Abstract
A decrease in the abundance and biodiversity of intestinal bacteria within the dominant phylum Firmicutes has been observed repeatedly in Crohn disease (CD) patients. In this study, we determined the composition of the mucosa-associated microbiota of CD patients at the time of surgical resection and 6 months later using FISH analysis. We found that a reduction of a major member of Firmicutes, Faecalibacterium prausnitzii, is associated with a higher risk of postoperative recurrence of ileal CD. A lower proportion of F. prausnitzii on resected ileal Crohn mucosa also was associated with endoscopic recurrence at 6 months. To evaluate the immunomodulatory properties of F. prausnitzii we analyzed the anti-inflammatory effects of F. prausnitzii in both in vitro (cellular models) and in vivo [2,4,6-trinitrobenzenesulphonic acid (TNBS)-induced] colitis in mice. In Caco-2 cells transfected with a reporter gene for NF-κB activity, F. prausnitzii had no effect on IL-1β-induced NF-κB activity, whereas the supernatant abolished it. In vitro peripheral blood mononuclear cell stimulation by F. prausnitzii led to significantly lower IL-12 and IFN-γ production levels and higher secretion of IL-10. Oral administration of either live F. prausnitzii or its supernatant markedly reduced the severity of TNBS colitis and tended to correct the dysbiosis associated with TNBS colitis, as demonstrated by real-time quantitative PCR (qPCR) analysis. F. prausnitzii exhibits anti-inflammatory effects on cellular and TNBS colitis models, partly due to secreted metabolites able to block NF-κB activation and IL-8 production. These results suggest that counterbalancing dysbiosis using F. prausnitzii as a probiotic is a promising strategy in CD treatment.
Keywords: IBD, microbiota, probiotic
Inflammatory bowel disease (IBD) is a group of disorders characterized by a chronic and relapsing inflammation of the gastrointestinal tract frequent in Western countries (1). The two most common forms of IBD are Crohn disease (CD) and ulcerative colitis. The pathogenesis involves an inappropriate and ongoing activation of the mucosal immune system driven by the presence of the intestinal microbiota in a genetically predisposed patient. In CD in particular, the intestinal microbiota is strongly suspected to play a role in initiating and triggering the immune system, leading to characteristic inflammation (2). Diversion of the fecal stream prevents postoperative recurrence of ileal CD (3). Two antagonistic strategies were proposed to define the role of microorganisms in IBD: “the candidate microorganism strategy” (4) and the “global description strategy” (5–7). We previously described the global specificities of the fecal microbiota in CD (6, 8). Using a metagenomic approach, we revealed a global decrease in the biodiversity of the fecal microbiota in CD (8). This was essentially due to a markedly reduced diversity of Firmicutes, and in particular of the Clostridium leptum phylogenetic group. Moreover, analysis by FISH combined with flow cytometry showed a significant quantitative reduction in the C. leptum group in CD patients compared with healthy subjects (6, 8). Using different molecular methods, other studies reported that Faecalibacterium prausnitzii was particularly depleted in IBD patients' ileocolonic mucosa-associated microbiota (MAM) (9, 10).
In this study, we first analyzed the composition of the ileal MAM of CD patients at the time of surgical resection for active disease and 6 months later by FISH. We observed that the proportion of Firmicutes, and in particular of F. prausnitzii, which is the major bacterium of the C. leptum group, was low in patients that exhibited endoscopic recurrence 6 months after surgery. We hypothesized that counterbalancing dysbiosis using this deficient commensal bacterium as a probiotic in CD could be beneficial. Then, we evaluated the potential role of F. prausnitzii on intestinal inflammation using cellular and animal models.
Results
A Lower Proportion of F. prausnitzii on Resected Ileal Crohn Mucosa Is Associated with Endoscopic Recurrence.
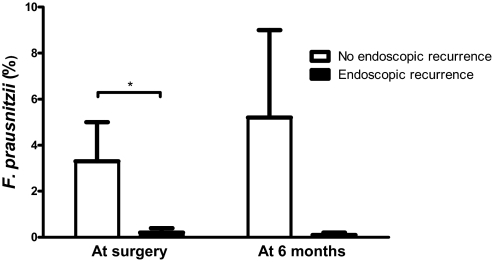
Previously, we demonstrated that administration of Lactobacillus johnsonii LA1 is ineffective for prophylaxis of postoperative endoscopic recurrence in CD (11). We did not observe any significant difference in the composition of the MAM between L. johnsonii LA1 and placebo groups at the time of surgery or 6 months after in surgical and biopsy samples from 21 human volunteers (data not shown). Focusing on endoscopic recurrence at 6 months (Rutgeerts score ≥2 for 13 patients, LA1 n = 6 and placebo n = 7), we observed: (i) a significantly lower proportion of F. prausnitzii at the time of surgery, consistently associated with endoscopic relapse, and (ii) a lower proportion of Firmicutes (i.e., C. coccoides and F. prausnitzii) 6 months after surgery in CD patients with endoscopic recurrence when compared with CD patients who were still in remission [Fig. 1, supporting information (SI) Table S1, and SI Materials and Methods]. Based on these observations, we hypothesized that remission might be associated with the presence of F. prausnitzii, and probably with its anti-inflammatory effects.
Fig. 1.
F. prausnitzii proportions in the ileal MAM using FISH at the time of surgery and at 6 months according to the endoscopic recurrence status. *, Significant difference, P = 0.03.
F. prausnitzii Exerts Anti-inflammatory Effects in Peripheral Blood Mononuclear Cells (PBMCs).
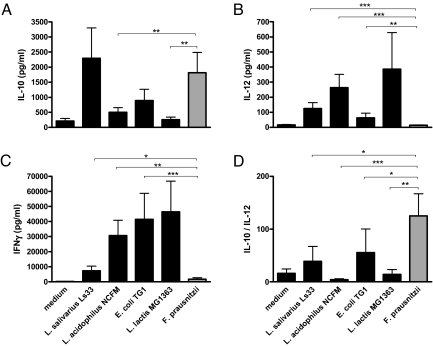
Because the cytokine profile released by PBMCs has been previously shown to correlate the in vitro and in vivo immunomodulation potential of different bacteria (29), we analyzed the capacities of F. prausnitzii to induce cytokine production in PBMCs (SI Materials and Methods). In addition, the immunostimulation by four other strains having well-documented in vitro and in vivo anti-inflammatory properties (Lactobacillus salivarius Ls33) (12), or lacking any anti-inflammatory activities (L. acidophilus NCFM, Lactococcus lactis MG1363, and Escherichia coli TG1) were also tested (Fig. 2). Interestingly, F. prausnitzii and L. salivarius Ls33 were the weakest inducers of proinflammatory/Th1 cytokines (e.g., IL-12 and IFN-γ) and the highest inducers of anti-inflammatory IL-10 compared with the other tested strains. The ratio of IL-10 to IL-12, frequently used to distinguish between strains exhibiting a proinflammatory (low ratio) vs. an anti-inflammatory (high ratio) profile, reveals that F. prausnitzii exhibit the highest anti-inflammatory profile (Fig. 2D).
Fig. 2.
IL-10 (A), IL-12 (B), and IFN-γ (C) response of human PBMCs of six distinct individual donors to stimulation with RPMI medium and five bacterial strains. The values are expressed as the mean ± SEM in pg/ml for cytokines and in percentages for IL-10/IL-12 ratio (D). Different asterisks (*) indicate significant differences (*, P < 0.05; **, P < 0.01; ***, P < 0.003).
F. prausnitzii Supernatant Reduces IL-8 Secretion by Caco-2 Cells.
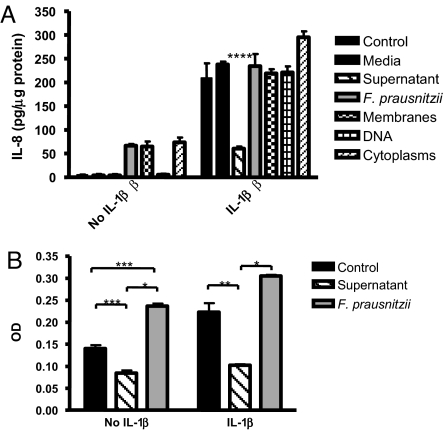
We examined the effects of UV-killed F. prausnitzii, bacterial fractions, supernatants, or sterile medium on IL-8 secretion in Caco-2 cells, with or without IL-1β stimulation (SI Materials and Methods and Fig. 3A). Either UV-killed F. prausnitzii, membrane extracts, or cytoplasmic extracts, but not DNA or sterile medium, induce low levels of IL-8 secretion. UV-killed F. prausnitzii, bacterial fractions, and sterile medium did not inhibit IL-8 secretion induced by IL-1β, compared with negative control. In contrast, supernatant from F. prausnitzii cultures significantly reduces IL-8 secretion induced by IL-1β.
Fig. 3.
F. prausnitzii supernatant exerts antiinflammatory effects on Caco-2 cells. (A) Modulation of IL-1β-induced IL-8 secretion by Caco-2 cells in contact with F. prausnitzii, its component, or its supernatant. Cells were stimulated or not with IL-1β at 15 ng/ml. IL-8 secretion is expressed as picograms per micgrogram of proteins. The values are expressed as the mean ± SEM in pg/μg of proteins. (B) Effects of F. prausnitzii on Caco2 cells stably transfected with an NF-κB SEAP reporter gene with or without stimulation with IL-1β. SEAP activity is expressed as optical density (OD). Different asterisks (*) indicate significant differences (*, P < 0.05; **, P < 0.01; ***, P < 0.003; ****, P < 0.001).
F. prausnitzii Supernatant Abolishes NF-κB Activation in Caco-2 Reporter Cell Lines.
To further explore the immunomodulatory properties of F. prausnitzii, we tested the effects of this bacterium and its supernatant on NF-κB activation using a Caco-2 reporter cell line (SI Materials and Methods). In Caco-2 cells, F. prausnitzii stimulates an NF-κB-dependent secreted alkaline-phosphatase (SEAP) production (Fig. 3B), whereas the supernatant decreases the basal NF-κB activity. After stimulation with IL-1β, NF-κB activity increases significantly. No significant effect on IL-1β stimulation was observed with UV-killed bacteria, whereas the F. prausnitzii supernatant strongly inhibits NF-κB activation by IL-1β in Caco-2 cells.
Because F. prausnitzii produces high amounts of butyrate, which has well known anti-inflammatory effects (13), we evaluated the effect of butyrate in Caco-2 reporter cell lines at the concentration present in the F. prausnitzii supernatant (i.e., 40 mM). Butyrate did not reproduce the supernatant inhibitory effect after IL-1β stimulation, but in contrast strongly increased NF-κB activation (data not shown).
F. prausnitzii Supernatant Did Not Display In Vitro Antibacterial Effect.
We investigated antibacterial properties of F. prausnitzii supernatant. Using two different in vitro techniques (critical dilutions of Mayr-Harting et al., ref. 14; and bacteriocin activity assay, ref. 15), we did not reveal any antibacterial effect on several anaerobic and aerobic bacterial species (see description in Materials and Methods).
Severity of TNBS-Induced Colitis in Mice Is Reduced by F. prausnitzii and Its Supernatant.
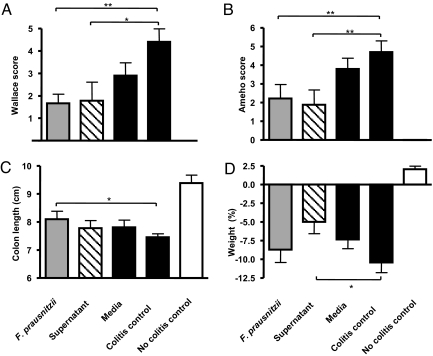
We further explored the relevance of these observations in vivo by testing the ability of F. prausnitzii, its DNA and membrane fractions, and its supernatant to prevent acute colitis induced by TNBS in BALB/c mice (SI Materials and Methods and Fig. 4). A severe colitis was observed in the colitis control group. No improvement in colitis severity was observed with either UV-killed F. prausnitzii, its DNA, or its membrane (data not shown). In contrast, daily intragastric administration of living F. prausnitzii (5 × 109 cfu) or its supernatant resulted in a marked attenuation of colitis with reduced weight loss, partial colon length normalization, and improvement of Wallace and Ameho scores. No significant protective effect was observed in mice receiving sterile culture medium.
Fig. 4.
Effects of intragastric administration of F. prausnitzii and its supernatant on TNBS-induced colitis in BALB/c mice considering (A) Wallace macroscopic score, (B) Ameho histologic score, (C) colon length, and (D) weight. Each group included 9 or 10 mice. The values are expressed as the mean ± SEM. Different asterisks (*) indicate significant differences (*, P < 0.05; **, P < 0.01).
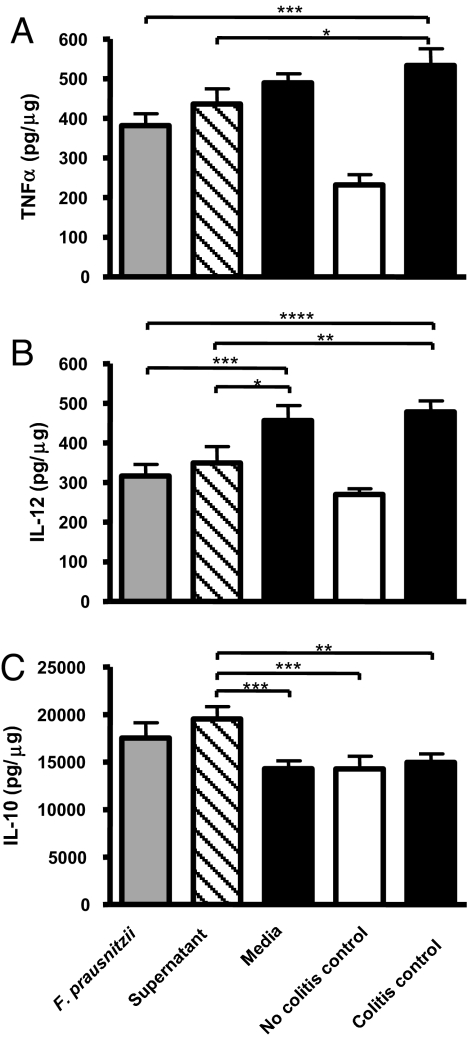
F. prausnitzii and Its Supernatant Induce an Increased Secretion of IL-10 and a Decreased Secretion of TNF-α and IL-12 in TNBS-Induced Colitis in Mice.
Forty-eight hours after TNBS instillation, colonic proinflammatory TNF-α and IL-12 cytokines and anti-inflammatory IL-10 were quantified by ELISA (SI Materials and Methods and Fig. 5). Compared with the no-colitis control group, TNF-α and IL-12 levels were increased, whereas IL-10 levels were barely modified. In the two TNBS groups treated with either F. prausnitzii or its supernatant, the secretion of TNF-α and IL-12 was significantly lower than in the colitis control group. Interestingly, IL-10 secretion was induced in the colon of mice treated with F. prausnitzii or its supernatant.
Fig. 5.
Quantification using ELISA of TNF-α (A), IL-12 (B), and IL-10 (C) in colons obtained 48 h after TNBS colitis induction. The values are expressed in pg/μg of total proteins as the mean ± SEM. Different asterisks (*) indicate significant differences (*, P < 0.05; **, P < 0.01; ***, P < 0.003; ****, P < 0.001).
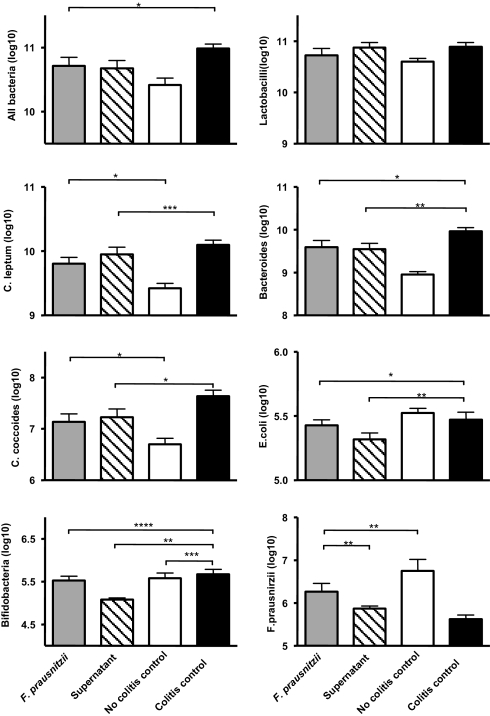
F. prausnitzii and Its Supernatant Tend to Counterbalance the TNBS-Induced Dysbiosis in Colitic Mice.
To validate our initial hypothesis, we determined the composition of the fecal microbiota of mice from the colitis (TNBS-treated) and no-colitis control, the F. prausnitzii-treated, and the supernatant-treated groups by real-time qPCR (SI Materials and Methods, Table S2, and Fig. 6). A higher concentration of total bacteria, bacteria from the C. leptum group, the Bacteroides group, and bacteria from the C. coccoides group was measured in the fecal microbiota of the colitis control mice compared with the no-colitis control mice. No difference was observed concerning the Lactobacillus group and E. coli species. F. prausnitzii was the only species diminished in colitis control mice. Treatment with either F. prausnitzii or its supernatant tended to counterbalance the dysbiosis observed in colitis control mice for all bacteria: C. leptum, C. coccoides, Bacteroides, and F. prausnitzii (Fig. 6). Strikingly, F. prausnitzii occurrence was nearly normalized in the F. prausnitzii-treated group.
Fig. 6.
Quantification using real-time qPCR of dominant and subdominant bacteria in the fecal microbiota of mice 48 h after TNBS induction of colitis. Noncolitis control group received PBS intragastrically and 0.9% NaCl/ethanol (50:50 vol/vol) intrarectally. The values are expressed as the mean of Log10 bacteria per gram of stool ± SEM. Different asterisks (*) indicate significant differences (*, P < 0.05; **, P < 0.01; ***, P < 0.003; ****, P < 0.001).
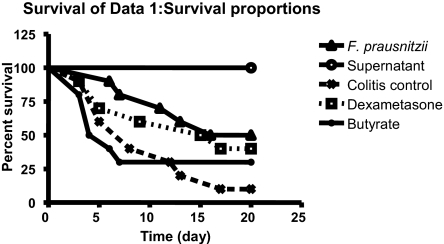
F. prausnitzii and Its Supernatant Can Act by a Gut-Independent Route.
To investigate whether F. prausnitzii or its supernatant could be active by a gut-independent pathway, we tested i.p. injection in a TNBS-induced colitis model. The survival rates 20 days after colitis induction were 100% and 10% in the no-colitis and colitis control groups, respectively (Fig. 7). In a third control group treated with dexamethasone, the survival rate was 40% at 20 days. In the groups treated with live F. prausnitzii or the supernatant, the mortality rate was dramatically decreased. The group treated by live F. prausnitzii had a significantly better survival curve than the colitis control group (P = 0.039), with a survival rate of 50% at 20 days, close to the rate observed in the dexamethasone group. The mortality rate in the group treated with the culture supernatant was reduced to 0 (versus positive control, P < 10−4; vs. dexamethasone group, P = 0.004). Interestingly, this strong anti-inflammatory effect was not due to butyrate presence, as the group of mice treated with butyrate (at the same concentration as supernatant) had a mortality rate similar to that in the colitis control group.
Fig. 7.
Protective effects of i.p. administration of F. prausnitzii and its supernatant on mice after TNBS challenge. Each group included 10 mice. Statistical analysis was performed using the log rank test.
Discussion
In this study, we showed that a low proportion of F. prausnitzii on resected ileal Crohn mucosa is associated with endoscopic recurrence at 6 months. Moreover, this bacterium, deficient in CD patients' microbiota, has anti-inflammatory effects in vitro as well as in vivo. On Caco-2 cells, secreted metabolites blocked NF-κB activation and IL-8 secretion. Moreover, on PBMCs, F. prausnitzii induced very low levels of IL-12 and IFN-γ, whereas high levels of IL-10 were obtained compared with other bacterial strains tested. Considering the IL10/IL12 ratio measured on PBMCs, F. prausnitzii displays the most anti-inflammatory properties. These in vitro effects were confirmed in vivo on TNBS-induced colitis.
It is now well established that microbial components of the resident microbiota can regulate gut inflammation (16). Moreover, some bacterial strains, like Bacteroides thetaiotaomicron, have been particularly studied and their participation in the gut immune homeostasis elucidated (17). In parallel, the specificities of the gut microbiota composition in IBD patients have been assessed using molecular methods. Nevertheless, this recent knowledge has not yet been used to find new anti-inflammatory bacteria within the normal gut microbiota, to date.
An original and rational process led us to select F. prausnitzii as a candidate anti-inflammatory bacterium. Comparing any gut microbiota of healthy subjects and of CD patients, we observed that the C. leptum group within the dominant Firmicutes phylum was defective in CD patients' microbiota (6, 8). This deficiency was quantitative and qualitative, as the biodiversity of the C. leptum was restricted. F. prausnitzii is one of the major members of the C. leptum group (18, 19) and one of the most prevalent bacteria within the human gut. In the present study, we showed that a low proportion of F. prausnitzii in the ileal MAM of CD patients at the time of a surgical resection for active disease was a risk factor for endoscopic recurrence 6 months later. Taken together, all these results suggested that F. prausnitzii could be crucial to the gut homeostasis and could have anti-inflammatory properties.
F. prausnitzii and its components were then tested on Caco-2 cells stimulated with IL-1β. After stimulation by IL-1β, IL-8 secretion was not modified by F. prausnitzii and reduced by its supernatant. To better understand the mechanisms involved in this effect, we tested the bacterium components on Caco-2 cells stably expressing a reporter gene for NF-κB. We observed that F. prausnitzii increased the IL-1β-induced NF-κB activity, whereas the supernatant abolished it. It is noticeable, that F. prausnitzii DNA did not exhibit any inhibitor effect on either IL-8 secretion or NF-κB activation. Moreover, we showed that this blocking effect on NF-κB activation was not due to butyrate.
Besides, it is questionable whether the anti-inflammatory effect of the supernatant is shared by an F. prausnitzii-secreted metabolite(s) or due to an F. prausnitzii-induced change in product(s) of the culture medium. The fact that living F. prausnitzii given without supernatant exerts an anti-inflammatory effect in the in vivo experiments favors the hypothesis of secreted metabolite(s)-induced effect. Identification of the active molecule(s) involved in this protective effect is needed to specify this point.
Our results favor a secreted metabolite, which could be involved in inflammatory pathway control. Several targets can be evoked from the lumen to immune cells in the lamina propria, and we first speculated on an antimicrobial peptide (bacteriocin) able to modify the gut barrier. However, our in vitro tests did not reveal any antibacterial effect on several anaerobic and aerobic bacteria species. Antibacterial mode of action also could be mediated by stimulation of defensin secretion. In fact, it has been shown that a defect in HD-5 and HD-6 α-defensin secretion by Paneth cells (specialized epithelial cells from ileum) plays a crucial role in ileal CD and could be linked to NOD2 frameshift mutation (20, 21). The clinical part of our study focused on ileal CD patients requiring surgical resection. Thus, the dysbiosis in F. prausnitzii appears attractive regarding the α-defensin deficiency concept. As some probiotics have been shown to induce defensin secretion (22), a step further will be to investigate the ability of F. prausnitzii to induce such a secretion. Beside a gut barrier effect, our results suggest that a diffusible molecule (modulin) could block NF-κB activation. More precisely, our data showed that F. prausnitzii supernatant acts as a regulator of the NF-κB induction after stimulation by IL-1β. Nevertheless, the mechanism is probably more complex, because NF-κB activation has been shown to play a crucial role in gut homeostasis (23). Indeed, F. prausnitzii itself without its supernatant exerts NF-κB induction effect on Caco-2 cells (Fig. 3B). However, our data did not support that this mechanism is Toll-like receptor-dependent, because in vivo and in vitro experiments testing different bacteria components (DNA, membranes, cytosol) did not show any anti-inflammatory activity. Finally, F. prausnitzii could drive the immune responses toward Th1 pathway inhibition.
To evaluate the potential immunomodulatory capacity of F. prausnitzii, we measured the cytokine response of human PBMCs stimulated by the bacterium. F. prausnitzii had an anti-inflammatory profile because it induced very low secretion levels of IL-12 and IFN-γ and high secretion levels of IL-10. Moreover, F. prausnitzii has a higher IL10/IL12 ratio than the probiotic L. salivarius Ls33 strain known to have anti-inflammatory effects.
To confirm in vivo the anti-inflammatory effects observed in vitro, we tested F. prausnitzii in a murine TNBS-induced colitis model. F. prausnitzii and its supernatant had a protective effect in this colitis model, on macroscopic and histologic criteria as well as on colonic cytokine secretion profile. Mice treated with either F. prausnitzii or its supernatant had a reduced IL-12 and an elevated IL-10 colonic concentration compared with the colitis control group. Interestingly, this effect on cytokine profile is close to the one observed in vitro on PBMCs.
To go back to our initial hypothesis, we analyzed the composition of the mouse fecal microbiota by culture-independent methods. We observed that the dysbiosis associated with TNBS-induced colitis was partially counterbalanced by F. prausnitzii or its supernatant administration.
Several authors reported that probiotics could act by systemic routes of administration (24–26). To investigate whether F. prausnitzii can also be active by a gut-independent pathway, we tested it i.p. on a TNBS-induced colitis model. Systemic delivery of F. prausnitzii and, above all, of its supernatant significantly improved the survival curve related to TNBS-induced colitis. Here again, this effect was not related to butyrate presence.
In summary, a rational process led us to select F. prausnitzii as an anti-inflammatory bacterial candidate. Analysis revealed that F. prausnitzii exhibited anti-inflammatory effects, partly due to secreted metabolites blocking NF-κB activation and IL-8 secretion. Moreover, in vivo effects were associated with a decrease in proinflammatory colonic cytokine synthesis and with the induction of anti-inflammatory cytokine secretion. Counterbalancing dysbiosis using the commensal bacterium F. prausnitzii as a candidate probiotic agent appears to be a promising strategy in CD treatment. Nevertheless, clinical studies should establish diagnostic tools to define the best population of patients for this probiotic species, and especially whether the clinical benefit is more pronounced in patients with low levels of endogenous Firmicutes.
Materials and Methods
Patients.
The present study was part of a double-blind controlled trial that compared the efficiency of the probiotic L. johnsonii LA1 strain and a placebo to decrease endoscopic recurrence after curative surgery for CD (11). Although the whole human trial included 98 patients, the present study, which assessed the MAM, included only a subset of these patients.
Tissues, Histological Examination, FISH, and Quantification of Bacteria.
Surgical samples and mucosal biopsy samples were processed directly in the operating room as previously described (27).
Bacterial Culture.
F. prausnitzii A2–165 (DSM 17677), isolated from human fecal stool, was grown at 37°C in LYHBHI medium [Brain–heart infusion medium supplemented with 0.5% yeast extract (Difco) and 5 mg/liter hemin] supplemented with cellobiose (1 mg/ml; Sigma–Aldrich), maltose (1 mg/ml; Sigma), and cysteine (0.5 mg/ml; Sigma) in an anaerobic chamber.
Butyrate Quantification.
Butyrate was quantified in supernatant and culture medium using a gas–liquid chromatograph (Autosystem XL; Perkin–Elmer) as described (28).
Antibacterial Assay.
Antibacterial effect of F. prausnitzii supernatant was investigated in vitro using two different techniques: the critical dilutions of Mayr-Harting (14) and the bacteriocin activity assay as described (15). This antibacterial effect was tested on six bacterial species: three aerobic bacteria (E. coli Nissle 1917, E. coli DH10B, and Listeria monocytogenes 11765), one facultative anaerobic bacteria (Lactococcus subsp cremoris MG1363), and two obligate anaerobic bacteria (Clostridium perfringens ATCC13124 and Bifidobacterium infantis DSM20088/ATCC15697). Antibiotic rifampicine and colistine were used as positive controls, whereas LYHBHI medium was used as negative control.
PBMC Isolation and Stimulation.
PBMCs were isolated from the peripheral blood of healthy donors as described (29).
TNBS-Induced Colitis.
After acclimatization, bacterial suspensions (109-1010 cfu in 500 μl), bacterial supernatant, or control medium was administered to mice daily by intragastric gavage from day 5 before to day 1 after induction of colitis. For colitis induction, mice were anesthetized with isofluran gas. TNBS (Sigma–Aldrich) was dissolved in 0.9% NaCl/ethanol (50:50 vol/vol), and 50 μl solution (at a dose of 100 mg/kg body weight) was administered intrarectally (using a 3.5 French catheter; Solomon Scientific), and the mice then were kept in a vertical position for 30 seconds. No-colitis control mice received PBS intragastrically and 0.9% NaCl/ethanol (50:50 vol/vol) intrarectally (30). Colitis control mice received PBS intragastrically and received TNBS intrarectally. Treated mice received bacteria (or bacterial components) intragastrically and TNBS intrarectally. Inflammation was monitored 48 h after TNBS administration. Mice were weighed before TNBS administration and at killing by cervical dislocation.
The i.p. administration route experiments were performed using a protocol derived from the one previously described by Foligné et al. (24). The protective effect of a single i.p. injection of live microorganisms (109-1010 cfu in 200 μl), supernatant (200 μl), dexamethasone (10 mg/kg), or butyrate (40 mM) was evaluated. Bacteria or components were injected 1 h before TNBS-colitis induction. The mortality rate was monitored day by day for 20 days.
Inflammation Score Assessment of TNBS Colitis.
The colon was removed, dissected free of fat and mesentery, carefully opened, and cleaned with PBS. Colon length was measured. Colonic damage and inflammation were assessed blindly according to the Wallace criteria (31).
Histological Assessment of TNBS-induced Colitis.
For histological assessment, a colon sample located in the most inflamed area was fixed in 4% paraformaldehyde acid and embedded in paraffin. Four-micrometer sections were stained with hematoxylin/eosin and examined blindly according to the Ameho criteria (32) (Fig. S1).
Supplementary Material
Acknowledgments.
We thank Alexandra Gruss, Sylvie Rabot, and Maarten van de Guchte for critical reading of this manuscript. We also thank Anne Lavergne-Slove for histological support. We thank the Groupe d'Etude Thérapeutique des Affections Inflammatoires du Tube Digestif members who participated in the sample collections for the clinical study. H.S. received a grant from Assistance Publique-Hôpitaux de Paris to achieve this work.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
See Commentary on page 16413.
This article contains supporting information online at www.pnas.org/cgi/content/full/0804812105/DCSupplemental.
References
- 1.Loftus EV., Jr Clinical epidemiology of inflammatory bowel disease: Incidence, prevalence, and environmental influences. Gastroenterology. 2004;126:1504–1517. doi: 10.1053/j.gastro.2004.01.063. [DOI] [PubMed] [Google Scholar]
- 2.Seksik P, et al. Review article: The role of bacteria in onset and perpetuation of inflammatory bowel disease. Aliment Pharmacol Ther. 2006;24:11–18. doi: 10.1111/j.1365-2036.2006.03053.x. [DOI] [PubMed] [Google Scholar]
- 3.Rutgeerts P, et al. Effect of faecal stream diversion on recurrence of Crohn's disease in the neoterminal ileum. Lancet. 1991;338:771–774. doi: 10.1016/0140-6736(91)90663-a. [DOI] [PubMed] [Google Scholar]
- 4.Darfeuille-Michaud A, et al. Presence of adherent Escherichia coli strains in ileal mucosa of patients with Crohn's disease. Gastroenterology. 1998;115:1405–1413. doi: 10.1016/s0016-5085(98)70019-8. [DOI] [PubMed] [Google Scholar]
- 5.Sokol H, Lepage P, Seksik P, Dore J, Marteau P. Molecular comparison of dominant microbiota associated with injured versus healthy mucosa in ulcerative colitis. Gut. 2007;56:152–154. doi: 10.1136/gut.2006.109686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sokol H, et al. Specificities of the fecal microbiota in inflammatory bowel disease. Inflamm Bowel Dis. 2006;12:106–111. doi: 10.1097/01.MIB.0000200323.38139.c6. [DOI] [PubMed] [Google Scholar]
- 7.Swidsinski A, Weber J, Loening-Baucke V, Hale LP, Lochs H. Spatial organization and composition of the mucosal flora in patients with inflammatory bowel disease. J Clin Microbiol. 2005;43:3380–3389. doi: 10.1128/JCM.43.7.3380-3389.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Manichanh C, et al. Reduced diversity of faecal microbiota in Crohn's disease revealed by a metagenomic approach. Gut. 2006;55:205–211. doi: 10.1136/gut.2005.073817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Frank DN, et al. Molecular-phylogenetic characterization of microbial community imbalances in human inflammatory bowel diseases. Proc Natl Acad Sci USA. 2007;104:13780–13785. doi: 10.1073/pnas.0706625104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Martinez-Medina M, Aldeguer X, Gonzalez-Huix F, Acero D, Garcia-Gil LJ. Abnormal microbiota composition in the ileocolonic mucosa of Crohn's disease patients as revealed by polymerase chain reaction-denaturing gradient gel electrophoresis. Inflamm Bowel Dis. 2006;12:1136–1145. doi: 10.1097/01.mib.0000235828.09305.0c. [DOI] [PubMed] [Google Scholar]
- 11.Marteau P, et al. Ineffectiveness of Lactobacillus johnsonii LA1 for prophylaxis of postoperative recurrence in Crohn's disease: A randomised, double blind, placebo controlled GETAID trial. Gut. 2006;55:842–847. doi: 10.1136/gut.2005.076604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Daniel C, et al. Selecting lactic acid bacteria for their safety and functionality by use of a mouse colitis model. Appl Environ Microbiol. 2006;72:5799–5805. doi: 10.1128/AEM.00109-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Segain JP, et al. Butyrate inhibits inflammatory responses through NFkappaB inhibition: Implications for Crohn's disease. Gut. 2000;47:397–403. doi: 10.1136/gut.47.3.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mayr-Harting A, Hedges AJ, Berkeley RCW. Methods for studying bacteriocins. Methods Microbiol. 1972;7A:315–422. [Google Scholar]
- 15.Dabard J, et al. Ruminococcin A, a new lantibiotic produced by a Ruminococcus gnavus strain isolated from human feces. Appl Environ Microbiol. 2001;67:4111–4118. doi: 10.1128/AEM.67.9.4111-4118.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rakoff-Nahoum S, Paglino J, Eslami-Varzaneh F, Edberg S, Medzhitov R. Recognition of commensal microflora by toll-like receptors is required for intestinal homeostasis. Cell. 2004;118:229–241. doi: 10.1016/j.cell.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 17.Kelly D, et al. Commensal anaerobic gut bacteria attenuate inflammation by regulating nuclear-cytoplasmic shuttling of PPAR-gamma and RelA. Nat Immunol. 2004;5:104–112. doi: 10.1038/ni1018. [DOI] [PubMed] [Google Scholar]
- 18.Barcenilla A, et al. Phylogenetic relationships of butyrate-producing bacteria from the human gut. Appl Environ Microbiol. 2000;66:1654–1661. doi: 10.1128/aem.66.4.1654-1661.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Suau A, et al. Direct analysis of genes encoding 16S rRNA from complex communities reveals many novel molecular species within the human gut. Appl Environ Microbiol. 1999;65:4799–4807. doi: 10.1128/aem.65.11.4799-4807.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wehkamp J, et al. NOD2 (CARD15) mutations in Crohn's disease are associated with diminished mucosal alpha-defensin expression. Gut. 2004;53:1658–1664. doi: 10.1136/gut.2003.032805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wehkamp J, et al. Reduced Paneth cell alpha-defensins in ileal Crohn's disease. Proc Natl Acad Sci USA. 2005;102:18129–18134. doi: 10.1073/pnas.0505256102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wehkamp J, et al. NF-kappaB- and AP-1-mediated induction of human beta defensin-2 in intestinal epithelial cells by Escherichia coli Nissle 1917: A novel effect of a probiotic bacterium. Infect Immun. 2004;72:5750–5758. doi: 10.1128/IAI.72.10.5750-5758.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nenci A, et al. Epithelial NEMO links innate immunity to chronic intestinal inflammation. Nature. 2007;446:557–561. doi: 10.1038/nature05698. [DOI] [PubMed] [Google Scholar]
- 24.Foligné B, Grangette C, Pot B. Probiotics in IBD: Mucosal and systemic routes of administration may promote similar effects. Gut. 2005;54:727–728. [PMC free article] [PubMed] [Google Scholar]
- 25.Rachmilewitz D, et al. Toll-like receptor 9 signaling mediates the anti-inflammatory effects of probiotics in murine experimental colitis. Gastroenterology. 2004;126:520–528. doi: 10.1053/j.gastro.2003.11.019. [DOI] [PubMed] [Google Scholar]
- 26.Sheil B, et al. Is the mucosal route of administration essential for probiotic function? Subcutaneous administration is associated with attenuation of murine colitis and arthritis. Gut. 2004;53:694–700. doi: 10.1136/gut.2003.027789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vasquez N, et al. Patchy distribution of mucosal lesions in ileal Crohn's disease is not linked to differences in the dominant mucosa-associated bacteria: A study using fluorescence in situ hybridization and temporal temperature gradient gel electrophoresis. Inflamm Bowel Dis. 2007;13:684–692. doi: 10.1002/ibd.20084. [DOI] [PubMed] [Google Scholar]
- 28.Lan A, et al. Survival and metabolic activity of selected strains of Propionibacterium freudenreichii in the gastrointestinal tract of human microbiota-associated rats. Br J Nutr. 2007;97:714–724. doi: 10.1017/S0007114507433001. [DOI] [PubMed] [Google Scholar]
- 29.Foligne B, et al. Correlation between in vitro and in vivo immunomodulatory properties of lactic acid bacteria. World J Gastroenterol. 2007;13:236–243. doi: 10.3748/wjg.v13.i2.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Foligne B, et al. Recommendations for improved use of the murine TNBS-induced colitis model in evaluating anti-inflammatory properties of lactic acid bacteria: technical and microbiological aspects. Dig Dis Sci. 2006;51:390–400. doi: 10.1007/s10620-006-3143-x. [DOI] [PubMed] [Google Scholar]
- 31.Wallace JL, MacNaughton WK, Morris GP, Beck PL. Inhibition of leukotriene synthesis markedly accelerates healing in a rat model of inflammatory bowel disease. Gastroenterology. 1989;96:29–36. doi: 10.1016/0016-5085(89)90760-9. [DOI] [PubMed] [Google Scholar]
- 32.Ameho CK, et al. Prophylactic effect of dietary glutamine supplementation on interleukin 8 and tumour necrosis factor alpha production in trinitrobenzene sulphonic acid induced colitis. Gut. 1997;41:487–493. doi: 10.1136/gut.41.4.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.