S. Zigler (Johns Hopkins University School of Medicine, Baltimore, MD) for the generous gift of polyclonal antibodies against α-, β-, and γ-crystallin and major intrinsic lens protein; B. Wang (Case Western Reserve University School of Medicine, Cleveland, OH) for EphA2 expression construct, X. Gong, G. Smith, and S. Chen for their expert advice; and J. Zheng for his stimulating discussions on the project and indispensable help in imaging. This work was supported in part by National Science Foundation Grant 0548561 and National Institutes of Health Grant P01-HD23315 (to R.Z.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0808987105/DCSupplemental.
References
- 1.World Health Organization. WHO Fact Sheet. Vol. 282. Geneva: WHO; 2004. Magnitude and causes of visual impairment. [Google Scholar]
- 2.Asbell PA, et al. Age-related cataract. Lancet. 2005;365:599–609. doi: 10.1016/S0140-6736(05)17911-2. [DOI] [PubMed] [Google Scholar]
- 3.Kuszak JR, Costello MJ. In: Development of the Ocular Lens. Lovicu FJ, Robinson ML, editors. Cambridge: Cambridge Univ. Press; 2004. pp. 71–118. [Google Scholar]
- 4.Kleiman NJ, Worgul BV. Lens. In: Tasman W, Jaeger EA, editors. Duane's Foundations of Clinical Opthalmology. vol. 1. Philadelphia: JB Lippincott; 1994. pp. 1–39. [Google Scholar]
- 5.Goodenough DA. The crystalline lens: a system networked by gap junctional intercellular communication. Semin Cell Biol. 1992;3:49–58. doi: 10.1016/s1043-4682(10)80007-8. [DOI] [PubMed] [Google Scholar]
- 6.Kistler J, et al. Ocular lens gap junctions: protein expression, assembly, and structure-function analysis. Microsc Res Tech. 1995;31:347–356. doi: 10.1002/jemt.1070310504. [DOI] [PubMed] [Google Scholar]
- 7.Gong X, et al. Disruption of alpha3 connexin gene leads to proteolysis and cataractogenesis in mice. Cell. 1997;91:833–843. doi: 10.1016/s0092-8674(00)80471-7. [DOI] [PubMed] [Google Scholar]
- 8.White TW, Goodenough DA, Paul DL. Targeted ablation of connexin50 in mice results in microphthalmia and zonular pulverulent cataracts. J Cell Biol. 1998;143:815–825. doi: 10.1083/jcb.143.3.815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.White TW, Paul DL. Genetic diseases and gene knockouts reveal diverse connexin functions. Annu Rev Physiol. 1999;61:283–310. doi: 10.1146/annurev.physiol.61.1.283. [DOI] [PubMed] [Google Scholar]
- 10.Wei CJ, Xu X, Lo CW. Connexins and cell signaling in development and disease. Annu Rev Cell Dev Biol. 2004;20:811–838. doi: 10.1146/annurev.cellbio.19.111301.144309. [DOI] [PubMed] [Google Scholar]
- 11.Harding JJ, Crabbe JC. The lens. In: Davson H, editor. The Eye. vol. 1b. New York: Academic; 1984. pp. 207–492. [Google Scholar]
- 12.Donaldson P, Kistler J, Mathias RT. Molecular solutions to mammalian lens transparency. News Physiol Sci. 2001;16:118–123. doi: 10.1152/physiologyonline.2001.16.3.118. [DOI] [PubMed] [Google Scholar]
- 13.Bagchi M, Katar M, Lewis J, Maisel H. Associated proteins of lens adherens junction. J Cell Biochem. 2002;86:700–703. doi: 10.1002/jcb.10258. [DOI] [PubMed] [Google Scholar]
- 14.Lo WK, Shaw AP, Paulsen DF, Mills A. Spatiotemporal distribution of zonulae adherens and associated actin bundles in both epithelium and fiber cells during chicken lens development. Exp Eye Res. 2000;71:45–55. doi: 10.1006/exer.2000.0848. [DOI] [PubMed] [Google Scholar]
- 15.Straub BK, et al. A novel cell-cell junction system: the cortex adhaerens mosaic of lens fiber cells. J Cell Sci. 2003;116:4985–4995. doi: 10.1242/jcs.00815. [DOI] [PubMed] [Google Scholar]
- 16.Beebe DC, Vasiliev O, Guo J, Shui YB, Bassrett S. Changes in adhesion complexes define stages in the differentiation of lens fiber cells. Invest Ophthalmol Vis Sci. 2001;42:727–734. [PubMed] [Google Scholar]
- 17.Pasquale EB. Eph receptor signalling casts a wide net on cell behaviour. Nat Rev Mol Cell Biol. 2005;6:462–475. doi: 10.1038/nrm1662. [DOI] [PubMed] [Google Scholar]
- 18.Zhou R. The Eph family receptors and ligands. Pharmacol Ther. 1998;77:151–181. doi: 10.1016/s0163-7258(97)00112-5. [DOI] [PubMed] [Google Scholar]
- 19.Pfeiffenberger C, et al. Ephrin-As and neural activity are required for eye-specific patterning during retinogeniculate mapping. Nat Neurosci. 2005;8:1022–1027. doi: 10.1038/nn1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zigler JS, Jr, Horwitz J. Immunochemical studies on the major intrinsic polypeptides from human lens membrane. Invest Ophthalmol Vis Sci. 1981;21:46–51. [PubMed] [Google Scholar]
- 21.Reddy VN, Lin LR, Arita T, Zigler JS, Jr, Huang QL. Crystallins and their synthesis in human lens epithelial cells in tissue culture. Exp Eye Res. 1988;47:465–478. doi: 10.1016/0014-4835(88)90057-7. [DOI] [PubMed] [Google Scholar]
- 22.Chepelinsky AB. The ocular lens fiber membrane specific protein MIP/Aquaporin 0. J Exp Zoolog A Comp Exp Biol. 2003;300:41–46. doi: 10.1002/jez.a.10307. [DOI] [PubMed] [Google Scholar]
- 23.Andley UP. Crystallins in the eye: function and pathology. Prog Retin Eye Res. 2007;26:78–98. doi: 10.1016/j.preteyeres.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 24.Robinson ML, Overbeek PA. Differential expression of alpha A- and alpha B-crystallin during murine ocular development. Invest Ophthalmol Vis Sci. 1996;37:2276–2284. [PubMed] [Google Scholar]
- 25.Wang X, Garcia CM, Shui YB, Beebe DC. Expression and regulation of alpha-, beta-, and gamma-crystallins in mammalian lens epithelial cells. Invest Ophthalmol Vis Sci. 2004;45:3608–3619. doi: 10.1167/iovs.04-0423. [DOI] [PubMed] [Google Scholar]
- 26.Nielsen PA, et al. Lens connexins alpha3Cx46 and alpha8Cx50 interact with zonula occludens protein-1 (ZO-1) Mol Biol Cell. 2003;14:2470–2481. doi: 10.1091/mbc.E02-10-0637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mellitzer G, Xu Q, Wilkinson DG. Eph receptors and ephrins restrict cell intermingling and communication. Nature. 1999;400:77–81. doi: 10.1038/21907. [DOI] [PubMed] [Google Scholar]
- 28.Davy A, Bush JO, Soriano P. Inhibition of gap junction communication at ectopic Eph/ephrin boundaries underlies craniofrontonasal syndrome. PLoS Biol. 2006;4:e315. doi: 10.1371/journal.pbio.0040315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Matsunami H, Takeichi M. Fetal brain subdivisions defined by R- and E-cadherin expressions: evidence for the role of cadherin activity in region-specific, cell-cell adhesion. Dev Biol. 1995;172:466–478. doi: 10.1006/dbio.1995.8029. [DOI] [PubMed] [Google Scholar]
- 30.Miyashita Y, Ozawa M. Increased internalization of p120-uncoupled E-cadherin and a requirement for a dileucine motif in the cytoplasmic domain for endocytosis of the protein. J Biol Chem. 2007;282:11540–11548. doi: 10.1074/jbc.M608351200. [DOI] [PubMed] [Google Scholar]
- 31.Paterson AD, Parton RG, Ferguson C, Stow JL, Yap AS. Characterization of E-cadherin endocytosis in isolated MCF-7 and chinese hamster ovary cells: the initial fate of unbound E-cadherin. J Biol Chem. 2003;278:21050–21057. doi: 10.1074/jbc.M300082200. [DOI] [PubMed] [Google Scholar]
- 32.Kon S, Tanabe K, Watanabe T, Sabe H, Satake M. Clathrin dependent endocytosis of E-cadherin is regulated by the Arf6GAP isoform SMAP1. Exp Cell Res. 2008;314:1415–1428. doi: 10.1016/j.yexcr.2007.11.006. [DOI] [PubMed] [Google Scholar]
- 33.Arikkath J, Reichardt LF. Cadherins and catenins at synapses: roles in synaptogenesis and synaptic plasticity. Trends Neurosci. 2008 doi: 10.1016/j.tins.2008.07.001. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Marcus RC, Matthews GA, Gale NW, Yancopoulos GD, Mason CA. Axon guidance in the mouse optic chiasm: retinal neurite inhibition by ephrin “A”-expressing hypothalamic cells in vitro. Dev Biol. 2000;221:132–147. doi: 10.1006/dbio.2000.9660. [DOI] [PubMed] [Google Scholar]
- 35.Lilien J, Balsamo J. The regulation of cadherin-mediated adhesion by tyrosine phosphorylation/dephosphorylation of beta-catenin. Curr Opin Cell Biol. 2005;17:459–465. doi: 10.1016/j.ceb.2005.08.009. [DOI] [PubMed] [Google Scholar]
- 36.Yap AS, Brieher WM, Gumbiner BM. Molecular and functional analysis of cadherin-based adherens junctions. Annu Rev Cell Dev Biol. 1997;13:119–146. doi: 10.1146/annurev.cellbio.13.1.119. [DOI] [PubMed] [Google Scholar]
- 37.Lawrenson ID, et al. Ephrin-A5 induces rounding, blebbing and de-adhesion of EphA3-expressing 293T and melanoma cells by CrkII and Rho-mediated signalling. J Cell Sci. 2002;115:1059–1072. doi: 10.1242/jcs.115.5.1059. [DOI] [PubMed] [Google Scholar]
- 38.Cain S, et al. Differential requirement for beta-catenin in epithelial and fiber cells during lens development. Dev Biol. 2008 doi: 10.1016/j.ydbio.2008.07.002. in press. [DOI] [PubMed] [Google Scholar]
- 39.Kreslova J, et al. Abnormal lens morphogenesis and ectopic lens formation in the absence of beta-catenin function. Genesis. 2007;45:157–168. doi: 10.1002/dvg.20277. [DOI] [PubMed] [Google Scholar]
- 40.Smith AN, Miller LA, Song N, Taketo MM, Lang RA. The duality of beta-catenin function: a requirement in lens morphogenesis and signaling suppression of lens fate in periocular ectoderm. Dev Biol. 2005;285:477–489. doi: 10.1016/j.ydbio.2005.07.019. [DOI] [PubMed] [Google Scholar]
- 41.Noren NK, Foos G, Hauser CA, Pasquale EB. The EphB4 receptor suppresses breast cancer cell tumorigenicity through an Abl-Crk pathway. Nat Cell Biol. 2006;8:815–825. doi: 10.1038/ncb1438. [DOI] [PubMed] [Google Scholar]
- 42.Cortina C, et al. EphB-ephrin-B interactions suppress colorectal cancer progression by compartmentalizing tumor cells. Nat Genet. 2007;39:1376–1383. doi: 10.1038/ng.2007.11. [DOI] [PubMed] [Google Scholar]
- 43.Zantek ND, et al. E-cadherin regulates the function of the EphA2 receptor tyrosine kinase. Cell Growth Differ. 1999;10:629–638. [PubMed] [Google Scholar]
- 44.Hess AR, Margaryan NV, Seftor EA, Hendrix MJ. Deciphering the signaling events that promote melanoma tumor cell vasculogenic mimicry and their link to embryonic vasculogenesis: role of the Eph receptors. Dev Dyn. 2007;236:3283–3296. doi: 10.1002/dvdy.21190. [DOI] [PubMed] [Google Scholar]
- 45.Orsulic S, Kemler R. Expression of Eph receptors and ephrins is differentially regulated by E-cadherin. J Cell Sci. 2000;113:1793–1802. doi: 10.1242/jcs.113.10.1793. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.