Abstract
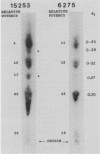
We investigated whether the striking difference in severity of coagulopathy observed between bacterial sepsis involving Neisseria meningitidis and Neisseria gonorrhoeae species is related to species-dependent abilities to directly activate coagulation. Using lipooligosaccharide (LOS)-activated gelation of Limulus amebocyte lysate, we compared the relative abilities of outer membrane LOS of 10 N. meningitidis and 10 N. gonorrhoeae strains to initiate coagulation. A wide range of procoagulant potencies was observed for each species, and there was significant overlap of potencies between species. Relative biological activities did not correlate with the oligosaccharide components as defined by LOS molecular weight or specific antigenic epitopes. Purified lipid A of two LOS strains of different potency demonstrated relative procoagulant biological activities similar to those of their parent LOSs. When these lipid A preparations were further separated by thin-layer chromatography, the most polar component of each lipid A possessed the majority of the procoagulant activity. We concluded that the ability of neisserial LOS to initiate coagulation of Limulus lysate is a property of the lipid A portion of the molecule and is most likely determined by fine structural differences in the lipid A which are independent of species.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersen B. M. Endotoxin release from neisseria meningitidis. Relationship between key bacterial characteristics and meningococcal disease. Scand J Infect Dis Suppl. 1989;64:1–43. doi: 10.3109/inf.1989.21.suppl-64.01. [DOI] [PubMed] [Google Scholar]
- Apicella M. A., Gagliardi N. C. Antigenic heterogeneity of the non-serogroup antigen structure of Neisseria gonorrhoeae lipopolysaccharides. Infect Immun. 1979 Dec;26(3):870–874. doi: 10.1128/iai.26.3.870-874.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandtzaeg P., Kierulf P., Gaustad P., Skulberg A., Bruun J. N., Halvorsen S., Sørensen E. Plasma endotoxin as a predictor of multiple organ failure and death in systemic meningococcal disease. J Infect Dis. 1989 Feb;159(2):195–204. doi: 10.1093/infdis/159.2.195. [DOI] [PubMed] [Google Scholar]
- Bryn K., Solberg O., Andersen B. M. Endotoxin liberation studied by biological and chemical methods. Chemical characterization of six meningococcal lipopolysaccharides. APMIS. 1989 May;97(5):429–435. doi: 10.1111/j.1699-0463.1989.tb00811.x. [DOI] [PubMed] [Google Scholar]
- Cooper M. D., McGraw P. A., Melly M. A. Localization of gonococcal lipopolysaccharide and its relationship to toxic damage in human fallopian tube mucosa. Infect Immun. 1986 Feb;51(2):425–430. doi: 10.1128/iai.51.2.425-430.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DITTMER J. C., LESTER R. L. A SIMPLE, SPECIFIC SPRAY FOR THE DETECTION OF PHOSPHOLIPIDS ON THIN-LAYER CHROMATOGRAMS. J Lipid Res. 1964 Jan;5:126–127. [PubMed] [Google Scholar]
- Davis C. E., Arnold K. Role of meningococcal endotoxin in meningococcal purpura. J Exp Med. 1974 Jul 1;140(1):159–171. doi: 10.1084/jem.140.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudas K. C., Apicella M. A. Selection and immunochemical analysis of lipooligosaccharide mutants of Neisseria gonorrhoeae. Infect Immun. 1988 Feb;56(2):499–504. doi: 10.1128/iai.56.2.499-504.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erwin A. L., Mandrell R. E., Munford R. S. Enzymatically deacylated Neisseria lipopolysaccharide (LPS) inhibits murine splenocyte mitogenesis induced by LPS. Infect Immun. 1991 Jun;59(6):1881–1887. doi: 10.1128/iai.59.6.1881-1887.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galanos C., Lüderitz O., Westphal O. Preparation and properties of antisera against the lipid-A component of bacterial lipopolysaccharides. Eur J Biochem. 1971 Dec 22;24(1):116–122. doi: 10.1111/j.1432-1033.1971.tb19661.x. [DOI] [PubMed] [Google Scholar]
- Gregg C. R., Melly M. A., Hellerqvist C. G., Coniglio J. G., McGee Z. A. Toxic activity of purified lipopolysaccharide of Neisseria gonorrhoeae for human fallopian tube mucosa. J Infect Dis. 1981 Mar;143(3):432–439. doi: 10.1093/infdis/143.3.432. [DOI] [PubMed] [Google Scholar]
- Griffiss J. M., Brandt B. L., Broud D. D., Goroff D. K., Baker C. J. Immune response of infants and children to disseminated infections with Neisseria meningitidis. J Infect Dis. 1984 Jul;150(1):71–79. doi: 10.1093/infdis/150.1.71. [DOI] [PubMed] [Google Scholar]
- Griffiss J. M., Jarvis G. A., O'Brien J. P., Eads M. M., Schneider H. Lysis of Neisseria gonorrhoeae initiated by binding of normal human IgM to a hexosamine-containing lipooligosaccharide epitope(s) is augmented by strain-specific, properdin-binding-dependent alternative complement pathway activation. J Immunol. 1991 Jul 1;147(1):298–305. [PubMed] [Google Scholar]
- Griffiss J. M., O'Brien J. P., Yamasaki R., Williams G. D., Rice P. A., Schneider H. Physical heterogeneity of neisserial lipooligosaccharides reflects oligosaccharides that differ in apparent molecular weight, chemical composition, and antigenic expression. Infect Immun. 1987 Aug;55(8):1792–1800. doi: 10.1128/iai.55.8.1792-1800.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helin H. Macrophage procoagulant factors--mediators of inflammatory and neoplastic tissue lesions. Med Biol. 1986;64(4):167–176. [PubMed] [Google Scholar]
- Homma R., Kuratsuka K., Akama K. Application of the LAL test and the chromogenic substrate test to the detection of endotoxin in human blood products. Prog Clin Biol Res. 1982;93:301–317. [PubMed] [Google Scholar]
- Kim J. J., Mandrell R. E., Griffiss J. M. Neisseria lactamica and Neisseria meningitidis share lipooligosaccharide epitopes but lack common capsular and class 1, 2, and 3 protein epitopes. Infect Immun. 1989 Feb;57(2):602–608. doi: 10.1128/iai.57.2.602-608.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEVIN J., BANG F. B. A DESCRIPTION OF CELLULAR COAGULATION IN THE LIMULUS. Bull Johns Hopkins Hosp. 1964 Oct;115:337–345. [PubMed] [Google Scholar]
- Levin J., Bang F. B. Clottable protein in Limulus; its localization and kinetics of its coagulation by endotoxin. Thromb Diath Haemorrh. 1968 Mar 31;19(1):186–197. [PubMed] [Google Scholar]
- Mandrell R. E., Griffiss J. M., Macher B. A. Lipooligosaccharides (LOS) of Neisseria gonorrhoeae and Neisseria meningitidis have components that are immunochemically similar to precursors of human blood group antigens. Carbohydrate sequence specificity of the mouse monoclonal antibodies that recognize crossreacting antigens on LOS and human erythrocytes. J Exp Med. 1988 Jul 1;168(1):107–126. doi: 10.1084/jem.168.1.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandrell R. E., Kim J. J., John C. M., Gibson B. W., Sugai J. V., Apicella M. A., Griffiss J. M., Yamasaki R. Endogenous sialylation of the lipooligosaccharides of Neisseria meningitidis. J Bacteriol. 1991 May;173(9):2823–2832. doi: 10.1128/jb.173.9.2823-2832.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandrell R. E., Lesse A. J., Sugai J. V., Shero M., Griffiss J. M., Cole J. A., Parsons N. J., Smith H., Morse S. A., Apicella M. A. In vitro and in vivo modification of Neisseria gonorrhoeae lipooligosaccharide epitope structure by sialylation. J Exp Med. 1990 May 1;171(5):1649–1664. doi: 10.1084/jem.171.5.1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandrell R. E., Zollinger W. D. Lipopolysaccharide serotyping of Neisseria meningitidis by hemagglutination inhibition. Infect Immun. 1977 May;16(2):471–475. doi: 10.1128/iai.16.2.471-475.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandrell R., Schneider H., Apicella M., Zollinger W., Rice P. A., Griffiss J. M. Antigenic and physical diversity of Neisseria gonorrhoeae lipooligosaccharides. Infect Immun. 1986 Oct;54(1):63–69. doi: 10.1128/iai.54.1.63-69.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison D. C., Cochrane C. G. Direct evidence for Hageman factor (factor XII) activation by bacterial lipopolysaccharides (endotoxins). J Exp Med. 1974 Sep 1;140(3):797–811. doi: 10.1084/jem.140.3.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prior R. B., Spagna V. A. Improved utility of Gonoscreen, a Limulus amoebocyte lysate assay, in the evaluation of urethral discharges in men. J Clin Microbiol. 1985 Aug;22(2):141–144. doi: 10.1128/jcm.22.2.141-144.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proctor R. A., Textor J. A. Activation and inhibition of Limulus amebocyte lysate coagulation by chemically defined substructures of lipid A. Infect Immun. 1985 Aug;49(2):286–290. doi: 10.1128/iai.49.2.286-290.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quagliarello V. J., Scheld W. M. Recent advances in the pathogenesis and pathophysiology of bacterial meningitis. Am J Med Sci. 1986 Nov;292(5):306–309. doi: 10.1097/00000441-198611000-00010. [DOI] [PubMed] [Google Scholar]
- Rietschel E. T., Wollenweber H. W., Zähringer U., Lüderitz O. Lipid A, the lipid component of bacterial lipopolysaccharides: relation of chemical structure to biological activity. Klin Wochenschr. 1982 Jul 15;60(14):705–709. doi: 10.1007/BF01716559. [DOI] [PubMed] [Google Scholar]
- Saubolle M. A., Jorgensen J. H. Use of the Limulus amebocyte lysate test as a cost-effective screen for gram-negative agents of meningitis. Diagn Microbiol Infect Dis. 1987 Jul;7(3):177–183. doi: 10.1016/0732-8893(87)90002-2. [DOI] [PubMed] [Google Scholar]
- Schneider H., Griffiss J. M., Williams G. D., Pier G. B. Immunological basis of serum resistance of Neisseria gonorrhoeae. J Gen Microbiol. 1982 Jan;128(1):13–22. doi: 10.1099/00221287-128-1-13. [DOI] [PubMed] [Google Scholar]
- Shafer W. M., Joiner K., Guymon L. F., Cohen M. S., Sparling P. F. Serum sensitivity of Neisseria gonorrhoeae: the role of lipopolysaccharide. J Infect Dis. 1984 Feb;149(2):175–183. doi: 10.1093/infdis/149.2.175. [DOI] [PubMed] [Google Scholar]
- Spagna V. A., Prior R. B., Perkins R. L. Rapid presumptive diagnosis of gonococcal cervicitis by the limulus lysate assay. Am J Obstet Gynecol. 1980 Jul 1;137(5):595–599. doi: 10.1016/0002-9378(80)90702-4. [DOI] [PubMed] [Google Scholar]
- Takada H., Kotani S., Tanaka S., Ogawa T., Takahashi I., Tsujimoto M., Komuro T., Shiba T., Kusumoto S., Kusunose N. Structural requirements of lipid A species in activation of clotting enzymes from the horseshoe crab, and the human complement cascade. Eur J Biochem. 1988 Aug 15;175(3):573–580. doi: 10.1111/j.1432-1033.1988.tb14230.x. [DOI] [PubMed] [Google Scholar]
- Takayama K., Qureshi N., Hyver K., Honovich J., Cotter R. J., Mascagni P., Schneider H. Characterization of a structural series of lipid A obtained from the lipopolysaccharides of Neisseria gonorrhoeae. Combined laser desorption and fast atom bombardment mass spectral analysis of high performance liquid chromatography-purified dimethyl derivatives. J Biol Chem. 1986 Aug 15;261(23):10624–10631. [PubMed] [Google Scholar]
- Takayama K., Qureshi N., Raetz C. R., Ribi E., Peterson J., Cantrell J. L., Pearson F. C., Wiggins J., Johnson A. G. Influence of fine structure of lipid A on Limulus amebocyte lysate clotting and toxic activities. Infect Immun. 1984 Aug;45(2):350–355. doi: 10.1128/iai.45.2.350-355.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takayama K., Qureshi N., Ribi E., Cantrell J. L. Separation and characterization of toxic and nontoxic forms of lipid A. Rev Infect Dis. 1984 Jul-Aug;6(4):439–443. doi: 10.1093/clinids/6.4.439. [DOI] [PubMed] [Google Scholar]
- Tramont E. C., Sadoff J. C., Wilson C. Variability of the lytic susceptibility of Neisseria gonorrhoeae to human sera. J Immunol. 1977 May;118(5):1843–1851. [PubMed] [Google Scholar]
- Waage A., Brandtzaeg P., Halstensen A., Kierulf P., Espevik T. The complex pattern of cytokines in serum from patients with meningococcal septic shock. Association between interleukin 6, interleukin 1, and fatal outcome. J Exp Med. 1989 Jan 1;169(1):333–338. doi: 10.1084/jem.169.1.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waage A., Halstensen A., Shalaby R., Brandtzaeg P., Kierulf P., Espevik T. Local production of tumor necrosis factor alpha, interleukin 1, and interleukin 6 in meningococcal meningitis. Relation to the inflammatory response. J Exp Med. 1989 Dec 1;170(6):1859–1867. doi: 10.1084/jem.170.6.1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weary M., Pearson F. C., 3rd, Bohon J., Donohue G. The activity of various endotoxins in the USP rabbit test and in three different LAL tests. Prog Clin Biol Res. 1982;93:365–379. [PubMed] [Google Scholar]
- Yamasaki R., Bacon B. E., Nasholds W., Schneider H., Griffiss J. M. Structural determination of oligosaccharides derived from lipooligosaccharide of Neisseria gonorrhoeae F62 by chemical, enzymatic, and two-dimensional NMR methods. Biochemistry. 1991 Oct 29;30(43):10566–10575. doi: 10.1021/bi00107a028. [DOI] [PubMed] [Google Scholar]
- Yamasaki R., Schneider H., Griffiss J. M., Mandrell R. Epitope expression of gonococcal lipooligosaccharide (LOS). Importance of the lipoidal moiety for expression of an epitope that exists in the oligosaccharide moiety of LOS. Mol Immunol. 1988 Aug;25(8):799–809. doi: 10.1016/0161-5890(88)90116-2. [DOI] [PubMed] [Google Scholar]