Abstract
The ubiquitin ligase Siah2 has been shown to regulate prolyl hydroxylase 3 (PHD3) stability with concomitant effect on HIF-1α availability. Because HIF-1α is implicated in tumorigenesis and metastasis, we used SW1 mouse melanoma cells, which develop primary tumors with a propensity to metastasize, in a syngeneic mouse model to assess a possible role for Siah2 in these processes. Inhibiting Siah2 activity by expressing a peptide designed to outcompete association of Siah2-interacting proteins reduced metastasis through HIF-1α without affecting tumorigenesis. Conversely, inhibiting Siah2 activity by means of a dominant-negative Siah2 RING mutant primarily reduced tumorigenesis through the action of Sprouty 2, a negative regulator of Ras signaling. Consistent with our findings, reduced expression of PHD3 and Sprouty2 was observed in more advanced stages of melanoma tumors. Using complementary approaches, our data establish the role of Siah2 in tumorigenesis and metastasis by HIF-dependent and -independent mechanisms.
Keywords: HIF, hypoxia, Siah, Sprouty, prolyl hydroxylase
The RING finger ubiquitin ligase Siah2 mediates efficient ubiquitination and degradation of substrates that play an important role in diverse stress and cytokine-activated signaling pathways (1). Initially identified and studied in Drosophila, the Siah homologue sina was shown to require the adaptor protein phyllopod (PHYL) to mediate its activities (2). Somewhat similar to PHYL is Siah-interacting protein (SIP), which interacts with Siah within the same structural domain (3, 4). In mammals, two isoforms, Siah1 and Siah2 (5), have been shown to control the stability of several substrates, including nuclear corepressor, β-catenin, TRAF2, α-ketoglutarate dehydrogenase, and prolyl hydroxylase 3 (PHD3), thereby affecting diverse cellular functions, such as signaling, survival, and mitochondrial biogenesis (6–10). We have demonstrated that by regulating PHD3 stability, Siah2 contributes to the abundance of hypoxia-inducible factor (HIF)-1α, thereby playing an important role in the cellular response to hypoxia (9).
Two major isoforms of HIF-α, HIF-1α and HIF-2α, have been implicated in regulating hypoxia-responsive genes. Important in the control of HIF abundance is their hydroxylation on proline residue(s) by HIF-prolyl hydroxylases (also called PHD or EGLN), which is required for their association with and degradation by the pVHL-Elongin B, C ubiquitin ligase complex (11, 12). At low oxygen concentrations, PHDs, which require dioxygen molecules for function, exhibit between 50% and 10% of their hydroxylase activity toward their substrates (13–15). Along these lines, hydroxylated HIF-1α protein is present even under 0.5% oxygen in RCC cells with functional VHL (16), suggesting that PHDs can still hydroxylate substrates under hypoxic conditions, although with lowered efficiency. Consistent with these findings, active degradation of PHD1/3 by Siah2 increases HIF-1α stability under hypoxia (9). The tight regulation of PHD activity and stability under hypoxic conditions contributes to the availability of HIF-1α protein, enabling transactivation of its target genes, among which VEGF, c-met, CXCR4, and lysyl oxidase have been implicated in tumor growth and metastasis (17–20).
Given the impact of Siah on PHD3 stability and consequent HIF-1α levels, we asked whether it played a potential role in tumor development and metastasis.
Results
Inhibiting Siah2 Activity in a SW1 Melanoma Model Reduces Tumor Metastasis.
Two approaches were used to inhibit Siah2 activity: (i) blocking its association with scaffold proteins, which are required for degradation of some but not all substrates (21); and (ii) inhibition by means of a RING mutant, dominant-negative form of the protein. Some Siah substrates appear to require adaptors, others appear to bind directly (by virtue of the AXVXP motif), whereas some substrates are targeted independent of the AXVXP motif (e.g., Sprouty2).
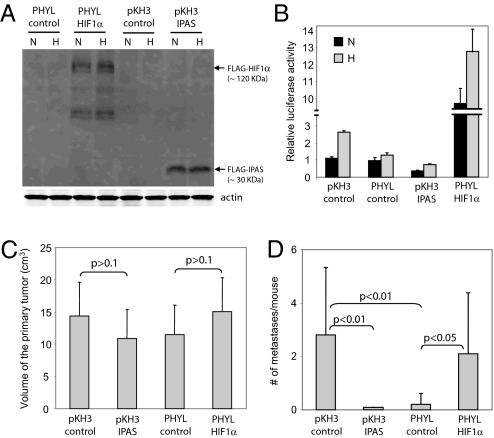
The interference strategy outcompeting the binding of adaptor proteins was achieved by overexpressing a PHYL peptide (22). Although derived from the Drosophila sequence, PHYL associates with Siah at the same structural domain as does SIP (4) but binds with much higher affinity (3) thus blocking Siah's ability to degrade substrates containing AXVXP motif or requiring adaptor protein (refs. 22 and 36). SW1 cells stably expressing PHYL peptide (SW1PHYL) exhibited reduced HIF-1α levels (Fig. 1A), but limited effect on HIF-2α levels (data not shown). Because Siah2 targets PHD3 degradation under hypoxia, PHD3 levels were reduced in these cells with functional Siah (a certain level of PHD3 protein seen under hypoxia is due to its HIF-mediated transcription). Accordingly, expression of PHD3 in SW1PHYL cells was not attenuated under hypoxia, compared with control SW1 cells expressing empty pKH3 vector (Fig. 1A). PHD2 levels, which were positively correlated with levels of HIF-1α, increased under hypoxia in pKH3 control cells but not in the SW1PHYL cells (Fig. 1A), possibly because PHD2 is a transcriptional target of HIF-1α (23). By contrast, levels of PHD1 (Fig. 1A) or other reported Siah substrates such as β-catenin and TRAF2 (data not shown) were unchanged in SW1PHYL cells, indicating tissue-selective and context-dependent targeting by Siah2. To further test whether reduced HIF-1α seen in SW1PHYL cells is dependent on PHD3, we inhibited PHD3 expression in SW1PHYL cells by transient transfection with PHD3 shRNA [supporting information (SI) Fig. S1]. PHD3 knockdown restored HIF-1α expression under hypoxia in the SW1PHYL cells. These data suggest that expression of the PHYL peptide attenuates the hypoxia response by inhibiting the Siah2/PHD3/HIF-1α axis.
Fig. 1.
Reduced tumor metastasis in PHYL-expressing SW1 melanoma tumors associated with reduced levels of VEGF expression, endothelial cell migration, and endothelial tube formation. (A) PHYL expression alters the PHD–HIF pathway in SW1 cells. Expression of HIF-1α and PHDs was detected by Western blotting in SW1 control (pKH3 empty vector) and PHYL-expressing cells. Cells were treated with normoxia (N) or hypoxia (H) (1% O2) for 5 h and total cell lysate was used for the analysis. (B) Tumor formation by SW1 cells. SW1 cells (pKH3 control and PHYL) were injected s.c. into the back of strain C3H mice, and tumor size was monitored weekly (n = 12 for each group). (C) Reduced lung metastasis in SW1PHYL tumors. The number of lung metastases was determined by H&E staining of 10 serial lung sections (100-μm interval between adjacent sections) derived from SW1 tumors (pKH3 control and PHYL) after 6 weeks of s.c. injection (n = 12 for each group). (D) Decreased HIF-1α expression, vasculogenesis, and cell proliferation as well as enhanced cell death in metastatic lesions of PHYL-expressing tumor cells. Immunohistochemistry of HIF-1α (i and ii), Glut-1 (iii and iv), CD31 (v and vi), PCNA (vii and viii), and TUNEL (ix and x) was performed on sections of metastatic lesions obtained from lungs of controls and PHYL-expressing tumor cells. (E) Decreased VEGF secretion from SW1PHYL cells under hypoxia. Control and PHYL-expressing cells were treated with normoxia or hypoxia (1% O2) for 24 h. VEGF levels in melanoma cell-conditioned media were measured by using a mouse VEGF ELISA kit. (F) Reduced endothelial cell migration toward conditioned media derived from 24-h, hypoxia-treated SW1PHYL cells. The number of migrated endothelial cells was determined in five random fields under a 10× objective. (G) Reduced ability of conditioned media derived from 24-h, hypoxia-treated SW1PHYL cells to induce endothelial tube formation. Tube formation was quantified by counting the number of endothelial tube junctions in 10 random fields under a 10× objective.
To assess the impact of these changes on tumor and metastasis development, we injected SW1PHYL or control SW1 cells into syngeneic C3H mice. We did not observe changes in primary tumor size (Fig. 1B) or weight (Fig. S2). Interestingly, however, analysis of lungs from injected mice revealed a marked reduction in the number of metastases in lungs of SW1PHYL compared with the controls (Fig. 1C and Table S1). These findings indicate that inhibiting Siah E3 ligase activity by PHYL, which competes for binding of adaptor protein(s), is sufficient to reduce metastasis without altering tumorigenesis.
Decreased Vasculogenesis, Reduced Cell Proliferation, and Increased Apoptosis in Metastatic Lesions of PHYL-Expressing SW1 Tumor Cells.
Immunohistochemistry of the few lung metastatic lesions from SW1PHYL tumors revealed markedly reduced levels of HIF-1α (Fig. 1D i and ii) and its target gene Glut-1 (Fig. 1D iii and iv). Consistent with the importance of HIF-1α in vasculogenesis, a decreased density of CD31-positive vessels was seen in metastatic lesions of SW1PHYL tumors (Fig. 1D v and vi, and Table S2). Furthermore, decreased PCNA (Fig. 1D vii and viii, and Table S2) and increased TUNEL (Fig. 1D ix and x, and Table S2) staining were observed in SW1PHYL metastatic lesions, indicating reduced proliferation and enhanced apoptosis, respectively. These findings support the role of Siah2 in regulating HIF-1α, which is reduced after expression of PHYL, thereby associating lower HIF-1α levels with decreased tumor metastasis. Although SW1PHYL primary tumors also displayed reduced levels of HIF-1α and Glut-1, no changes in CD31, PNCA, or TUNEL staining were detected (Fig. S3 and Table S2). These results suggest that other factors, such as tumor microenvironment, which differs between the primary and metastatic sites, are required to determine tumor cell ability to grow at the primary site or to metastasize.
Consistent with reduced HIF-1α expression and vasculogenesis, VEGF transcription was decreased in SW1PHYL cells under hypoxia, as revealed by semiquantitative RT-PCR (Fig. S4). Furthermore, reduced levels of VEGF protein were observed in conditioned medium collected from SW1PHYL cells subjected to hypoxia, compared with control SW1 cells (Fig. 1E). Importantly, conditioned medium obtained from SW1PHYL cells also exhibited reduced capacity to induce chemotactic migration (Fig. 1F, and Fig. S5) and tube formation (Fig. 1G and Fig. S6) of human lung microvessel endothelial cells. These differences were abolished upon addition of VEGF-neutralizing antibody to the conditioned media (Fig. 1 F and G), suggesting that the changes are VEGF-dependent and that reduced secretion of VEGF by SW1PHYL cells inhibits angiogenesis in vitro. Collectively, these findings suggest that PHYL-mediated Siah2 inhibition and concomitant reduction in HIF-1α levels attenuate vasculogenesis, resulting in accelerated apoptosis and lowered proliferation associated with reduced lung metastasis.
Effect of Siah2 on Metastasis of SW1 Melanoma Cells Is HIF-1α-Dependent.
We next performed two parallel experiments to determine whether reduced metastasis seen after Siah2 inhibition was mediated by HIF-1α. In the first, we expressed an inhibitor of all HIF transcription factors in SW1 cells and monitored changes in tumorigenicity and metastasis. Inhibitory Per/Arnt/Sim (IPAS) domain protein is a spliced form of HIF-3α that effectively inhibits HIF-mediated transcription (24, 25). SW1 cells stably expressing IPAS were generated (SW1IPAS; Fig. 2A), and inhibition of HIF-1α activity was confirmed by an HRE-luciferase assay (Fig. 2B). Because IPAS inhibits both HIF-1α and HIF-2α, we have used corresponding siRNA to inhibit each of the HIF proteins. Inhibition of HIF-1α, but not HIF-2α, caused effective inhibition of HRE-luciferase activity (data not shown), suggesting that HIF-1α mediates Hypoxia Response Element (HRE)-dependent transcription in these melanoma cells. Injection of SW1IPAS cells into C3H mice did not alter primary tumor growth (Fig. 2C and Fig. S7) but caused a complete inhibition of metastasis (Fig. 2D and Table S3), suggesting that HIF is important in metastasis but not tumorigenesis of this tumor type. Overall, inhibition of HIF activities by IPAS results in phenotypes resembling those observed upon inhibition of Siah2 by PHYL (Fig. 1 B and C).
Fig. 2.
Reexpression of HIF-1α restores and IPAS expression blocks melanoma metastasis. (A) Ectopic HIF-1α and IPAS expression in SW1PHYL cells and SW1 pKH3 cells, respectively. Cells were treated with normoxia (N) or hypoxia (H) (1% O2) for 5 h and total cell lysates were analyzed by Western blotting with anti-FLAG mAb. (B) HIF-1α or IPAS expression alters HIF-1α-dependent gene transcription in SW1PHYL or control cells. Cells were transfected with a HRE-luciferase construct and treated with normoxia or hypoxia before assaying luciferase activity. A β-gal construct was cotransfected to control transfection efficiency. Luciferase activity of SW1 pKH3 control cells under normoxia was normalized to 1. (C) HIF-1α or IPAS expression has a minimal effect on the size of primary tumors. SW1 transfectants were injected s.c. into the back of C3H mice and tumor size was measured after 6 weeks (n = 10 for each group). (D) HIF-1α or IPAS expression affects lung metastasis. The number of lung metastases was determined 6 weeks after s.c. injection of tumor cells (n = 10 for each group).
To confirm the role of HIF in mediating changes elicited by Siah2, we asked whether HIF-1α reexpression would rescue the meta-static potential of SW1PHYL cells or affect their tumorigenicity. When HIF-1α was overexpressed in SW1PHYL cells (SW1PHYL+HIF-1α) (Fig. 2A), we observed a marked increase in HRE-luciferase activity compared with SW1 control and SW1PHYL cells (Fig. 2B). Subcutaneous injection of SW1PHYL+HIF-1α cells into C3H mice did not alter primary tumor growth (Fig. 2C and Fig. S7) but restored high levels of lung metastasis, which were inhibited in SW1PHYL tumors (Fig. 2D and Table S3). These findings provide direct support for the role of HIF-1α in mediating Siah2's effect on metastasis of SW1 melanoma tumors.
Regulation of SW1 Tumor Growth Is Siah2-Dependent and HIF-1α-Independent.
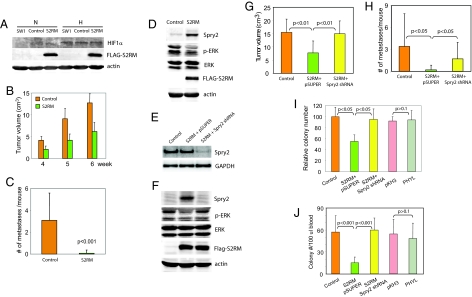
We next determined whether tumorigenesis and metastasis of melanoma cells would be affected upon inhibition of Siah2 ligase activity by a RING mutant (S2RM), a potent dominant-negative form of Siah2 (6). Stable expression of S2RM in SW1 cells (SW1S2RM) was confirmed in immunoblot analysis (Fig. 3A). Although on its own S2RM expression did not reduce HIF-1α levels (Fig. 3A) or increase PHD3 levels (Fig. S8), coexpression of S2RM with S1RM reduced HIF-1α levels in SW1 melanoma cells (data shown and discussed below). Noteworthy was the observation that injection of SW1S2RM cells into C3H mice inhibited both tumorigenesis (tumor size and weight; Fig. 3B and Fig. S9) and metastasis (Fig. 3C and Table S4). Because neither PHYL nor HIF-1α alters tumorigenesis in this mouse model and because S2RM alone does not alter levels of either PHD3 or HIF-1α, we hypothesized that inhibition of tumorigenesis by S2RM may be mediated by a HIF-independent pathway. Among Siah2 targets is Sprouty2 (Spry2) (26), an inhibitor of the Ras/Raf signaling pathway shown to play an important role in tumorigenesis (27). Analysis of SW1S2RM cells revealed a marked increase in Spry2 expression. Changes in Spry2 expression coincide with reduced levels of Ras signaling, as determined by monitoring p-ERK levels (Fig. 3D). These findings suggest that inhibiting Siah2 also up-regulates Spry2 expression with concomitant attenuation of Ras signaling. To confirm that altered Ras signaling is due to Siah2 effect on Spry2 we stably expressed Spry2 shRNA in SW1S2RM cells (Fig. 3 E and F). Spry2 knockdown was confirmed at both mRNA (Fig. 3E) and protein (Fig. 3F) levels. Inhibition of Spry2 expression by using shRNA for Spry2 attenuated S2RM-mediated inhibition of Ras signaling as reflected in restored p-ERK levels (Fig. 3F). Changes in levels of Spry2 expression and p-ERK were also seen in SW1S2RM tumors (Fig. S10).
Fig. 3.
Inhibition of Siah2 activity by a RING mutant affects tumorigenesis and metastasis by means of Spry2, independent of HIF. (A) Expression of Siah2 RING mutant (S2RM) has a negligible effect on HIF-1α levels under hypoxia. Expression of HIF-1α and S2RM was detected by Western blotting in SW1 parental, control (pcDNA empty vector), and FLAG-S2RM-expressing cells after normoxia (N) or hypoxia (H) (1% O2) treatment for 5 h. (B) S2RM expression reduces size of primary tumors. SW1 control (pcDNA) or S2RM-expressing cells were s.c. injected into C3H mice. The size of primary tumors was measured after 4, 5, and 6 weeks (n = 12 for each group). (C) S2RM expression reduces lung metastasis. Metastasis was determined 6 weeks after s.c. injection of SW1 control or S2RM-expressing cells (n = 12 for each group). (D) SW1 S2RM-expressing cells exhibit altered levels of Spry2 and p-ERK. Total cell lysates from SW1 control (pcDNA) or S2RM-expressing cells were analyzed by Western blotting for the indicated proteins. (E) Spry2 knockdown in SW1S2RM cells. SW1S2RM cells were stably transfected with pSUPER empty vector or Spry2 shRNA. Changes in Spry2 mRNA levels were determined by semiquantitative RT-PCR (see Materials and Methods). (F) Spry2 knockdown in SW1S2RM cells rescues ERK activation. SW1S2RM cells were stably transfected with pSUPER empty vector or Spry2 shRNA. Changes in Spry2 and p-ERK expression were analyzed by Western blotting and compared with SW1 pcDNA control. The labeling of lanes in F is the same as shown in E. (G) Inhibiting Spry2 expression in SW1S2RM cell restores primary tumor size. The size of primary tumors was measured 6 weeks after s.c. injection (n = 10 for each group). (H) Inhibiting Spry2 expression in SW1S2RM cells causes partial rescue of lung metastasis. Metastasis was examined 6 weeks after s.c. injection (n = 10 for each group). (I) Knockdown of Spry2 in SW1S2RM cells increases colony number in soft agar assays. The indicated SW1 transfectants were subjected to a soft agar assay, and the number of colonies was recorded after 4 weeks. Total number of colonies in 10 high-power fields (40× objective) of each triplicate plate was quantified. The number of colonies for the SW1 control was normalized to 100. (J) Quantification of colony number of tumor cells in blood. Blood was collected from C3H mice 6 weeks after s.c. injection of SW1 tumor cells expressing S2RM, PHYL, or S2RM + Spry2 shRNA. Blood was cultured in melanoma cell growth medium containing G418 for 1 week to allow tumor cells in blood to form colonies. Total colony numbers on the plates were determined and normalized to blood volume.
Significantly, inhibiting Spry2 expression in SW1S2RM cells completely restored tumorigenesis (Fig. 3G and Fig. S11) and partially restored the degree of tumor metastasis (Fig. 3H and Table S5). SW1S2RM tumors showed reduced cell proliferation as indicated by lower levels of PCNA staining, which were restored after expression of Spry2 shRNA (Fig. S12 a–c and Table S6). However, no difference was seen in apoptosis and vasculogenesis between SW1S2RM and control tumors as revealed by TUNEL (Fig. S12 d–f and Table S6) and CD31 staining (Fig. S12 g–i and Table S6), respectively. These data suggest that inhibition of Siah2 by S2RM reduces SW1 melanoma cell proliferation and tumorigenesis by Spry2-dependent control of Ras/ERK signaling. SW1S2RM cells exhibited efficient inhibition of tumorigenesis and metastasis, whereas SW1PHYL cells displayed only impaired metastasis. Therefore, we assessed the ability of PHYL and S2RM-expressing SW1 cultures to form colonies in soft agar. Both number and size of colonies exhibiting anchorage-independent growth were reduced in SW1S2RM cells, whereas inhibiting Spry2 expression by shRNA antagonized this effect (Fig. 3I and Fig. S13). Such inhibition was not seen in PHYL-expressing SW1 cells (Fig. 3I and Fig. S13). Consistent with these observations, intravasation of SW1 cells, monitored by the number of colonies formed from blood obtained from mice injected s.c. with the corresponding cell lines, was not affected in PHYL-expressing cells but was inhibited in SW1S2RM cells and rescued after expression of Spry2 shRNA (Fig. 3J and Fig. S14). These data indicate that regulation of Spry2 by Siah2 is important for anchorage-independent growth and intravasation, thereby affecting both tumorigenesis and metastatic capacity of SW1 melanoma cells.
Siah2 Regulation of HIF-1α and Spry2 Is Mediated by Two Distinct Mechanisms.
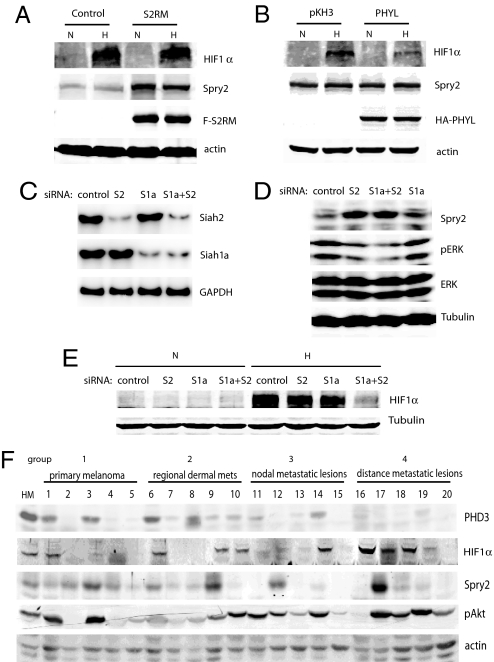
Because expression of the Siah2 RING mutant altered tumorigenesis and metastasis by means of Spry2 without affecting HIF-1α levels (Fig. 3), whereas expression of PHYL primarily affected HIF pathway (Fig. 1), we compared changes in HIF-1α and Spry2 levels in SW1 cultures expressing either PHYL or S2RM. Indeed, expression of S2RM markedly increased Spry2 expression and had little effect on HIF-1α expression (Fig. 4A), whereas PHYL expression did not affect Spry2 but reduced HIF-1α expression (Fig. 4B). These data support the conclusion that Siah2 uses distinct mechanisms to regulate Spry2 and PHD3/HIF-1α levels.
Fig. 4.
Differential effect of S2RM and PHYL on Spry2 and HIF-1α levels. (A and B) Total lysates from SW1 cells expressing S2RM (A) or PHYL (B) were analyzed by Western blotting for the indicated proteins. (C) Semiquantitative RT-PCR of Siah2 and Siah1a for SW1 melanoma cells transfected with siRNAs against Siah2 (S2), Siah1a (S1a), or both Siah1a and Siah2 (S1a+S2). (D) Siah2 knockdown elevates Spry2 levels. Melanoma siRNA transfectants described in C were analyzed by Western blotting of indicated proteins. (E) Double Siah2/Siah1a knockdown decreases HIF-1α levels under hypoxia. SW1 siRNA transfectants described in C were analyzed by Western blotting for HIF-1α. (F) Decreased PHD3 and Spry2 in metastatic melanoma samples from human patients. Total proteins were extracted from 20 human melanoma samples and analyzed by Western blotting of the indicated proteins. The 20 samples are divided into four categories each containing five samples. To assess statistical significance, we compared mean values of expression levels (linear densitometry data obtained by using the LiCOR system) in groups 1 + 2 with 3 + 4, and groups 1 + 2 + 3 with 4, by using a t test. Comparison of PHD3 and SPRY2 expression in groups 1 + 2 with 3 + 4 revealed that the lower expression is significant (P = 0.0065 for PHD3 and P = 0.025 for SPRY2 in one-tailed, two-sample, unequal variance t tests).
Although expression of S2RM alone had little effect on HIF-1α levels, coexpression of S2RM and S1aRM significantly inhibited HIF-1α induction under hypoxic conditions (Fig. S15). The latter effect resembles phenotypes seen after PHYL expression, which inhibits both Siah2 and Siah1 activity toward substrates requiring adaptor proteins (Fig. 4B). These results suggest a requirement for both Siah2 and Siah1a in regulating HIF-1α in melanoma cells. To further test this possibility, we used siRNAs to specifically silence Siah2, Siah1a, or both (Fig. 4C). Indeed, knockdown of Siah2, but not of Siah1a, was sufficient to up-regulate Spry2 and inhibit ERK activity (Fig. 4D). On the other hand, knockdown of Siah2 or Siah1a alone had little effect on HIF-1α levels, whereas double knockdown of both Siah2 and Siah1a significantly attenuated the HIF-1α induction under hypoxia (Fig. 4E), indicating cooperation between Siah2 and Siah1a in regulating HIF-1α levels.
Our results with Siah RING mutants and siRNAs demonstrate that although Siah2 alone is sufficient to regulate Spry2/ERK, both Siah2 and Siah1a are able to regulate PHD3/HIF-1α in melanoma cells. Because PHYL interacts with both Siah2 and Siah1a, expression of PHYL peptide affects levels of PHD3/HIF-1α (Fig. 1A) as expected. However, PHYL expression had no effect on Spry2 levels (Fig. 4B). This difference may be attributed to direct interaction of SPRY2 and Siah2, apparently independent of the binding pocket that the PHYL peptide targets (26).
Altered Expression of Spry2, HIF-1α, and PHD3 in Melanoma Tumor Samples.
The mouse SW1 melanoma model harbors the N-Ras mutation, which is found in 15% of human melanomas. To extend our observations in the SW1 melanoma model we analyzed the Siah2 regulatory axis in tumor samples from melanoma patients. Western blot analysis was performed on 20 samples, including primary melanomas, regional dermal metastatic lesions, nodal metastatic lesions, and distant metastatic lesions (Fig. 4F). This analysis revealed reduced expression of the Siah2 substrates PHD3 and Spry2 in more advanced metastatic melanoma samples, with a corresponding increase in HIF-1α (but not HIF2α; data not shown) expression levels. Of note, pAkt levels increased in more advanced melanoma tumor samples (Fig. 4F). Decreased expression of both Spry2 and PHD3 correlating with severity of disease was statistically significant (P = 0.0065 for PHD3 and P = 0.025 for Spry2 when comparing expression levels in groups 1 + 2 with groups 3 + 4; Fig. 4F).
Although Spry2 levels are lowered in the distant metastatic group compared with other groups, p-ERK levels did not exhibit correlation with any of the groups (data not shown). This might be because 50–70% of human melanomas show activating mutations in BRAF (28), and Spry2 may not be able to suppress that activity (29). Indeed, expression of S2RM in Lu1205 cells, a human melanoma line harboring the activating V599E BRAF mutant, increased Spry2 levels without affecting ERK activity (Fig. S16). This finding implies that Spry2 or its ligase Siah2 cannot affect ERK signaling in melanoma carrying BRAF mutations. Expression of Siah2 cannot be detected because of its extremely low expression level, attributed to its self-degradation. Overall, this analysis confirms that Siah2 substrates Spry2 and PHD3 are deregulated in the more advanced melanomas, consistent with the role of Siah2 in their regulation and the implication of such regulatory role in the corresponding SW1 melanoma model.
Discussion
The present study uses a melanoma tumor model to test the importance of Siah2 in tumorigenesis and metastasis. We show that Siah2 acts in both HIF-dependent and -independent pathways by affecting distinct substrates, through either direct or adaptor-mediated targeting. First, Siah2 affects the stability of PHD3 with a consequent increase in HIF-1α stability. For this function, Siah2 requires possible adaptor proteins whose identity remains to be determined. Second, Siah2 affects Spry2 stability, a function that does not require AXVXP motif or adaptor proteins. Whereas Spry2 primarily affects tumorigenesis, HIF-1α mainly affects metastasis in the SW1 melanoma model used here. Interestingly, HIF-2α was not found to be affected by Siah2, nor was it found to affect HRE-luciferase activity in the SW1 melanoma cells. Consistent with the data presented here for the SW1 melanoma cells, cross of Siah2 KO mice with TRAMP prostate tumor model or with polyoma middle T mammary tumor model resulted in a marked inhibition of tumorigenicity and metastasis (Qi et al., Moeller et al., unpublished observations).
The use of two Siah2 inhibitors, the PHYL peptide and Siah2 RING mutant, enabled us to identify two pathways controlled by Siah2 that contribute to tumorigenesis and metastasis. Both Siah2 and Siah1 promote degradation of PHD3 (9), whereas Siah2 but not Siah1 primarily targets Spry2 (Fig. 4 and ref. 26). Furthermore, Siah2 and Siah1 interact with PHD3 by means of adaptor proteins, whereas Siah2 interacts with Spry2 directly (26). Expression of the PHYL peptide competes with adaptor protein binding to both Siah2 and Siah1, thereby attenuating Siah's interaction with and degradation of PHD3. However, the PHYL peptide does not affect Spry2 levels because Spry2 does not contain the AXVXP motif found on other Siah2 substrates/adaptor proteins and because the Siah2/Spry2 interaction is mediated through a domain that is distant to the adaptor association region of Siah2 (3, 22, 26). Consistent with these conclusions are data obtained in in vitro protein binding experiments in which PHYL disrupts the Siah2/PHD3 interaction but has little effect on the Siah2/Spry2 interaction (data not shown).
Expression of S2RM is sufficient to inhibit Spry2 degradation, which is mainly promoted by Siah2. However, when Siah2 activity is inhibited by S2RM, Siah1 may still elicit degradation of PHD3, a substrate common to Siah2 and Siah1, and stabilize HIF-1α under hypoxia. This possibility is supported by our findings that coexpression of S2RM and S1aRM or siRNA knockdown of Siah2 and Siah1a efficiently inhibits HIF stabilization under hypoxia. Analysis of other Siah2 substrates, including TRAF2 and β-catenin, did not reveal changes in steady-state levels, indicating that cell type (melanoma versus fibroblasts) and conditions used for analysis (such as types of cellular stress) play key roles in Siah ligase substrate specificity.
Our studies point to a clear distinction between Siah2 substrates PHD3/HIF and Spry2/Ras, which function differently in tumorigenesis and metastasis. HIF-1α does not play a role in tumorigenesis in the melanoma model studied here or in some tumor models (30, 31), whereas it was shown to play a role in others (32–34). The finding that Siah2 plays a key role in metastasis of melanoma tumors (this study), prostate tumors (J.Q., K.N., R. Cardiff, D.B., Z.R., unpublished work) and breast tumors (36) is consistent with a recent report demonstrating that conditional deletion of HIF-1α in the mammary epithelium of a transgenic mouse model of metastatic breast cancer resulted in reduced pulmonary metastasis and had a limited impact on primary tumor formation (35). Changes in HIF-1α levels are expected to affect targets associated with metastatic capacity, as demonstrated for VEGF and Glut-1. Reduced metastasis also was seen after expression of S2RM, which primarily inhibits Spry2, possibly because of cross talk between Ras and VEGF signaling, which may be HIF-independent. Identification of Siah2's role in tumor development and metastasis offers insight into the importance of this ubiquitin ligase on key biological processes that are mediated by distinct substrates both dependent on and independent of cellular hypoxia responses.
Materials and Methods
SW1 Cell Culture and Injection into Syngeneic C3H Background Mice.
SW1 cells (a metastatic variant of K1735p melanoma cells) were cultured in DMEM with 10% FBS and antibiotics. SW1 cells that stably express PHYL peptide, HIF-1α, IPAS, Siah2 RING mutant, or Spry2 shRNA were generated by transfecting specified vectors followed by selection with G418 (2.5 mg/ml), puromycin (2 μg/ml), or blasticidin (30 μg/ml). Protein expression was verified by Western blot analysis. Polyclonal stable transfectants (1 × 106 cells per 100 μl of PBS) were injected s.c. into the back of C3H background mice. Tumor growth was monitored every week for up to 6 weeks, at which time animals were killed and analyzed.
Antibodies.
Antibodies to HIF-1α (monoclonal) and HIF-2α (polyclonal) (Novus), to VEGF (R&D), to PHD1 and PHD3 (Bethly Laboratories), to Glut-1 (Chemicon), to ERK, p-ERK, and PCNA (Cell Signaling), to CD31 (Santa Cruz Biotechnology), to PHD2 (BD Bioscience), and to FLAG, hemagglutinin, tubulin, and β-actin (Sigma) were used according to manufacturers' recommendations. HIF-1α rabbit polyclonal Ab was provided by Robert Abraham and Gary Chiang (Burnham Institute, La Jolla, CA). Spry2 rabbit polyclonal Ab was raised by immunizing rabbits.
siRNA Transfections.
siRNAs purchased from Ambion were as follows: Siah2 siRNA-1 (#150805), Siah2 siRNA-2 (#64554), Siah1a siRNA-1 (#64368), and Siah1a siRNA-2 (#64461). To effectively knockdown Siah2 or Siah1a, two respective specific siRNAs (50 nM each) were pooled for transfection of 3 × 106 cells in a 6-well plate by using Lipofectamine RNAiMAX (Invitrogen) according to the manufacturer's protocol. Cells were analyzed 48 h after transfection. Additional methods can be found in SI Methods.
Supplementary Material
Acknowledgments.
We thank members of Ronai Laboratory for helpful discussions. We also thank Drs. Lorenz Poellinger (Karolinska Institute, Stockholm Sweden), Robert Abraham, Gary Chiang, Andreas Moeller, and Collin House (Peter McCallum Cancer Center, Melbourne, Australia) for expression vectors and antibodies. This work was supported by National Cancer Institute Grant CA111515 (to Z.R.). J.Q. is supported by a Canadian Institutes of Health Research postdoctoral fellowship.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. D.E.F. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/cgi/content/full/0804063105/DCSupplemental.
References
- 1.Reed JC, Ely KR. Degrading liaisons: Siah structure revealed. Nat Struct Biol. 2002;9:8–10. doi: 10.1038/nsb0102-8. [DOI] [PubMed] [Google Scholar]
- 2.Li S, Xu C, Carthew RW. Phyllopod acts as an adaptor protein to link the sina ubiquitin ligase to the substrate protein tramtrack. Mol Cell Biol. 2002;22:6854–6865. doi: 10.1128/MCB.22.19.6854-6865.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.House CM, et al. Elucidation of the substrate binding site of Siah ubiquitin ligase. Structure. 2006;14:695–701. doi: 10.1016/j.str.2005.12.013. [DOI] [PubMed] [Google Scholar]
- 4.Santelli E, et al. Structural analysis of Siah1-Siah-interacting protein interactions and insights into the assembly of an E3 ligase multiprotein complex. J Biol Chem. 2005;280:34278–34287. doi: 10.1074/jbc.M506707200. [DOI] [PubMed] [Google Scholar]
- 5.Della NG, Senior PV, Bowtell DD. Isolation and characterisation of murine homologues of the Drosophila seven in absentia gene (sina) Development. 1993;117:1333–1343. doi: 10.1242/dev.117.4.1333. [DOI] [PubMed] [Google Scholar]
- 6.Habelhah H, et al. Stress-induced decrease in TRAF2 stability is mediated by Siah2. EMBO J. 2002;21:5756–5765. doi: 10.1093/emboj/cdf576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Habelhah H, et al. Regulation of 2-oxoglutarate (alpha-ketoglutarate) dehydrogenase stability by the RING finger ubiquitin ligase Siah. J Biol Chem. 2004;279:53782–53788. doi: 10.1074/jbc.M410315200. [DOI] [PubMed] [Google Scholar]
- 8.Matsuzawa SI, Reed JC. Siah-1, SIP, and Ebi collaborate in a novel pathway for beta-catenin degradation linked to p53 responses. Mol Cell. 2001;7:915–926. doi: 10.1016/s1097-2765(01)00242-8. [DOI] [PubMed] [Google Scholar]
- 9.Nakayama K, et al. Siah2 regulates stability of prolyl-hydroxylases, controls HIF1alpha abundance, and modulates physiological responses to hypoxia. Cell. 2004;117:941–952. doi: 10.1016/j.cell.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 10.Zhang J, Guenther MG, Carthew RW, Lazar MA. Proteasomal regulation of nuclear receptor corepressor-mediated repression. Genes Dev. 1998;12:1775–1780. doi: 10.1101/gad.12.12.1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ivan M, et al. HIFalpha targeted for VHL-mediated destruction by proline hydroxylation: implications for O2 sensing. Science. 2001;292:464–468. doi: 10.1126/science.1059817. [DOI] [PubMed] [Google Scholar]
- 12.Jaakkola P, et al. Targeting of HIF-alpha to the von Hippel-Lindau ubiquitylation complex by O2-regulated prolyl hydroxylation. Science. 2001;292:468–472. doi: 10.1126/science.1059796. [DOI] [PubMed] [Google Scholar]
- 13.Stiehl DP, et al. Increased prolyl 4-hydroxylase domain proteins compensate for decreased oxygen levels. Evidence for an autoregulatory oxygen-sensing system. J Biol Chem. 2006;281:23482–23491. doi: 10.1074/jbc.M601719200. [DOI] [PubMed] [Google Scholar]
- 14.Tuckerman JR, et al. Determination and comparison of specific activity of the HIF-prolyl hydroxylases. FEBS Lett. 2004;576:145–150. doi: 10.1016/j.febslet.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 15.Wirthner R, et al. Determination and modulation of prolyl-4-hydroxylase domain oxygen sensor activity. Methods Enzymol. 2007;435:43–60. doi: 10.1016/S0076-6879(07)35003-9. [DOI] [PubMed] [Google Scholar]
- 16.Chan DA, Sutphin PD, Yen SE, Giaccia AJ. Coordinate regulation of the oxygen-dependent degradation domains of hypoxia-inducible factor 1 alpha. Mol Cell Biol. 2005;25:6415–6426. doi: 10.1128/MCB.25.15.6415-6426.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Erler JT, et al. Lysyl oxidase is essential for hypoxia-induced metastasis. Nature. 2006;440:1222–1226. doi: 10.1038/nature04695. [DOI] [PubMed] [Google Scholar]
- 18.Pennacchietti S, et al. Hypoxia promotes invasive growth by transcriptional activation of the met protooncogene. Cancer Cell. 2003;3:347–361. doi: 10.1016/s1535-6108(03)00085-0. [DOI] [PubMed] [Google Scholar]
- 19.Semenza GL. Targeting HIF-1 for cancer therapy. Nat Rev Cancer. 2003;3:721–732. doi: 10.1038/nrc1187. [DOI] [PubMed] [Google Scholar]
- 20.Staller P, et al. Chemokine receptor CXCR4 downregulated by von Hippel-Lindau tumour suppressor pVHL. Nature. 2003;425:307–311. doi: 10.1038/nature01874. [DOI] [PubMed] [Google Scholar]
- 21.Cooper SE, Murawsky CM, Lowe N, Travers AA. Two modes of degradation of the tramtrack transcription factors by Siah homologues. J Biol Chem. 2008;283:1076–1083. doi: 10.1074/jbc.M707765200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.House CM, et al. A binding motif for Siah ubiquitin ligase. Proc Natl Acad Sci USA. 2003;100:3101–3106. doi: 10.1073/pnas.0534783100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Berchner-Pfannschmidt U, Yamac H, Trinidad B, Fandrey J. Nitric oxide modulates oxygen sensing by hypoxia-inducible factor 1-dependent induction of prolyl hydroxylase 2. J Biol Chem. 2007;282:1788–1796. doi: 10.1074/jbc.M607065200. [DOI] [PubMed] [Google Scholar]
- 24.Makino Y, et al. Inhibitory PAS domain protein is a negative regulator of hypoxia-inducible gene expression. Nature. 2001;414:550–554. doi: 10.1038/35107085. [DOI] [PubMed] [Google Scholar]
- 25.Makino Y, Kanopka A, Wilson WJ, Tanaka H, Poellinger L. Inhibitory PAS domain protein (IPAS) is a hypoxia-inducible splicing variant of the hypoxia-inducible factor-3alpha locus. J Biol Chem. 2002;277:32405–32408. doi: 10.1074/jbc.C200328200. [DOI] [PubMed] [Google Scholar]
- 26.Nadeau RJ, Toher JL, Yang X, Kovalenko D, Friesel R. Regulation of Sprouty2 stability by mammalian Seven-in-Absentia homolog 2. J Cell Biochem. 2007;100:151–160. doi: 10.1002/jcb.21040. [DOI] [PubMed] [Google Scholar]
- 27.Shaw AT, et al. Sprouty-2 regulates oncogenic K-ras in lung development and tumorigenesis. Genes Dev. 2007;21:694–707. doi: 10.1101/gad.1526207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gray-Schopfer V, Wellbrock C, Marais R. Melanoma biology and new targeted therapy. Nature. 2007;445:851–857. doi: 10.1038/nature05661. [DOI] [PubMed] [Google Scholar]
- 29.Tsavachidou D, et al. SPRY2 is an inhibitor of the ras/extracellular signal-regulated kinase pathway in melanocytes and melanoma cells with wild-type BRAF but not with the V599E mutant. Cancer Res. 2004;64:5556–5559. doi: 10.1158/0008-5472.CAN-04-1669. [DOI] [PubMed] [Google Scholar]
- 30.Mack FA, et al. Loss of pVHL is sufficient to cause HIF dysregulation in primary cells but does not promote tumor growth. Cancer Cell. 2003;3:75–88. doi: 10.1016/s1535-6108(02)00240-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Maranchie JK, et al. The contribution of VHL substrate binding and HIF1-alpha to the phenotype of VHL loss in renal cell carcinoma. Cancer Cell. 2002;1:247–255. doi: 10.1016/s1535-6108(02)00044-2. [DOI] [PubMed] [Google Scholar]
- 32.Gao P, et al. HIF-dependent antitumorigenic effect of antioxidants in vivo. Cancer Cell. 2007;12:230–238. doi: 10.1016/j.ccr.2007.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gordan JD, Bertout JA, Hu CJ, Diehl JA, Simon MC. HIF-2alpha promotes hypoxic cell proliferation by enhancing c-myc transcriptional activity. Cancer Cell. 2007;11:335–347. doi: 10.1016/j.ccr.2007.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kondo K, Klco J, Nakamura E, Lechpammer M, Kaelin WG., Jr Inhibition of HIF is necessary for tumor suppression by the von Hippel-Lindau protein. Cancer Cell. 2002;1:237–246. doi: 10.1016/s1535-6108(02)00043-0. [DOI] [PubMed] [Google Scholar]
- 35.Liao D, Corle C, Seagroves TN, Johnson RS. Hypoxia-inducible factor-1alpha is a key regulator of metastasis in a transgenic model of cancer initiation and progression. Cancer Res. 2007;67:563–572. doi: 10.1158/0008-5472.CAN-06-2701. [DOI] [PubMed] [Google Scholar]
- 36.Moeller A, et al. Inhibition of Siah ubiquitin ligase function. Oncogene. 2008 doi: 10.1038/onc.2008.382. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.