Abstract
Submergence-tolerant rice maintains viability during complete submergence by limiting underwater elongation until floodwaters recede. Acclimation responses to submergence are coordinated by the submergence-inducible Sub1A, which encodes an ethylene-responsive factor-type transcription factor (ERF). Sub1A is limited to tolerant genotypes and sufficient to confer submergence tolerance to intolerant accessions. Here we evaluated the role of Sub1A in the integration of ethylene, abscisic acid (ABA), and gibberellin (GA) signaling during submergence. The submergence-stimulated decrease in ABA content was Sub1A-independent, whereas GA-mediated underwater elongation was significantly restricted by Sub1A. Transgenics that ectopically express Sub1A displayed classical GA-insensitive phenotypes, leading to the hypothesis that Sub1A limits the response to GA. Notably Sub1A increased the accumulation of the GA signaling repressors Slender Rice-1 (SLR1) and SLR1 Like-1 (SLRL1) and concomitantly diminished GA-inducible gene expression under submerged conditions. In the Sub1A overexpression line, SLR1 protein levels declined under prolonged submergence but were accompanied by an increase in accumulation of SLRL1, which lacks the DELLA domain. In the presence of Sub1A, the increase in these GA signaling repressors and decrease in GA responsiveness were stimulated by ethylene, which promotes Sub1A expression. Conversely, ethylene promoted GA responsiveness and shoot elongation in submergence-intolerant lines. Together, these results demonstrate that Sub1A limits ethylene-promoted GA responsiveness during submergence by augmenting accumulation of the GA signaling repressors SLR1 and SLRL1.
Keywords: abscisic acid, ethylene, flooding, GRAS-domain protein
Rice (Oryza sativa L.) is a semiaquatic plant species that is well adapted to a partially flooded environment. However, flash flooding of fields can cause complete submergence, which results in catastrophic losses in rice production. Complete submergence imposes a complex stress due to a 10,000-fold reduction in the diffusion of oxygen and carbon dioxide as well as a restriction in light availability. Deepwater rice grown in wetlands adapts to gradual flooding by accelerating the elongation of submerged internodes to maintain aerial tissue above the air–water interface (1). If rapidly submerged, deepwater and most lowland varieties hasten internode and/or leaf elongation to escape the inundation but die within 10–14 days if aerial tissue remains underwater (2, 3). By contrast, submergence-tolerant lowland varieties including Flood Resistant 13A (FR13A) overcome complete submergence through a restriction in shoot elongation and carbohydrate consumption, thereby conserving energy reserves to enable recommencement of development upon desubmergence (3–6).
Map-based cloning of the Submergence-1 (Sub1) locus identified a polygenic region that encodes 2 or 3 paralogous ethylene responsive factor (ERF) DNA binding proteins that are induced at the transcript level during submergence (3, 6). A correlation between Sub1 locus haplotype and submergence tolerance led to the finding that Sub1A-1, present only in tolerant accessions, is necessary and sufficient to confer tolerance (3). Through evaluation of a pair of near-isogenic lines that differ in Sub1 haplotype we uncovered that submergence-induced expression of Sub1A-1 is correlated with restrained induction of genes associated with cell elongation and carbohydrate consumption (6).
The phytohormones ethylene, abscisic acid (ABA), and gibberellin (GA) orchestrate the acclimation response to submergence in rice and the semiaquatic dicot Rumex palustris (1, 7–9). However, the molecular intricacies of the submergence response signaling pathway are poorly understood. The physical entrapment and biosynthesis of ethylene upon submergence are thought to initiate the growth response. Interestingly, Sub1A-1 expression is induced by low levels of ethylene but also limits ethylene production during submergence (6). In deepwater rice, submergence triggers degradation of ABA, an antagonist of GA, leading to enhancement of responsiveness to GA and promotion of elongation growth (10). A decrease in ABA and an increase in GA biosynthesis were demonstrated in response to submergence in R. palustris and were shown to promote the elongation growth of shoots necessary for leaf emergence from the water (11). The treatment of FR13A rice with GA3 during submergence promoted elongation growth and compromised survival, indicating that GA-regulated processes negatively impact tolerance to prolonged submergence (2, 5). Conversely, treatment of intolerant cultivars with paclobutrazol, an inhibitor of GA biosynthesis, restricted underwater elongation and enhanced submergence survival. We observed that the near-isogenic submergence-intolerant and -tolerant lines M202 and M202(Sub1), respectively, develop at the same rate and augment elongation growth to the same extent when treated with GA3 under normal conditions (6). This suggests that inhibition of GA-mediated elongation growth is manifested specifically during submergence, when Sub1A expression is enhanced.
The dampening of GA-mediated elongation growth is beneficial during prolonged submergence because it delays the exhaustion of carbohydrates that ultimately compromises cell viability due to a deficiency in ATP. To elucidate the hormone-mediated mechanism that promotes elongation growth during submergence, we evaluated the influence of Sub1A-1 on the interplay among ethylene, ABA, and GA using the introgression line M202(Sub1), which contains submergence-inducible Sub1A-1 from FR13A, and transgenic lines that ectopically express Sub1A-1. We showed previously that Sub1A-1 mRNA levels are enhanced in aerial tissue by submergence or ethylene exposure but not by GA3 treatment (6). The transgenic lines that constitutively express Sub1A-1 afforded the opportunity to examine directly whether this gene regulates responsiveness to GA. Although inundation stimulated a similar reduction in ABA and its derivatives in tolerant and intolerant lines, both induced and constitutive Sub1A-1 expression counteracted the resultant elevation in GA responsiveness by promoting the accumulation of 2 repressors for GA signaling. Thus, submergence-induced expression of Sub1A-1 suppresses GA action, thereby limiting underwater elongation, prolonging submergence endurance, and sustaining the capacity for regrowth upon desubmergence.
Results
Constitutive and Submergence-Induced Expression of Sub1A-1 Confers Similar Growth Restriction and Survival of Prolonged Submergence.
Two pairs of near-isogenic lines were used to evaluate the role of Sub1A-1 in the determination of submergence tolerance [M202 and M202(Sub1); Liaogeng (LG) and Ubi:Sub1A]. M202 and LG are submergence-intolerant japonica cultivars that encode Sub1B-2 and Sub1C-2 at the Sub1 locus. M202(Sub1) is submergence-tolerant and has an ≈180-kb region containing Sub1A-1, Sub1B-1, and Sub1C-1 introgressed from FR13A (6). The Ubi:Sub1A transgenic lines were derived by transformation of LG with Sub1A-1 driven by the maize Ubiquitin-1 promoter (3). Sub1A-1 is ubiquitously expressed in the Ubi:Sub1A transgenics, whereas the gene is induced by submergence and ethylene in M202(Sub1) (3, 6).
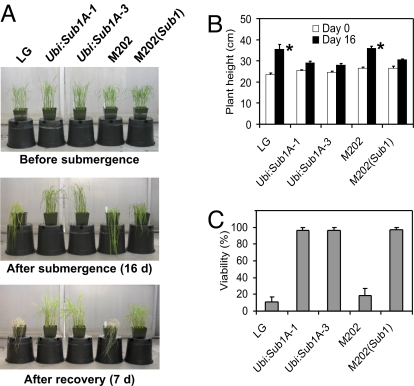
We confirmed that ectopic expression of Sub1A-1 significantly diminished plant height in 2 independent transgenic lines with a single insertion of Ubi:Sub1A-1 (homozygous T3 lines; Ubi:Sub1A-1 and Ubi:Sub1A-3) [supporting information (SI) Fig. S1A]. Ubi:Sub1A plants grown for 21 days had a height similar to that of LG plants grown for 14 days (Fig. 1 A and B). To accurately evaluate the effect of Sub1A on elongation of aerial tissue and survival of prolonged submergence, LG and transgenics of similar height and with the same leaf number were subjected to submergence along with the previously characterized genotypes M202 and M202(Sub1) (6). The increase in plant height after 16 days of submergence was significantly greater in LG and M202 than in Ubi:Sub1A and M202(Sub1) (Fig. 1 A and B). Plant viability was evaluated on the basis of the emergence and growth of new leaves over 7 days after desubmergence (Fig. 1 A and C). Nearly 100% of the Sub1A-1 overexpression and M202(Sub1) plants survived the prolonged inundation, as compared with only 10.7% and 18.7% of the LG and M202 plants, respectively. The survival of the transgenics was consistent with limited induction of gene transcripts associated with carbohydrate consumption and cell elongation over a 14-day period of submergence, as compared with LG (Fig. S2), in agreement with the dampened induction of the same genes in M202(Sub1) (6). Based on these observations, we suggest that ectopic Sub1A-1 expression confers submergence tolerance via a limitation of carbohydrate consumption and restriction of elongation, as confirmed for the introgression line M202(Sub1) (6).
Fig. 1.
Constitutive and submergence-induced expression of Sub1A confers similar growth restriction and survival of prolonged submergence. (A) Plants before submergence (Top), after 16 days of submergence (Middle), and after 7 days of recovery from 16 days of submergence (Bottom). Fourteen-day-old [LG, M202, and M202(Sub1)] and 21-day-old (Ubi:Sub1A) plants at a similar developmental age were subjected to submergence tests. (B) Plant height before and after submergence. (C) Viability of plants after 16 days of submergence and 7 days of recovery. In (B) and (C), data represent mean ± SD from 3 independent biological replicates (n = 75) and an asterisk indicates a significant difference between LG vs. Ubi:Sub1A lines and M202 vs. M202(Sub1) (P < 0.05).
ABA Degradation Is Enhanced by Submergence in a Sub1A-Independent Manner.
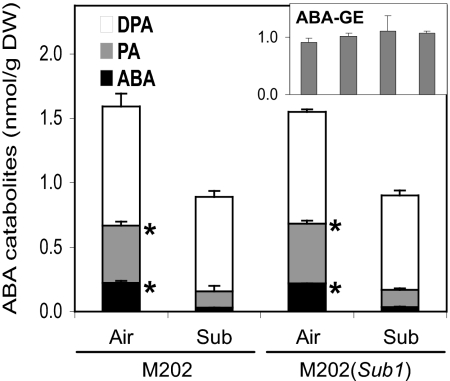
Submergence promotes a rapid decrease in endogenous ABA, leading to an increase in the response to GA in deepwater rice (1). We considered that the presence of Sub1A could affect the process of ABA degradation during the stress. The major pathway for ABA degradation is hydroxylation of ABA to the unstable intermediate 8′-hydroxy ABA by ABA 8′-hydroxylase (ABA8ox), which is spontaneously converted to phaseic acid (PA) (12). PA has slight ABA activity and can be further reduced to inactive dihydrophaseic acid (DPA). We found that 1 day of submergence decreased ABA content to ≈15% of the control and PA to ≈29% of the control in both M202 and M202(Sub1) but had no effect on the abundance of endogenous DPA (Fig. 2). There was no significant difference in ABA, PA, or DPA contents of the 2 genotypes when levels from the same condition were compared. In addition to the ABA8ox pathway, ABA can be inactivated by conjugation with glucose (12). However, the level of the ABA glucose ester (ABA-GE) was unaltered by submergence in either genotype. This analysis confirms that hydroxylation of ABA via ABA8ox is the major pathway for ABA catabolism during submergence and is promoted in a Sub1A-independent manner.
Fig. 2.
Rapid decline in endogenous ABA during submergence is Sub1A-independent. Endogenous levels of ABA and its catabolites were quantified in nonsubmerged and submerged aerial organs of M202 and M202(Sub1) by tandem mass spectrometry. Air: 14-day-old plants grown in air; Sub: 14-day-old plants submerged for 1 day. PA, phaseic acid; DPA, dihydrophaseic acid; ABA-GE, ABA glucose ester. The data represent mean ± SD from 3 independent biological replicates. An asterisk indicates a significant difference in metabolite content in air and after submergence (P < 0.01).
To determine whether ABA influences the accumulation of Sub1 gene transcripts, we monitored the abundance of Sub1A/B/C mRNAs in aerial tissue of M202 and M202(Sub1) after treatment with 5 or 50 μM ABA (Fig. S3A). Application of ABA decreased the abundance of all Sub1 gene mRNAs in both lines. A similar effect of ABA on Sub1 gene transcript accumulation was observed in LG and Ubi:Sub1A seedlings, although as expected the constitutively expressed Sub1A mRNA did not decline in the transgenic line (Fig. S3B). This indicates that a reduction in ABA content may be a prerequisite for increased accumulation of Sub1 gene transcripts during submergence.
Sub1A Negatively Regulates GA Responsiveness.
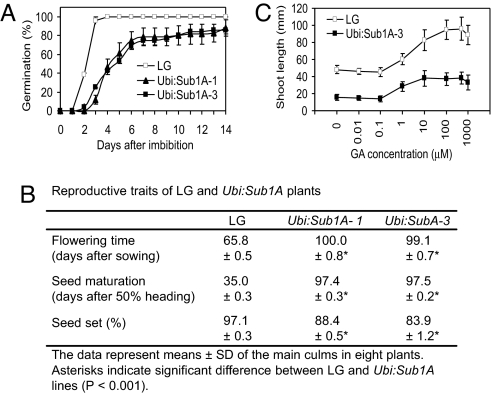
Ubi:Sub1A transgenics displayed hallmarks of defects in GA biosynthesis, signaling, or response including delayed seed germination (Fig. 3A), semidwarf stature (Fig. S1 A and B), late flowering, delayed seed maturation, and reduced seed set (Fig. 3B). Confirmation that Sub1A expression limited GA responsiveness was achieved by evaluation of a dose response to GA3 in pregerminated LG and Ubi:Sub1A seeds (Fig. 3C). After 7 days of treatment, the shoots of Ubi:Sub1A-3 were considerably shorter than those of LG. Although submicromolar concentrations (0.01–0.1 μM) of GA3 did not promote shoot elongation, concentrations >1 μM promoted elongation in both genotypes in a dose-dependent manner. The steeper slope of the GA3 response curve (1–100 μM) for LG than Ubi:Sub1A-3 seedlings indicates that ectopic expression of Sub1A reduces the response to bioactive GA. This indicates that Sub1A diminishes GA signaling and thereby negatively regulates GA-dependent processes.
Fig. 3.
Sub1A restricts GA-dependent processes and GA response. (A) Germination percentages of LG and Ubi:Sub1A seeds. Twenty-five seeds of each genotype were germinated on wet filter paper for 14 days. (B) Reproductive traits of LG and Ubi:Sub1A lines. The data represent mean ± SD of the main culms of 8 plants. (C) GA-induced shoot elongation in seedlings of LG and Ubi:Sub1A. Germinating seeds with 1-mm shoot (15 seeds) were placed on wet filter paper containing mock (0.1% DMSO) or GA (0.1–1,000 μM GA3 in 0.1% DMSO) solution for 7 days. After incubation, the shoot length of each seedling was recorded. Data in A and C represent mean ± SD from 3 independent biological replicates.
Sub1A Restrains GA-Mediated Underwater Elongation via SLR1 and SLRL1.
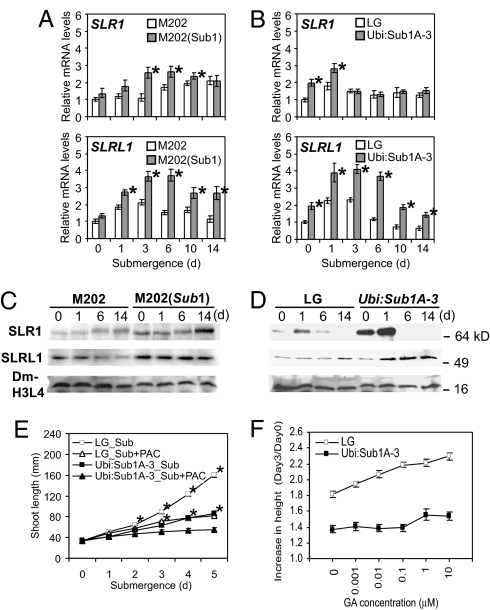
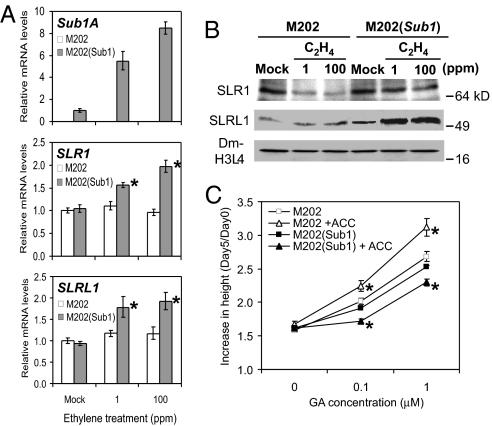
SLENDER RICE-1 (SLR1) and SLR1 like-1 (SLRL1) are nuclear-localized GRAS domain proteins that function as the central suppressors of GA signaling in rice (13–17). SLRL1 is distinguished from SLR1 by the absence of the N-terminal DELLA domain that is responsible for 26S proteosome-mediated degradation of this protein in a GA-dependent manner. Overexpression of SLR1 or SLRL1 gives rise to constitutive GA insensitivity (15, 16). To discern the role of Sub1A in the regulation of GA signaling during submergence, the accumulation of SLR1 and SLRL1 transcripts was evaluated in aerial tissue of M202 and M202(Sub1) over a 14-day time course of submergence (Fig. 4A). SLR1 mRNA was induced during submergence in the 2 genotypes, but the increase was more rapid and pronounced in M202(Sub1). SLRL1 mRNA was also submergence-inducible in both genotypes, but to a significantly greater extent in M202(Sub1) over the time course of submergence. The Ubi:Sub1A transgenics displayed increased basal levels of SLR1 and SLRL1 transcripts under normal growth conditions, along with a more pronounced induction during submergence (Fig. 4B). These data confirm that Sub1A promotes accumulation of SLR1 and SLRL1 transcripts in aerial tissue under submerged conditions. The use of antisera that specifically recognize SLR1 and SLRL1 confirmed that submergence also perturbed the accumulation of these proteins (Fig. 4C). The level of SLR1 was notably lower in M202 than in M202(Sub1) over the submergence time course. A decrease in the electrophoretic mobility of SLR1 was evident after 6 days of submergence in both genotypes, presumably because of O-linked GlcNAc (O-GlcNAc) modification or phosphorylation (18, 19). Similar to SLR, the abundance of SLRL1 was lower in M202 than M202(Sub1) during submergence. The accumulation of these repressors was also differentially regulated by submergence stress in LG and Ubi:Sub1A plants (Fig. 4D). The increase in SLRL1 transcript consistently coincided with higher levels of this GA signaling repressor during submergence. These data demonstrate that Sub1A acts upstream of a key repressor of GA signaling, SLRL1, which lacks the DELLA domain.
Fig. 4.
Sub1A dampens GA response during submergence. (A and B) Relative transcript levels of SLR1 and SLRL1 in aerial tissue during submergence. Fourteen-day-old [M202, M202(Sub1), and LG] and 21-d-old (Ubi:Sub1A-3) plants were submerged for up to 14 days. Aerial tissue was collected at the specific time points and subjected to quantitative RT-PCR analysis. Relative levels of individual transcripts were calculated by comparison to the nonsubmerged control (M202 or LG at day 0). Data represent mean ± SD from 3 independent biological replicates, and an asterisk indicates a significant difference between M202 vs. M202(Sub1) and LG vs. Ubi:Sub1A-3 (P < 0.01). (C and D) Immunoblot analysis of SLR1 and SLRL1 protein accumulation in aerial tissue during submergence. Tissue samples used for A and B were analyzed by Western blotting. Crude protein (5 μg) was fractionated by electrophoresis in a 10% (wt/vol) SDS/PAGE gel. The level of dimethyl-Histone H3 (Lys-4) (Dm-H3L4) was used as a loading control. (E) Effect of the GA biosynthesis inhibitor paclobutrazol on underwater elongation. Five-day-old (LG) and 10-day-old (Ubi:Sub1A-3) plants were submerged in mock (0.1% DMSO) or paclobutrazol (50 μM in 0.1% DMSO) solution for up to 5 days. Data represent mean ± SE from 3 independent biological replicates (n = 45), and an asterisk indicates a significant difference between mock and paclobutrazol treatment (P < 0.05). (F) GA-induced elongation of aerial tissue during submergence. Five-day-old (LG) and 10-day-old (Ubi:Sub1A-3) seedlings were submerged in a series of GA3 solutions (0.001–10 μM GA3 in 0.1% DMSO) containing 200 μM paclobutrazol for 3 days. Data represent mean ± SE from 3 independent biological replicates (n = 45).
The effect of endogenous GA on underwater elongation was compared in LG (5-day-old) and Ubi:Sub1A-3 (10-day-old) plants of similar maturity (Fig. 4E). The shoot length of both genotypes was almost identical at day 0, but LG elongated significantly more than Ubi:Sub1A-3 after 5 days of complete submergence. The presence of the GA biosynthesis inhibitor paclobutrazol restrained elongation in both genotypes, confirming a role for GA biosynthesis in elongation growth during submergence. To determine whether Sub1A restricts GA response during submergence, LG and Ubi:Sub1A-3 plants were submerged in a series of GA3 solutions for 3 days (Fig. 4F). Both mock and GA3 solutions contained 200 μM paclobutrazol to inhibit production of bioactive GAs. Shoot elongation was promoted in LG by all GA3 concentrations tested (0.001–10 μM). By contrast, Ubi:Sub1A-3 plants responded only to 1 and 10 μM GA3 under submergence, consistent with enhanced levels of SLRL1 (Fig. 4D) and reduced elongation of aerial tissue during submergence (Figs. 1B and 4E).
Secondary confirmation that Sub1A overexpression increases SLR1 and SLRL1 transcript abundance and decreases GA responsiveness was obtained by evaluating embryo-less half seeds (Fig. S4). A repression of GA3-promoted accumulation of α-amylase mRNAs by Sub1A was observed in accordance with the low germination percentage and limited GA response in the Sub1A overexpression lines (Fig. 3 A and B).
Sub1A Reverses the Effect of Ethylene on GA Responsiveness During Submergence.
The GA-mediated elongation in semiaquatic plants is triggered by ethylene accumulated during submergence (1, 7–9). To discern the effect of ethylene on the repressors of GA signaling, mRNA levels of SLR1 and SLRL1 were evaluated in 14-day-old M202 and M202(Sub1) plants after treatment with 1 or 100 ppm ethylene (Fig. 5A). As shown previously (6), ethylene dramatically increased the level of Sub1A mRNA in M202(Sub1). SLR1 and SLRL1 transcripts were not induced by application of ethylene in M202 but rose significantly in M202(Sub1). The levels of SLR1 and SLRL1 proteins were also monitored in ethylene-treated plants (Fig. 5B). Ethylene reduced the abundance of SLR1 in M202, but the protein was maintained in M202(Sub1). By contrast, ethylene had little effect on SLRL1 in M202 but markedly increased the protein abundance in M202(Sub1). These data demonstrate that ethylene-mediated Sub1A expression promotes the accumulation of SLR1 and SLRL1 transcripts and proteins.
Fig. 5.
Ethylene-inducible Sub1A restricts GA-mediated elongation via SLR1/SLRL1. (A) Relative transcript levels of Sub1A, SLR1, and SLRL1 in response to ethylene. Fourteen-day-old plants were treated with 1 or 100 ppm of ethylene for 6 h, and aerial tissue was subjected to quantitative RT-PCR analysis. Relative levels of SLR1 and SLRL1 mRNAs were calculated in comparison to nontreated M202. For Sub1A mRNA, nontreated M202(Sub1) was used because M202 lacks Sub1A. Data represent mean ± SD from 3 independent biological replicates, and an asterisk indicates a significant difference between M202 and M202(Sub1) (P < 0.01). (B) Immunoblot analysis of SLR1 and SLRL1 protein accumulation in response to ethylene. Tissue samples used for A were analyzed by Western blotting. (C) Effect of ACC on GA-mediated shoot elongation. Five-day-old seedlings were treated with combinations of ACC (0 or 10 μM) and/or GA3 (0, 0.1, or 1 μM) solutions for 5 days. All solutions used for this experiment additionally contained 200 μM paclobutrazol. Data represent mean ± SE from 3 independent biological replicates (n = 45), and an asterisk indicates a significant difference between the presence and absence of ACC in each genotype (P < 0.05).
To further evaluate the influence of ethylene on the GA response, 5-day-old M202 and M202(Sub1) plants were treated with combinations of the ethylene precursor 1-aminocyclopropane-1-carboxylic acid (ACC) and GA3 under normal growth conditions and growth over 5 days was recorded (Fig. 5C). All treatments additionally included 200 μM paclobutrazol. As we reported previously (6), the shoot elongation response to GA3 was similar in nonsubmerged M202 and M202(Sub1). The application of 10 μM ACC did not affect growth of aerial tissue in the 2 lines. However, the addition of ACC to GA3 solutions further increased GA-mediated elongation of aerial tissue in M202, consistent with observations for deepwater rice (1). Notably, the response to GA3 was significantly reduced by ACC in M202(Sub1). These results suggest that, in the absence of Sub1A, ethylene augments GA responsiveness by lowering levels of SLR1. Conversely, ethylene-induced Sub1A maintains and further increases the abundance of SLR1 and SLRL1, respectively, manifesting a suppression of GA-mediated elongation growth.
Discussion
Submergence tolerance in rice can be conferred by a Sub1A-dependent restriction of carbohydrate consumption and elongation growth (3, 6). To determine the functional mechanism of Sub1A in submergence tolerance, we first considered that modulation of the balance between GA and ABA might underlie distinctions in Sub1A-mediated submergence tolerance. However, we found that the rapid catabolism of endogenous ABA and PA occurs in a Sub1A-independent manner in response to submergence (Fig. 2). Consistent with our results, submergence reduced the endogenous ABA content to a similar extent within 1 day in seedlings of both deepwater and submergence-intolerant lowland rice (20). This shows that the tolerance of prolonged inundation provided by Sub1A is mediated downstream of ABA catabolism and upstream of GA responses.
The expression of Sub1A was correlated with a significant increase in SLR1 and SLRL1 mRNAs during submergence in aerial tissue of M202(Sub1) and Ubi:Sib1A-3 (Fig. 4 A and B) and was accompanied by maintenance of higher levels of SLR1 and SLRL1 proteins under submerged conditions (Fig. 4 C and D). Promoter regions (2,000 bp upstream of the translation start site) of japonica SLR1 and SLRL1 do not contain the GCC motif to which Arabidopsis ERF proteins bind, suggesting that these genes may not be direct targets of SUB1A (data not shown). The primary distinction between the 2 GA signaling repressors is that SLR1 possesses an N-terminal DELLA domain that interacts with the GA receptor GA INSENSITIVE DWARF1 (GID1) in a GA-dependent manner and promotes binding to an F-box protein that facilitates SLR1 ubiquitination (16, 17). The subsequent destruction of SLR1 by the 26S proteosome stimulates GA-activated gene expression. A conserved leucine repeat present in all SLR1 orthologs mediates an interaction between an Arabidopsis SLR1 ortholog (REPRESSOR OF ga1–3, RGA) and the basic helix–loop–helix DNA binding domain of the transcription factors PHYTOCHROME INTERACTING FACTOR 3 and 4, thereby inhibiting their role in transcriptional activation (21, 22). This suggests that SLR1 orthologs can repress gene expression by sequestering DNA binding proteins from their targets. Interestingly, Arabidopsis lacks a SLRL1 equivalent because its 5 SLR1 orthologs possess a DELLA domain. In rice, SLRL1 functionally complements an slr1 mutant, indicating that these proteins serve similar roles (16). Both SLR1 and SLRL1 overexpression lines of rice are semidwarf and recalcitrant to GA signaling (15, 16). Consistent with the constitutive elevation of SLR1 in aerial tissue of Ubi:Sub1A, ectopic expression of Sub1A delayed seed maturation and germination, reduced plant stature, prolonged vegetative growth, and delayed floral development and seed production (Fig. 3 A and B). Moreover, Sub1A restrained the elongation response to GA3 under aerial and submerged conditions (Figs. 3C and 4F). Together, these findings demonstrate that Sub1A elevates SLR1 and SLRL1 levels, thereby limiting GA-mediated developmental and physiological responses. Future studies might address the importance of timing and amplitude as well as cell type and regional specificity of Sub1A and Sub1C expression in orchestration of the responses to submergence.
Modulation of SLR1 and SLRL1 proteins in response to submergence was notably distinct in the 4 rice genotypes evaluated (Fig. 4 C and D). In M202 and M202(Sub1), SLR1 increased in abundance and appeared to be posttranslationally modified (18, 19). By contrast, SLR1 was constitutively elevated in Ubi:Sub1A, consistent with the manifestation of phenotypes associated with defects in GA signaling. SLR1 levels dropped below the detection limit at the later time points of submergence in the LG and Ubi:Sub1A genotypes, with a notable acceleration of this processing in the transgenic line. The constitutive elevation of SLR1 mRNA and protein may disturb the endogenous GA homoeostasis in Ubi:Sub1A plants under non-stress conditions. It has been reported that GA-insensitive rice mutants, gid1 and gid2, overproduce endogenous GAs (23, 24). It is feasible that higher levels of bioactive GA or enhanced GA biosynthesis in response to submergence could be responsible for the marked decline in SLR1 in the Ubi:Sub1A transgenic. An alternative may be that the japonica cultivar LG has higher levels of bioactive GAs after submergence than M202, enhancing proteosome-mediated degradation of SLR1. The elongation of aerial tissue was significantly restricted in Ubi:Sub1A lines despite the decline of SLR1 during submergence (Figs. 1B and 4 E and F). We postulate that this is because of complementation of SLR1 activity by SLRL1. This model is particularly attractive because SLRL1 is not degraded via the 26S proteosome pathway in response to an increase in GA because of its lack of a DELLA domain (16). Indeed, the level of SLRL1 progressively increased under submerged conditions in Ubi:Sub1A (Fig. 4D). The decrease in SLR1 was not observed in M202(Sub1) over the submergence time course (Fig. 4C), demonstrating that the dampening of GA responsiveness is mediated by maintenance of both SLR1 and SLRL1 when the endogenous Sub1A promoter is induced by the stress. In both of the submergence-tolerant lines studied, the restriction of GA responsiveness and underwater elongation corresponded to a higher level of 1 or both of these repressors. In conclusion, Sub1A augments SLR1 and SLRL1 mRNA and protein levels, which counteracts the elevated responsiveness to GA promoted by the increase in ethylene and decline in ABA. Strikingly, the DELLA-lacking SLRL1 serves as a backup to limit GA responsiveness after SLR1 degradation.
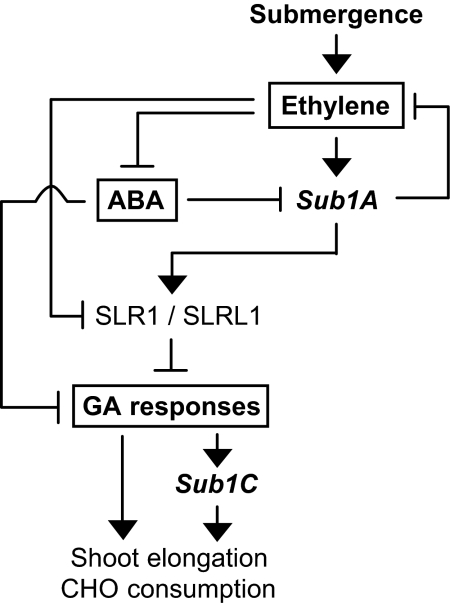
We propose a model for Sub1A-dependent hormonal regulation of submergence responses in rice (Fig. 6). First, submergence increases cellular ethylene content, which independently triggers the catabolic degradation of ABA via the ABA 8′ hydroxylase pathway (25). In the absence of Sub1A, this increase in ethylene leads to reduced levels of SLR1 protein (Fig. 5B). In turn, the decline in ABA and SLR1 increases the responsiveness to GA, which gives rise to the promotion of elongation growth accompanied by carbohydrate consumption. Submergence-tolerant genotypes counteract this scenario via the action of Sub1A. The rise in ethylene during submergence is likely to be the key regulatory switch for Sub1A expression (Fig. 5A) (6). Sub1A acts directly or indirectly to promote the accumulation of the negative regulators for GA signaling, SLR1 and SLRL1 (Figs. 4 and 5), which subsequently dampen accumulation of GA-inducible mRNAs including Sub1C (Figs. S2 and S4). The level of Sub1C mRNA is positively correlated with the extent of underwater elongation in rice, indicating that the involvement of Sub1C in GA-mediated gene regulation is important for adaptive responses to the stress (3, 6). However, GA responses are also likely to be regulated in a Sub1C-independent manner during submergence because the transcript levels of GA-responsive expansin A genes are unaltered when Sub1C is induced by GA treatment under normal conditions (6). Another function of Sub1A is to restrict ethylene production during submergence, resulting in the limitation of ethylene-induced enhancement of GA responsiveness (Fig. 5C) (6). All together, the Sub1A-dependent regulation of GA signaling and ethylene production suppresses energy-consuming responses, causing restriction in carbohydrate consumption and quiescence in elongation growth that correlate with the capacity for regrowth upon desubmergence. This strategy of response to submergence provided by a naturally occurring Sub1 haplotype is particularly advantageous when the inundation is deep and prolonged, as occurs in severe periods of floods in India and Southeast Asia.
Fig. 6.
Model for Sub1A-dependent hormonal regulation of submergence responses. Ethylene accumulation, ABA degradation, and increased GA response occur sequentially in response to submergence in rice. Submergence-tolerant rice carrying naturally or ectopically expressed Sub1A-1 restrains ethylene production and GA signaling in a Sub1A-dependent manner, resulting in the limitation of energy-consuming responses [shoot elongation and carbohydrate (CHO) consumption]. This quiescence response to submergence allows the plant to economize carbohydrate reserves under the stress, which facilitates regrowth upon desubmergence, even after inundation for 16 days or longer.
Methods
Plant Materials, Growth Conditions, and Phenotype Analyses.
Rice (O. sativa L.) cv. Liaogeng (LG; japonica), cv. M202 (japonica), transgenic lines with constitutively expressed Sub1A, Ubi:Sub1A-1, and Ubi:Sub1A-3 (background genotype; LG), and the Sub1 introgression line M202(Sub1) (accession no. DX236-17-2-4) were analyzed in this study (3, 6). Plants were grown as described in ref. 6. After 14 days, plants were transferred to 7.5 L pots (2 plants per pot) and cultured in a greenhouse until seed harvest. Seed germination, flowering time, seed maturation, and seed set percentage were recorded as described in SI Text.
Submergence and Hormone Treatments.
All submergence and hormone treatments were replicated in at least 3 independent biological experiments. Submergence and ethylene treatments were carried out as described in ref. 6. For ABA treatment, aerial tissue of 14-day-old [LG, M202, and M202(Sub1)] or 21-day-old (Ubi:Sub1A-3) plants was obtained by excision at the base of the stem and immediately placed into 20 mL of mock [0.1% (vol/vol) DMSO] or ABA solution (5 or 50 μM in 0.1% DMSO) in a 250-mL glass beaker for 24 h in the light (50 μmol/m2/s). For GA treatment, embryos were removed from dehulled seeds by use of a razor blade and the embryo-less half seeds were soaked in mock (0.1% DMSO) or GA3 (0.1 or 10 μM GA3 in 0.1% DMSO) solution for 24 h at room temperature in the light (50 μmol/m2/s). After treatments, tissue was frozen immediately in liquid nitrogen.
Measurement of Shoot Elongation.
Fifteen seeds with 1-mm coleoptiles were placed on wet filter paper containing mock [0.1% (vol/vol) DMSO] or a series of GA3 solutions (0.01–1,000 μM GA3 in 0.1% DMSO) at 25 °C in the light (50 μmol/m2/s). After a 7-day incubation, the entire shoot length was recorded. Dehulled seeds were surface sterilized in 70% (vol/vol) ethanol for 10 min and in 2% (vol/vol) sodium hypochlorite for 20 min. After rinsing with sterilized deionized water, each seed was grown on MS medium [1× Murashige and Skoog medium, pH 5.7/0.33% (wt/vol) phytagel (Sigma–Aldrich)] in test tubes for 5 days [LG, M202, and M202(Sub1)] or 10 days (Ubi:Sub1A-3) as described in ref. 6. Each test tube was filled with mock [0.1% (vol/vol) DMSO] or paclobutrazol (50 μM) solutions and closed with a loose plastic cap. The plants were grown under submerged conditions for up to 5 days. Plants grown in test tubes were also completely submerged in a series of GA3 solutions (0–10 μM GA3) containing 200 μM paclobutrazol and 0.1% (vol/vol) DMSO for 3 days. Five-day-old plants grown in test tubes were carefully removed from MS media and immediately placed into 15 mL of ACC (0 or 10 μM) and/or GA3 (0, 0.1, or 1 μM) solution containing 200 μM paclobutrazol and 0.1% (vol/vol) DMSO in a plastic culture box (6 cm length × 6 cm width × 10 cm height) for 5 days. For each experiment, plants were incubated at 20 °C in the periodic photoperiod (16 h light/8 h dark; light level, 50 μmol/m2/s). The entire shoot length was recoded at the time point specified.
RNA Extraction, RT-PCR, and Quantitative RT-PCR.
Total RNA was extracted from aerial tissue by using the RNeasy plant mini kit. After DNase treatment cDNA synthesis was performed by following the methods of ref. 6. Quantitative RT-PCR measurements were performed with 20 μL of reaction solution containing 2 μL of cDNA, 0.5 μM of each primer, and 10 μL of iQ SYBR Green supermix by using a MyiQ real-time PCR detection system (Bio-Rad). Relative transcript abundance was calculated by using the comparative CT method. Actin1 was used as a normalization control. Sequences of primer pairs and PCR conditions are listed in Table S1. Procedures of RNA extraction from embryo-less half seeds and RT-PCR are described in SI Text.
Western Blot Analysis.
Crude protein was extracted from aerial tissue (50 mg) in an extraction buffer (0.2 mL) containing 50 mM Tris·HCl (pH 8.0), 150 mM NaCl, 1% (vol/vol) Igepal CA-630, 1 mM EDTA, 0.5% (wt/vol) sodium deoxycholate, 0.1% (wt/vol) SDS, and 1 mM phenylmethylsulfonyl fluoride. Western blotting was performed as described in ref. 26. Protein sample (5 μg) was fractionated in a 10% (wt/vol) SDS/PAGE gel. Rabbit antisera against SLR1 and SLRL1 were kindly provided by Makoto Matsuoka (Nagoya University, Japan). The blots were incubated in a 1:10,000 dilution of anti-SLR1, anti-SLRL1, or anti-dimethyl-Histone H3 (Lys-4) (Millipore) for 1 h at 25 °C and then in a 1:7,000 dilution of goat anti-rabbit IgG horseradish peroxidase-conjugated secondary antibody for 1 h at 25 °C. Peroxidase activity was detected by ECL Plus (GE Healthcare).
Quantification of Endogenous Hormones.
Lyophilized aerial tissue (100 mg dry weight) was subjected to quantification of ABA and ABA catabolites. Each metabolite was separated by high-performance liquid chromatography and quantified by tandem mass spectrometry. The hormone analysis was performed by the Plant Hormone Profiling team at the National Research Council Plant Biotechnology Institute (Saskatoon, Canada) following the method described in ref. 27.
Supplementary Material
Acknowledgments.
We thank Pamela Ronald, David Mackill, Sigrid Heuer, Abdelbagi Ismail, and Angelika Mustroph for valuable comments and discussion. We are also grateful to Dave Mackill (International Rice Research Institute, Philippines) for the M202(Sub1) line, Pamela Ronald (University of California, Davis) for the Ubi:Sub1A lines, and Makoto Matsuoka for anti-SLR1 and anti-SLRL1 antibodies. This work was supported by the U.S. Department of Agriculture National Research Initiative Competitive Grant Program (2006-35100-17288) and by U.S. Agency for International Development Linkage Project Funds from the International Rice Research Institute.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0807821105/DCSupplemental.
References
- 1.Kende H, van der Knaap E, Cho H-T. Deepwater rice: A model plant to study stem elongation. Plant Physiol. 1998;118:1105–1110. doi: 10.1104/pp.118.4.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Setter TL, Laureles EV. The beneficial effect of reduced elongation growth on submergence tolerance of rice. J Exp Bot. 1996;47:1551–1559. [Google Scholar]
- 3.Xu K, et al. Sub1A is an ethylene-response-factor-like gene that confers submergence tolerance to rice. Nature. 2006;442:705–708. doi: 10.1038/nature04920. [DOI] [PubMed] [Google Scholar]
- 4.Singh HP, Singh BB, Ram PC. Submergence tolerance of rainfed lowland rice: Search for physiological marker traits. J Plant Physiol. 2001;158:883–889. [Google Scholar]
- 5.Das KK, Sarkar RK, Ismail AM. Elongation ability and non-structural carbohydrate levels in relation to submergence tolerance in rice. Plant Sci. 2005;168:131–136. [Google Scholar]
- 6.Fukao T, Xu K, Ronald PC, Bailey-Serres J. A variable cluster of ethylene response factor-like genes regulates metabolic and developmental acclimation responses to submergence in rice. Plant Cell. 2006;18:2021–2034. doi: 10.1105/tpc.106.043000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Voesenek LACJ, et al. How plants cope with complete submergence. New Phytol. 2006;170:213–226. doi: 10.1111/j.1469-8137.2006.01692.x. [DOI] [PubMed] [Google Scholar]
- 8.Bailey-Serres J, Voesenek LACJ. Flooding stress: Acclimations and genetic diversity. Annu Rev Plant Biol. 2008;59:313–339. doi: 10.1146/annurev.arplant.59.032607.092752. [DOI] [PubMed] [Google Scholar]
- 9.Fukao T, Bailey-Serres J. Ethylene—A key regulator of submergence responses in rice. Plant Sci. 2008;175:43–51. [Google Scholar]
- 10.Hoffmann-Benning S, Kende H. On the role of abscisic acid and gibberellin in the regulation of growth in rice. Plant Physiol. 1992;99:1156–1161. doi: 10.1104/pp.99.3.1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Benschop JJ, et al. Long-term submergence-induced elongation in Rumex palustris requires abscisic acid-dependent biosynthesis of gibberellin1. Plant Physiol. 2006;141:1644–1652. doi: 10.1104/pp.106.082636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cutler AJ, Krochko JE. Formation and breakdown of ABA. Trends Plants Sci. 1999;4:472–478. doi: 10.1016/s1360-1385(99)01497-1. [DOI] [PubMed] [Google Scholar]
- 13.Hartweck LM, Olszewski NE. Rice GIBBERELLIN INSENSITIVE DWARF1 is a gibberellin receptor that illuminates and raises questions about GA signaling. Plant Cell. 2006;18:278–282. doi: 10.1105/tpc.105.039958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ikeda A, et al. slender rice, a constitutive gibberellin response mutant, is caused by a null mutation of the SLR1 gene, an ortholog of the height-regulating gene GAI/RGA/RHT/D8. Plant Cell. 2001;13:999–1010. doi: 10.1105/tpc.13.5.999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Itoh H, et al. The gibberellin signaling pathway is regulated by the appearance and disappearance of SLENDER RICE1 in nuclei. Plant Cell. 2002;14:57–70. doi: 10.1105/tpc.010319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Itoh H, et al. Overexpression of a GRAS protein lacking the DELLA domain confers altered gibberellin responses in rice. Plant J. 2005;44:669–679. doi: 10.1111/j.1365-313X.2005.02562.x. [DOI] [PubMed] [Google Scholar]
- 17.Ueguchi-Tanaka M, et al. Molecular interactions of a soluble gibberellin receptor, GID1, with a rice DELLA protein, SLR1, and gibberellin. Plant Cell. 2007;19:2140–2155. doi: 10.1105/tpc.106.043729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thornton TM, Swain SM, Olszewski NE. Gibberellin signal transduction presents … the SPY who O-GlcNAc'd me. Trends Plants Sci. 1999;4:424–428. doi: 10.1016/s1360-1385(99)01485-5. [DOI] [PubMed] [Google Scholar]
- 19.Jiang C, Fu X. GA action: Turning on de-DELLA repressing signaling. Curr Opin Plant Biol. 2007;10:461–465. doi: 10.1016/j.pbi.2007.08.011. [DOI] [PubMed] [Google Scholar]
- 20.Van der Straeten D, et al. A comparative molecular-physiological study of submergence response in lowland and deepwater rice. Plant Physiol. 2001;125:955–968. doi: 10.1104/pp.125.2.955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.de Lucas M, et al. A molecular framework for light and gibberellin control of cell elongation. Nature. 2008;451:480–484. doi: 10.1038/nature06520. [DOI] [PubMed] [Google Scholar]
- 22.Feng S, et al. Coordinated regulation of Arabidopsis thaliana development by light and gibberellins. Nature. 2008;451:475–479. doi: 10.1038/nature06448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sasaki A, et al. Accumulation of phosphorylated repressor for gibberellin signaling in an F-box mutant. Science. 2003;299:1896–1898. doi: 10.1126/science.1081077. [DOI] [PubMed] [Google Scholar]
- 24.Ueguchi-Tanaka M, et al. GIBBERELLIN INSENSITIVE DWARF1 encodes a soluble receptor for gibberellin. Nature. 2005;437:693–698. doi: 10.1038/nature04028. [DOI] [PubMed] [Google Scholar]
- 25.Saika H, et al. Ethylene promotes submergence-induced expression of OsABA8ox1, a gene that encodes ABA 8′-hydroxylase in rice. Plant Cell Physiol. 2007;48:287–298. doi: 10.1093/pcp/pcm003. [DOI] [PubMed] [Google Scholar]
- 26.Williams AJ, Werner-Fraczek J, Chang I-F, Bailey-Serres J. Regulated phosphorylation of 40S ribosomal protein S6 in root tips of maize. Plant Physiol. 2003;132:2086–2097. doi: 10.1104/pp.103.022749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chiwocha SDS, et al. The etr1–2 mutation in Arabidopsis thaliana affects the abscisic acid, auxin, cytokinin and gibberellin metabolic pathways during maintenance of seed dormancy, stratification and germination. Plant J. 2005;42:35–48. doi: 10.1111/j.1365-313X.2005.02359.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.