Abstract
In the prefrontal cortex, NMDA receptors are important for normal prefrontal functions such as working memory, and their dysfunction plays a key role in the pathological processes of psychiatric disorders such as schizophrenia. Little is known, however, about the synaptic properties of NMDA receptors in the local circuits of recurrent excitation, a leading candidate mechanism underlying working memory. We investigated the NMDA receptor-mediated currents at monosynaptic connections between pairs of layer 5 pyramidal neurons. We found that NMDA receptor-mediated currents at prefrontal synapses in the adult, but not young, rats exhibit a twofold longer decay time-constant and temporally summate a train of stimuli more effectively, compared to those in the primary visual cortex. Experiments with pharmacological, immunocytochemical, and biochemical approaches further suggest that, in the adult animals, neurons express significantly more NR2B subunits in the prefrontal cortex than the visual cortex. The NR2B-rich synapses in the prefrontal circuitry may be critically implicated in online cognitive computations and plasticity in learning, as well as psychiatric disorders.
Keywords: cortical development, persistent activity, working memory, recurrent excitation
What are the microcircuit properties that enable the prefrontal cortex (PFC) to subserve cognitive functions such as working memory and decision making in contrast to early sensory coding and processing in primary sensory areas? To address this central question, physiologists have focused on a salient feature of PFC, namely self-sustained persistent activity, as a candidate neural mechanism for short-term working memory in primates (1–3) and rodents (4). It has been hypothesized that persistent activity is generated by sufficiently strong recurrent excitation among prefrontal neurons (5). The N-methyl-D-aspartate receptors (NMDARs) may be critically involved in persistent activity, as indicated by the findings that NMDAR antagonists impaired performance on delayed response tasks in rat PFC (6, 7). Recently, computational models confirmed that only the slow kinetics of NMDARs could stabilize the active maintenance of memory trace (8–10), and their voltage dependences could enhance the stimulus selectivity of persistent activity (11). Therefore, modeling work suggests that a distinctive feature of PFC is its slow reverberating neural dynamics that depend on the NMDARs in the local recurrent circuits.
This raises the question of whether PFC neurons are endowed with a substantially higher number of NMDARs, or with NMDARs that express distinct biophysical properties, compared with those in a sensory area such as the primary visual cortex V1 (10). One anatomical study has reported a higher level of mRNA for NMDARs in PFC than in other cortices in human postmortem brain tissues (12). Physiologically, little is known about the functional properties of NMDARs in the prefrontal local recurrent circuits. Because prefrontal functions mature more slowly than the visual cortex, and this maturation extends to early adulthood in humans, direct measurement of NMDAR-mediated transmissions at local recurrent synapses in adult animals is needed to address these questions. We investigated the functional properties of the NMDA current at the recurrent synapses between pyramidal cells with multiple patch clamp recordings in the PFC and V1 of adult rats, accompanied with immunocytochemistry and Western blot. We show that NMDARs at PFC recurrent excitatory synapses are distinct from those in V1, with significantly higher expression of NR2B subunits in the prefrontal synapses.
Results
NMDAR-Mediated Current Exhibits a Slower Decay and Larger Charge Transfer at Layer 5 Recurrent Synapses in PFC Compared with V1.
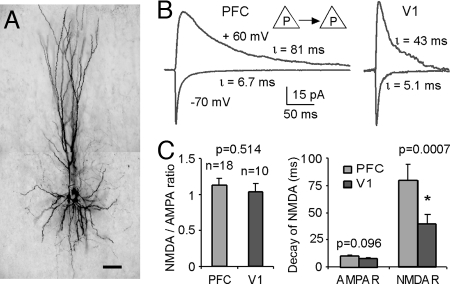
To measure excitatory postsynaptic currents (EPSCs) of recurrent synaptic connections between pyramidal neurons in medial PFC, simultaneous patch clamp recordings were used in layer 5 pyramidal neurons. Fig. 1A shows an example of a quadruple recording of layer 5 pyramidal cells in adult (3–4 months) rat medial PFC. The EPSCs in neuronal connections between layer 5 pyramidal (P-P) neurons were recorded when an action potential was evoked in the presynaptic neuron. At membrane potential −70 mV the EPSCs were dominated by AMPA receptors. The NMDA receptor-mediated currents were recorded at positive potentials +60 mV with both AMPA and GABAA channels were blocked with 6-Cyano-7-nitroquinoxaline-2,3-dione (CNQX) and picrotoxin added to the bathing media (Fig. 1). The reversal potential of the EPSCs was shown to be about +10 mV under our recording conditions [supporting information (SI) Fig. S1].
Fig. 1.
NMDA receptor-mediated currents of excitatory recurrent synapses exhibited a slower decay in PFC versus V1. (A) microphotograph of the biocytin-labeled PFC layer 5 pyramidal neurons from a multiple-cell recording. (Scale bar, 100 μm.) (B) examples of single-pulse recordings of layer 5 P-P pairs from PFC and V1, respectively. When membrane potentials were held at −70 mV, the currents are predominantly mediated by AMPA receptors; whereas at + 60 mV with CNQX and picrotoxin in the bath solution blocking the AMPA and GABAA channels, respectively, the currents were largely mediated by NMDAR channels. The NMDA currents of PFC recurrent synapses showed significantly slower decays compared to those in V1. (C) Summary graph showing the significant differences of NMDAR decays in the two cortical areas (P = 0.0007). In contrast, the NMDA/AMPA receptor ratios were similar, without statistically significant difference (P = 0.514).
Twenty-eight layer 5 connected pairs from adult rats were recorded, 18 from PFC and 10 from V1. Fig. 1B show examples of the AMPA and NMDA receptor-mediated currents at P-P connections in PFC and V1, respectively. In these connections, we observed a similar NMDAR/AMPAR ratio of peak currents in PFC and V1, without significant differences (P = 0.514), in agreement with a previous study conducted in the young (postnatal days 16–21) rat mPFC (13). Amplitudes of both AMPA-EPSCs (16.4 ± 3.69 pA in PFC versus 19.5 ± 5.25 pA in V1, P = 0.526) and NMDA-EPSCs (17.8 ± 2.32 pA in PFC vs. 20.2 ± 4.49 pA in V1, P = 0.172) were slightly lower in PFC recurrent excitatory synapses, without significant difference. On the other hand, the NMDA currents in the prefrontal synapses exhibit a slower decay by more than twofold compared with those in V1 (P = 0.0007). At −70 mV, the EPSCs at PFC recurrent synapses also show a slower decay although not significantly different from those in V1 (P = 0.094, Fig. 1).
In preliminary studies, we recorded some layer 5 P-P connections in the young adult (3–4.5 months) ferret PFC (n = 6) and V1 (n = 6). The NMDA-EPSC of ferret PFC neurons also exhibited significantly slower decay (P < 0.05) and larger charge (P < 0.01) compared to V1, as well as similar NMDA/AMPA peak current ratio (P = 0.674) (see Fig. S2). These results are entirely consistent with findings from adult rat mPFC and V1, suggesting that the synaptic properties of cortical recurrent synapses are consistent across mammalian species.
Temporal Summation in Responses to 20 Hz Repetitive Stimulation at Recurrent Excitatory Synapses in PFC and V1.
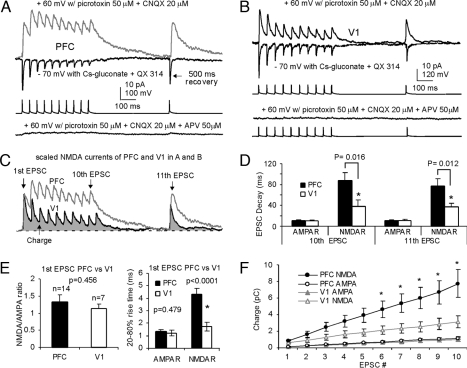
Modeling work (8, 9) has predicted that NMDARs are especially suited for sustaining persistent activity at physiological firing rates. Delay period persistent firing rates of PFC neurons observed in behaving monkeys are in the range of 5–40 Hz and average 15–25 Hz (2, 3). To reveal whether the slower decaying NMDA current would lead to better summation at physiological firing rates as observed in vivo, we applied a 10-pulse train at 20 Hz followed by a recovery pulse (11th). When this protocol was applied to presynaptic neurons (n = 14 in PFC and n = 7 in V1), the NMDAR-EPSCs at PFC recurrent synapses exhibited considerably slower decay and better temporal summation of responses (Table S1). Fig. 2 illustrates examples from PFC and V1, respectively. The NMDA-EPSCs were recorded at +60 mV and were confirmed by bath application of APV (50 μM). Although the AMPA-EPSCs were similar to the PFC connections, the NMDAR-mediated current of the V1 connection (Fig. 2B) exhibited faster decay and smaller charge (time integral) than those in PFC (Fig. 2C). The absolute amplitudes of individual EPSCs exhibited short-term depression for both AMPAR- and NMDAR-mediated components in the two cortical regions, without clear difference (P > 0.05, data not shown). Fig. 2 D–F shows population data with the repetitive stimulation protocol. Much slower decay of PFC NMDA currents were observed compared with those of V1 in the majority of connections. We found that in only 4 out of 14 PFC connections (4/14 = 28.6%), the decay time of the 11th EPSC was shorter than 40 ms (range from 27.1 ms to 138.5 ms), compared with 4 out of 7 V1 connections (4/7 = 57.1%, range from 22.3 ms to 68.1 ms; P = 0.046). The decay time constant of the NMDA currents was significantly slower in PFC for both the 10th (P = 0.016) and the 11th EPSC (P = 0.012, Fig. 2D). The fast decaying NMDA current observed in V1 is in line with a previous study in which an average of ≈40 ms decay time of NMDA current in adult mouse V1 (postnatal day 52) was reported (14). In addition, the 20–80% rise time of NMDA currents in PFC was also significantly slower than that of V1 (P < 0.001), whereas rise time of AMPA currents were similar (P = 0.479, Fig. 2E).
Fig. 2.
Examples and summary showing the population data for the layer 5 P-P connections in PFC (n = 14) and V1 (n = 7) with a 10-pulse train at 20 Hz followed by a recovery pulse. (A and B) a sample P-P connection of PFC (A) and a P-P pair from V1 (B). The NMDA EPSCs were confirmed with APV application in the bath solution. (C) scaled NMDA currents from samples in A and B. Although both AMPA currents in PFC and V1 synapses exhibited short-term depression, the charge carried by PFC synapse was significantly larger than that in V1 synapse. (D and E) summary graphs showing that, although NMDAR/AMPAR ratios of the 1st EPSC are similar (P = 0.456), the decays of the 10th and 11th EPSC of NMDA currents in PFC are significantly slower (P < 0.05). In addition, the rise times of the 1st NMDA currents (20–80%) of the PFC synapses are also significantly slower (P < 0.0001) compared to V1. (F) because of the slower decay of the PFC NMDA current, the EPSCs show a significantly larger charge transfer compared with those from V1 (*, P < 0.05), while the AMPA currents in the two cortical areas were almost identical.
Because of imperfect spatial clamp conditions, the dendritic location of synapses could affect the amplitude and shape of the EPSCs recorded from soma, we wondered if the decay differences of NMDA currents were due to the potential differences of somatodendritic morphologies of PFC and V1 pyramidal neurons (15). This did not seem likely because such a filtering should also change the kinetics of AMPA currents, whereas we found no significant difference in the decay time or rise time in AMPA currents between the two regions. There was also no difference between cortical areas in the latencies of EPSC onset for both AMPA (0.96 ± 0.11 ms in PFC vs. 0.81 ± 0.14 ms in V1; P = 0.127) and NMDA currents (1.33 ± 0.13 PFC vs. 1.16 ± 0.14 V1; P = 0.155). Nevertheless, we calculated the synaptic charges of the EPSCs, which are less affected by dendritic filtering and thus provide a relatively filtering-independent assessment of synaptic conductance (16). The charge transfers of the NMDA and AMPA currents were measured as the integral of the time course of these currents, hence represent direct temporal summation in response to a stimulus train. Because of the slower decays, the charge transfers of the 10-pulse NMDA-EPSCs (20 Hz) in PFC recurrent connections were significantly greater than those in the V1 (P < 0.05 from the 5th to 10th EPSC, Fig. 2F), whereas the charges carried by AMPA currents were nearly identical in PFC and V1 (P > 0.05).
Higher Proportion of NR2B-Containing NMDARs at PFC Recurrent Synapses in Adult but Not Young Rats.
Since the duration of NMDA current is largely controlled by the receptor subunit compositions and the decay time constant of synaptic responses generated by NR1-NR2B receptors is much slower compared to NR1-NR2A receptors (17), we speculated that the ratio of NMDAR subtypes might differ between the two cortical areas. To test this hypothesis, we applied ifenprodil (3 μM, bath) to the perfusion media of the prefrontal (n = 8) and visual (n = 6) cortical preparations. Ifenprodil is a noncompetitive antagonist of the NMDA receptors with higher affinity for NR1/NR2B channels (IC50 = 0.34 μM) compared with NR1/NR2A receptors (IC50 = 146 μM). Ifenprodil blocked 58.5 ± 4.23% of the NMDA currents in prefrontal synapses, compared with only 32.8 ± 6.06% in V1 (P < 0.05, Fig. 3). The finding in V1 is in agreement with a recent study that reported that the NMDA current mediated by NR1/NR2B in V1 neurons declined to approximately 30% in postnatal day 60–70 rats (18). Indeed, NR2A normally dominates the subunit composition of NMDARs in adult V1 neurons (19). In addition, as shown in the EPSC traces scaled by peak amplitude (Fig. 3C), ifenprodil also cause a significant decrease in the NMDA current decay in PFC neurons (τ = 79.2 ± 6.83 ms in control vs. τ = 38.9 ± 4.13 ms in ifenprodil, P < 0.001) and a smaller but significant decrease in the V1 neurons (τ = 40.0 ± 2.13 ms in control vs. τ = 32.3 ± 1.61 ms in ifenprodil, P < 0.05). The scaled EPSCs observed during ifenprodil applications exhibited similar time constant in PFC and V1 (Fig. 3C inset, P = 0.339).
Fig. 3.
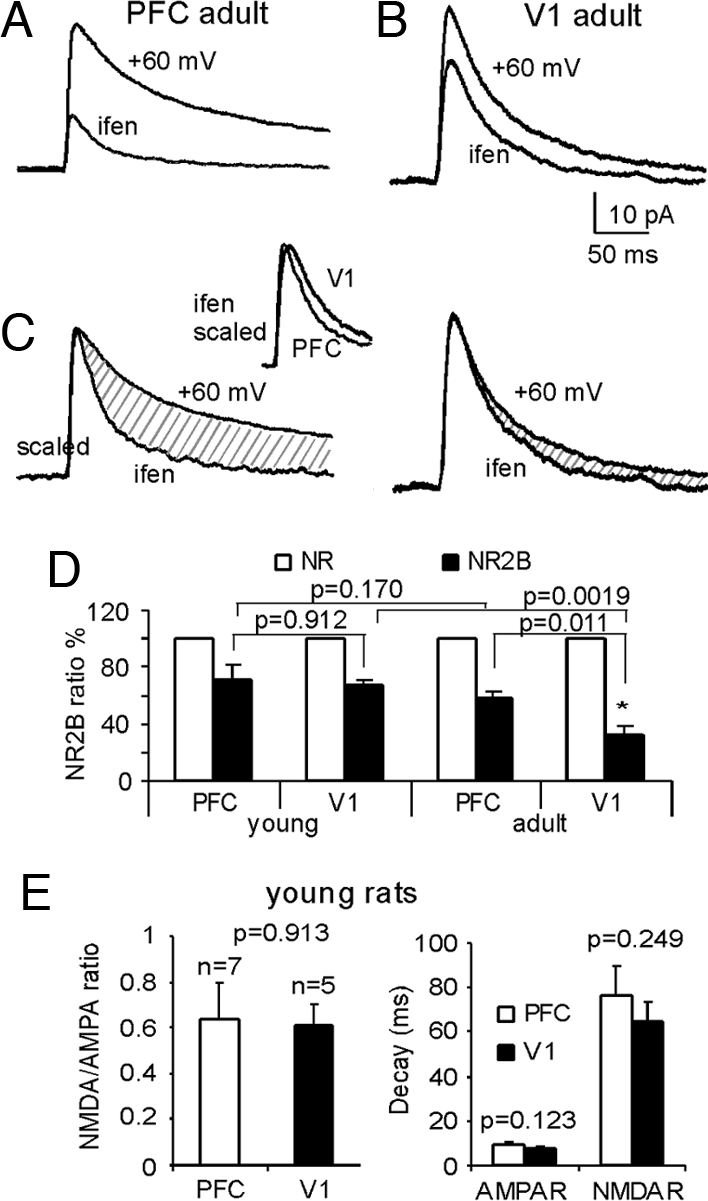
Adult, but not young, PFC recurrent synapses exhibit a higher proportion of NR2B subunits compared to V1. (A and B) the NR2B antagonist ifenprodil (3 μM, bath application) is more effective at blocking the NMDA current in PFC than in V1. (C) EPSCs traces scaled by the peak amplitude, from control and ifenprodil, exhibited significantly reduced decays in both PFC and V1. However, the scaled ifenprodil-EPSCs showed very similar dynamics (inset). (D) summary graph showing a significant decrease of ifenprodil-sensitive currents of NMDA channels in adult V1 cortex (n = 6) but not in PFC (n = 8) synapses. (E) NMDA/AMPA ratios and the decay time constants of NMDA currents are not significantly different in the young rat PFC and V1 (P > 0.05).
This result is different from a recent study that reported no difference between the decay time constant of NMDA current in young (postnatal day 13–29) PFC versus V1 (13). However, virtually all previous studies were conducted using young rats (postnatal age at postnatal day 13–29 and in one study at postnatal day 22–40, see ref 13). We therefore speculated that the differences in the NMDA current decay between PFC and V1 neurons observed in the present study might be attributable to our use of adult animals. To check this possibility, we examined the properties of layer 5 P-P connections of young (postnatal day 13–23) rats using the same procedure as reported in Myme, et al. (13). The neurons were clamped at −60 mV, and magnesium was omitted from the ACSF perfusion solution. With the addition of CNQX and picrotoxin blocking AMPA and GABAA channels, respectively, no epileptic activity appeared, and thus NMDA currents could be recorded. Under this condition, we examined 7 pairs of layer 5 P-P connections in PFC and 5 pairs in V1. As shown in Fig. 3, we again did not observe a significant difference in the NMDAR/AMPAR peak current ratio (P = 0.913). More importantly, in contrast to our observations from the adult rats, in young animals there was also no difference in the decay of NMDA currents (P = 0.249) or AMPA currents (P = 0.123) between PFC and V1 connections. The same holds true for the charge transfer (see Fig. S3). These results are not only consistent with a previous comparison between PFC and V1 in young rats (13) but are also in agreement with numerous previous reports in sensory cortices, particularly in V1, where sensory experience drives characteristic age-dependent changes in the NR2A/NR2B subunit ratio (14, 20). Further experiments showed that the ifenprodil sensitive components in the NMDA currents were similar in the young PFC and V1 (P = 0.912, Fig. 3). This result suggests that, unlike V1 where the NR2B significantly declined in the adult rats (67.3 ± 3.49% versus 32.8 ± 6.06%, P = 0.0019), excitatory recurrent synapses of layer 5 PFC neurons exhibit a small decrease in NR2B expression without significant difference (71.1 ± 10.4% versus 58.5 ± 4.23%, P = 0.171).
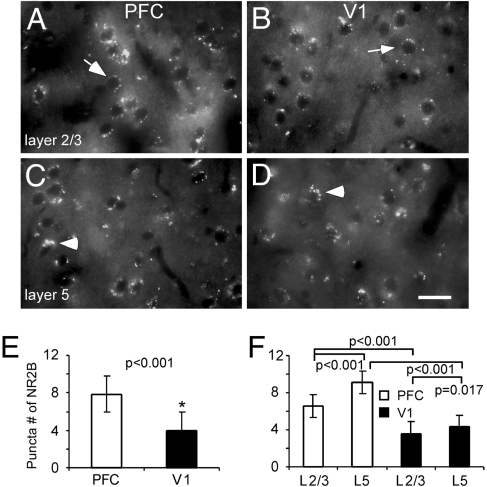
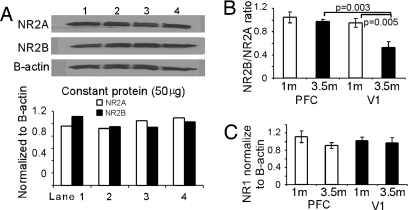
Because laminar differences of NMDAR subunits have been reported in the V1 (21), it is possible that layer 2/3 neurons in the PFC may also show different biophysical properties compared with layer 5 neurons as reported in a recent study in the monkey PFC (22). In other words, the NR2B/NR2A ratio may be layer-specific. If this were true, would overall NR2B levels in the PFC remain significantly higher than in the V1? To address these questions, we have conducted some additional experiments. First, we did immunofluorescence staining to assess NR2B subunit levels in the mPFC and V1 of adult rats (p120 or 4 months) with NR1 as control. The overall pattern of NR2B staining in the mPFC exhibited significant higher density of receptor puncta in each labeled neurons compared with those in V1 (P < 0.001, cell number = 100; Fig. 4). In fact, we also found that layer 5 neurons do express significantly higher levels of NR2B subunits than do layer 2/3 cells in both PFC and V1. Nevertheless, NR2B puncta number in the layer 2/3 neurons in PFC remains significantly higher than that observed in V1 (P < 0.05; n = 50 for each layer). In contrast, the overall distributions of NR1 subunits appeared to be similar in PFC and V1, without significant difference (see Fig. S4). However, again, we observed significant laminar differences in NR1 staining in both cortical areas (P < 0.001). The overall NR2B levels and the developmental changes of individual NR2 subunits in animals at different ages were further analyzed in the PFC and V1 brain tissues with Western blot using NR2A and NR2B antibodies, and beta-actin as the control. Fig. 5 shows the protein expressions of NR1 and NR2 subunits in the rat PFC and V1. Band densities were measured with Image J (NIH image) and the optical densities of the PFC and V1 were normalized to that of beta-actin. Fig. 5A shows the repeated loadings of constant proteins from the same tissue sample of the PFC (3.5 months). It is clear that the constant loadings of the same amount of proteins resulted in a reproducible and proportional NR2A and NR2B subunits in the adult PFC tissue. Fig. 5B summarized the relative ratios of NR2A and NR2B protein expression in the PFC and V1 in two age groups. The NR2A and NR2B protein levels in the PFC and V1 were similar in the young rats (1 month) but significantly differed in the adults (3.5 months), with V1 exhibiting significantly decreased NR2B (P = 0.005) but not the PFC (P = 0.492). In contrast, the NR1 subunits were comparable, without significant differences between young and adult animals in both PFC and V1 (P > 0.05 for all comparisons, Fig. 5C), suggesting that PFC neurons are not endowed with higher amounts of NMDARs but only exhibit a differential developmental changes in the NR2 subunits compared with those in V1 neurons. These data support the findings of both physiology and immunocytochemistry and are consistent with numerous previous studies in the V1 (14, 20, 21).
Fig. 4.
Immunofluorescence labeling of NR2B subunits in the PFC and V1 of adult rats. (A–D) in both layer 2/3 and layer 5, the NR2B-FITC in the PFC exhibited higher density of puncta compared with those in V1. Arrows indicate sample cells selected for quantifying measurements, whereas arrowheads point the cluster of puncta. (Scale bar, 20 μm.) (E and F) summary graphs showing the significant differences of puncta number (cell number = 100, P < 0.01) between PFC and V1. The immunofluorescence puncta were manually counted from individual labeled neurons and 50 neurons were selected for quantification in each cortical layer. In both PFC and V1, layer 5 neurons express significantly more puncta and clusters compared with those in layer 2/3.
Fig. 5.
Unlike V1 where NR2B subunit exhibits significant decrease in the adult, PFC shows similar levels of NR2B protein expression in both young and adult animals. (A) Western blot with constant protein loadings from same PFC tissue sample (3.5 months) results in reproducible proportions of NR2A subunit and NR2B subunit. (B) summary of NR2B/NR2A ratio showing the significant decrease of NR2B in the V1 in the adult rats (P = 0.005) but not in PFC (P = 0.492), indicating a relative unchanged NR2A and NR2B subunit expressions in both young and adult rats in the PFC. (C) in contrast, the NR1 subunits are comparable, without significant differences between young and adult animals in both PFC and V1 (P > 0.05).
Discussion
In this study, we demonstrated that while the NMDA/AMPA peak current ratios were similar in the PFC and V1 cortices, the NMDA currents at prefrontal recurrent excitatory synapses exhibited a twofold longer decay and hence a more effective temporal summation compared with those in the V1. Furthermore, our results suggested a greater contribution of NR2B subunits in the adult PFC than that in V1 and a likely regional and laminar difference in developmental change of NR2B subunits in these two cortical areas.
It is known that the PFC is a key brain region critically involved in working memory function (5). The involvement of the PFC in this cognitive process is supported by a subpopulation of neurons exhibiting persistent activity in the absence of external stimuli (2, 23, 24). From a mechanistic point of view, the critical question is what cellular and circuit mechanisms generate the self-sustained mnemonic persistent activity within the PFC network. One candidate mechanism is dependent upon the reverberation mediated by mutual excitation among pyramidal neurons; once activated, this process provides the necessary positive feedback to a participating neuronal network after the cessation of a transient stimulus (5, 10, 25). In biophysically-based neural circuit models, NMDARs have been shown to play an essential role in maintaining synaptic activity within the recurrent neuronal network because of its significantly longer time constant decay (9, 10, 25). The data documented in this study have provided supporting experimental evidence for this model prediction. We found that the time constants of NMDA currents in PFC recurrent connections are twofold slower than those in V1, even though the NMDAR/AMPAR peak current ratios are similar in the two cortical areas. Previous studies indicated that multiple mechanisms might contribute to the slow process of persistent activity in prefrontal circuit. For instance, a subset of PFC recurrent excitatory synapses exhibiting pronounced short-term facilitation (26, 27) and augmentation (28) can play a role in persistent activity. However, we found that most of the layer 5 P-P connections exhibited similar short-term depression in both PFC and V1 (28) although some connections in PFC exhibited larger recovery EPSCs (see Fig. 2C, 11th EPSC) than those in V1, as previous reported (26). Because NMDARs are highly localized near the soma and proximal dendrites (29), we believe that the possible difference of somatodendritic morphologies of pyramidal neurons in PFC and V1 (26) would not cause such a distinct difference in rise time and decay time constants in the NMDA currents (16). In addition, any voltage-clamp imperfection should also affect the AMPA currents, whereas we found that the rise time and the charge, as well as the latency of AMPA-EPSCs in both PFC and V1, were similar. Finally, the peak amplitudes of NMDA EPSCs in PFC were slightly smaller than those in V1, and smaller responses are expected to be better clamped and exhibit faster rise times (30). In our view, the slower time constant of the PFC NMDA current is likely to be the result of higher expression of NR2B subunits in PFC neurons, because NMDA current durations are largely controlled by the subunit composition of NMDARs [17, 31–33]. Indeed, we found that the proportion of the NMDAR current blocked by the NR2B antagonist ifenprodil was twofold higher in PFC than V1. The results of the immunostaining and Western blot also confirmed that PFC exhibited only a slight decrease of NR2B subunit, resulting in a relatively higher proportion of NR2B expression in the adult animals compared with that in V1. These findings are consistent with numerous previous reports that suggesting the subunit composition of NMDARs in visual cortex of adult animals is dominated by NR2A (14, 20, 21).
It is well known that maturation of cortical circuitry depends critically on experience that normally occurs with age (20). This experience-dependent maturation is coincident with NR2A/2B subunit changes that occur at the onset of the critical period of cortical development. Nevertheless, little is known about how PFC neural circuits mature in the transition to adulthood. Since cognitive functions that depend on PFC and NMDA receptors, such as working memory and critical thinking, are acquired and refined during adolescence, we speculated that changes in the subunit composition of NMDA receptors might be delayed to complement the development of prefrontal function. This important hypothesis has, however, never been tested and little is known about the expression of NMDAR subunits in the PFC. Our data suggest that while the relative contribution of NR2B subunits decrease significantly with age in V1, there is no significant difference in the NR2B-sensitive NMDA current in PFC between prepubertal and adult rats, at least for layer 5 neurons. Interestingly, we found that the NMDAR subunits in both PFC and V1 might be laminar-specific, with layer 2/3 neurons exhibiting relative less NR2B subunits (21, 22). Despite the difference between layer 2/3 and layer 5 neurons, the overall distribution of NR2B subunits in the PFC synapses was significantly higher than that in the V1. We speculate that these differences are important for prefrontal functions, not only for normal function such as working memory but also for dysfunctions in psychiatric disorders, such as schizophrenia. In other words, the higher fraction of NR2B could be a double-edged sword for PFC neurons. On one hand, higher levels of NR2B are needed for normal PFC functions (34) and for learning flexible behavior (35); on the other hand, neurons with more NR2B would be more fragile and vulnerable to excessive glutamate release (36). In this respect, our results suggest that even in adulthood, the neural circuits may be especially plastic in the PFC, a brain region believed to be crucial for learning flexible behavior. More importantly, we propose that a higher quantity of NR2B subunits is important for online processing of PFC functions, as previous modeling predicted (10), because only the slow-decayed NR2B expression could allow greater temporal integration of asynchronous synaptic inputs (33) and cross talk among multiple synapses (30) than those with NR2A subunits. Indeed, a recent study reported that NMDAR channels with a higher proportion of NR2B subunits are critical for network activity in the prefrontal circuitry because hallucinogen-induced upstate in the PFC could be blocked by application of NR2B antagonist (37).
In summary, a high expression of NR2B-containing NMDARs at intrinsic synapses in adulthood may be a characteristic of the PFC circuitry that is critical for generating the slow recurrent dynamics needed for normal prefrontal functions such as working memory and decision making. Instead, the faster decay of NMDA currents in sensory cortical areas may be better suited for high fidelity signal transduction that is necessary for rapid coding of continuous streams of sensory information. Our finding of these specialized NMDAR properties in the PFC circuits and its potential role on the slow active neural dynamics have thus provided new insights into the understanding the role of NMDAR signaling in normal prefrontal functions as well as in cognitive impairments associated with NMDAR dysfunction in schizophrenia and other psychiatric disorders.
Experimental Procedures (see SI Text for detail)
Multiple Whole Cell Recordings in PFC Slice.
The results were obtained from experiments using adult (postnatal day 85–110) or young (postnatal day 13–23) rats. Detailed procedures can be found elsewhere (38). Rats were anesthetized with Euthasol (0.2 ml/kg, i.p.) and euthanized through decapitation. The brains were placed in ice-cold (< 4 °C) sucrose solution. Neocortex containing mPFC was trimmed and glued to stage (< 4 °C) of a Vibratome (Vibratome Co.). Horizontal brain slices of 300 μm were cut and incubated in oxygenated Ringer solution until being transferred to a recording chamber.
Whole cell recordings were obtained from multiple pyramidal neurons simultaneously. The recordings were conducted at 35–36° C. The resistance of the recording pipette was 5–9 MΩ. Monosynaptic connections between layer 5 pyramidal neurons were recorded with one cell (presynaptic neuron) filled with K+ based intracellular solution and the remaining neurons (postsynaptic neurons) filled with Cs+ solution. The signals were acquired with pCLAMP 9.2 (Molecular Devices). Access resistances were continuously monitored and neurons with more than 20% change of series resistance were excluded from data analysis.
Data Analysis of Electrophysiology.
The EPSC amplitudes were measured by from the onset to peak of EPSCs. The amplitudes of 2nd and following EPSCs were measured from onset to peak. The normalized ratios of the 2nd divided by the 1st EPSC, as well as the ratios of nth/1st, were calculated to show the dynamic of synaptic transmission at 20 Hz train. The decay time course was fitted with either a single exponential or a sum of two exponentials using standard exponential formulas in Clampfit 9.2. The integrated EPSC areas (charge) were measured and expressed as picocoulomb (pC). The contribution of NR2B to the overall NMDA current was measured as control trace—ifenprodil trace)/control × 100. All data were presented as mean ± standard error (SE), with Student's t test or ANOVA for statistical significance.
Immunocytochemistry.
Animals were euthanized and perfused with 0.1 mM PBS (pH 7.4) followed by 4% paraformaldehyde. Coronal sections of 30 μm were processed for immunofluorescence staining. Sections were rinsed, blocked nonspecific binding, and then transferred into goat anti-NR2B and anti-NR1 (1:800; Santa Cruz Biotechnology) for incubation at 4° C, 48–72 h. After thorough rinsing, bovine anti-goat IgG-FITC at a dilution of 1:800 was used as the secondary antibody. We demonstrated the specificity by preblocking with serum and by including primary antibody-free controls in each experiment. The specificity of the staining was further evidenced by the fact that only certain cell types showed immunolabeling with this antibody. To quantify the staining, sections from three animals were used for quantitative analysis. We manually counted the puncta numbers in labeled neurons and more than one section per brain region was analyzed. Data from a previous study of Nissl-stained neurons was used to delineate the cortical layers. The data were analyzed with Student's t test for statistical significance and are expressed as means ± SE.
Western Blot.
Tissues containing PFC or V1 were dissected and homogenized in lysis buffer. Equal amounts of protein samples were separated and electrotransferred onto nitrocellulose membranes (Invitrogen), which are probed with anti-NR2A and anti-NR2B (Millipore), with β-actin as a loading control (Sigma-Aldrich). The secondary antibody is an HRP-conjugated goat anti-rabbit (or mouse) IgG (ECL plus) diluted at 1:2,000. Signals are detected with ECL Western Blotting System (Amersham Bioscience). Band densities are measured with Image J (NIH) and are normalized to those of β-actin, with background subtraction.
Supplementary Material
Acknowledgments.
We thank B.D. Waterhouse, B.D. Clark, M.C. Crair, and T. Koos for comments on the manuscript. I am also grateful to J. Coburn, M. Pappy, and W. Yan for technical support. This work was supported by a Drexel University College of Medicine, NARSAD young investigator award and by a National Institute of Health R21 grant MH232307 to W-J Gao and by NIH R01 MH062349 to X-J Wang.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0804318105/DCSupplemental.
References
- 1.Fuster JM, Alexander GE. Neuron activity related to short-term memory. Science. 1971;173:652–654. doi: 10.1126/science.173.3997.652. [DOI] [PubMed] [Google Scholar]
- 2.Funahashi S, Bruce CJ, Goldman-Rakic PS. Mnemonic coding of visual space in the monkey's dorsolateral prefrontal cortex. J Neurophysiol. 1989;61:331–349. doi: 10.1152/jn.1989.61.2.331. [DOI] [PubMed] [Google Scholar]
- 3.Miller EK, Erickson CA, Desimone R. Neural mechanisms of visual working memory in prefrontal cortex of the macaque. J Neurosci. 1996;16:5154–5167. doi: 10.1523/JNEUROSCI.16-16-05154.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jung MW, et al. Firing characteristics of deep layer neurons in prefrontal cortex in rats performing spatial working memory tasks. Cereb Cortex. 1998;8:437–450. doi: 10.1093/cercor/8.5.437. [DOI] [PubMed] [Google Scholar]
- 5.Goldman-Rakic PS. Cellular basis of working memory. Neuron. 1995;14:477–85. doi: 10.1016/0896-6273(95)90304-6. [DOI] [PubMed] [Google Scholar]
- 6.Verma A, Moghaddam B. NMDA receptor antagonists impair prefrontal cortex function as assessed via spatial delayed alternation performance in rats: modulation by dopamine. J Neurosci. 1996;16:373–379. doi: 10.1523/JNEUROSCI.16-01-00373.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aura J, Riekkinen PJ. Blockade of NMDA receptors located at the dorsomedial prefrontal cortex impairs spatial working memory in rats. Neuroreport. 1999;10:243–248. doi: 10.1097/00001756-199902050-00008. [DOI] [PubMed] [Google Scholar]
- 8.Wang X-J. Synaptic basis of cortical persistent activity: The importance of NMDA receptors to working memory. J Neurosci. 1999;19:9587–9603. doi: 10.1523/JNEUROSCI.19-21-09587.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Compte A, et al. Synaptic mechanisms and network dynamics underlying spatial working memory in a cortical network model. Cereb Cortex. 2000;10:910–923. doi: 10.1093/cercor/10.9.910. [DOI] [PubMed] [Google Scholar]
- 10.Wang X-J. Synaptic reverberation underlying mnemonic persistent activity. Trends Neurosci. 2001;24:455–463. doi: 10.1016/s0166-2236(00)01868-3. [DOI] [PubMed] [Google Scholar]
- 11.Lisman JE, Fellous JM, Wang X-J. A role for NMDA-receptor channels in working memory. Nat Neurosci. 1998;1:273–275. doi: 10.1038/1086. [DOI] [PubMed] [Google Scholar]
- 12.Scherzer CR, et al. Expression of N-methyl-D-aspartate receptor subunit mRNAs in the human brain: hippocampus and cortex. J Comp Neurol. 1998;390:75–90. doi: 10.1002/(sici)1096-9861(19980105)390:1<75::aid-cne7>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 13.Myme CI, et al. The NMDA-to-AMPA ratio at synapses onto layer 2/3 pyramidal neurons is conserved across prefrontal and visual cortices. J Neurophysiol. 2003;90:771–779. doi: 10.1152/jn.00070.2003. [DOI] [PubMed] [Google Scholar]
- 14.Fagiolini M, et al. Separable features of visual cortical plasticity revealed by N-methyl-D-aspartate receptor 2A signaling. Proc Natl Acad Sci USA. 2003;100:2854–2859. doi: 10.1073/pnas.0536089100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Elston GN. Cortex, cognition and the cell: new insights into the pyramidal neuron and prefrontal function. Cereb Cortex. 2003;13:1124–1138. doi: 10.1093/cercor/bhg093. [DOI] [PubMed] [Google Scholar]
- 16.Magee JC, Cook EP. Somatic EPSP amplitude is independent of synapse location in hippocampal pyramidal neurons. Nat Neurosci. 2000;3:895–903. doi: 10.1038/78800. [DOI] [PubMed] [Google Scholar]
- 17.Cull-Candy S, Brickley S, Farrant M. NMDA receptor subunits: diversity, development and disease. Curr Opin Neurobiol. 2001;11:327–335. doi: 10.1016/s0959-4388(00)00215-4. [DOI] [PubMed] [Google Scholar]
- 18.Yoshimura Y, Ohmura T, Komatsu Y. Two forms of synaptic plasticity with distinct dependence on age, experience, and NMDA receptor subtype in rat visual cortex. J Neurosci. 2003;23:6557–6566. doi: 10.1523/JNEUROSCI.23-16-06557.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Roberts EB, Ramoa AS. Enhanced NR2A subunit expression and decreased NMDA receptor decay time at the onset of ocular dominance plasticity in the ferret. J Neurophysiol. 1999;81:2587–2591. doi: 10.1152/jn.1999.81.5.2587. [DOI] [PubMed] [Google Scholar]
- 20.Quinlan EM, Olstein DH, Bear MF. Bidirectional, experience-dependent regulation of N-methyl-D-aspartate receptor subunit composition in the rat visual cortex during postnatal development. Proc Natl Acad Sci USA. 1999;96:12876–12880. doi: 10.1073/pnas.96.22.12876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Erisir A, Harris JL. Decline of the critical period of visual plasticity is concurrent with the reduction of NR2B subunit of the synaptic NMDA receptor in layer 4. J Neurosci. 2003;23:5208–5218. doi: 10.1523/JNEUROSCI.23-12-05208.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gonzalez-Burgos G, et al. Functional maturation of excitatory synapses in layer 3 pyramidal neurons during postnatal development of the primate prefrontal cortex. Cereb Cortex. 2008;18:626–637. doi: 10.1093/cercor/bhm095. [DOI] [PubMed] [Google Scholar]
- 23.Romo R, et al. Neuronal correlates of parametric working memory in the prefrontal cortex. Nature. 1999;399:470–473. doi: 10.1038/20939. [DOI] [PubMed] [Google Scholar]
- 24.Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annu Rev Neurosc. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- 25.Durstewitz D, Seamans JK, Sejnowski TJ. Neurocomputational models of working memory. Nat Neurosci. 2000;3(Suppl):1184–1191. doi: 10.1038/81460. [DOI] [PubMed] [Google Scholar]
- 26.Wang Y, et al. Heterogeneity in the pyramidal network of the medial prefrontal cortex. Nat Neurosci. 2006;9:534–542. doi: 10.1038/nn1670. [DOI] [PubMed] [Google Scholar]
- 27.Mongillo G, Barak O, Tsodyks M. Synaptic Theory of Working Memory. Science. 2008;319:1543–1546. doi: 10.1126/science.1150769. [DOI] [PubMed] [Google Scholar]
- 28.Hempel CM, et al. Multiple forms of short-term plasticity at excitatory synapses in rat medial prefrontal cortex. J Neurophysiol. 2000;83:3031–3041. doi: 10.1152/jn.2000.83.5.3031. [DOI] [PubMed] [Google Scholar]
- 29.Dodt HU, et al. NMDA and AMPA receptors on neocortical neurons are differentially distributed. Eur J Neurosci. 1998;10:3351–3357. doi: 10.1046/j.1460-9568.1998.00338.x. [DOI] [PubMed] [Google Scholar]
- 30.Scimemi A, et al. NR2B-containing receptors mediate cross talk among hippocampal synapses. J Neurosc. 2004;24:4767–4777. doi: 10.1523/JNEUROSCI.0364-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Flint AC, et al. NR2A subunit expression shortens NMDA receptor synaptic currents in developing neocortex. J Neurosci. 1997;17:2469–2476. doi: 10.1523/JNEUROSCI.17-07-02469.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tovar KR, Sprouffske K, Westbrook GL. Fast NMDA receptor-mediated synaptic currents in neurons from mice lacking the epsilon2 (NR2B) subunit. J Neurophysiol. 2000;83:616–620. doi: 10.1152/jn.2000.83.1.616. [DOI] [PubMed] [Google Scholar]
- 33.Erreger K, et al. Subunit-specific gating controls rat NR1/NR2A and NR1/NR2B NMDA channel kinetics and synaptic signalling profiles. J Physiol. 2005;563:345–358. doi: 10.1113/jphysiol.2004.080028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhao M-G, et al. Roles of NMDA NR2B subtype receptor in prefrontal long-term potentiation and contextual fear memory. Neuron. 2005;47:859–872. doi: 10.1016/j.neuron.2005.08.014. [DOI] [PubMed] [Google Scholar]
- 35.Tang YP, et al. Genetic enhancement of learning and memory in mice. Nature. 1999;401:63–69. doi: 10.1038/43432. [DOI] [PubMed] [Google Scholar]
- 36.Hardingham GE, Bading H. The Yin and Yang of NMDA receptor signalling. Trends Neurosci. 2003;26:81–89. doi: 10.1016/S0166-2236(02)00040-1. [DOI] [PubMed] [Google Scholar]
- 37.Lambe EK, Aghajanian GK. Hallucinogen-induced up states in the brain slice of rat prefrontal cortex: role of glutamate spillover and NR2B-NMDA receptors. Neuropsychopharmacology. 2006;31:1682–1689. doi: 10.1038/sj.npp.1300944. [DOI] [PubMed] [Google Scholar]
- 38.Gao WJ, Krimer LS, Goldman-Rakic PS. Presynaptic regulation of recurrent excitation by D1 receptors in prefrontal circuits. Proc Natl Acad Sci USA. 2001;98:295–300. doi: 10.1073/pnas.011524298. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.