Abstract
The opportunistic pathogen Pseudomonas aeruginosa causes infections that are difficult to treat by antibiotic therapy. This bacterium can cause biofilm infections where it shows tolerance to antibiotics. Here we report the novel use of a metallo-complex, desferrioxamine-gallium (DFO-Ga) that targets P. aeruginosa iron metabolism. This complex kills free-living bacteria and blocks biofilm formation. A combination of DFO-Ga and the anti-Pseudomonas antibiotic gentamicin caused massive killing of P. aeruginosa cells in mature biofilms. In a P. aeruginosa rabbit corneal infection, topical administration of DFO-Ga together with gentamicin decreased both infiltrate and final scar size by about 50% compared to topical application of gentamicin alone. The use of DFO-Ga as a Trojan horse delivery system that interferes with iron metabolism shows promise as a treatment for P. aeruginosa infections.
Keywords: antimicrobials, biofilms, chronic infections, iron uptake, keratitis
Bacteria can exist in a free-living planktonic state and also in a biofilm state. Biofilms are groups of bacteria associated with a surface and embedded in a self-produced extracellular matrix (1, 2). As a consequence of the biofilm lifestyle, bacteria can tolerate exposure to antibiotics; biofilm infections are notoriously difficult to treat and often impossible to cure (1, 3). Various approaches to inhibit bacterial growth and biofilm formation include novel antibiotics, anti-quorum sensing molecules, and modulation of iron availability (4, 5). Iron is an especially intriguing target as it is an essential element for growth of most organisms, including bacteria. In fact, maintaining free iron concentrations at extremely low levels (<10−18 M) is a first line of host defense against invading pathogens (6, 7).
For Pseudomonas aeruginosa, an opportunistic pathogen that can cause both acute and chronic infections (1, 8), iron is not only a necessary element for growth but also a cue in biofilm formation (9, 10). Thus, interfering with bacterial iron homeostasis may serve as a potential therapeutic target that can block P. aeruginosa virulence in both the free-living and biofilm states. Targeting bacterial iron metabolism to treat infections has been explored previously. Published reports describe the use of siderophore-antibiotic conjugates (11, 12) and attempts to replace the active Fe(III) moiety with a metabolically-inactive metal ion such as Sc(III), In(III), or Ga(III) (4, 13, 14). Both approaches yielded promising results when applied to free-living bacteria. Most recently, Kaneko et al. (4) examined the use of Ga(NO3)3 to eradicate P. aeruginosa biofilm cells. Gallium inhibited P. aeruginosa growth, prevented biofilm formation, and showed bactericidal activity against free-living as well as biofilm cells. Furthermore, Ga was effective in two different experimental animal infections simulating acute lethal pneumonia and a chronic airway biofilm infection.
Here we report a novel approach for delivery of gallium to P. aeruginosa. We use a metallo-complex that combines a strong siderophore, desferrioxamine (DFO) with gallium, a redox-inactive metal ion. Because Ga(III) ligand chemistry shares similarity with that of Fe(III), it interferes with iron metabolism (4, 14). The DFO-Ga complex was originally designed as an antioxidant that can act by “push and pull” mechanisms, sequestering ferric ions (the siderophore effect) and, in turn, releasing gallium ions that further compete with ferric ions at iron binding sites of proteins (15, 16). Here we exploit the chemical and structural similarities between DFO-Fe and DFO-Ga to interfere with iron homeostasis in P. aeruginosa. We chose DFO as a siderophore carrier of Ga because P. aeruginosa is thought to possess two uptake systems for DFO-Fe (10). Thus DFO-Ga could serve as a Trojan horse that delivers toxic gallium to P. aeruginosa cells via either of the two DFO uptake systems.
Results
DFO-Ga Kills planktonic P. aeruginosa Cells.
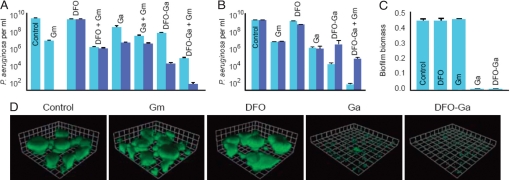
We first examined the effect of DFO-Ga on planktonic P. aeruginosa in a low iron medium. The minimal inhibitory concentration (MIC) of DFO-Ga under these conditions was 0.032 mM. A similar result was obtained for Ga(III) alone (GaCl3). As expected, DFO did not inhibit growth, even at a concentration of 1 mM. Like many other bacterial species, when in the stationary phase, P. aeruginosa is not effectively eradicated by antibiotics (17), and part of the explanation for biofilm resistance to antibiotic treatment is that a large fraction of the bacterial cells in a biofilm are likely to be in a stationary phase-like state. We thus tested the ability of DFO-Ga to kill stationary phase cells (Fig. 1). After a long (24 h) incubation period, gentamicin (Gm) (10 μg per ml, more than 10 times the MIC) reduced P. aeruginosa viability by three log units, while DFO-Ga (1 mM) was 10–100 fold more effective. Because DFO-Ga and Gm exert their antimicrobial effects via different mechanisms, we examined whether they have an additive effect when administered together. The combined treatment resulted in a remarkable six-log reduction in the number of viable cells, suggesting synergy between DFO-Ga and Gm (Fig. 1A).
Fig. 1.
DFO-Ga is effective against P. aeruginosa stationary phase cells and blocks biofilm formation. (A) Survival of P. aeruginosa stationary phase cells treated for 24 h with desferrioxamine (DFO), gentamicin (Gm), gallium (Ga), desferrioxamine-gallium complex (DFO-Ga), or combinations of these agents as indicated. The concentrations used for each compound were 0.1 mM (light blue) or 1 mM (dark blue) for DFO, Ga, or DFO-Ga, and 10 μg per ml for Gm. (B) Survival of a P. aeruginosa mutant with defects in the two putative metallo-DFO receptor systems (see Materials and Methods). These experiments were performed as described for A, at the 1 mM concentration. The wild-type parent is light blue and the double mutant, dark blue. (C) Biofilm growth (18 h) in polyvinylchloride microtiter dish wells with the indicated compounds (concentrations were 0.001 mM for DFO, Ga, and DFO-Ga, and 0.1 μg/ml for Gm). Attached biofilm biomass was determined by measuring crystal violet binding. (D) Biofilm formation in flow cells with subinhibitory concentrations of DFO (0.001 mM), Ga (0.001 mM), DFO-Ga (0.001 mM), or Gm (0.1 μg/ml). Shown are 3D-reconstructed confocal microscope images of six-day biofilms grown in the presence of the indicated agent (a side of each square on the grids is 23 μm). P. aeruginosa cells are expressing GFP.
To examine the role of the previously identified putative DFO receptors (10) in killing by DFO-Ga, we obtained two single mutant strains and generated a double mutant. The mutations were in either PA0470, PA2466, or both genes. We then compared the sensitivities of these mutants to Ga and DFO-Ga (Fig. 1B). The single mutants and wild-type were equally sensitive to Ga and DFO-Ga (data not shown), whereas the double mutant showed a reduced sensitivity to DFO-Ga but not to Ga (Fig. 1B). The mutant analysis indicates that PA0470 and PA2466 code for components of two different independent metallo-DFO uptake systems and that either is sufficient to confer DFO-Ga sensitivity to P. aeruginosa.
DFO-Ga Blocks Biofilm Formation and Kills P. aeruginosa in Mature Biofilms.
To evaluate the ability of DFO-Ga to inhibit biofilm development, we first examined biofilm formation under static growth conditions in microtiter dish wells. Subinhibitory concentrations of Gm that do not affect planktonic growth (0.1 μg per ml), or DFO (0.001 mM) alone did not prevent biofilm formation. In contrast, when subinhibitory concentrations of DFO-Ga (0.001 mM) or Ga alone (0.001 mM) were used, biofilm formation was effectively blocked (Fig. 1C). We also followed the development of biofilms under a continuous flow of medium in microscope observation chambers. Under our experimental conditions P. aeruginosa developed mushroom-like structures containing cells embedded in a self-produced matrix (Fig. 1D). The presence of DFO-Ga (0.001 mM) completely blocked biofilm formation in this system.
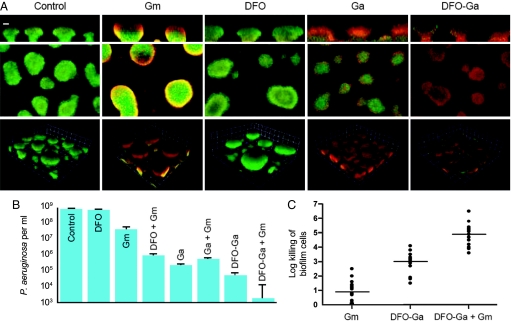
To examine whether DFO-Ga is also effective against established biofilms, we further used the continuous flow chamber and applied the complex only after biofilms had formed. We used propidium iodide, which stains non-viable cells, to show that DFO-Ga causes death of cells throughout the mushroom-like biofilm structures. Addition of DFO alone did not appear to cause a similar effect, and addition of Gm (50 μg/ml) or gallium (1 mM) resulted in cell death that was mainly restricted to the outer rim of biofilm structures (as detected by an increase in red propidium iodide staining) (Fig. 2A). To provide further evidence for the activity of DFO-Ga against mature biofilms, we examined its influence on biofilms by using a different system, a spinning disk reactor that exposes biofilms to continuous high shear (Fig. 2B). Spinning disk biofilms treated with Gm (50 μg per ml) showed a 2-log decrease in cell counts. DFO-Ga (1 mM) caused a 3–4-log decrease in cell counts. When used together, DFO-Ga and Gm again showed a much larger 6 log reduction in viable cells with only a few live cells surviving treatment.
Fig. 2.
DFO-Ga kills cells in established biofilms of P. aeruginosa PAO1 and other clinical isolates of P. aeruginosa. (A) Survival of P. aeruginosa in mature biofilms treated with Gm (50 μg/ml), DFO (1 mM), Ga (1 mM), or DFO-Ga (1 mM). Six-day-old biofilms were treated for 24 h and stained with propidum iodide. Live cells are shown in green, and dead cells are red. The top images are saggital reconstructions, the middle images horizontal sections, and the bottom images 3D reconstructions. The marker bar on the top left image is 25 μm. The images on the top and middle panels are at the same magnification. For the bottom images, the squares are 23 μm on each side. (B) Spinning disk reactor biofilms were treated for 24 h with either DFO, Ga, DFO-Ga (each at 1 mM), or 50 μg/ml Gm. (C) P. aeruginosa clinical isolates from eyes, wounds and sputum from CF patients with chronic P. aeruginosa infections were grown as biofilms and treated for 24 h with Gm (50 μg/ml), DFO-Ga (1 mM) or combination of both. The horizontal lines represent means, all experiments were carried out in duplicates.
Is killing by DFO-Ga specific to the P. aeruginosa laboratory strain PAO1? We screened 15 clinical isolates obtained from lungs of cystic fibrosis (CF) patients, eyes with keratitis, and infected skin wounds. These isolates included mucoid strains and strains with various drug resistance profiles. All strains showed higher sensitivities to DFO-Ga (with a mean of 3 log killing) and DFO-Ga+Gm (mean of 5 log killing) as compared to Gm treatment alone (mean of 1 log killing) (Fig. 2C). The in vitro experiments described above indicate that DFO-Ga is an effective antimicrobial agent that kills P. aeruginosa cells growing either planktonically or in biofilms. The experiments also show that DFO-Ga and Gm, when used together, are particularly effective in killing biofilm bacteria.
DFO-Ga Is an Effective Treatment for P. aeruginosa Infections of Rabbit Eyes.
The above results led us to examine whether DFO-Ga was also efficacious in an in vivo animal model of infection. To this end, we used an experimental rabbit cornea infection model that closely resembles keratitis caused by P. aeruginosa in humans.
Ulcerative keratitis is a rapidly progressive inflammatory response to a bacterial infection of the cornea (18). Due to its potential to permanently impair vision or even cause blindness, bacterial keratitis is considered an ophthalmologic emergency necessitating rapid initiation of topical antibiotics at high concentrations with frequent dosing. Increased use of soft contact lenses has led to a dramatic rise in the occurrence of bacterial keratitis, particularly due to P. aeruginosa infections (19). We consider these P. aeruginosa eye infections to represent biofilm infections where the bacteria grow in large aggregates attached to the surface of the cornea. That is not to say that this infection exhibits characteristics similar to chronic biofilm infections such as those, which occur in lungs of people with CF. Because the eye is accessible and can be treated by washing with high concentrations of topical antibiotics, it is often possible to cure these surface-associated infections and prevent the devastating complication of endophthalmitis. However, scarring and loss of corneal clarity are frequent sequelae, which may lead to visual impairment. Thus, a secondary aim of treatment is to limit the residual area of corneal scarring and opacity to a minimum.
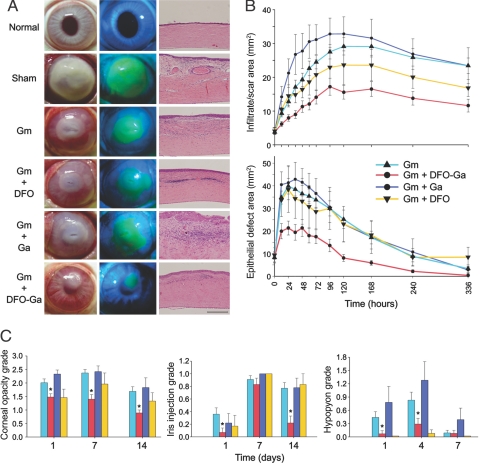
A rabbit “scratched cornea” model, which closely resembles keratitis in contact lens wearers, was designed, combining an initial insult to the corneal epithelium and outer stroma followed by application of an infected contact lens. Once threshold levels of infiltrate and epithelial erosion developed, intensive topical treatment was initiated (described in Materials and Methods). In sham-treated eyes, infection spread rapidly and within 48 h total opacification of the cornea occurred and endophthalmitis developed in most cases. In all Gm-treated groups the course of disease was less severe (Fig. 3 and Figs. S2 and S3). Notably, the most prominent rescue effect was observed in Gm+DFO-Ga treated eyes, where infiltration and final scar area were reduced by 50–60% compared to Gm alone, a difference that was statistically significant. Addition of either DFO or GaCl3 to Gm yielded an effect that did not differ significantly from Gm alone (Fig. 3 and Fig. S3). The extent of the corneal epithelial defect was also significantly reduced in the Gm+DFO-Ga treated group, with a 43% smaller area of epithelial erosion after 12 h as compared to treatment with Gm alone. This difference persisted throughout follow-up, until the epithelial defect ultimately healed in all Gm-treated groups (in approximately 10–14 days). The protective effect of Gm+DFO-Ga treatment as compared to Gm alone was statistically significant, while the Gm+DFO and Gm+GaCl3 treatments did not significantly differ from Gm alone (Fig. 3 and Fig. S3).
Fig. 3.
DFO-Ga reduces severity of keratitis in a rabbit infection model. (A) Representative images of P. aeruginosa infected corneas following different treatment regimens. (Left column) Photographs showing the extent of infection and infiltrate 96 h after treatment initiation. (Middle column) Fluorescein-staining of the same eyes showing the extent of epithelial injury. (Right column) Histopathology of corneas from the different experimental groups (bar is 250 μm). (B) Combined treatment of Gm plus DFO-Ga markedly reduces keratitis progression. (Top) Extent and evolution of corneal infiltrate and (bottom) epithelial defect over time in the different treatment groups (sham group progression depicted in supporting information Fig. S3). Eyes treated with Gm plus DFO-Ga fared better than eyes treated with Gm alone at all time points. The other treatment groups did not significantly differ from Gm alone. Each point represents mean area ± SEM. The method of quantification and number of eyes at each point are detailed in Table S1 and Fig. S1. (C) Disease severity parameters are reduced in Gm plus DFO-Ga treated eyes. (Left) Corneal opacity, (Middle) Iris injection and (Right) Hypopyon level. Numbers of eyes graded at each time point are detailed in Table S1. Asterisks denote a statistically significant difference between Gm+DFO-Ga and Gm alone. Bars represent means ± SEM.
The reduction in corneal injury following Gm+DFO-Ga treatment was also evident in histological specimens (Fig. 3A). Sham-treated eyes were characterized by prominent scarring, irregular thickening of the corneal epithelium, disruption of the corneal stroma with ragged appearance of stromal lamellae, leukocyte infiltration, and severe neovascularization. Gm-treated eyes showed notable scar tissue and prominent leukocyte infiltration, while treatment with Gm+DFO-Ga was associated with relatively mild changes, including slight stromal disturbance, minor neovascularization, and scarce leukocytes within the tissue. Additional parameters of disease, i.e., corneal opacity, iris injection, and hypopyon formation were also reduced by Gm+DFO-Ga treatment (Fig. 3C). These data indicate that combined treatment of DFO-Ga with gentamicin results in a less aggressive infection and allows for faster healing of Pseudomonas keratitis in the rabbit model.
Discussion
Both DFO and gallium are approved agents for clinical use in humans. High-dose DFO is used for chelation therapy in thalassemia and hemochromatosis. Gallium is used to treat hypercalcemia and in nuclear medicine. These two medicines have been combined into a DFO-Ga adduct that shows antioxidant properties in mammalian cells (15, 16). Recent findings that iron is critical for P. aeruginosa biofilm formation (9, 10), that Ga has anti-biofilm activity (4), and that P. aeruginosa has two DFO-Fe transport systems (10) led us to hypothesize that DFO-Ga might readily enter P. aeruginosa cells where gallium would exert its toxic effect on iron metabolism. In agreement with this, we show that DFO-Ga kills P. aeruginosa under conditions where antibiotics have only limited efficacy, namely stationary phase and in biofilm conditions. DFO-Ga together with gentamicin, an antibiotic used routinely to treat P. aeruginosa infections, showed enhanced, apparently synergistic bacterial killing. The activity of DFO-Ga could be dependent on bacterial strains or culture conditions. However, we examined a number of clinical isolates, and we examined biofilms grown in three different ways. DFO-Ga was effective under all conditions tested. These results suggest that DFO-Ga may show efficacy in P. aeruginosa infections. Our rabbit keratitis experiments bore this out. In this surface-associated P. aeruginosa infection, DFO-Ga plus gentamicin treatment resulted in a better outcome as compared to treatment with gentamicin alone. Before clinical application of metallo-complexes, further experimentation is required including comparison of efficacy versus the dual aminoglycoside and cephalosporin antibiotic therapeutic regimen currently considered to be the standard of care. Importantly, the role of the cephalosporins in the clinical regimen is to cover Gram-positive pathogens, and therefore we expect that cephalosporins would not confer additional benefit in the context of P. aeruginosa infection. Whether DFO-Ga will show similar efficacy in other types of P. aeruginosa infections remains to be determined. However, the fact that P. aeruginosa clinical isolates from wounds and CF sputum showed similar sensitivity in vitro suggests it might.
In a recent study, Kaneko et al. (4) reported on efficacy of gallium against P. aeruginosa planktonic and biofilm cells. We believe that DFO-Ga provides an additional advantage because evidence indicates that P. aeruginosa possesses two high affinity uptake systems for metallo-DFO complexes (10, 20). Thus DFO-Ga can function as a “Trojan Horse” where receptors on the bacterial surface will bind DFO-Ga and actively take up the chelated poison. Because there are two DFO transport systems (10, 20, and Fig. 1B), evolution of resistance to this complex should be considerably more difficult than evolution of resistance to a single target. We hasten to point out that we have not measured DFO-Ga transport, and experimental details about how this molecule interacts with P. aeruginosa to kill it await further studies. There is also little known about transport of gallium ion into bacterial cells. Furthermore, the results of our experiments and those of Kaneko et al. (4) are not directly comparable. Different conditions and different formulations of gallium (gallium chloride in our experiments and gallium nitrate in the prior study) were used.
The in vitro findings support the notion that DFO-Ga acts as an antimicrobial agent in different forms and stages of P. aeruginosa growth. However, we cannot conclude that the treatment effect in the rabbit kerititis model results from, or solely from, antimicrobial activity of DFO-Ga. DFO-Ga has been reported to reduce formation of reactive oxygen species (ROS), which have been implicated in promoting corneal injury during infection. This ability to curb formation of ROS interrelates with the affect of the complex on iron availability (16). In the Fenton reaction, conversion and formation of reactive hydroxyl radicals apparently depends on the presence of trace amounts of redox-active metal ions. The two components of DFO-Ga serve to reduce the availability and activity of these metal ions via two mechanisms: (i) chelation by DFO and (ii) displacement of redox-active iron ions by the relatively inert gallium ion at specific sites. The ability of the complex (and a similar complex, DFO-zinc) to reduce formation of free radicals has been previously demonstrated in a number of experimental systems including models of chemical corneal injury (21, 22).
Our discovery of the antimicrobial activity of DFO-Ga warrants further investigation. The study was designed first to test the ability of DFO-Ga to kill P. aeruginosa under conditions where this bacterium can tolerate high levels of antibiotics and second to show a proof of concept by using a simple animal infection model to test whether DFO-Ga can enhance treatment of P. aeruginosa infections. The results are a step toward the possible use of DFO-Ga complexes as an adjuvant to antibiotic therapy for difficult to treat bacterial infections.
Materials and Methods
Bacterial Strains and Culture Conditions.
We used P. aeruginosa PAO1 (23), PAO1 derivatives, and various P. aeruginosa eye, wound, and CF isolates. For flow cell biofilm experiments we used strains containing the GFP expression vector pMRP9–1 (24). Both flow cell and disk reactor biofilms were grown in 1% Tryptic Soy Broth (TSB) (Becton Dickinson) as were planktonic cultures. All cultures were incubated at 37°C unless otherwise indicated. For the static biofilm formation assay we used M63 minimal medium (25) supplemented with glucose (0.2%), arginine (0.4%) and MgSO4 (1 mM).
Construction of P. aeruginosa DFO Uptake Double Mutant.
PA0470 and PA2466 mutants of strain PAO1 were obtained from the Comprehensive P. aeruginosa Transposon Mutant Library at the University of Washington Genome Center (26). To construct a PA2466-PA0470 double mutant with a 63-bp insertion in PA0470 and an ISlacZ/hah insertion in PA2466 we used a genomic transformation procedure described previously (27). To first obtain the unmarked PAO1 63 bp PA0470 mutant we used the cre-recombinase system (28). The PA0470 mutant from the library was mated with Escerichia coli carrying pCre1. Transconjugants were selected on Pseudomonas Isolation Agar. A TcS strain was isolated and the 63-bp insertion was confirmed by PCR. This strain was than transformed with PTL11509 genomic DNA (50–90 μg/μl), and a transformant was selected. All mutations were confirmed by PCR analysis.
Effects of DFO-Ga on Planktonic P. aeruginosa.
We determined minimal inhibitory concentrations (MICs) of various agents by using 96-well plates. Each well contained 100 μl of 1% TSB plus a test compound. The inoculum was 5 × 105 P. aeruginosa cells per well. To test the effect of agents on survival of stationary phase cells we used cultures inoculated with 105 cells per ml and incubated for 18 h at 37°C with aeration. The 18-h cultures were centrifuged. The pelleted cells were washed twice and suspended in a volume of fresh medium equal to the original culture volume. The cells were incubated at 37°C for an additional 2 h with shaking and then exposed to test compounds at the indicated concentrations for 24 h. Viability was determined by plating dilutions on LB agar.
Biofilm Experiments.
We studied P. aeruginosa biofilms in three different ways. Biofilms were grown under static conditions in 96-well microtiter dishes as previously described (29) for 18 h at 37°C in M63 medium with or without added agents as indicated. Attached biomass was stained with crystal violet and the degree of crystal violet staining was measured as described elsewhere (29). To visualize biofilms grown under a continuous flow of medium we used flow cells and confocal microscopy at an incubation temperature of 25°C (10, 30). To determine whether an agent prevented biofilm formation it was added to the medium at the beginning of an experiment. To determine the affect of an agent on mature biofilms the biofilm was allowed to grow for six days before addition of the test compound. To discriminate live and dead cells we used propidium iodide (PI, 30 μM, Sigma Chemical Co.) as described elsewhere (31). We also used a spinning disk reactor as a third method to study biofilms (32). In this system biofilms are grown under a flow of medium and at high shear forces. After 24 h under flow the polycarbonate chips with attached biofilm bacteria were removed from the spinning disk and washed 3 times in PBS. All incubations for spinning disk experiments were at 37°C. The spinning disk biofilms were then exposed to the indicated agents in water for 24 h without shear (31). To estimate the number of remaining attached biofilm cells, we placed the discs in 1 ml PBS and dispersed the cells by using a tissue homogenizer (Brinkman Homogenizer). Viable cell numbers were determined by plating on LB agar.
Animals.
New Zealand albino rabbits weighing 2.5 to 3.5 kg were used in this study. All experiments were conducted in accordance with the Association for Research in Vision and Ophthalmology statement for the use of animals in Ophthalmic and Vision Research and approved by the Hadassah-Hebrew University Animal Ethics and Welfare Committee. For induction of keratitis, animals were anesthetized. At the end of the experiment, animals were euthanized and corneas were removed at the corneoscleral limbus and then processed for histopathological evaluation.
Induction of P. aeruginosa Keratitis.
After induction of anesthesia, a 2 mm long, 160 μm-deep incision was performed in the center of the cornea using a diamond keratome (45′ Micrometer Diamond Keratome, HUCO). To prepare the contaminated contact lens, sterile soft contact lenses (Platinum Everyday Super, St. Shine) were suspended in a bacterial culture (109 cell/ml) of the same main P. aeruginosa strain used in the in vitro experiments for 2 h at 37°C and then washed to remove non-adherent bacteria. The contaminated contact lens (with about 105 bacterial cells) was then applied to the ocular surface, and the eyelids were transiently sutured shut. The sutures were released after 10 h, the contact lens removed, and the animals were followed until the corneal infiltrate and erosion reached the threshold size for treatment initiation, defined as a combination of an infiltrate with a surface area of 3.6–4 mm2 and epithelial erosion equaling 8.0–8.4 mm2. The rabbit keratitis model developed as part of this study combines accurate and reproducible corneal injury with application of an infected contact lens. It resembles the mechanism of corneal infection in contact-lens users and may be useful for future investigations in this field.
Treatment Groups.
Once threshold infiltrate and erosion size was reached, infected eyes were randomly assigned to one of five topical treatment groups; a control group was sham treated with ophthalmic artificial tears (Hadassah Medical Center Pharmacy, Jerusalem, Israel), which also served as the dilution vehicle for the other treatment preparations. The four other groups received artificial tears eye drops containing 0.5% Gm Sulfate (Gentamicin-IKA, TEVA), either alone or in combination with 3.5 mM DFO Mesylate (Desferal®, CIBA-GEIGY), 3.5 mM GaCl3 (Aldrich Chemical Co.), or 3.5 mM DFO-Ga. This concentration of the metallo-DFO complex (and its constituents as additional controls) was based on previous studies showing its safety and efficacy in a rabbit model of nitrogen mustard-induced corneal injury (22, 33). The numbers of eyes in each treatment group at the different time points are summarized in Table S1.
Treatment Regimen.
The antibiotic used in this study was gentamicin, the leading anti-Pseudomonas ocular agent. The accepted first line of treatment in clinical bacterial keratitis usually also includes a first or second generation cephalosporine (often Cefazolin or Cefuroxime 5%) (34). However, the cephalosporins are used in this context to cover Gram-positive bacteria and not to enhance the action of aminoglycosides on Gram-negative rods. Therefore, they were not used in this study. The concentration of Gm we used was based upon a series of calibration trials. The common clinically used concentration of Gm for treatment of Pseudomonas induced corneal ulcers in humans is significantly higher (34). However, we sought to reach a concentration at which Gm itself would not be sufficient to rapidly control the infection, thus allowing a window for examining the possible beneficial synergistic effect of DFO-Ga. Treatment protocols were designed to achieve maximal similarity to the regimen applied in the clinical setting: topical antibiotics q15–60 min around the clock for 48 h and then a slow taper until healing over 7–14 d (35, 36). The exact treatment regimen is detailed in SI Text.
Measurements of Disease Progression.
To document the clinical progression of the infectious process, serial digital color photographs were taken: conventional color photographs were used to monitor the corneal infiltrate, degree of diffuse corneal opacity and scarring, level of hypopyon in the anterior chamber of the eye, and extent of iris injection as described previously (22, 33). Photographs under blue light following application of fluorescein to the cornea were used to measure the area of corneal epithelial erosion. Measurements and grading were performed in a masked fashion by two independent observers. A detailed description of the quantification process is provided in SI Text and Fig. S1.
Statistical Analysis.
Analysis of lesion progression was performed using a repeated measures ANOVA model with the Greenhouse-Geisser test for assessing time effect and interactions between time and treatment groups. The Scheffé posthoc test was applied for determining which pairs of treatment groups contributed to the overall treatment group effect. For analysis of graded parameters and microbiological data, Kruskal-Wallis (non-parametric one-way analysis of variance) and Mann-Whitney tests with Bonferroni correction were used. All analyses were performed using the SPSS 7.1 program (SPSS Inc.).
Supplementary Material
Acknowledgments.
We thank Dr. Alexey Obolensky for his assistance in developing the rabbit keratitis model and Dr. Shiri Navon-Venezia for providing bacterial strains. This research was supported by AI30040 from National Institutes of Allergy and Infectious Diseases (E.P.G.) the US Cystic Fibrosis Foundation (E.P.G.), the Bar-Ilan Nanotechnology Start-up Fund, and Israel Science Foundation Grant 366/07 (Ehud Banin), a Yedidut Research Grant (Eyal Banin), Israel Science Foundation Grants 316/05 and 585/02, and the Dr. Moshe and Paulina Bergman Memorial Fund (M.C.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0808608105/DCSupplemental.
References
- 1.Costerton JW, Stewart PS, Greenberg EP. Bacterial biofilms: A common cause of persistent infections. Science. 1999;284:1318–1322. doi: 10.1126/science.284.5418.1318. [DOI] [PubMed] [Google Scholar]
- 2.O'Toole G, Kaplan HB, Kolter R. Biofilm formation as microbial development. Annu Rev Microbiol. 2000;54:49–79. doi: 10.1146/annurev.micro.54.1.49. [DOI] [PubMed] [Google Scholar]
- 3.Davies D. Understanding biofilm resistance to antibacterial agents. Nat Rev Drug Discov. 2003;2:114–122. doi: 10.1038/nrd1008. [DOI] [PubMed] [Google Scholar]
- 4.Kaneko Y, Thoendel M, Olakanmi O, Britigan BE, Singh PK. The transition metal gallium disrupts Pseudomonas aeruginosa iron metabolism and has antimicrobial and antibiofilm activity. J Clin Invest. 2007;117:877–888. doi: 10.1172/JCI30783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rasmussen TB, Givskov M. Quorum-sensing inhibitors as anti-pathogenic drugs. Int J Med Microbiol. 2006;296:149–161. doi: 10.1016/j.ijmm.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 6.Ratledge C, Dover LG. Iron metabolism in pathogenic bacteria. Annu Rev Microbiol. 2000;54:881–941. doi: 10.1146/annurev.micro.54.1.881. [DOI] [PubMed] [Google Scholar]
- 7.Schaible UE, Kaufmann SH. Iron and microbial infection. Nat Rev Microbiol. 2004;2:946–953. doi: 10.1038/nrmicro1046. [DOI] [PubMed] [Google Scholar]
- 8.Livermore DM. Multiple mechanisms of antimicrobial resistance in Pseudomonas aeruginosa: Our worst nightmare? Clin Infect Dis. 2002;34:634–640. doi: 10.1086/338782. [DOI] [PubMed] [Google Scholar]
- 9.Singh PK, Parsek MR, Greenberg EP, Welsh MJ. A component of innate immunity prevents bacterial biofilm development. Nature. 2002;417:552–555. doi: 10.1038/417552a. [DOI] [PubMed] [Google Scholar]
- 10.Banin E, Vasil ML, Greenberg EP. Iron and Pseudomonas aeruginosa biofilm formation. Proc Natl Acad Sci USA. 2005;102:11076–11081. doi: 10.1073/pnas.0504266102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Budzikiewicz H. Siderophore-antibiotic conjugates used as trojan horses against Pseudomonas aeruginosa. Curr Top Med Chem. 2001;1:73–82. doi: 10.2174/1568026013395524. [DOI] [PubMed] [Google Scholar]
- 12.Ghosh A, et al. Iron transport-mediated drug delivery using mixed-ligand siderophore-beta-lactam conjugates. Chem Biol. 1996;3:1011–1019. doi: 10.1016/s1074-5521(96)90167-2. [DOI] [PubMed] [Google Scholar]
- 13.Rogers HJ, Woods VE, Synge C. Antibacterial effect of the scandium and indium complexes of enterochelin on Escherichia coli. J Gen Microbiol. 1982;128:2389–2394. doi: 10.1099/00221287-128-10-2389. [DOI] [PubMed] [Google Scholar]
- 14.Stojiljkovic I, Kumar V, Srinivasan N. Non-iron metalloporphyrins: Potent antibacterial compounds that exploit haem/Hb uptake systems of pathogenic bacteria. Mol Microbiol. 1999;31:429–442. doi: 10.1046/j.1365-2958.1999.01175.x. [DOI] [PubMed] [Google Scholar]
- 15.Chevion M. Protection against free radical-induced and transition metal-mediated damage: the use of “pull” and “push” mechanisms. Free Radic Res Commun. 1991;12–13(Pt 2):691–696. doi: 10.3109/10715769109145848. [DOI] [PubMed] [Google Scholar]
- 16.Karck M, et al. The push-and-pull mechanism to scavenge redox-active transition metals: A novel concept in myocardial protection. J Thorac Cardiovasc Surg. 2001;121:1169–1178. doi: 10.1067/mtc.2001.113325. [DOI] [PubMed] [Google Scholar]
- 17.Spoering AL, Lewis K. Biofilms and planktonic cells of Pseudomonas aeruginosa have similar resistance to killing by antimicrobials. J Bacteriol. 2001;183:6746–6751. doi: 10.1128/JB.183.23.6746-6751.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hazlett LD. Corneal response to Pseudomonas aeruginosa infection. Prog Retin Eye Res. 2004;23:1–30. doi: 10.1016/j.preteyeres.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 19.Cheng KH, et al. Incidence of contact-lens-associated microbial keratitis and its related morbidity. Lancet. 1999;354:181–185. doi: 10.1016/S0140-6736(98)09385-4. [DOI] [PubMed] [Google Scholar]
- 20.Llamas MA, et al. The heterologous siderophores ferrioxamine B and ferrichrome activate signaling pathways in Pseudomonas aeruginosa. J Bacteriol. 2006;188:1882–1891. doi: 10.1128/JB.188.5.1882-1891.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Banin E, Berenshtein E, Kitrossky N, Pe'er J, Chevion M. Gallium-desferrioxamine protects the cat retina against injury after ischemia and reperfusion. Free Radic Biol Med. 2000;28:315–323. doi: 10.1016/s0891-5849(99)00227-0. [DOI] [PubMed] [Google Scholar]
- 22.Banin E, et al. Injury induced by chemical warfare agents: Characterization and treatment of ocular tissues exposed to nitrogen mustard. Invest Ophthalmol Vis Sci. 2003;44:2966–2972. doi: 10.1167/iovs.02-1164. [DOI] [PubMed] [Google Scholar]
- 23.Holloway BW, Krishnapillai V, Morgan AF. Chromosomal genetics of Pseudomonas. Microbiol Rev. 1979;43:73–102. doi: 10.1128/mr.43.1.73-102.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Davies DG, et al. The involvement of cell-to-cell signals in the development of a bacterial biofilm. Science. 1998;280:295–298. doi: 10.1126/science.280.5361.295. [DOI] [PubMed] [Google Scholar]
- 25.Pardee AB, Jacob F, Monod J. The genetic control and cytoplasmic expression of ‘inducibility’ in the synthesis of β-galactosidase in Escerichia coli. J Mol Biol. 1959;28:165–178. [Google Scholar]
- 26.Jacobs MA, et al. Comprehensive transposon mutant library of Pseudomonas aeruginosa. Proc Natl Acad Sci USA. 2003;100:14339–14344. doi: 10.1073/pnas.2036282100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schuster M, Hawkins AC, Harwood CS, Greenberg EP. The Pseudomonas aeruginosa RpoS regulon and its relationship to quorum sensing. Mol Microbiol. 2004;51:973–985. doi: 10.1046/j.1365-2958.2003.03886.x. [DOI] [PubMed] [Google Scholar]
- 28.Bailey J, Manoil C. Genome-wide internal tagging of bacterial exported proteins. Nat Biotechnol. 2002;20:839–842. doi: 10.1038/nbt715. [DOI] [PubMed] [Google Scholar]
- 29.O'Toole GA, Kolter R. Initiation of biofilm formation in Pseudomonas fluorescens WCS365 proceeds via multiple, convergent signaling pathways: A genetic analysis. Mol Microbiol. 1998;28:449–461. doi: 10.1046/j.1365-2958.1998.00797.x. [DOI] [PubMed] [Google Scholar]
- 30.Parsek MR, Greenberg EP. Quorum sensing signals in development of Pseudomonas aeruginosa biofilms. Methods Enzymol. 1999;310:43–55. doi: 10.1016/s0076-6879(99)10005-3. [DOI] [PubMed] [Google Scholar]
- 31.Banin E, Brady KM, Greenberg EP. Chelator-induced dispersal and killing of Pseudomonas aeruginosa cells in a biofilm. Appl Environ Microbiol. 2006;72:2064–2069. doi: 10.1128/AEM.72.3.2064-2069.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hentzer M, et al. Alginate overproduction affects Pseudomonas aeruginosa biofilm structure and function. J Bacteriol. 2001;183:5395–5401. doi: 10.1128/JB.183.18.5395-5401.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Morad Y, et al. Treatment of ocular tissues exposed to nitrogen mustard: Beneficial effect of zinc desferrioxamine combined with steroids. Invest Ophthalmol Vis Sci. 2005;46:1640–1646. doi: 10.1167/iovs.04-1165. [DOI] [PubMed] [Google Scholar]
- 34.Bennett HG, Hay J, Kirkness CM, Seal DV, Devonshire P. Antimicrobial management of presumed microbial keratitis: Guidelines for treatment of central and peripheral ulcers. Br J Ophthalmol. 1998;82:137–145. doi: 10.1136/bjo.82.2.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bourcier T, Thomas F, Borderie V, Chaumeil C, Laroche L. Bacterial keratitis: Predisposing factors, clinical and microbiological review of 300 cases. Br J Ophthalmol. 2003;87:834–838. doi: 10.1136/bjo.87.7.834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schaefer F, Bruttin O, Zografos L, Guex-Crosier Y. Bacterial keratitis: A prospective clinical and microbiological study. Br J Ophthalmol. 2001;85:842–847. doi: 10.1136/bjo.85.7.842. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.