Abstract
Purinergic ionotropic P2X7 receptors (P2X7Rs) are closely associated with excitotoxicity and nociception. Inhibition of P2X7R activation has been considered as a potentially useful strategy to improve recovery from spinal cord injury and reduce inflammatory damage to trauma. The physiological functions of P2X7Rs, however, are poorly understood, even though such information is essential for making the P2X7R an effective therapeutic target. We show here that P2X7Rs in satellite cells of dorsal root ganglia tonically inhibit the expression of P2X3Rs in neurons. Reducing P2X7R expression using siRNA or blocking P2X7R activity by antagonists elicits P2X3R up-regulation, increases the activity of sensory neurons responding to painful stimuli, and evokes abnormal nociceptive behaviors in rats. Thus, contrary to the notion that P2X7R activation is cytotoxic, P2X7Rs in satellite cells play a crucial role in maintaining proper P2X3R expression in dorsal root ganglia. Studying the mechanism underlying the P2X7R–P2X3R control, we demonstrate that activation of P2X7Rs evokes ATP release from satellite cells. ATP in turn stimulates P2Y1 receptors in neurons. P2Y1 receptor activation appears to be necessary and sufficient for the inhibitory control of P2X3R expression. We further determine the roles of the P2X7R–P2Y1–P2X3R inhibitory control under injurious conditions. Activation of the inhibitory control effectively prevents the development of allodynia and increases the potency of systemically administered P2X7R agonists in inflamed rats. Thus, direct blocking P2X7Rs, as proposed before, may not be the best strategy for reducing pain or lessening neuronal degeneration because it also disrupts the protective function of P2X7Rs.
Keywords: dorsal root ganglia, neuron–glia communication, P2Y1, pain, inflammation
P2X3 receptors (P2X7Rs) and P2X7Rs receptors (P2X7Rs) are expressed in the spinal cord and dorsal root ganglia (DRGs) (1, 2) and are involved in neuron–glia communication (3, 4). P2X3Rs have been linked to nociceptive signaling (5–10) and become sensitized after inflammation or nerve injury (11–13). Most P2X7Rs are found in immune or glial cells, although limited neuronal expression of P2X7R has been documented (14–16). P2X7Rs play a prominent role in excitotoxicity and nociception (15–19). Activation of P2X7Rs has been associated with the maturation and release of proinflammatory cytokines from glial cells (20), which can cause an increase in neuronal excitability (4, 21) and exaggerated nociception (22, 23). Spinal cord injury induces ATP release in the spinal cord and elicits apoptosis (15, 24). Application of P2X7R antagonists to the spinal cord promotes cell survival and improves the locomotive behavioral score of injured rats (15). P2X7R knock-out mice fail to develop hyperalgesia or allodynic pain after inflammation, anti–collagen-induced arthritis, or nerve ligation (22, 23). It is therefore suggested that blocking P2X7R activation is a valid strategy to treat spinal cord injuries and control chronic pain (15, 23). A majority of the studies of P2X7R functions were conducted under pathological conditions (16). Few have addressed the physiological function of P2X7R activation. We therefore determined P2X7R–P2X3R interactions in DRGs, which contain a group of primary sensory neurons responsible for transmitting somatosensory information including touch, itch, and pain to the spinal cord. Studying the expression of P2X3Rs and P2X7Rs in DRGs, we found, contrary to previous observations, that P2X7R activation in satellite cells down-regulates P2X3R expression in neurons. Disrupting the P2X7R–P2X3R inhibitory control exacerbates abnormal pain behaviors in rats. Purinergic 2Y1 receptor (P2Y1R) activation in neurons is necessary and sufficient for P2X7R inhibitory regulation.
Results
P2X7R Activation in Satellite Cells Down-Regulates P2X3R Expression in Neurons.
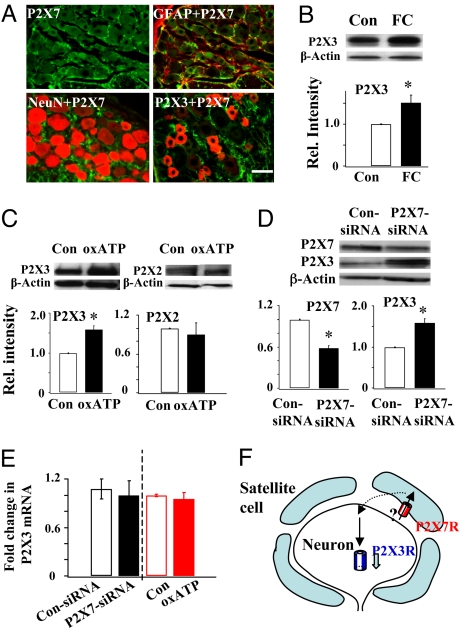
We first determined the location of P2X7Rs and P2X3Rs in DRGs by double labeling them with the respective antibody and the glia marker glial fibrillary acid protein (GFAP) or the neuronal marker neuronal nuclei (NeuN). P2X7Rs were found only in GFAP-labeled satellite cells and not in NeuN-labeled neurons (Fig. 1A). In contrast, P2X3Rs were expressed only in neurons, in agreement with previous studies (2, 25). This mutually exclusive expression greatly facilitates our study of interactions between neurons and satellite cells. We next studied the P2X3R expression following disruption of the activity of satellite cells using the glia Kreb cycle inhibitor fluorocitrate (FC) (26), P2X3R expression became significantly higher (Fig. 1B) after the FC treatment, suggesting that satellite cell activity profoundly affects the expression of P2X3Rs in DRG neurons. To determine if P2X7Rs play any role in P2X3R expression, we blocked P2X7R activation in L4–5 DRGs using the irreversible P2X7R antagonist oxidized ATP (oxATP) and examined the P2X2R and P2X3R expression. The block had no effect on the P2X2R expression but significantly increased P2X3R expression (Fig. 1C). We further confirmed the observations by studying the effect of intrathecal P2X7-siRNA on P2X3R expression. Compared with saline solution- and control siRNA–treated DRGs [supporting information (SI) Fig. S1], P2X7R expression in P2X7-siRNA rats was significantly reduced (Fig. 1D). The activity of P2X7Rs in DRGs of P2X7-siRNA rats, tested with Ca2+ imaging analyses, was also greatly diminished (Fig. S2). The P2X3R expression, conversely, became substantially up-regulated (Fig. 1D). This P2X3R up-regulation is not a result of a change in transcription because P2X3R mRNA was not altered by P2X7-siRNA or by oxATP treatment (Fig. 1E). These results suggest that tonic activation of P2X7Rs exerts an inhibitory control specifically on the expression of P2X3Rs in DRGs (Fig. 1F).
Fig. 1.
Activation of P2X7Rs in satellite cells down-regulates P2X3R expression in neurons. (A) Immunocytochemical analyses of P2X7R and P2X3R expression in DRGs. P2X7Rs (labeled green) were found only in GFAP-labeled (red) satellite cells, but not in NeuN-labeled (red) neurons. P2X3Rs (labeled red) were found only in DRG neurons, not co-localized with P2X7Rs. (B) Western analysis showed that blocking satellite glial cells with the glia Kreb cycle inhibitor FC significantly increased P2X3R expression in DRGs (FC/control = 1.52 ± 0.18, n = 3, *P < 0.05). (C) After blocking P2X7R activity with oxATP, P2X3R expression was significantly higher (P2X3: oxATP/control = 1.59 ± 0.10, n = 8, *P < 0.05), whereas P2X2R expression was unchanged (P2X2: oxATP/control = 0.90 ± 0.18, n = 3; P > 0.05). (D) Down-regulating P2X7Rs with P2X7-siRNA (P2X7: P2X7-siRNA/control siRNA = 0.58 ± 0.04, n = 4, *P < 0.05) resulted in an increase in P2X3R expression (P2X3: P2X7-siRNA/control siRNA 1.65 ± 0.23, n = 5, *P < 0.05). (E) P2X3 mRNA did not change after treatment with P2X7-siRNA (control siRNA: 1.08 ± 0.12, n = 5; P2X7-siRNA: 1.00 ± 0.18, n = 7, P > 0.05) or oxATP (control 1.00 ± 0.01, n = 4; oxATP: 0.95 ± 0.08, n = 6, P > 0.05). (F) A schematic showing the inhibitory control of P2X3R expression by P2X7Rs.
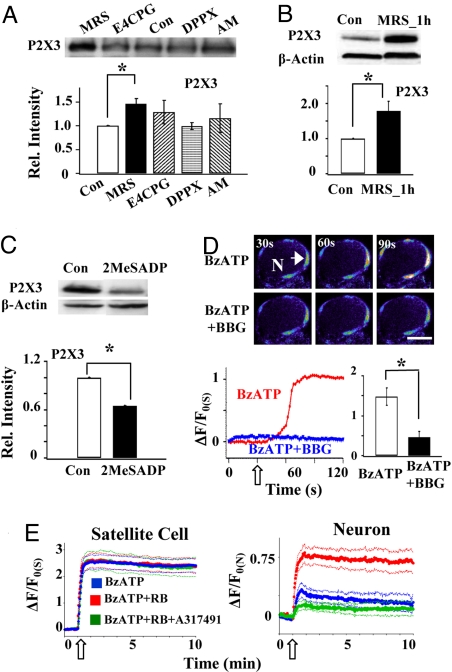
To determine the mechanism underlying P2X7R–P2X3R control, the participation of purinergic 2Y1 (P2Y1), adenosine A1, group I/II metabotropic glutamate, and cannabinoid CB1 receptors was tested by studying the P2X3R expression following a 3-h incubation of their respective antagonists (Fig. 2A). We found that only the P2Y1R antagonist MRS2179 significantly increased the P2X3R expression. The increase could be observed even after 1 h of MRS2179 treatment (Fig. 2B). These observations suggest the involvement of P2Y1Rs in the P2X3R expression. Consistent with the finding, we found that the P2Y1R agonist 2-methylthio-ADP (2MeSADP) inhibited the expression of P2X3Rs (Fig. 2C). The functional effect of P2YR activation was studied using Ca2+ imaging analyses in whole ganglia. Activation of P2X7Rs by the P2X7R agonist 2′-3′-O-(4-benzoylbenzoyl)-ATP (BzATP) elicited a large increase in intracellular Ca2+ ([Ca2+]i) in satellite cells that was inhibited by the P2X7R antagonist brilliant blue G (BBG; Fig. 2D). Following preincubation of DRGs with the P2YR antagonist reactive blue 2 (RB), BzATP did not further increase [Ca2+]i in satellite cells. Conversely, in the presence of RB, [Ca2+]i in neurons increased significantly. The enhancement was mediated by P2X3R because the P2X3R antagonist A317491 blocked the increase (Fig. 2E). These results are all consistent with the idea that blocking P2Y1Rs up-regulates P2X3R expression.
Fig. 2.
Activation of P2Y1Rs inhibits the expression and activity of P2X3Rs. (A) Treating DRGs with the P2Y1R antagonist MRS2179 (60 μM) for 3 h increased the P2X3R expression (1.46 ± 0.11, n = 3, *P < 0.05). Conversely, P2X3R expression was not affected by 3 h of incubation of the group I/II metabotropic glutamate antagonist E4CPBG (1 mM; 1.29 ± 0.25, n = 3, P > 0.05), the adenosine A1 receptor antagonist 1,3-diporpyl-8-phenylxanthine (DPPX, 200 nM; 0.99 ± 0.07, n = 3, P > 0.05) or the cannabinoid CB1 antagonist AM251 (2 μM; 1.16 ± 0.30, n = 3, P > 0.05). (B) MRS2179 (60 μM) could change P2X3R expression within 1 h of its incubation (1.79 ± 0.28, n = 4, P < 0.05). (C) Incubating DRGs with the P2Y1R agonist 2MeSADP (100 μM) reduced the expression of P2X3Rs in DRGs (0.65 ± 0.10, n = 3, *P < 0.05). (D) The P2X7 agonist BzATP (30 μM) increased [Ca2+]i in satellite cells. Pseudocolor images of a DRG neuron (N) with surrounding satellite cells showed a large fluorescence increase in satellite cells following the application of BzATP. The number at the upper left corner in each frame indicates the time at which the image was taken. Below is the time course of relative fluorescence changes in one of the satellite cells (arrow), i.e., (ΔF/Fo(S)) = [(F-Fo)/Fo] × 100 where Fo is the basal fluorescence in the satellite cell before drug application. The empty arrow indicates the starting time of BzATP application. Bar = 20 μm. The bar graph indicates that activation of P2X7Rs by BzATP increased [Ca2+]i in satellite cells, and the increase was inhibited by 20 min preincubation with BBG (1 μM) (BzATP: 148 ± 22%; BzATP + BBG: 46 ± 14%, n = 23, *P < 0.05). (E) BzATP (100 μM) elicited a large ΔF/Fo(S) increase in satellite cells, but a rather small change in ΔF/Fo(N) in neurons. A 1-h preincubation of the DRG with the P2YR antagonist RB (1 μM) did not further increase [Ca2+]i in satellite cells. Conversely, [Ca2+]i in neurons increased significantly in the presence of RB. This large increase in neuronal [Ca2+]i was blocked when the P2X3 antagonist A317491 (60 μM) was applied with BzATP to the DRG, suggesting P2X3Rs mediate the increase. The thick lines are the average [Ca2+]i responses of 10 to 15 neurons or those of 16 to 26 satellite cells. The thin lines represent SEs of the average values. Empty arrows indicate the starting time of drug applications.
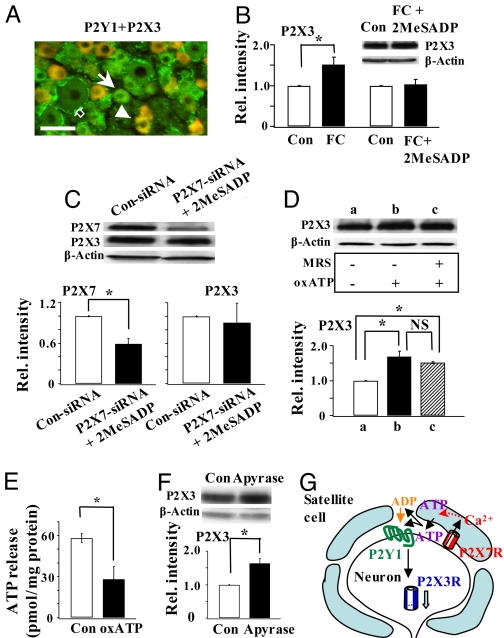
Immunocytochemical studies showed that many small neurons co-expressed P2X3 and P2Y1 receptors. Only a few satellite cells were immunostained for P2Y1Rs (Fig. 3A). To determine if P2Y1Rs in neurons mediate the 2MeSADP action, we disrupted the activity of satellite cells by FC. Although FC alone up-regulated P2X3Rs (Fig. 1B), FC, in the presence of 2MeSADP, no longer could affect P2X3R expression (Fig. 3B). Thus, 2MeSADP exerted its inhibitory control even when satellite cell activity was destroyed, suggesting that P2Y1Rs in neurons, not the P2Y1Rs in satellite cells, participate in the regulation of P2X3Rs. Because FC treatment blocked the function of P2X7Rs in satellite cells, we studied the effect of 2MeSADP on P2X3R expression in P2X7-siRNA rats. Down-regulation of P2X7Rs increased P2X3R expression (Fig. 1D). However, P2X3Rs were no longer up-regulated in the presence of 2MeSADP (Fig. 3C), suggesting that neuronal P2Y1R activation alone is sufficient to inhibit P2X3R expression. Consistent with this conclusion, we found that a 1-h treatment of DRG neurons with the agonist 2MeSADP produced a long-lasting decrease in the amplitude of α,β-methylene ATP (meATP)–induced currents without altering the kinetics of the currents (SI Materials and Methods, Fig. S3).
Fig. 3.
P2Y1R activation is necessary and sufficient for the regulation of P2X3R expression by P2X7Rs. (A) P2Y1Rs were co-expressed with P2X3Rs in a number of small DRG neurons (arrowhead). Some neurons expressed only P2Y1Rs (arrow). Only a few satellite cells were found to express P2Y1Rs (empty arrow). Bar = 50 μm. (B) P2X3R expression increased after FC treatment (FC/control = 1.52 ± 0.18, n = 3, *P < 0.05; data were re-plotted from Fig. 1B). In the presence of 2MeSADP, FC could no longer change P2X3R expression ([FC + 2MeSADP]/control:1.03 ± 0.12, n = 3, P > 0.05). (C) Activation of P2Y1R is sufficient for the inhibitory control of P2X3R expression. Down-regulation of P2X7Rs no longer elicited an increase in P2X3R expression when rats were treated with 2MeSADP (P2X7R: 0.59 ± 0.08, n = 3, *P < 0.05; P2X3R: 0.9 ± 0.29, n = 3, P > 0.05). (D) In the presence of oxATP, P2X3R expression was up-regulated (oxATP/control 1.68 ± 0.16, *P < 0.05, n = 2). Blocking the activity of P2Y1R by MRS could not further affect the expression of P2X3Rs ([MRS + oxATP]/control: 1.51 ± 0.03, *P < 0.05, n = 2; oxATP/[MRS + oxATP] = 1.11 ± 0.09, n = 2, P > 0.05). (E) P2X7Rs are involved in the basal ATP release. Basal ATP release was much reduced when P2X7R activity was blocked by oxATP (control: 58.03 ± 3.56 pmol/mg protein, oxATP: 28.13 ± 9.16 pmol/mg, n = 3, *P < 0.05). (F) Degrading ATP and ADP by apyrase (20 U/ml) significantly relieved the inhibitory control of P2X3R expression (apyrase/control: 1.64 ± 0.15, n = 3,*P < 0.05). (G) Schematic shows the involvement of P2Y1Rs in P2X7R–P2X3R inhibitory control.
We then determined if activation of P2Y1R is dependent on the endogenous activity of P2X7Rs. The effect of P2Y1R inhibitor MRS2179 on P2X3R expression was studied when the activation of P2X7R was blocked by oxATP (Fig. 3D). MRS2179 no longer could affect the expression of P2X3Rs under this condition (Fig. 3D). This observation suggests that P2Y1R inhibitory control of P2X3R expression requires the activation of P2X7Rs. If the P2Y1R action occurs downstream of P2X7R activation, ATP should be released following the activation of P2X7Rs. To test this, the release of endogenous ATP from ganglia was measured with a luciferase assay. Treatment of oxATP greatly reduced the basal ATP release (Fig. 3E), suggesting that P2X7Rs are endogenously active and mediate a significant portion of ATP release from satellite cells. To determine the consequence of the release, we used the degrading enzyme apyrase to lower extracellular ATP and ADP levels and found that apyrase relieved the inhibition of P2X3R expression (Fig. 3F). The extent of P2X3R up-regulation in the absence of both P2X7R and P2Y1R activation following apyrase application (Fig. 3F) was indistinguishable from the up-regulation following the block of only P2X7Rs by oxATP (Figs. 1C and 3D). Taken together, these results are consistent with the idea that ATP released from endogenously active P2X7Rs elicits the activity of neuronal P2Y1Rs to control the P2X3R expression. Because blocking P2Y1Rs prevents the inhibitory control of P2X3Rs by P2X7Rs (Fig. 2 A and B), P2Y1R activation is required for the down-regulation of P2X3Rs. These results (Figs. 2 A and B, 3 B and C) have led us to suggest that P2Y1R activation is downstream of P2X7R signaling. Its activation is necessary and sufficient for the P2X7R inhibitory control of P2X3R expression (Fig. 3G).
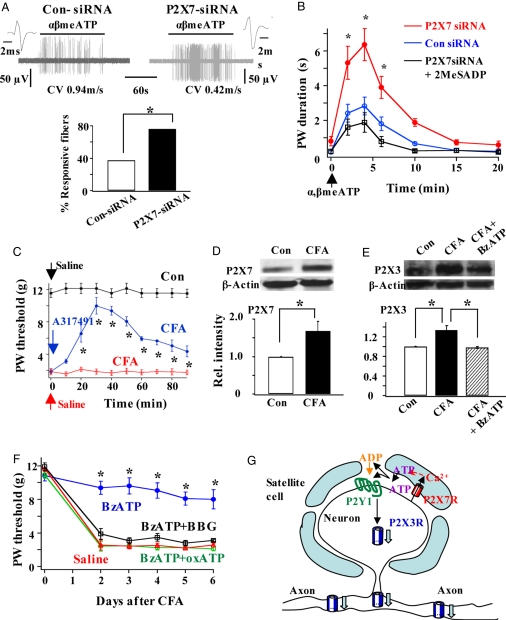
The functional consequences of P2X7R–P2X3R inhibitory control were explored. Following their synthesis in DRGs, P2X3Rs are transported to peripheral and central nerve terminals for sensory signaling. The responses of nerve terminals to α,β-meATP in a skin-nerve preparation isolated from control siRNA and P2X7-siRNA rats were compared (Fig. 4A). We found that a much higher percentage of nociceptive fibers responded to α,β-meATP when P2X7Rs were down-regulated. The behavioral consequence of P2X7R down-regulation under physiological conditions was also examined. Application of α,β-meATP to the rat hind paw elicited repetitive lifting of the paw (i.e., flinching; Fig. 4B), which is known to be P2X3R–mediated (13). Following knock-down of P2X7Rs, nociceptive flinching responses were greatly exaggerated. The exaggerated responses were blocked when P2Y1Rs were activated by 2MeSADP. Thus, the activation of P2X7Rs and P2Y1Rs significantly blunts the P2X3R-elicited nociceptive responses.
Fig. 4.
Functional significance of the inhibitory control of P2X3R expression by P2X7Rs. (A) The percentage of fibers responding to α,β-meATP increased in P2X7-siRNA rats. (Upper) Examples of the activity of fibers isolated from a skin-nerve preparation of a control siRNA rat and of a P2X7R-siRNA rat. (Lower) In 5 of 13 cases (38.4%) Aδ and C fibers recorded in skin-nerve preparations isolated from control siRNA rats responded to α,β-meATP. A much higher percentage of fibers (16 of 21; 76.2%) isolated from P2X7-siRNA rats were sensitive to α,β-meATP (χ2 test, *P < 0.05). (B) Flinch behavioral responses to α,β-meATP were much enhanced in P2X7-siRNA rats. The enhanced responses were reversed in rats treated with intrathecal P2X7-siRNA + 2MeSADP (n = 4–7; ANOVA, *P < 0.05). (C) CFA-produced allodynia was P2X3R-mediated. Compared with the PBS solution-injected rats, the paw withdrawal threshold to von Frey stimulation in CFA rats was much reduced. The allodynia in CFA rats was reversed when the P2X3R activation was blocked by an intraplantar injection of the P2X3R antagonist A317491 (6 mM, 50 μl) into the tested paw (*P < 0.05 vs. CFA rat group; n = 5). Arrows indicate the time of saline solution or A317491 injection. (D) P2X7R expression was enhanced in rats inflamed with CFA (P2X7: CFA/control = 1.68 ± 0.26, n = 9, *P < 0.05). (E) Although P2X3R expression was higher after inflammation (CFA/control = 1.34 ± 0.09, n = 3, *P < 0.05), P2X3R expression in CFA + BzATP rats was not different from that in control rats ([CFA + BzATP]/control = 0.98 ± 0.02, n = 3, P > 0.05). (F) Activation of P2X7R by pretreatment of rats with BzATP effectively blocked the development of CFA-induced allodynia. When the P2X7R activation was blocked by BBG or oxATP, BzATP could no longer prevent the development of allodynia. Six animals were tested in each rat group (*P < 0.05 vs. saline solution, BzATP + BBG or BzATP + oxATP rat group). (G) The proposed P2X7R–P2Y1R–P2X3R inhibitory control results in a decrease in P2X3Rs at afferent terminals and thus a reduction in nociception.
We then asked if maintaining a proper control of P2X3R expression by P2X7R activation is a valid therapeutic strategy for chronic pain control. The roles of P2X3R and P2X7R in nociception were studied in complete Freund adjuvant (CFA)-induced inflamed rats. As the P2X3R antagonist A317491 reversed the reduction of paw withdrawal threshold to von Frey filament stimulation induced by CFA (Fig. 4C), P2X3Rs clearly mediate the inflammation-elicited allodynia. When the expression of P2X3R and P2X7R were studied in CFA rats, we found that P2X7R level was significantly elevated (Fig. 4D). Immunocytochemical studies of P2X7R expression indicate that increase could be attributed mostly to an increase in P2X7Rs in satellite cells (Fig. S4). As shown before (12), inflammation enhances P2X3R expression in DRGs. P2X3R expression was then investigated in P2X7-siRNA-treated CFA rats. Down-regulation of P2X7Rs again increased P2X3R expression (Fig. S5), suggesting that endogenous P2X7R-P2X3R inhibitory control remains after inflammation. We observed an increase in P2X3R in CFA rats, even though P2X7R expression was increased (Fig. 4E). The endogenous P2X7R activity clearly was not sufficient to block all of the up-regulation of P2X3R induced by CFA. We therefore activated P2X7Rs further by treating CFA rats with BzATP and found that P2X3R expression returned to the control level (Fig. 4E). To determine the behavioral consequence of the BzATP treatment, we studied the development of allodynia in CFA rats. When P2X7Rs were activated by BzATP, allodynia failed to develop (Fig. 4F). This anti-allodynic effect of BzATP was blocked when BzATP was applied with the P2X7 antagonist BBG or oxATP (Fig. 4F). Thus, activation of P2X7Rs effectively blocks inflammation-induced allodynia. These observations suggest that activation of P2X7R-P2X3R is likely a new strategy to control abnormal nociceptive responses under chronic inflammatory conditions.
To determine the impact of the P2X7R–P2X3R inhibitory control on the antinociceptive effect of the systemically applied P2X7R antagonist oxATP, the dose-response curves of i.p. applied oxATP in the absence and presence of the intrathecal P2Y1R agonist 2MeSADP were examined in CFA rats. Activation of P2Y1R in DRGs increased the potency of i.p. oxATP by 3.57 fold (SI Materials and Methods and Fig. S6). We conclude that the P2X7R–P2Y1R–P2X3R inhibitory control observed in DRGs not only affects peripheral nociception, but also influences the systemic use of P2X7 antagonists in pain treatment.
Discussion
Thus far, P2X7R has been considered as a receptor participating in cell injury. Its activation exacerbates nociceptive behavioral responses (23) and produces apoptosis (15). It is therefore unexpected to find that activation of P2X7Rs in DRGs results in the inhibition of P2X3R expression (Fig. 1) and reduces α,β-meATP-elicited nociception (Fig. 4B) under physiological conditions. This inhibitory control is an effective protective mechanism used by sensory ganglia to maintain an optimal P2X3R activity. Even more significant, we found that activation of P2X7Rs by BzATP in DRGs largely abolishes inflammation-induced allodynia (Fig. 4F) and activation of P2Y1Rs in DRG neurons substantially increases the potency of i.p. P2X7R antagonist for pain control (SI Materials and Methods and Fig. S6). The reason for the opposite consequence of P2X7R activation on nociception in the spinal cord (23) and in DRGs has yet to be determined. There are obvious differences in the location of P2X7Rs and the way the receptor exerts its effects in the spinal cord and DRGs. P2X7Rs have been found in glial cells (27) and in numerous glutamatergic presynaptic terminals of dorsal and ventral horn neurons in the spinal cord (14, 15, 24, 28). Their activation is likely to facilitate the release of glutamate, thus producing neurotoxicity in neurons (14). Conversely, in DRGs, P2X7Rs are located in the satellite glial cells but not in neurons (Fig. 1A). Therefore, P2X7R exerts its effects almost exclusively through satellite–neuron interactions in the ganglia.
We have shown that the P2Y1R is a critical link in the P2X7R–P2X3R inhibitory control (Fig. 3). P2Y1Rs are expressed mostly in small and medium DRG neurons (Fig. 3A). Many of them are co-localized with P2X3Rs. These results are consistent with the 2003 report by Ruan and Burnstock (29), who found that P2X3Rs and P2Y1Rs are co-localized in more than 80% of DRG neurons. Our results suggest that neuronal rather than glial P2Y1Rs regulate the expression in P2X3Rs (Fig. 3B). A block of P2Y1Rs can lead to an increase in P2X3R expression in 1 h (Fig. 2B). Because the P2X3R mRNA level does not change (Fig. 1E), rapid translation and/or posttranslation modifications of P2X3Rs would have to occur. Recent studies of metabotropic glutamate receptor-mediated long-term depression have shown that rapid translation of activity-regulated cytoskeleton associated protein can occur within 10 min of metabotropic glutamate receptor activation in the hippocampus (30). The question of how the P2Y1R controls the expression of P2X3Rs remains to be established. P2Y1Rs have been shown to inhibit P2X3R currents by facilitating current desensitization (31, 32). This mechanism does not appear to participate in the functional change observed here because treatment of DRG neurons with the P2Y1R agonist 2-methylthioadenosine diphosphate does not alter the desensitization of α,β-meATP currents (SI Materials and Methods and Fig. 7). One cannot eliminate the possibility, however, that other mechanisms, such as phosphorylation of P2X3Rs or altering the trafficking of P2X3Rs, contribute to changes in [Ca2+]i (Fig. 2E) or P2X3R-mediated currents (Fig. S3) following a change in P2Y1R activity.
The mechanism underlying the endogenous activation of P2X7Rs remains to be determined. In contrast to P2X3Rs and P2Y1Rs, P2X7Rs require a much higher concentration of ATP for their activation (1, 33). How can ATP activate P2X7Rs without first stimulating P2X3Rs and P2Y1Rs? How do P2X7Rs maintain tonically active without producing excitotoxicity or desensitizing P2X3Rs? The answers to these questions have yet to be explored. We have shown in a previous study that nerve stimulation can elicit vesicular ATP release from somata of DRG neurons to activate P2X7Rs, thus triggering the communication between neuronal somata and satellite cells (4). The soma of each DRG neuron is tightly wrapped by a layer of satellite glial cells. The gap between the soma and their satellite cells is narrow (≈20 nm) (34). If P2X7Rs are close to the release sites whereas P2X3Rs and P2X7Rs are located further away, the concentration of ATP released from somata in the vicinity of release sites could reach a sufficiently high level (5–500 μM) (35) to locally activate P2X7Rs without activating P2X3Rs or P2Y1Rs. Only after P2X7Rs are activated and a significant ATP is released from satellite cells, the extracellular ATP at P2X3Rs and P2Y1Rs could reach a level to activate them. This is consistent with our observation that the inhibitory control of P2X3R up-regulation by P2Y1Rs depends almost entirely on the activation of P2X7Rs in satellite glial cells (Fig. 3 D–F). The kinetic properties of P2X3R-mediated currents are unusual. Although the development of desensitization of P2X3Rs is independent of temperature and external ATP concentrations, the recovery from desensitization is highly temperature-dependent (36) and sensitive to use-dependent inhibition of nanomolar ATP (36, 37). P2X3R-mediated currents are therefore likely to recover rather quickly from desensitization near body temperature, thus allowing the receptor to be repetitively activated. In addition, the widespread expression of ecto-ATPase and ecto-apyrase (38) prevents extracellular ATP concentration from being elevated for a long period to cause excitotoxic injury.
Nature appears to use the P2X7R–P2X3R negative control to curb excessive activity of P2X3Rs, thus exerting significant antinociceptive effects under normal and injured conditions (Fig. 4 and Fig. S6). The observation that activation of P2Y1Rs increases the potency of i.p. oxATP (Fig. S6) suggests that systematically applied P2X7 antagonists not only block the deleterious effects of P2X7R activation, but also inhibit the protective effects of P2Y1R–P2X3R inhibitory control. We therefore suggest that direct inhibition of P2X7Rs, as proposed before (15, 23), may not be the most effective therapeutic strategy for controlling pain or cell degeneration. Superior strategies would be to aim at P2X7R-associated proteins such as pannexin-1 hemichannels (39, 40), which are directly involved in the damaging effects of P2X7R activation, such as eliciting cytokine release (4, 20) or large pore formation (1, 18), while preserving the protective effects of P2X7R activation observed here.
Materials and Methods
Animals.
All experiments were performed on adult male Spargue-Dawley rats in accordance with the guidelines of National Institutes of Health and were approved by the Institutional Animal Care and Use Committee at the University of Texas Medical Branch. Inflammation was induced by injection of CFA (10 mg/ml, 50 μl peanut oil/saline solution emulsion) into the plantar surface of the rat left hind paw.
Drug Application.
Intrathecal applications of siRNA or drugs were done through a PE10 tube, which was inserted into the subarachnoid space at the level of the S1–S2 vertebrae and threaded rostrally until the tip of the tube reached the lumbar cistern between the L4 and L5 vertebrae. The applied chemicals were limited at the L4–L5 DRG levels by using a small volume (≈10 μl) of the injectant and limiting the rate of injection (3.3 μl/min).
Western Blotting.
Western blot analyses were performed according to the method described by Chen et al (13).
Immunohistochemistry.
Experiments were conducted using the previous described methods of Chen et al (13). Metamorph Image v. 6.1 or ImageJ (National Institutes of Health) was used for analyses.
Calcium Imaging.
Calcium imaging was done on whole L4 or L5 DRGs as described by Zhang et al (4).
Patch Recordings.
L4 and L5 DRG neurons were isolated for whole-cell current recordings using the methods described by Xu and Huang (12).
Single Fiber Recordings.
Fiber activity of sensory neurons was recorded from the medial and lateral plantar nerves in a rat skin-nerve preparation (41).
ATP Measurements.
ATP released from L4–L5 DRGs was measured using the method previously described by Chen et al (13).
Behavioral Experiments.
Nocifensive (i.e., flinching) and allodynia behavioral studies were conducted in rats using a procedure described by Chen et al (13).
Data Analyses.
Data are expressed as mean ± SEM. Differences between two means were analyzed with Student unpaired t tests. Changes in the percentage of responsive fibers were analyzed with the χ2 test. Comparisons between multiple means were done with one-way ANOVA followed by a Tukey post-hoc test. On Hill equation A, P value less than 0.05 was considered significant.
See the SI Materials and Methods for detailed description of animals, drug application, siRNA, real-time PCR, Western blotting, immunohistochemistry, Ca2+ imaging, patch recordings, single-fiber recordings, ATP release measurements, and behavioral experiments.
Supplementary Material
Acknowledgments.
We thank Drs. S. Carlton and J. Du for their help in the skin-nerve preparation. The work was supported by grants from the National Institute of Neurological Disorders and Stroke and National Institute of Dental and Craniofacial Research.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0801793105/DCSupplemental.
References
- 1.North RA. Molecular physiology of P2X receptors. Physiol Rev. 2002;82:1013–1067. doi: 10.1152/physrev.00015.2002. [DOI] [PubMed] [Google Scholar]
- 2.Kobayashi K, et al. Differential expression patterns of mRNAs for P2X receptor subunits in neurochemically characterized dorsal root ganglion neurons in the rat. J Comp Neurol. 2005;481:377–390. doi: 10.1002/cne.20393. [DOI] [PubMed] [Google Scholar]
- 3.Fields RD, Burnstock G. Purinergic signalling in neuron-glia interactions. Nat Rev Neurosci. 2006;7:423–436. doi: 10.1038/nrn1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang X, Chen Y, Wang C, Huang LY. Neuronal somatic ATP release triggers neuron-satellite glial cell communication in dorsal root ganglia. Proc Natl Acad Sci USA. 2007;104:9864–9869. doi: 10.1073/pnas.0611048104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cook SP, Vulchanova L, Hargreaves KM, Elde R, McCleskey EW. Distinct ATP receptors on pain-sensing and stretch-sensing neurons. Nature. 1997;387:505–508. doi: 10.1038/387505a0. [DOI] [PubMed] [Google Scholar]
- 6.Cockayne DA, et al. Urinary bladder hyporeflexia and reduced pain-related behaviour in P2X3-deficient mice. Nature. 2000;407:1011–1015. doi: 10.1038/35039519. [DOI] [PubMed] [Google Scholar]
- 7.Souslova V, et al. Warm-coding deficits and aberrant inflammatory pain in mice lacking P2X3 receptors. Nature. 2000;407:1015–1017. doi: 10.1038/35039526. [DOI] [PubMed] [Google Scholar]
- 8.Engelman HS, MacDermott AB. Presynaptic ionotropic receptors and control of transmitter release. Nat Rev Neurosci. 2004;5:135–145. doi: 10.1038/nrn1297. [DOI] [PubMed] [Google Scholar]
- 9.Liu XJ, Salter MW. Purines and pain mechanisms: recent developments. Curr Opin Investig Drugs. 2005;6:65–75. [PubMed] [Google Scholar]
- 10.Nakatsuka T, Gu JG. P2X purinoceptors and sensory transmission. Pflugers Arch. 2006;452:598–607. doi: 10.1007/s00424-006-0057-6. [DOI] [PubMed] [Google Scholar]
- 11.Hamilton SG, McMahon SB, Lewin GR. Selective activation of nociceptors by P2X receptor agonists in normal and inflamed rat skin. J Physiol. 2001;534:437–445. doi: 10.1111/j.1469-7793.2001.00437.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xu GY, Huang LY. Peripheral inflammation sensitizes P2X receptor-mediated responses in rat dorsal root ganglion neurons. J Neurosci. 2002;22:93–102. doi: 10.1523/JNEUROSCI.22-01-00093.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen Y, Li GW, Wang C, Gu Y, Huang LY. Mechanisms underlying enhanced P2X receptor-mediated responses in the neuropathic pain state. Pain. 2005;119:38–48. doi: 10.1016/j.pain.2005.09.007. [DOI] [PubMed] [Google Scholar]
- 14.Deuchars SA, et al. Neuronal P2X7 receptors are targeted to presynaptic terminals in the central and peripheral nervous systems. J Neurosci. 2001;21:7143–7152. doi: 10.1523/JNEUROSCI.21-18-07143.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang X, et al. P2X7 receptor inhibition improves recovery after spinal cord injury. Nat Med. 2004;10:821–827. doi: 10.1038/nm1082. [DOI] [PubMed] [Google Scholar]
- 16.Sperlagh B, Vizi ES, Wirkner K, Illes P. P2X7 receptors in the nervous system. Prog Neurobiol. 2006;78:327–346. doi: 10.1016/j.pneurobio.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 17.Le Feuvre R, Brough D, Rothwell D. Extracellular ATP and P2X7 receptors in neurodegeneratiom. Eur J Pharmacol. 2002;447:261–269. doi: 10.1016/s0014-2999(02)01848-4. [DOI] [PubMed] [Google Scholar]
- 18.Khakh BS, North RA. P2X receptors as cell-surface ATP sensors in health and disease. Nature. 2006;442:527–532. doi: 10.1038/nature04886. [DOI] [PubMed] [Google Scholar]
- 19.Donnelly-Roberts DL, Jarvis MF. Discovery of P2X7 receptor-selective antagonists offers new insights into P2X7 receptor function and indicates a role in chronic pain states. Br J Pharmacol. 2007;151:571–579. doi: 10.1038/sj.bjp.0707265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Colomar A, et al. Maturation and release of interleukin-1beta by lipopolysaccharide-primed mouse Schwann cells require the stimulation of P2X7 receptors. J Biol Chem. 2003;278:30732–30740. doi: 10.1074/jbc.M304534200. [DOI] [PubMed] [Google Scholar]
- 21.Schafers M, Lee DH, Brors D, Yaksh TL, Sorkin LS. Increased sensitivity of injured and adjacent uninjured rat primary sensory neurons to exogenous tumor necrosis factor-alpha after spinal nerve ligation. J Neurosci. 2003;23:3028–3038. doi: 10.1523/JNEUROSCI.23-07-03028.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Labasi JM, et al. Absence of the P2X7 receptor alters leukocyte function and attenuates an inflammatory response. J Immunol. 2002;168:6436–6445. doi: 10.4049/jimmunol.168.12.6436. [DOI] [PubMed] [Google Scholar]
- 23.Chessell IP, et al. Disruption of the P2X7 purinoceptor gene abolishes chronic inflammatory and neuropathic pain. Pain. 2005;114:386–396. doi: 10.1016/j.pain.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 24.Deng Z, Fyffe RE. Expression of P2X7 receptor immunoreactivity in distinct subsets of synaptic terminals in the ventral horn of rat lumbar spinal cord. Brain Res. 2004;1020:53–61. doi: 10.1016/j.brainres.2004.06.014. [DOI] [PubMed] [Google Scholar]
- 25.Zhang XF, Han P, Faltynek CR, Jarvis MF, Shieh CC. Functional expression of P2X7 receptors in non-neuronal cells of rat dorsal root ganglia. Brain Res. 2005;1052:63–70. doi: 10.1016/j.brainres.2005.06.022. [DOI] [PubMed] [Google Scholar]
- 26.Gordon GR, et al. Norepinephrine triggers release of glial ATP to increase postsynaptic efficacy. Nat Neurosci. 2005;8:1078–1086. doi: 10.1038/nn1498. [DOI] [PubMed] [Google Scholar]
- 27.Suadicani SO, Brosnan CF, Scemes E. P2X7 receptors mediate ATP release and amplification of astrocytic intercellular Ca2+ signaling. J Neurosci. 2006;26:1378–1385. doi: 10.1523/JNEUROSCI.3902-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Atkinson L, et al. Differential co-localisation of the P2X7 receptor subunit with vesicular glutamate transporters VGLUT1 and VGLUT2 in rat CNS. Neuroscience. 2004;123:761–768. doi: 10.1016/j.neuroscience.2003.08.065. [DOI] [PubMed] [Google Scholar]
- 29.Ruan HZ, Burnstock G. Localisation of P2Y1 and P2Y4 receptors in dorsal root, nodose and trigeminal ganglia of the rat. Histochem Cell Biol. 2003;120:415–426. doi: 10.1007/s00418-003-0579-3. [DOI] [PubMed] [Google Scholar]
- 30.Waung MW, Pfeiffer BE, Nosyreva ED, Ronesi JA, Huber KM. Rapid translation of Arc/Arg3.1 selectively mediates mGluR-dependent LTD through persistent increases in AMPAR endocytosis rate. Neuron. 2008;59:84–97. doi: 10.1016/j.neuron.2008.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gerevich Z, Muller C, Illes P. Metabotropic P2Y1 receptors inhibit P2X3 receptor-channels in rat dorsal root ganglion neurons. Eur J Pharmacol. 2005;521:34–38. doi: 10.1016/j.ejphar.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 32.Gerevich Z, et al. Metabotropic P2Y receptors inhibit P2X(3) receptor-channels via G protein-dependent facilitation of their desensitization. Br J Pharmacol. 2007;151:226–236. doi: 10.1038/sj.bjp.0707217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nahum V, et al. Adenosine 5′-O-(1-boranotriphosphate) derivatives as novel P2Y(1) receptor agonists. J Med Chem. 2002;45:5384–5396. doi: 10.1021/jm020251d. [DOI] [PubMed] [Google Scholar]
- 34.Pannese E. The satellite cells of the sensory ganglia. Adv Anat Embryol Cell Biol. 1981;65:1–111. doi: 10.1007/978-3-642-67750-2. [DOI] [PubMed] [Google Scholar]
- 35.Pankratov Y, Lalo U, Verkhratsky A, North RA. Vesicular release of ATP at central synapses. Pflugers Arch. 2006;452:589–597. doi: 10.1007/s00424-006-0061-x. [DOI] [PubMed] [Google Scholar]
- 36.Khmyz V, Maximyuk O, Teslenko V, Verkhratsky A, Krishtal O. P2X(3) receptor gating near normal body temperature. Pflugers Arch. 2008;456:339–347. doi: 10.1007/s00424-007-0376-2. [DOI] [PubMed] [Google Scholar]
- 37.Pratt EB, Brink TS, Bergson P, Voigt MM, Cook SP. Use-dependent inhibition of P2X3 receptors by nanomolar agonist. J Neurosci. 2005;25:7359–7365. doi: 10.1523/JNEUROSCI.5189-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang TF, Guidotti G. Widespread expression of ecto-apyrase (CD39) in the central nervous system. Brain Res. 1998;790:318–322. doi: 10.1016/s0006-8993(97)01562-x. [DOI] [PubMed] [Google Scholar]
- 39.Pelegrin P, Surprenant A. Pannexin-1 mediates large pore formation and interleukin-1beta release by the ATP-gated P2X7 receptor. EMBO J. 2006;25:5071–5082. doi: 10.1038/sj.emboj.7601378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Locovei S, Scemes E, Qiu F, Spray DC, Dahl G. Pannexin1 is part of the pore forming unit of the P2X(7) receptor death complex. FEBS Lett. 2007;581:483–488. doi: 10.1016/j.febslet.2006.12.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Du J, Koltzenburg M, Carlton SM. Glutamate-induced excitation and sensitization of nociceptors in rat glabrous skin. Pain. 2001;89:187–198. doi: 10.1016/s0304-3959(00)00362-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.