Abstract
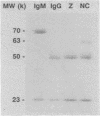
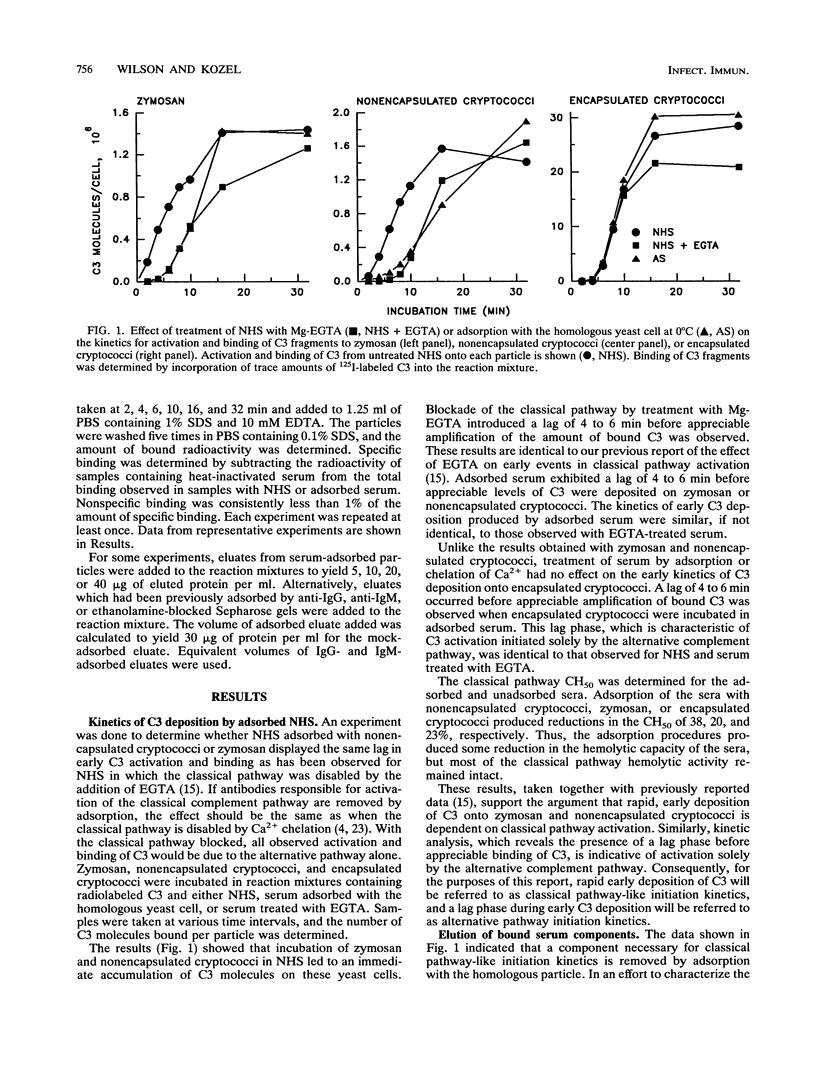
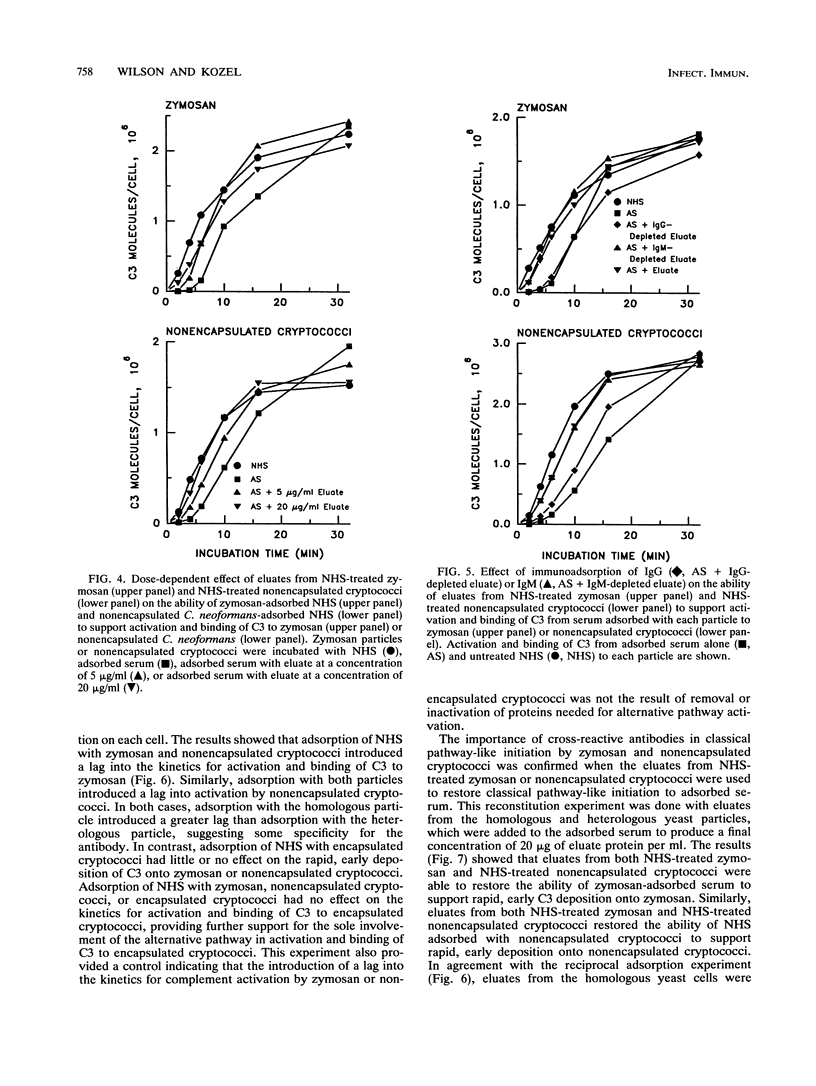
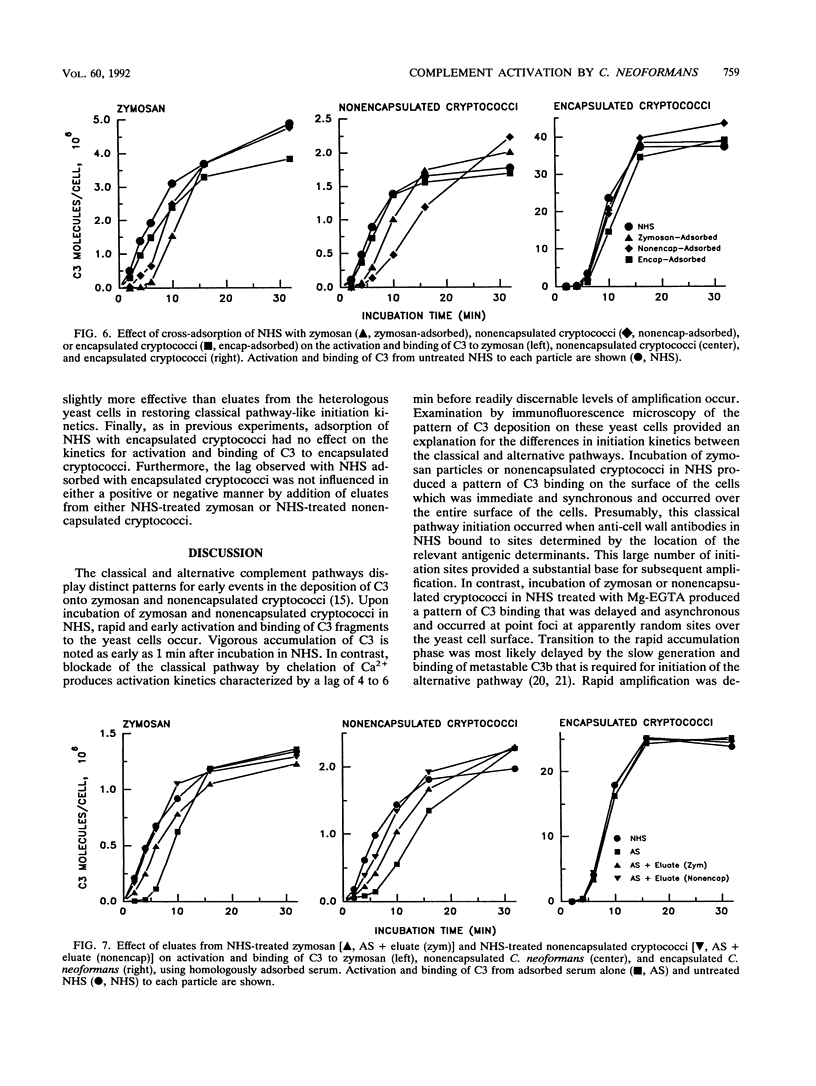
Encapsulated and nonencapsulated cryptococci differ in their activation of the complement system. Incubation of nonencapsulated cryptococci in normal human serum (NHS) initiates both the classical and alternative pathways. This activation is characterized by an immediate, synchronous activation and binding of C3 to the yeast cells. Encapsulated cryptococci activate only the alternative pathway. This activation is characterized by a delayed (4 to 5 min), asynchronous activation and binding of C3. We examined the properties of antibodies in NHS that mediate immediate, synchronous binding of C3 to nonencapsulated cryptococci and zymosan. Adsorption of NHS with nonencapsulated cryptococci or zymosan produced a 4- to 6-min delay in the kinetics for activation and binding of C3 from the adsorbed serum to each respective yeast cell. This delay was similar to the delay observed when nonencapsulated cryptococci or zymosan was incubated in NHS in which the classical pathway was blocked by chelation of Ca2+. Proteins bound to serum-treated nonencapsulated cryptococci or zymosan were eluted and found to be predominantly immunoglobulin G (IgG), with lesser amounts of IgM. The eluted IgG could restore to adsorbed serum the rapid early kinetics for activation and binding of C3 characteristic of classical pathway initiation. Cross-adsorption showed that there was considerable cross-reactivity between the antibodies which restored rapid, early activation kinetics to NHS adsorbed with zymosan or nonencapsulated cryptococci. Encapsulated cryptococci were unable to adsorb the antibodies from NHS that mediated the rapid, early activation and binding of C3 to zymosan and nonencapsulated cryptococci. The latter results show that occlusion of antigenic sites at the cryptococcal cell wall is a newly recognized property that can be added to the repertoire of biological activities of the cryptococcal capsule.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BLUM L. EVIDENCE FOR IMMUNOLOGICAL SPECIFICITY OF THE PROPERDIN SYSTEM; DEMONSTRATION, ISOLATION AND PROPERTIES OF A SERUM FACTOR WHICH INTERACTS WITH ZYMOSAN AND OTHER POLYSACCHARIDES AT 0 DEGREES CENTIGRADE. J Immunol. 1964 Jan;92:61–72. [PubMed] [Google Scholar]
- Bacon J. S., Farmer V. C., Jones D., Taylor I. F. The glucan components of the cell wall of baker's yeast (Saccharomyces cerevisiae) considered in relation to its ultrastructure. Biochem J. 1969 Sep;114(3):557–567. doi: 10.1042/bj1140557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fine D. P., Marney S. R., Jr, Colley D. G., Sergent J. S., Des Prez R. M. C3 shunt activation in human serum chelated with EGTA. J Immunol. 1972 Oct;109(4):807–809. [PubMed] [Google Scholar]
- Fraker P. J., Speck J. C., Jr Protein and cell membrane iodinations with a sparingly soluble chloroamide, 1,3,4,6-tetrachloro-3a,6a-diphrenylglycoluril. Biochem Biophys Res Commun. 1978 Feb 28;80(4):849–857. doi: 10.1016/0006-291x(78)91322-0. [DOI] [PubMed] [Google Scholar]
- James P. G., Cherniak R., Jones R. G., Stortz C. A., Reiss E. Cell-wall glucans of Cryptococcus neoformans Cap 67. Carbohydr Res. 1990 Apr 2;198(1):23–38. doi: 10.1016/0008-6215(90)84273-w. [DOI] [PubMed] [Google Scholar]
- Kohn J., Wilchek M. A new approach (cyano-transfer) for cyanogen bromide activation of Sepharose at neutral pH, which yields activated resins, free of interfering nitrogen derivatives. Biochem Biophys Res Commun. 1982 Aug;107(3):878–884. doi: 10.1016/0006-291x(82)90604-0. [DOI] [PubMed] [Google Scholar]
- Kozel T. R., Cazin J. Nonencapsulated Variant of Cryptococcus neoformans I. Virulence Studies and Characterization of Soluble Polysaccharide. Infect Immun. 1971 Feb;3(2):287–294. doi: 10.1128/iai.3.2.287-294.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozel T. R., Highison B., Stratton C. J. Localization on encapsulated Cryptococcus neoformans of serum components opsonic for phagocytosis by macrophages and neutrophils. Infect Immun. 1984 Feb;43(2):574–579. doi: 10.1128/iai.43.2.574-579.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozel T. R., McGaw T. G. Opsonization of Cryptococcus neoformans by human immunoglobulin G: role of immunoglobulin G in phagocytosis by macrophages. Infect Immun. 1979 Jul;25(1):255–261. doi: 10.1128/iai.25.1.255-261.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozel T. R., Pfrommer G. S. Activation of the complement system by Cryptococcus neoformans leads to binding of iC3b to the yeast. Infect Immun. 1986 Apr;52(1):1–5. doi: 10.1128/iai.52.1.1-5.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozel T. R., Pfrommer G. S., Guerlain A. S., Highison B. A., Highison G. J. Strain variation in phagocytosis of Cryptococcus neoformans: dissociation of susceptibility to phagocytosis from activation and binding of opsonic fragments of C3. Infect Immun. 1988 Nov;56(11):2794–2800. doi: 10.1128/iai.56.11.2794-2800.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozel T. R., Wilson M. A., Murphy J. W. Early events in initiation of alternative complement pathway activation by the capsule of Cryptococcus neoformans. Infect Immun. 1991 Sep;59(9):3101–3110. doi: 10.1128/iai.59.9.3101-3110.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozel T. R., Wilson M. A., Pfrommer G. S., Schlageter A. M. Activation and binding of opsonic fragments of C3 on encapsulated Cryptococcus neoformans by using an alternative complement pathway reconstituted from six isolated proteins. Infect Immun. 1989 Jul;57(7):1922–1927. doi: 10.1128/iai.57.7.1922-1927.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Law S. K., Lichtenberg N. A., Levine R. P. Evidence for an ester linkage between the labile binding site of C3b and receptive surfaces. J Immunol. 1979 Sep;123(3):1388–1394. [PubMed] [Google Scholar]
- Nelson B., Ruddy S. Enhancing role of IgG in lysis of rabbit erythrocytes by the alternative pathway of human complement. J Immunol. 1979 May;122(5):1994–1999. [PubMed] [Google Scholar]
- PHAFF H. J. CELL WALL OF YEASTS. Annu Rev Microbiol. 1963;17:15–30. doi: 10.1146/annurev.mi.17.100163.000311. [DOI] [PubMed] [Google Scholar]
- Pangburn M. K., Müller-Eberhard H. J. The alternative pathway of complement. Springer Semin Immunopathol. 1984;7(2-3):163–192. doi: 10.1007/BF01893019. [DOI] [PubMed] [Google Scholar]
- Pangburn M. K., Schreiber R. D., Müller-Eberhard H. J. Formation of the initial C3 convertase of the alternative complement pathway. Acquisition of C3b-like activities by spontaneous hydrolysis of the putative thioester in native C3. J Exp Med. 1981 Sep 1;154(3):856–867. doi: 10.1084/jem.154.3.856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platts-Mills T. A., Ishizaka K. Activation of the alternate pathway of human complements by rabbit cells. J Immunol. 1974 Jul;113(1):348–358. [PubMed] [Google Scholar]
- Polhill R. B., Jr, Newman S. L., Pruitt K. M., Johnston R. B., Jr Kinetic assessment of alternative complement pathway activity in a hemolytic system. II. Influence of antibody on alternative pathway activation. J Immunol. 1978 Jul;121(1):371–376. [PubMed] [Google Scholar]
- Schenkein H. A., Ruddy S. The role of immunoglobulins in alternative complement pathway activation by zymosan. I. Human IgG with specificity for Zymosan enhances alternative pathway activation by zymosan. J Immunol. 1981 Jan;126(1):7–10. [PubMed] [Google Scholar]
- Schenkein H. A., Ruddy S. The role of immunoglobulins in alternative pathway activation by zymosan. II. The effect of IgG on the kinetics of the alternative pathway. J Immunol. 1981 Jan;126(1):11–15. [PubMed] [Google Scholar]