Abstract
Targeting the oestrogen receptor, HER2 (human epidermal growth factor receptor 2) and vascular endothelial growth factor has markedly improved breast cancer therapy. New targeted therapeutic approaches to induction of apoptosis or inhibition of anti-apoptosis, cell cycle progression, signal transduction and angiogenesis are described. The molecular pathways and their inhibitory or repair mechanisms are discussed in the preclinical and clinical settings.
Introduction
Treatment of early-stage breast cancer requires a multimodality approach to eradicate residual cancer and prevent recurrent disease. Targeting the pathways that promote or sustain growth and invasion of carcinoma cells is critical to effective treatment of breast cancer [1,2].
Targeting the oestrogen receptor (ER) is the oldest molecular targeted therapy approach, and widespread use of the selective ER modulator tamoxifen in breast cancer is responsible for major improvements in cure rates, quality of life and disease prevention during the past 25 years. Targeting both HER2 (human epidermal growth factor receptor 2) with trastuzumab and the vascular endothelial growth factor (VEGF) with bevacizumab in combination with chemotherapy has become a further milestone of molecular targeted therapy [3-5]. However, intrinsic and acquired resistance to endocrine and/or cytostatic treatments is still a common feature that limits the benefits of these novel therapeutic strategies. Therefore, clinical trials of endocrine or cytotoxic therapies combined with growth factor pathway inhibitors or their downstream signalling elements are warranted; such approaches may allow us to improve upon the current standard of care for breast cancer patients [6]. Unfortunately, despite encouraging preclinical data, some of these combinations have yielded disappointing results in the clinical setting [7].
This review describes and critically discusses targeted therapies for induction of apoptosis or inhibition of anti-apoptosis, cell cycle progression, signal transduction and angiogenesis (Fig. 1). Table 1 summarizes both finished and ongoing studies in this area.
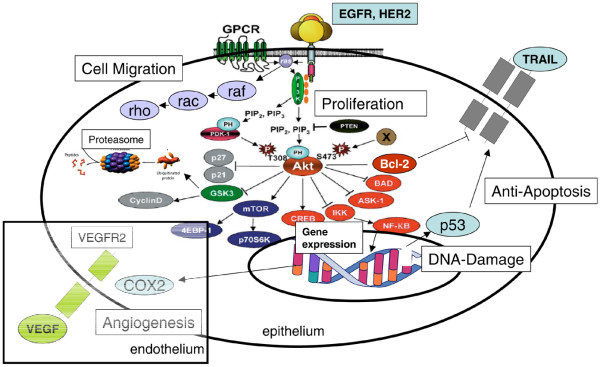
Figure 1.
Cell signalling pathways: targets for breast cancer treatment. EGFR, epidermal growth factor receptor; GPCR, G-protein-coupled receptors; HER, human epidermal growth factor receptor; IKK, inhibitor of NF-κB kinase; mTOR, mammalian target of rapamycin; NF-κB, nuclear factor-κB; TRAIL, tumour necrosis factor-related apoptosis inducing ligand; VEGF, vascular endothelial growth factor; VEGFR, vascular endothelial growth factor receptor.
Table 1.
Clinical studies of targeted therapy with anti-sense nucleotides, antibodies, kinase inhibitors and other agents in breast cancer
| Cellular target | Agent | Application | Clinical study | References |
| TRAIL receptors | TRAIL | BC, gynaecologic malignancies | Phase I | [9,14] |
| 26S proteasome | Bortezomib | Metastatic BC | Phase II | [22] |
| Bortezomib | Metastatic BC | Phase II | [23] | |
| Bortezomib/trastuzumab | Metastatic BC | Phase I | [24] | |
| Bortezomib/capecitabine | Metastatic BC | Phase I/II | [25] | |
| mTOR | Everolimus (RAD-001) | Primary BC, neoadjuvant | Phase III, GEPARquinto, GBG 44 | [88] |
| Everolimus (RAD-001) | Metastatic BC, bone | Phase II, GBG 41 | [89] | |
| metastases | ||||
| p53 | Ad5CMV-p53 and docetaxel/doxorubicin | Primary BC, neoadjuvant | Phase II | [42] |
| EGFR | Cetuximab and paclitaxel | Advanced BC | Phase I | [44] |
| Erlotinib | Primary BC, neoadjuvant | Phase I | [50] | |
| Trastuzumab and capecitabine versus capecitabine | BC, beyond progression | Phase II, GBG 26, | [90] | |
| EGFR/HER2 | Lapatinib and capecitabine versus capecitabine | Advanced BC | Phase III | [60,61] |
| Lapatinib and paclitaxel | Inflammatory BC, neoadjuvant | Phase II | [62] | |
| Lapatinib and paclitaxel/trastuzumab | Primary BC, HER2+, neoadjuvant | Phase III, GBG 47, NeoAltto | [91] | |
| Lapatinib and trastuzumab | BC, HER2+ | Phase III, GBG 46, ALTTO | [92] | |
| Ras, farnesyl transferase | Tipifarnib and gemcitabine | Metastatic BC | Phase II | [63] |
| Tipifarnib and letrozole | Advanced BC | Phase II | [64] | |
| Lonafarnib and anastrozole | Metastatic BC | Phase II | [63] | |
| COX-2 | Celecoxib | BC adjuvant | Phase III, GBG 27 | [93] |
| VEGF | Bevacizumab | Metastatic BC | Phase I/II | [68] |
| Bevacizumab | Metastatic BC | Phase II | [69] | |
| Bevacizumab and vinorelbine | Metastatic BC | Phase II | [70] | |
| Bevacizumab and vinorelbine | Metastatic BC | Phase II | [71] | |
| Bevacizumab, docetaxel | Metastatic BC | Phase II | [72] | |
| Bevacizumab/trastuzumab, carboplatin/nab-paclitaxel versus trastuzumab carboplatin/nab-paclitaxel | HER-2 positive metastatic BC | Phase II | [73] | |
| Bevacizumab, docetaxel | Neo-adjuvant, nonmetastatic, metastatic BC | Phase II | [75] | |
| Bevacizumab doxorubicin/docetaxel | Neo-adjuvant, inflammatory, locally advanced | Phase II | [76] | |
| Bevacizumab and capecitabine versus capecitabine | Advanced BC | Phase III | [74] | |
| Bevacizumab and paclitaxel versus paclitaxel | Advanced BC | Phase III | [5] | |
| Bevacizumab and trastuzumab | HER2+, metastatic BC | Phase II | [79] | |
| Bevacizumab and docetaxel/trastuzumab versus docetaxol/trastuzumab | HER2+, recurrent or metastatic BC | Phase III | [80] | |
| Bevacizumab and letrozole versus letrozole | BC, advanced and metastatic | Phase III, GEICAM/GBG 51 | [94] | |
| Bevacizumab and erlotinib | Metastatic BC | Phase II | [81] | |
| Bevacizumab and everolimus | Advanced solid tumours | Phase I | [82] |
BC, breast cancer; COX, cyclo-oxygenase; EGFR, epidermal growth factor receptor; HER, human epidermal growth factor receptor; mTOR, mammalian target of rapamycin; TRAIL, tumour necrosis factor-related apoptosis inducing ligand; VEGF, vascular endothelial growth factor.
Induction of apoptosis and inhibition of anti-apoptosis
Apoptosis is a precisely regulated and evolutionarily conserved programme of cell suicide, which plays important roles during embryogenesis and immunology. Disturbances in the physiological programme of apoptosis prolong the life of cells and thereby promote carcinogenesis. Consequently, apoptosis is frequently diminished in cancer cells, supposedly caused by a dominance of anti-apoptotic proteins in malignant tumours. Regulation of apoptosis is complex, but two distinct pathways can be identified: the intrinsic apoptotic pathway, also referred to as p53-mitochondrial pathway; and the extrinsic pathway, which is activated through 'death receptors' and their corresponding ligands (for example, the death-inducing cytokine TRAIL [tumour necrosis factor-related apoptosis inducing ligand]). TRAIL is a trans-membrane protein that is cleaved by proteases to release a soluble form. Although it is constitutively expressed in normal tissue, TRAIL preferentially induces apoptosis, with minimal adverse effects on normal cells. Therefore, targeting TRAIL and raising agonistic monoclonal antibodies directed against TRAIL receptor 1 or 2 have emerged as promising therapeutic approaches in cancer [8].
TRAIL receptor activating agents have been found to exhibit favourable in vitro and in vivo activity in treatment of several malignancies, including breast and gynaecological cancers. Preclinical and early phase I studies have provided some support to the assumption that these novel agents are safe, with increased target specificity for malignant cells. When these targeted agents are combined with conventional chemotherapy drugs or radiotherapy, they appear to increase cell death over single agent modalities [9] (Table 1).
Mitochondria-mediated apoptosis is regulated through anti-apoptotic (bcl-2) and pro-apoptotic (bax and bad) proteins of the bcl-2 family. Over-expression of bcl-2 occurs in 40% to 80% of human breast cancers. Most bcl-2 positive breast cancers express ER and/or progesterone receptor. This positive association of bcl-2 with hormone receptors in breast cancer may explain its apparent correlation with response to hormonal therapy. However, diminished apoptotic response caused by bcl-2 over-expression is associated with resistance of tumour cells to cytotoxic drugs. Downregulation of bcl-2 by antisense oligonucleotides has been shown to improve the efficacy of chemotherapy in experimental models. Phase I randomized clinical trials are ongoing in patients with solid tumours [10] using G3139 (oblimersen sodium), a phosphorothioate antisense oligodeoxynucleotide that targets bcl-2 mRNA and downregulates bcl-2 protein translation. Further studies should examine the molecular effect of the regimen as well as clinical responses of cancers in which taxanes are indicated to be beneficial [11].
Inhibitors of apoptosis proteins
Negative regulators called 'inhibitors of apoptosis proteins' (IAPs) prevent uncontrolled and excessive cell death in the final course of apoptotic signalling. An important member of this group is survivin, which can be detected in approximately 90% of breast tumours. Increased survivin levels are significantly associated with high nuclear grade, negative hormone receptor status, HER2 over-expression, VEGF expression, and high urokinase-type plasminogen activator and plasminogen activator inhibitor-1 levels. Therefore, patients with elevated levels of survivin have significantly worse disease-free survival and overall survival than do patients with lower levels of survivin. In a multivariate analysis the prognostic value of survivin in terms of disease-free and overall survival was found to be independent of both TNM stage and molecular parameters.
Survivin may be a suitable target in future therapeutic strategies [12]. Although anti-cancer drugs have been designed to inhibit the growth of tumour cells, chemotherapy frequently fails because of the development of multidrug resistance (MDR). Over-expression of survivin in MCF-7 cells due to transfection with survivin expression vectors results in decreasing sensitivity to cytotoxic drugs and activation of P-glycoprotein (MDR1), which exports drugs out of cells. Downregulation of survivin expression in MCF-7/adriamycin-resistant cells by RNA interference directed against survivin increased drug accumulation in cells because of inhibtion of P-glycoprotein. Downregulation of P-glycoprotein expression with the specific inhibitor verapamil markedly suppressed survivin mRNA expression, whereas no opposite effect of upregulated P-glycoprotein has been reported. These findings suggest that survivin may play a key role in MDR in the presence of P-glycoprotein, and this may represent a novel strategy for modulating MDR in cancer cells [13]. LY 2181308 is an antisense molecule directed against survivin that is already being studied in phase I clinical trials [14,15].
Nuclear transcription factor (nuclear factor-κB)
The nuclear transcription factor nuclear factor-κB (NF-κB) has an anti-apoptotic effect, activating IAPs. NF-κB may also work in a pro-apoptotic way by regulating 'interferon-regulated factor-1', the oncogene c-myc, p53 and caspase-1. A preclinical investigation into inhibition of NF-κB via the synthetic inhibitor PS-1.145 is underway [14]. It is already known that constitutive activation of NF-κB supports progression of breast cancer to hormone-independent growth [16] Furthermore, NF-κB inhibits extracellular signal-regulated kinase (ERK) activation to enhance cell survival during the development of tumour adaptive radioresistance [17].
Gemcitabine is a nucleoside analogue that is applied in the treatment of several solid tumours, including breast carcinoma. Despite its cytotoxic effect, clinical efficacy is impaired by the development of resistance. Immunohistochemical analysis of clinical samples of breast carcinoma validated the belief that neoadjuvant gemcitabine treatment induces NF-κB expression and downregulation of inhibitor of NF-κB (IκB). Gene expression patterns and findings of in vitro functional studies and analyses of tissue samples are in agreement that NF-κB plays a role in the induction of resistance to chemotherapy. These data give support to clinical strategies that combine gemcitabine with NF-κB inhibitors in breast cancer [18]. Increasing evidence supports a protective role for inhibitor of differentiation and DNA binding-1 (Jd-1) against anti-cancer drug induced apoptosis. Jd-1 expression results in increased number of viable MCF-7 breast cancer cells, reduced bax expression and enhanced bcl-2 expression, but no change in bcl-xL expression. Expression of NF-κB is augmented, whereas expressions of p53 and IκB are reduced. Finally, Jd-1 plays a protective role against taxol-induced apoptosis in breast cancer cells. Inactivation of Jd-1 is a potential therapeutic strategy that may inhibit breast cancer progression and anti-cancer drug resistance [19].
Ubiquitine-proteasome system
The ubiquitine-proteasome system regulates the cell cycle regulator p53, cyclins and cyclin-dependent kinases (CDKs), as well as proteins of the bcl family. Inhibitors of the proteasome system are responsible for the accumulation of pro-apoptotic proteins (for instance, BAX), delivering cytochrome from mitochondria and thereby activating intrinsic apoptotic signal transduction.
Inactivation of NF-κB by proteasome inhibition contributes to increased apoptosis induced by histone deacetylase inhibitors (HDACis). The HDACis constitute a novel class of anti-cancer agents that cause growth arrest, differentiation and/or apoptosis in many tumour cells, and they regulate the activity of the anti-apoptotic transcription factor NF-κB. The proteosome inhibitor MG-132 strongly reduces the activity of NF-κB. Moreover, MG-132 potentiates HDACi-induced cell death. Induction of the stress-related kinase JNK (c-Jun amino terminal kinase) and p38, and upregulation of p21 and p27 are also observed after co-treatment of cells with HDACi and MG-132. Thus, combined treatment with HDACi and proteasome inhibitors potentiates apoptosis in breast cancer cells, representing a novel potential therapeutic strategy in breast cancer [20].
Signalling pathways that converge on two different transcription factor complexes, NF-κB and activator protein (AP)-1, have been identified in ER-positive breast cancers that are resistant to the anti-oestrogen tamoxifen. The model cell lines (MCF-7/HER2 and BT 474) were treated with the IκB kinase inhibitor parthenolide or the proteasome inhibitor bortezomib (PS 341), alone and in combination with tamoxifen. Furthermore, expression microarray data available from UCSF node-negative ER-positive breast cancer patients with known clinical outcome were used to search for potential genes that signify upregulated NF-κB and AP-1 transcriptional activity in association with tamoxifen resistance. The association of these genes with patient outcome was further evaluated using node-negative ER-positive breast cancer patients identified from three other published datasets (Rotterdam, Amsterdam and Basel). Doses of parthenolide and bortezomib that were capable of sensitizing the two endocrine-resistant breast cancer models to tamoxifen can suppress NF-κB and AP-1 regulated gene expression in combination with tamoxifen. Transcript profiles from the UCSF breast cancer cases revealed three NF-κB and AP-1 upregulated genes (cyclin D1, urokinase-type plasminogen activator and VEGF) that can dichotomize node-negative ER-positive cases into early and late relapsing subsets, despite adjuvant tamoxifen therapy; they had the greatest ability to predict outcome in younger patients. Across the four independent sets of node-negative ER-positive breast cancer cases (UCSF, Rotterdam, Amsterdam and Basel [21]), high expression of all three NF-κB and AP-1 upregulated genes was associated with earliest metastatic relapse. These findings suggest that agents that can prevent activation of NF-κB and AP-1 genes may prove useful in restoring the endocrine responsiveness of high-risk ER-positive breast cancers [21].
Bortezomib is also a potent inhibitor of the 26S proteasome, with broad anti-tumour activity (Table 1). Twelve patients with metastatic breast cancer were treated with bortezomib in a phase II study [22]. No objective responses were observed, one patient had stable disease and 11 others experienced disease progression. The median survival time was 4.3 months. The most common grade 3 or 4 toxicities included fatigue (58%) and skin rash (33%). Although bortezomib was well tolerated, it exhibited only limited clinical activity against metastatic breast cancer when used as a single agent. The future development of this drug for the treatment of breast cancer should be guided by in vivo models that optimize activity in combination with other anti-tumour agents. Similar clinical experience was reported by Engel and coworkers [23], who conducted a single institution, phase II study of bortezomib in the treatment of patients with metastatic breast cancer. There were no observed objective responses in any of the 12 patients who received treatment with bortezomib. Furthermore, all 12 patients progressed while receiving therapy with bortezomib. The study was terminated after first stage because of lack of any objective response.
The two agents bortezomib and trastuzumab prevent NF-κB activation and induce nuclear accumulation of the CDK inhibitor p27kip1, suggesting that combining bortezomib with trastuzumab could increase the efficacy of trastuzumab. Bortezomib induced apoptosis in HER2-positive (SKBR-3, MDA-MB-453, HER2 transfected MCF-7) and HER2-negative breast cancer cells (MCF-7) in a dose-dependent and time-dependent manner. Sequential treatment (trastuzumab then bortezomib) induced either necrosis or apoptosis, depending on the trastuzumab pre-incubation time. The addition of bortezomib to trastuzumab increases the effect of trastuzumab in HER2-positive cell lines in a synergistic way. This effect probably results from the ability of these two drugs to target the NF-κB and p27 pathways. The potential clinical application of this drug combination is currently under evaluation in a phase I clinical trial [24].
A phase I/II including 35 patients [25] was recently conducted to evaluate the combination of capecitabine and bortezomib in anthracycline-pretreated and/or taxane-pre-treated patients with metastatic breast cancer. The treatment was generally well tolerated and associated with toxic effects that were consistent with the known side effects of the individual agents. The intent-to-treat overall response rate was 15%, and an additional 27% of patients had stable disease. Median time to progression and overall survival were 3.5 months and 7.5 months, respectively. The median duration of response was 4.4 months. Although bortezomib and capecitabine are well tolerated, the combination had only moderate anti-tumour activity in heavily pretreated patients.
Phosphatidyl-inositol 3-kinase
Phosphatidyl-inositol 3-kinase (PI3K) plays an important role in survival, proliferation, motility and neoangiogenesis in cancer cells. As a consequence of dysregulation of the cell cycle, PI3K is over-expressed and activates Akt, which-through phosphorylation of NF-κB, bad and caspase 9 – has anti-apoptotic influences. Also, epidermal growth factor receptor (EGFR), as well as HER2, activate PI3K and enhance cell growth via Akt.
Preclinical data identified a possible role for the PI3K/Akt pathway in docetaxel-induced apoptosis. Specifically, inhibition of one of the three isoforms of Akt, namely Akt 2, by the PI3K inhibitor LY294002 counteracted the activity of fibronectin to protect cells from apoptosis induced by docetaxel. Further investigations showed that Akt 2 activation protects against docetaxel-induced apoptosis by regulating survivin levels in a PI3K-dependent manner. Targeting the PI3K/Akt 2 pathway might be a promising strategy for enhancing sensitivity to docetaxel in breast cancer [26]. Inhibition of the PI3K/Akt pathway improves response of long-term oestrogen-deprived breast cancer xenografts to anti-oestrogens. The combination of wortmannin with tamoxifen or fulvestrant inhibited tumour growth of long-term oestrogen-deprived aromatase-transfected human ER-positive breast cancer cells (UMB-1Ca) better than did either drug alone. The combination of wortmannin plus fulvestrant was the most effective treatment, maintaining tumour regression for a prolonged period. These data suggest that blocking both ER and growth factor receptor pathways could provide effective control over tumour growth in long-term oestrogen-deprived human breast cancers [27]. Further data reveal that loss of the PTEN gene (which encodes a phosphatidylinositol phos-phatase, and therefore is a negative regulator of PI3K function) results in constitutive activation of the PI3K pathway and poor patient outcome [28]. The study indicates that aberrant PI3K pathway signalling is strongly associated with metastasis and poor survival, supporting the contention that inhibition of this pathway could improve prognosis.
The protein mammalian target of rapamycin (mTOR) is activated downstream by phosphorylated Akt. Thereby, mTOR promotes cancer cell proliferation, maintains blood and nutrient supply by neoangiogenesis, promotes tumour spread and metastasis, and inhibits cancer cell apoptosis. Inhibiting mTOR with the new drug CCI-779 (temsirolimus), which negatively influences these functions, is already being investigated in clinical studies. HER2 and PI3K pathway inhibitors were tested for their ability to inhibit breast cancer cell growth and tumour development in 20 human breast cancer lines [29]. In that study, rapamycin combined with trastuzumab was found to increase anti-tumour efficacy significantly in comparison with trastuzumab alone in HER2-over-expressing breast cancer cells. Rapamycin and trastuzumab significantly reduced levels of cyclins D1 and D3 and increased the cleavage of caspase 3, suggesting increased apoptosis. These findings suggest that rapamycin combined with trastuzumab has an enhanced anti-cancer effect, and this combination could be developed as an improved therapeutic regimen in breast cancer.
The German Breast Group is conducting a phase III study in primary breast cancer integrating mTOR antagonist evero-limus (RAD001), bevacizumab and lapatinib in the neo-adjuvant setting (GeparQuinto), and a randomized discontinuation phase II study to determine the efficacy of everolimus (RAD001) in breast cancer patients with bone metastases (RADAR; Table 1).
Cell cycle inhibition and cyclin-dependent kinase inhibitors
Interactions of cyclins with CDKs play an important role in regulating the cell cycle. CDKs promote phosphorylation of their target proteins, initiating progression of the cell cycle. Because cells begin to undergo cell division through mitogenic stimuli, there is induction of synthesis of cyclin D1, which is associated with the kinases CDK4 as well as CDK6. Cyclin D1 is essential for HER2-induced cell growth and is induced by growth factors through Ras-dependent and Ras-independent signalling pathways.
In a preclinical study [30], the CDK inhibitor flavopiridol was combined with several transduction inhibitors (cyclo-oxygenase-2 inhibitor SC236, protein kinase C kinase inhibitor, and PI3K inhibitor LY294002). In two breast cancer cell lines, one HER2 low expressing and another HER2 high expressing (MCF/neo and MCF/18, respectively), enhanced growth inhibition was observed predominantly in the high HER2 expressing cell line. These data suggest that combinations of flavopiridol and signal transduction inhibitors warrant further studies as treatments for breast tumours, and that HER2 expression may influence the choice of inhibitor to combine with flavopiridol.
Exposure of MCF-7 cells to adriamycin, taxol, or UVB results in fourfold to fivefold increased expression of survivin. Inhibition of survivin phosphorylation by flavopiridol resulted in loss of survivin expression, and nonphos-phorylatable survivin exhibited accelerated clearance [31]. Treatment with flavopiridol caused accumulation in the G1 phase of the cell cycle and induced apoptosis in breast cancer cell lines (SKBR-3 and MB-468). This was associated with downregulation of the levels of cyclins D1 and B1, and with inhibition of CDK1, CDK2 and CDK4. In MB-468 cells exhibiting over-expression of bcl-2, apoptosis was inhibited. Sequential treatment with a nontaxane tubuline polymerizing agent epothilone (Epo B) followed by flavopiridol induced significantly more apoptosis of MB-468 cells than treatment with the reverse sequence or treatment with either agent alone [32]. These findings suggest that the superior sequence-dependent anti-breast cancer activity of EpoB followed by flavopiridol may be due to flavopiridol-induced bax conformational change and downregulation of the anti-apoptotic IAP bcl-xL and Mcl-1 proteins, but this treatment may not overcome the resistance to apoptosis of breast cancer cells conferred by over-expression of bcl-2.
Human breast cancer cell lines, which express high levels of endogenous HER2 receptor, were treated with trastuzumab together with flavopiridol [33]. The combination synergistically inhibited DNA synthesis, cellular proliferation and contact-dependent growth. In SKBR3 cells, the combination of trastuzumab and flavopiridol inhibited the Ras/mitogen-activated protein kinase (MAPK)/Akt pathway, and decreased cyclin D1 abundance and kinase activity to a greater extent than either drug alone. Compared with single-agent treatment, combination treatment selectively inhibited Akt and RB1 phosphorylation. Cyclin D1 over-expression reverses drug treatment induced cell cycle arrest. Inhibition of Akt may prove to be a useful therapeutic strategy in combination with flavopiridol for HER2-positive tumours.
A mRNA expression signature including EGFR was found to predict response to the combination of trastuzumab and flavopiridol in array analysis [34]. Three lines of evidence support the contention that EGFR is a potential target of flavopiridol-trastuzumab synergy: EGFR protein was rapidly and completely lost after combination treatment; a cell line that expresses amplified levels of both HER2 and the EGFR was resistant to the combined drugs; and treatment with EGF prevented any therapeutic effects of flavopiridol and trastuzumab, either singly or in combination. The data clearly demonstrate that the interaction between HER2 and EGFR is not sufficiently addressed in targeted and combination breast cancer therapies [2] and should be studied extensively. In recent phase I clinical trials, flavopiridol has shown some promise in the treatment of a variety of human tumours. However, because of the severe toxicity observed, the use of less toxic doses in combination with other antiproliferative agents, as reported above from in vitro experiments, would be desirable. Consequently, phase I studies are underway to determine the toxicities and characterize the pharmacokinetics of docetaxel and flavopiridol in patients with meta-static breast cancer [35].
p53: a target for gene therapy
The p53 gene acts as a regulator of cell growth and DNA repair in normal cells; inactivation of the gene appears to lead to cancer. It is the most frequently mutated gene in human cancers; 35% of breast tumours have p53 mutations, of which 88% are located within exons 5 to 8 [36]. Mutations in the p53 gene occur more frequently in ER-negative, basal-like and HER2-amplified tumours than in luminal, ER-positive breast tumours [37]. p53 mutations occur in 24.5% of the axillary node-negative breast carcinomas and more frequently in cancers with HER2 amplification (38.9% versus 20.9% in those without HER2 amplification). Elevated risks for disease recurrence and mortality were identified in patients with both p53 mutation and HER2 amplification [38].
p53 mutations may help to identify a subset of very high risk breast cancer patients with worse prognosis [39]. To investigate the effect of specific inhibition of mutated p53 gene, breast cancer cells harbouring an inactivating p53 mutation were transfected with antisense RNA using a cationic liposome-mediated method. Forty-eight hours after transfection, the antisense RNA (Asp53 exon 8'RNA) had a significant retarding effect on p53-related proliferation inhibition, along with a decrease in p53 protein expression. Such an approach may be used as a therapeutic option in human malignancy [40]. Cells expressing p53 mutations are either more sensitive to cisplatin and melphelan or more resistant than untransfected cells, depending on the mutation. However, there is no difference in response to daunorubicin treatment. These data also suggest that the nature of the p53 mutation influences sensitivity to cytotoxic drugs [41].
Intratumoural administration of a nonreplicating adenoviral vector (Ad5) that contains the human wild-type p53 (Ad5CMV-p53) combined with chemotherapy could increase the efficacy of primary systemic chemotherapy (PST) as measured by pathological complete response (pCR) in the management of patients with locally advanced breast cancer (Table 1). In a prospective, open label, phase II trial [42], 13 patients with locally advanced breast cancer were treated with six 3-week cycles of PST, which consisted of intra-tumoural injections of Ad5CMV-p53 for 2 consecutive days plus docetaxel and doxorubicin followed by surgery. The trial was terminated early because none of the patients achieved pCR. Eight patients (73%) had a p53 mutation; serial biopsies showed increases in p53 mRNA and p21 (WAF1/Cip1 mRNA). All 12 evaluable patients achieved an objective clinical response. Surgical specimens revealed scattered tumour cells with extensive tumour infiltrate leucocytes, predominantly T lymphocytes. There was no increase in systemic toxicity. Ad5CMV-p53 combined with PST was safe, active and associated with local immune modulatory effects. The promising clinical activity of this combination deserves further investigation in randomized studies.
Monoclonal antibodies and tyrosine kinase inhibitors for EGFR and HER2
EGFR, HER2, HER3 and HER4 are members of the ErbB family of receptor thyrosine kinases. EGFR and HER2 are over-expressed in a variety of human tumours, and over-expression generally correlates with poor prognosis and decreased survival. Three of the four receptors comprise an extracellular amino-terminal domain to bind corresponding ligands (for example, EGF to EGFR; ligands to HER2 are not known) and an intracellular carboxyl-terminal domain lodging a tyrosine kinase with the exception of HER3. Binding of ligands induces dimerization of the receptor and activation of the kinase through autophosphorylation, stimulating PI3K/Akt and/or MAPK signalling, various transcription factors (such as STAT-3, c-fos and ELK-1) and enhanced production of VEGF. Proliferation, migration, adhesion and angiogenesis are favoured, whereas apoptosis is inhibited [43].
From 15% to 30% of breast cancers have been shown to express high levels of EGFR and HER2. Moreover, hormone-resistant disease is associated with an inreased expression of both EGFR and EGFR ligands. Therefore, EGFR and its downstream signalling pathways are promising anti-tumour targets.
Cetuximab, an IgG1 chimeric monoclonal antibody, competes with ligand binding to the EGFR ectodomain, resulting in an efficient blockade of the tumour-promoting downstream signalling pathway. Preclinical studies have indicated a synergistic effect for the combination of anti-EGFR therapy plus paclitaxel in breast cancer models (Table 1). The feasibility of this combination was evaluated in a dose-escalation phase I trial using cetuximab/paclitaxel in patients with metastatic breast cancer [44]. Treatment consisted of weekly cetuximab therapy and paclitaxel given every 3 weeks, with dose escalation of cetuximab until the maximum tolerated dose was reached. Twelve patients were enrolled into three treatment cohorts. Two out of six patients in the second cohort (cetuximab 100 mg/m2) developed dose-limiting toxicities, presenting as grade 3 rash. In the third cohort (cetuximab 100 mg/m2 and paclitaxel in a weekly schedule), one out of three patients developed grade 3 skin toxicity. Of 10 patients evaluable for response, two experienced stable disease and eight had disease progression. Because of prohibitive dermatological toxicity and disappointing preliminary efficacy, this combination of paclitaxel/cetuximab was not considered promising.
ZD 1839 (gefitinib) is an example of an orally active, selective EGFR tyrosine kinase inhibitor, which has shown extensive preclinical activity. Gefitinib has exhibited in vitro activity as both monotherapy and in combination with other agents such as paclitaxel and doxorubicin, inhibiting the growth of breast cancer cells that are resistant to endocrine agents such as tamoxifen [45].
To test whether continous or pulsatile inhibition of EGFR signalling is effective in inhibiting tumour proliferation, combinations of paclitaxel and gefitinib, using either intermittent or continous dosing schedules, were compared in mice [46]. In combination with paclitaxel, pulsatile gefitinib was significantly superior to continous dosing. When gefitinib was administered for 1 or 2 consecutive days before paclitaxel, much higher doses could be given safely. Two days of gefitinib treatment before paclitaxel was most effective, causing significantly greater mean tumour regression and higher percentage of complete responses than other schedules.
Preclinical studies conducted in human ductal carcinoma in situ (DCIS) xenografts in nude mice suggest a potential role for EGFR inhibitors. Gefitinib has been shown to produce a response in DCIS through a decrease in epithelial proliferation. These findings indicate that tyrosine kinase inhibitor (TKI) blockade of EGFR has potential in the treatment and chemoprevention of DCIS [47] (Table 1).
The spontaneous pulmonary metastasis mouse model was applied to evaluate the ability of the EGFR TKI erlotinib (OSI-774) to prevent pulmonary metastasis in curatively resected breast carcinoma [48,49]. The expression levels of EGF and EGFR were significantly higher in pulmonary metastatic nodules than in primary breast cancer tissue. Treatment of erlotinib given to mastectomized mice inhibited the incidence of pulmonary metastases. The number of metastatic pulmonary nodules was significantly reduced in the erlotinib-treated group compared with the control. The effects of treatment with erlotinib for 6 to 14 days was evaluated in a presurgical study conducted in 41 patients with breast cancer of stage I to III [50]. Grade 2 rash and diarrhoea were the main toxicities. Erlotinib inhibited tumour cell proliferation (Ki-67), and phosphorylation of EGFR and HER2. Treatment was associated with significant reductions in phosphorylated MAPK and Akt in ER-positive cancers. Inhibition of proliferation occurred in ER-positive but not in HER2-positive or triple-negative carcinomas.
Trastuzumab, a humanized monoclonal antibody that targets HER2, is of special importance in breast cancers that over-express HER2. When combined with standard cytotoxic chemotherapy, trastuzumab improves the outcome and survival in patients with metastatic disease [3]. Furthermore, over the past 3 years, studies incorporating trastuzumab in sequence or concurrently with taxane-based chemotherapy in the adjuvant setting have demonstrated considerable benefit in this subset of patients, and longer term findings regarding outcomes and late toxicity are expected during the next few years [51-55] (Table 1). Based on these impressive results, trastuzumab represents the standard of care in the adjuvant treatment of HER2 over-expressing breast cancers. The role of trastuzumab in the neoadjuvant setting is very promising but must be further evaluated in larger prospective randomized trials [56].
However, there is still a large proportion of patients over-expressing HER2 who do not respond to trastuzumab. Results from a gene expression analysis indicated that lower expression of genes involved in CD40 signalling is associated with a greater risk for residual cancer after trastuzumab-containing preoperative chemotherapy [57]. The optimal combination of trastuzumab with other agents requires further evaluation this patient cohort. The role of the novel antibody pertuzumab remains to be defined in breast cancer lacking HER2 amplification [58].
Heterodimerization, compensatory crosstalk and redundancy exist in the HER2/EGFR network, which provides a sound scientific rationale for dual inhibition of EGFR and HER2. Trials of approved agents in combination, for example trastuzumab and cetuximab, are underway. Preclinical studies conducted in cell lines (MCF-7/HER2 and BT474) showed that combined treatment with gefitinib, trastuzumab and pertuzumab in order to block signals from all HER homodimers and heterodimers inhibits growth of HER2 over-expressing xenografts significantly better than single agents and dual combinations [59]. There is also a new generation of small-molecule TKIs and monoclonal antibodies that target two or more ErbB receptors (Table 1). Lapatinib, a TKI of EGFR and HER2, has shown clinical benefit in trastuzumab-refractory breast cancer and is now approved by the US Food and Drug Administration [60]. A phase III trial demonstrated that lapatinib plus capecitabine is superior to capecitabine alone in women with HER2-positive advanced breast cancer that progressed after prior therapy including trastuzumab [61]. The addition of lapatinib prolonged time to progression significantly and resulted in a trend toward improved overall survival and fewer patients with central nervous system involvement at first progression. However, extracellular domain HER2 (HER2 ECD) baseline serum did not predict benefit from lapatinib. Paclitaxel monotherapy in combination with lapatinib could be a successful approach to managing inflammatory advanced breast cancers patients, because this type of breast cancer most commonly expresses EGFR and/or HER2 [62]. In a neoadjuvant phase II study, a clinical response rate of 77% (complete and partial responses) and a pCR of 17% were achieved in HER2-positive tumours. Eighty per cent of EGFR-positive patients exhibited a response.
Farnesyltransferase inhibitors
The Ras protein regulates genes that are involved with transcription, translation, cell growth, cell survival and cellular interactions, as well as development of the cytoskeleton. Because the gene is mutated in 30%, the regulation of Ras appears to be a further rational anti-tumour strategy. Activation of Ras is induced by farnesyl transferase ('farnesylation'). In preclinical studies inhibition of breast cancer cell growth through some farnesyl transferase inhibitors has been confirmed. The efficacy of the oral inhibitors tipifarnib and lonafarnib is currently being examined in phase II studies [63]. However, preliminary findings from randomized controlled studies are rather disappointing. Tipifarnib combined with letrozole did not show any benefit compared with letrozole alone [64].
Targeting tumour neoangiogenesis: VEGF and VEGF receptor
Tumour-associated macrophages (TAMs) are associated with tumour progression and metastasis. Pro-angiogenic factors were detected as being released by TAMs such as transforming growth factor-β, tumour necrosis factor-α, matrix metalloproteinase-9 and VEGF [65]. Besides, legumain (a member of the asparginyl endopeptidase family) is over-expressed by TAMs and provides a therapeutic target molecule. In murine models of metastatic breast, colon and non-small-cell lung cancers, legumain-based DNA vaccine induced a CD8+ T lymphocyte response against TAMs, which dramatically reduced their density in tumour tissue and resulted in a marked decrease in angiogenesis.
VEGF has emerged as a key target in the treatment of cancer. As the ligand to the VEGF receptor, it plays a central role in promoting tumour angiogenesis. Over-expression of VEGF leads to poor outcomes in patients with breast cancer and other tumours. Preclinical studies have shown that the humanized monoclonal antibody to VEGF bevacizumab can reduce tumour angiogenesis and inhibit the growth of solid tumours, either alone or in combination with chemotherapy [66,67]. A phase I/II dose escalating study in previously treated metastatic breast cancer [68] identified an overall response rate of 6.7% and a median duration of response of 5.5 months. Four of the 75 patients included experienced adverse events such as hypertensive encephalopathy, nephrotic syndrome and headache combined with nausea and vomiting, and therefore discontinued therapy. In a randomized phase II trial with single-agent bevacizumab at two dosages in 35 patients with pretreated metastatic breast cancer, one patient in the high dose arm (10 mg/kg) was shown to have a complete response [69]. In combination with established chemotherapies, such as vinorelbine [70,71], docetaxel [72], carboplatin and nab-paclitaxel [73], use of bevacizumab has yielded more encouraging results in phase II clinical trials in patients with refractory metastatic breast cancer.
In a randomized phase III trial conducted in heavily pretreated breast cancer patients [74], the addition of bevacizumab to capecitabine resulted in a significant increase in response rates (19.8% versus 9.1%) and was well tolerated. However, this did not translate into improved progression-free survival (4.86 months versus 4.17 months) or overall survival (15.1 months versus 14.5 months). However, in an open-label, randomized phase III trial [5], initial therapy for metastatic breast cancer with paclitaxel plus bevacizumab significantly prolonged progression-free survival (11.8 months versus 5.9 months), but not overall survival (26.7 months versus 25.2 months), as compared with paclitaxel alone. Unfortunately, adverse events were more frequent in patients receiving paclitaxel plus bevacizumab. However, bevacizu-mab as first-line therapy in metastatic breast cancer is under investigation in a confirmational phase III trial that is currently taking place in Europe (AVADO trial).
In the neoadjuvant setting bevacizumab has already been evaluated. In a first study 34 patients with nonmetastatic and metastatic unresectable breast tumours exhibited five complete clinical responses and 24 partial responses [75]. In another neoadjuvant trial [76] patients with inflammatory and locally advanced breast cancer received bevacizumab alone for the first cycle followed by six cycles of bevacizumab with doxorubicin and docetaxel. After completion of preoperative chemotherapy, eight out of 13 patients exhibited confirmed partial responses [76].
Konecny and coworkers [77] measured HER2 and VEGF levels in primary breast tumour tissue from 611 patients and found a positive association between their expression levels and a correlation between increased HER2/VEGF levels and worse clinical outcomes. In a phase I study initiated by Pegram and colleagues [78], they assessed the optimal dose schedule and safety of bevacizumab in combination with trastuzumab; this was the first trial to test a combination of multiple monoclonal antibodies in humans. Pharmacokinetic data from this study suggested that the combination did not alter the pharmacokinetics of either agent. The response rates and the tolerable safety profile of this study led to a phase II extension to evaluate this combination as first-line treatment for HER2-positive metastatic breast cancer [79]. Studies to test this combination are currently being performed, including a randomized, open-label, phase III study with trastuzumab plus a taxane with or without bevacizumab in HER2-positive locally recurrent or metastatic breast cancer [80]. Further investigations are evaluating the combination of bevacizumab and erlotinib [81] and combination therapy with bevacizumab and the mTOR inhibitor everolimus [82].
Future trials should not just focus on the combination partner; the timing of administration is also of major importance. Because tumour angiogenesis occurs in a very early stage of carcinogenesis, there is a strong rationale for earlier use of bevacizumab in the adjuvant setting [83].
Conclusion
Treatments of breast cancer continue to evolve rapidly. New scientific and clinical achievements are constantly changing the standard of care and have already led to substantial reductions in breast cancer mortality. Despite encouraging preclinical data, some of the molecular targeted agents ('biologicals') have yielded low response rates in the clinical setting [7]. A further issue is the successful management of side effects of these agents, such as acneform rash with anti-EGFR therapy, cardiac insufficiency with trastuzumab treatment, and hypertension and cerebrovascular ischaemia with bevacizumab therapy. New opportunities to select the right patient for a benefical therapy has resulted from knowledge of most of the genes in the human genome and the development of whole-genome gene expression analysis by array technology. Many studies have been performed and are still underway whose aim is to achieve improved matching of effective drug(s) to the molecular characteristics of the individual cancer patient [84-86]. Further studies must provide much needed data on predicting response to targeted therapies, revealing the mechanisms of resistance to such therapies and maximizing the patient's benefit [87].
Abbreviations
AP = activator protein; CDK = cyclin-dependent kinase; DCIS = ductal carcinoma in situ; EGFR = epidermal growth factor receptor; ER = oestrogen receptor; HDACi = histone deacetylase inhibitor; HER = human epidermal growth factor receptor; IAP = inhibitor of apoptosis proteins; IκB = inhibitor of NF-κB; Jd-1 = inhibitor of differentiation and DNA binding-1; MAPK = mitogen-activated protein kinase; MDR = multidrug resistance; mTOR = mammalian target of rapamycin; NF-κB = nuclear factor-κB; pCR = pathological complete response; PI3K = phosphatidyl-inositol 3-kinase; PST = primary systemic chemotherapy; TAM = tumour-associated macrophage; TKI = tyrosine kinase inhibitor; TRAIL = tumour necrosis factor-related apoptosis inducing ligand; VEGF = vascular endothelial growth factor.
Competing interests
The authors declare that they have no competing interests.
References
- Lin A, Rugo HS. The role of trastuzumab in early stage breast cancer: current data and treatment recommendations. Curr Treat Options Oncol. 2007;8:47–60. doi: 10.1007/s11864-007-0008-2. [DOI] [PubMed] [Google Scholar]
- Vogt U, Schlotter CM, Allgayer H. Biologicrational therapeutic strategies: targeted therapies. In: Schlotter CM, Bonk U, Brandt B Bremen, editor. Individualized concepts of neo-adjuvant and adjuvant therapy of breast cancer – gene and gene expression [in German] London, Boston: UNI-MED Verlag; 2007. [Google Scholar]
- Slamon D, Leyland-Jones B, Shak S, Fuchs H, Paton V, Bajamonde A, Fleming T, Eiermann W, Wolter J, Pegram M, Baselga J, Norton L. Use of chemotherapy plus monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344:783–792. doi: 10.1056/NEJM200103153441101. [DOI] [PubMed] [Google Scholar]
- Hortobagyi GN. Trastuzumab in the treatment of breast cancer. N Engl J Med. 2005;353:1734–1736. doi: 10.1056/NEJMe058196. [DOI] [PubMed] [Google Scholar]
- Miller K, Wang M, Gralow J, Dickler M, Cobleigh M, Perez EA, Shenkier T, Cella D, Davidson NE. Paclitaxel plus bevacizumab versus paclitaxel alone for metastatic breast cancer. N Engl J Med. 2007;357:2666–2676. doi: 10.1056/NEJMoa072113. [DOI] [PubMed] [Google Scholar]
- Massarweh S, Schiff R. Resistance to endocrine therapy in breast cancer: exploiting estrogen receptor/growth factor signaling crosstalk. Endocr Relat Cancer. 2006;13(suppl 1):S15–S24. doi: 10.1677/erc.1.01273. [DOI] [PubMed] [Google Scholar]
- Leary AF, Sirohi B, Johnston SR. Clinical trials update: endocrine and biological therapy combinations in the treatment of breast cancer. Breast Cancer Res. 2007;9:112. doi: 10.1186/bcr1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Wang H, Wang Z, Makhija S, Buchsbaum D, LoBuglio A, Kimberly R, Zhou T. Inducible resistance of tumor cells to tumor necrosis-related apoptosis-inducing ligand receptor2-mediated apoptosis by generation of a blockade at the death domain function. Cancer Res. 2006;66:8520–8528. doi: 10.1158/0008-5472.CAN-05-4364. [DOI] [PubMed] [Google Scholar]
- Kendrick JE, Estes JM, Straughn JM, Jr, Alvarez RD, Buchsbaum DJ. Gynecol Oncol. 2007;106:614–621. doi: 10.1016/j.ygyno.2007.05.035. [DOI] [PubMed] [Google Scholar]
- Nahta R, Esteva FJ. Bcl-2 antisense oligonucleotides: a potential novel strategy for the treatment of breast cancer. Semin Oncol. 2003;30:143–149. doi: 10.1053/j.seminoncol.2003.08.016. [DOI] [PubMed] [Google Scholar]
- Marshall J, Chen H, Yang D, Figueira M, Bouker KB, Ling H, Lippman M, Frankel SR, Hayes DF. A phase I trial of a Bcl-2 antisense (G3139) and weekly docetaxel in patients with advanced breast cancer and other solid tumors. Ann Oncol. 2004;15:1274–1283. doi: 10.1093/annonc/mdh317. [DOI] [PubMed] [Google Scholar]
- Ryan BM, Konecny GE, Kahlert S, Wang HJ, Untch M, Meng G, Pegram MD, Podratz KC, Crown J, Slamon DJ, Duffy MJ. Survivin expression in breast cancer predicts clinical outcome and is associated with HER2, VEGF, urokinase plasminogen activator and PAI-1. Ann Oncol. 2006;17:597–604. doi: 10.1093/annonc/mdj121. [DOI] [PubMed] [Google Scholar]
- Liu F, Xie ZH, Cai GPY, Jiang YY. The effect of surviving on multidrug resistance mediated by P-glycoprotein in MCF-7 and its adriamycin resistant cells. Biol Pharm Bull. 2007;30:2279–2283. doi: 10.1248/bpb.30.2279. [DOI] [PubMed] [Google Scholar]
- Ghobriall M, Witzig TE, Adjei AA. Targeting apoptosis pathways in cancer therapy. CA Cancer J Clin. 2005;55:178–194. doi: 10.3322/canjclin.55.3.178. [DOI] [PubMed] [Google Scholar]
- Reed JC. Apoptosis-targeted therapies for cancer. Cancer Cell. 2003;3:17–22. doi: 10.1016/S1535-6108(02)00241-6. [DOI] [PubMed] [Google Scholar]
- Nakshatri H, Bhat-Nakshatri P, Martin DA, Goulet RJ, Jr, Sledge GW., Jr Constitutive activation of NF-kappaB during progression of breast cancer to hormone-dependent growth. Mol Cell Biol. 1997;17:3629–3639. doi: 10.1128/mcb.17.7.3629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed KM, Dong S, Fan M, Li JJ. Nuclear factor-kappaB p65 inhibits mitogen-activated protein kinase signalling pathway in radioresistant breast cancer cells. Mol Cancer Res. 2006;4:945–955. doi: 10.1158/1541-7786.MCR-06-0291. [DOI] [PubMed] [Google Scholar]
- Hernandez-Vargas H, Rodriguez-Pinilla SM, Julian-Tendero M, Sanchez-Rovira P, Cuevas C, Anton A, Rios MJ, Palacios J, Moreno-Bueno G. Gene expression profiling of breast cancer cells in response to gemcitabine: NF-kappaB patway activation as a potential mechanism of resistance. Breast Cancer Res Treat. 2007;102:157–1572. doi: 10.1007/s10549-006-9322-9. [DOI] [PubMed] [Google Scholar]
- Kim H, Chung H, Kim HJ, Lee JY, Oh MY, Kim Y, Kong G. Id-1 regulates Bcl-2 and Bax expression through p53 and NF-kappaB in MCF-7 breast cancer cells. Breast Cancer Res Treat. 2007. [DOI] [PubMed]
- Domingo-Domenech J, Pippa R, Tapia M, Gascon P, Bachs O, Bosch M. Inactivation of NF-kappaB by proteasome inhibition contributes to increased apoptosis induced by histone deacetylase inhibitors in human breast cancer cells. Breast Cancer Res Treat. 2007 doi: 10.1007/s10549-007-9837-8. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Yau C, Gray JW, Chew K, Dairkee SH, Moore DH, Eppenberger U, Eppenberger-Castori S, Benz CC. Enhanced NF-kappaB and AP-1 transcriptional activity associated with antestrogen resistant breast cancer. BMC Cancer. 2007;7:59. doi: 10.1186/1471-2407-7-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang CH, Gonzalez-Angulo AM, Reuben JM, Booser DJ, Pusztai L, Krishnamurthy S, Esseltine D, Stec D, Broglio KR, Islam R, Hortobagyi GN, Cristofanilli M. Bortezomid (Velcade) in metastatic breast cancer: pharmacodynamics, biological effects, and prediction of clinical benefits. Ann Oncol. 2006;17:813–817. doi: 10.1093/annonc/mdj131. [DOI] [PubMed] [Google Scholar]
- Engel RH, Brown JA, Von Roenn JH, O'Regan RM, Bergan R, Badve S, Rademaker A, Gradishar WJ. A phase II study of single agent bortezomib in patients with metastatic breast cancer: a single institution experience. Cancer Invest. 2007;25:733–737. doi: 10.1080/07357900701506573. [DOI] [PubMed] [Google Scholar]
- Cardoso F, Durbecq V, Laes JF, Badran B, Lagneaux L, Bex F, Desmedt C, Willard-Gallo K, Ross JS, Burny A, Piccart M, Sotiriou C. Bortezomib (PS-341, Velcade) increases the efficacy of trastuzumab (Herceptin) in HER2 positive breast cancer cells in a synergistic manner. Mol Cancer Ther. 2006;5:3042–3051. doi: 10.1158/1535-7163.MCT-06-0104. [DOI] [PubMed] [Google Scholar]
- Schmid P, Kühnhardt D, Kiewe P, Lehenbauer-Dehm S, Schippinger W, Greil R, Lange W, Preiss J, Niederle N, Brossart P, Freier W, Kümmel S, Velde H Van de, Regierer A, Possinger K. A phase I/II study of bortezomib and capecitabine in patients with metastatic breast cancer previously treated with taxanes and/or anthracyclines. Ann Oncol. 2008;19:871–876. doi: 10.1093/annonc/mdm569. [DOI] [PubMed] [Google Scholar]
- Xing H, Weng D, Chen G, Tao W, Zhu T, Yang X, Meng L, Wang S, Lu Y, Ma D. Activation of fibronectin/PI-3K/Akt2 leads to chemoresistance to docetaxel by regulating survivin protein expression in ovarian and breast cancer cells. Cancer Lett. 2008;261:108–119. doi: 10.1016/j.canlet.2007.11.022. [DOI] [PubMed] [Google Scholar]
- Sabnis G, Goloubeva O, Jelovac D, Schayowitz A, Brodie A. Inhibition of long-term estrogen-deprived breast cancer xenografts to antiestrogens. Clin Cancer Res. 2007;13:2751–2757. doi: 10.1158/1078-0432.CCR-06-2466. [DOI] [PubMed] [Google Scholar]
- Saal LH, Johansson P, Holm K, Gruvberger-Saal SK, She Qb, Maurer M, Koujak S, Ferrando AA, Malmström P, Memeo L, Isola J, Bendahl PO, Rosen N, Hibshoosh H, Ringner M, Borg A, Parsons R. Poor prognosis in carcinoma is associated with a gene expression signature of aberrant PTEN tumor suppressor pathway activity. Proc Natl Acad Sci USA. 2007;104:7564–7569. doi: 10.1073/pnas.0702507104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang LH, Chan JL, Li W. Rapamycin together with herceptin significantly increased anti-tumor efficacy compared to either alone in ErbB2 overexpressing breast cancer cells. Int J Cancer. 2007;121:157–164. doi: 10.1002/ijc.22606. [DOI] [PubMed] [Google Scholar]
- Witters LM, Myers A, Lipton A. Combining flavopiridol with various signal transduction inhibitors. Oncol Rep. 2004;11:693–698. [PubMed] [Google Scholar]
- Wall NR, O'Connor DS, Plescia J, Pommier Y, Altieri DC. Suppression of survivin phosphorylation on Th34 by flavopiridol enhances tumor cell apoptosis. Cancer Res. 2003;63:230–235. [PubMed] [Google Scholar]
- Wittmann S, Bali P, Donapaty S, Nimmanapalli R, Guo F, Yamaguchi H, Huang M, Jove R, Wang HG, Bhalla K. Flavopiridol down-regulates anti-apoptotic proteins and sensitizes human breast cancer cells to epothilone B-induced apoptosis. Cancer Res. 2003;63:93–99. [PubMed] [Google Scholar]
- Wu K, Wang C, D'Amico M, Lee RJ, Albanese C, Pestell RG, Mani S. Flavopiridol and trastuzumam synergistically inhibit proliferation of breast cancer cells: association with selective cooperative inhibition of cyclin D1-dependent kinase and Akt signaling pathways. Mol Cancer Ther. 2002;1:695–706. [PubMed] [Google Scholar]
- Nahta R, Trent S, Yang C, Schmidt EV. Epidermal growth factor receptor expression is a candidate target of the synergistic combination of trastuzumab and flavopiridol in breast cancer. Cancer Res. 2003;63:3626–3631. [PubMed] [Google Scholar]
- Tan AR, Yang X, Berman A, Zhai S, Sparreboom A, Parr AL, Chow C, Brahim JS, Steinberg SM, Figg WD, Swain SM. Phase I trial of the cyclin-dependent kinase inhibitor flavopiridol in combination with docetaxel in patients with metastatic breast cancer. Clin Cancer Res. 2004;10:5038–5047. doi: 10.1158/1078-0432.CCR-04-0025. [DOI] [PubMed] [Google Scholar]
- Tennis H, Krishnan S, Bonner M, Ambrosone CB, Vena JE, Moysich K, Swede HY, McCann S, Hall P, Shields PG, Freudenheim JL. p53 Mutation analysis in breast tumors by a DNA microarray method. Cancer Epidemiol Biomarkers Prev. 2006;15:80–85. doi: 10.1158/1055-9965.EPI-05-0444. [DOI] [PubMed] [Google Scholar]
- Troester MA, Herschkowitz JI, Oh DS, He X, Hoadley KA, Barbier CS, Perou CM. Gene expression patterns associated with p53 status in breast cancer. BMC Cancer. 2006;6:276. doi: 10.1186/1471-2407-6-276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bull SB, Ozcelik H, Pinnaduwage D, Blackstein ME, Sutherland DA, Pritchard KI, Tzontcheva AT, Sidlofsky S, Hanna WM, Qizilbash AH, Tweeddale ME, Fine S, McCready DR, Andrulis IL. The combination of p53 mutation and neu/erbB-2 amplification is associated with poor survival in node-negative breast cancer. J Clin Oncol. 2004;22:86–96. doi: 10.1200/JCO.2004.09.128. [DOI] [PubMed] [Google Scholar]
- Marchetti P, Cannita K, Ricevuto E, De Galitiis F, Di Rocco ZC, Tessitore A, Bisegna R, Porzio G, De Rubeis GP, Ventura T, Martinetti S, Ficorella C. Prognostic value of p53 molecular status in high-risk primary breast cancer. Ann Oncol. 2003;14:704–708. doi: 10.1093/annonc/mdg197. [DOI] [PubMed] [Google Scholar]
- Wang YH, Sun YL, Xu SF, Zhang YY, Zhang L, Zhang B, Feng Ym, Niu RF, Fu L. Targeted down-regulation of p53 gene expression by individual antisense RNA in vitro. Zhonghua Bing Li Xue Za Zhi. 2007;36:544–549. [PubMed] [Google Scholar]
- Donninger H, Binder A, Bohm L, Parker MI. Differential effects of novel tumor-derived p53 mutation on the transformation of NIH-3T3 cells. Biol Chem. 2008;389:57–67. doi: 10.1515/BC.2008.010. [DOI] [PubMed] [Google Scholar]
- Cristofanilli M, Krishnamurthy S, Guerra L, Broglio K, Arun B, Booser DJ, Menander K, Van Wart Hood J, Valero V, Hortobagyi GN. A nonreplicating adenoviral vector that contains the wild-type p53 transgene combined with chemotherapy for primary breast cancer: safety, efficacy, and biologic activity of a novel gene-therapy approach. Cancer. 2006;107:935–9344. doi: 10.1002/cncr.22080. [DOI] [PubMed] [Google Scholar]
- Russo SM, Ove R. Molecular targets as therapeutic strategies in the management of breast cancer. Expert Opin Ther Targets. 2003;7:543–557. doi: 10.1517/14728222.7.4.543. [DOI] [PubMed] [Google Scholar]
- Modi S, D'Andrea G, Norton L, Yao TJ, Caravelli J, Rosen PP, Hudis C, Seidman AD. A phase I study of cetuximab/paclitaxel in patients with advanced-stage breast cancer. Clin Breast Cancer. 2006;7:270–277. doi: 10.3816/CBC.2006.n.040. [DOI] [PubMed] [Google Scholar]
- Feldner JC, Brandt BH. Cancer Cell motility: on the road from c-erbB-2 receptor steered signaling to actin reorganization. Exp Cell Res. 2002;272:93–108. doi: 10.1006/excr.2001.5385. [DOI] [PubMed] [Google Scholar]
- Solit DB, She Y, Lobo J, Kris MG, Scher HI, Rosen N, Sirotnak FM. Pulsatile administration of the epidermal growth factor receptor inhibitor gefitinib is significantly more effective than continous dosing for sensitizing tumors to paclitaxel. Clin Cancer Res. 2005;11:1983–1989. doi: 10.1158/1078-0432.CCR-04-1347. [DOI] [PubMed] [Google Scholar]
- Bundred NJ, Chan K, Anderson NG. Studies of epidermal growth factor receptor inhibition in breast cancer. Endocr Relat Cancer. 2001;8:183–189. doi: 10.1677/erc.0.0080183. [DOI] [PubMed] [Google Scholar]
- Choi YJ, Nam SJ, Son MJ, Kim DK, Kim JH, Yang JH, Kim MH, Song HS, Nam DH, Bang SI. Erlotinib prevents pulmonary metastasis in curatively resected breast carcinoma using a mouse model. Oncol Rep. 2006;16:119–122. [PubMed] [Google Scholar]
- Catania C, De Pas TM, Pelosi G, Manzotti M, Adamoli L, Nole F, Goldhirsch A. Erlotinib-induced breast cancer regression. Ann Pharmacother. 2006;40:2043–2047. doi: 10.1345/aph.1H252. [DOI] [PubMed] [Google Scholar]
- Guix M, Granja Nde M, Meszoely I, Adkins TB, Wieman BM, Frierson KE, Sanchez V, Sanders ME, Grau AM, Mayer IA, Pestano G, Shyr Y, Muthuswamy S, Calvo B, Krontiras H, Krop IE, Kelley MC, Arteaga CL. Short preoperative treatment with erlotinib inhibits tumor cell proliferation in hormone receptor positive breast cancers. J Clin Oncol. 2008;26:897–906. doi: 10.1200/JCO.2007.13.5939. [DOI] [PubMed] [Google Scholar]
- Romond EH, Perez EA, Bryant J, Suman VJ, Geyer CE, Jr, Davidson NE, Tan-Chiu E, Martino S, Paik S, Kaufman PA, Swain SM, Pisansky TM, Fehrenbacher L, Kutteh LA, Vogel VG, Visscher DW, Yothers G, Jenkins RB, Brown AM, Dakhil SR, Mamounas EP, Lingle WL, Klein PM, Ingle JN, Wolmark N. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med. 2005;353:1673–1684. doi: 10.1056/NEJMoa052122. [DOI] [PubMed] [Google Scholar]
- Slamon D, Eiermann W, Robert N, Pienkowski T, Martin M, Pawlicki M, Chan A, Smylie M, Liu M, Falkson C, Pinter T, Fornander T, Shiftan T, Valero V, Von Minckwitz G, Mackey J, Tabah-Fisch I, Buyse M, Lindsay MA, Riva A, Bee V, Pegram M, Press M, Crow J. Phase III randomized trial comparing Doxorubicin and cyclophosphamide followed by docetaxel (AC T) with doxorubicin and cyclophosphamide followed by docetaxel and trastuzumab (AC TH) with docetaxel, carboplatin and trastuzumab (TCH) in HER2 positive early breast cancer patients: BCIRG 006 study [abstract 1] Breast Cancer Res Treat. 2005;100(suppl 1):S5. [Google Scholar]
- Piccart-Gebhart MJ, Procter M, Leyland-Jones B, Goldhirsch A, Untch M, Smith I, Gianni L, Baselga J, Bell R, Jackisch C, Cameron D, Dowsett M, Barrios CH, Steger G, Huang CS, Andersson M, Inbar M, Lichinitser M, Láng I, Nitz U, Iwata H, Thomssen C, Lohrisch C, Suter TM, Rüschoff J, Suto T, Greatorex V, Ward C, Straehle C, McFadden E, et al. Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N Engl J Med. 2005;353:1659–1672. doi: 10.1056/NEJMoa052306. [DOI] [PubMed] [Google Scholar]
- Joensuu H, Kellokumpu-Lehtinen PL, Bono P, Alanko T, Kataja V, Asola R, Utriainen T, Kokko R, Hemminki A, Tarkkanen M, Turpeenniemi-Hujanen T, Jyrkkiö S, Flander M, Helle L, Ingalsuo S, Johansson K, Jääskeläinen AS, Pajunen M, Rauhala M, Kaleva-Kerola J, Salminen T, Leinonen M, Elomaa I, Isola J, FinHer Study Investigators Adjuvant docetaxel or vinorelbine with or without trastuzumab for breast cancer. N Engl J Med. 2006;354:809–820. doi: 10.1056/NEJMoa053028. [DOI] [PubMed] [Google Scholar]
- Tsakonas G, Kosmas C. Integration of novel targeted therapies of breast cancer – a review. J BUON. 2007;12:319–327. [PubMed] [Google Scholar]
- Buzdar AU, Ibrahim NK, Francis D, Booser DJ, Thomas ES, Theriault RL, Pusztai L, Green MC, Arun BK, Giordano SH, Cristofanilli M, Frye DK, Smith TL, Hunt KK, Singletary SE, Sahin AA, Ewer MS, Buchholz TA, Berry D, Hortobagyi GN. Significantly higher pathologic complete remission rate after neoadjuvant therapy with trastuzumab, paclitaxel, and epirubicin chemotherapy: results of a randomized trial in human epidermal growth factor receptor 2-positive operable breast cancer. J Clin Oncol. 2005;23:3676–3685. doi: 10.1200/JCO.2005.07.032. [DOI] [PubMed] [Google Scholar]
- Esteva FJ, Wang J, Lin F, Mejia JA, Yan K, Altundag K, Valero V, Buzdar AU, Hortobagyi GN, Symmans WF, Pusztai L. CD40 signaling predicts response to preoperative trastuzumab and concomitant paclitaxel followed by 5-fluorouracil, epirubicin, and cyclophosphamide in HER-2-overexpressing breast cancer. Breast Cancer Res. 2007;9:R87. doi: 10.1186/bcr1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer NG, Pestalozzi BC, Knuth A, Renner C. Potential use of humanized antibodies in the treatment of breast cancer. Expert Rev Anticancer Ther. 2006;6:1065–1074. doi: 10.1586/14737140.6.7.1065. [DOI] [PubMed] [Google Scholar]
- Arpino G, Gutierrez C, Weiss H, Rimawi M, Massarweh S, Bharwani L, De Placido S, Osborne CK, Schiff R. Treatment of human epidermal growth factor receptor 2-overexpressing breast cancer xenografts with multiagent HER-targeted therapy. J Natl Cancer Inst. 2007;99:694–705. doi: 10.1093/jnci/djk151. [DOI] [PubMed] [Google Scholar]
- Reid A, Vidal L, Shaw H, de Bono J. Dual inhibition of ErbB1 (EGFR/HER1) and ErbB2 (HER2/neu) Eur J Cancer. 2007;43:481–489. doi: 10.1016/j.ejca.2006.11.007. [DOI] [PubMed] [Google Scholar]
- Geyer CE, Forster J, Lindquist D, Chan S, Romieu CG, Pienkowski T, Jagiello-Gruszfeld A, Crown J, Chan A, Kaufman B, Skarlos D, Campone M, Davidson M, Berger M, Oliva C, Rubin SD, Stein S, Cameron D. Lapatinib plus capecitabine for HER2-positive advanced breast cancer. N Engl J Med. 2006;355:2733–2743. doi: 10.1056/NEJMoa064320. [DOI] [PubMed] [Google Scholar]
- Cristofanilli M, Boussen H, Baselga J, Lluch A, Ben Ayed F, Friaha M, Ben Ahmed S, Hurley J, Johnston S, Kaufman B, Findlay M, Olopade O, Shannon C, Harris J, Stein S, Spector N. A phase II combination study of lapatinib and paclitaxel as a neoadjuvant therapy in patients with newly diagnosed inflammatory breast cancer (IBC) San Antonio Breast Cancer Symposium Abstract Nr 1; General Session. 2006.
- Appels NM, Beijnen JH, Schellens JH. Development of farnesyl transferase inhibitors: a review. Oncologist. 2005;10:565–578. doi: 10.1634/theoncologist.10-8-565. [DOI] [PubMed] [Google Scholar]
- Gligorov J, Azria D, Namer M, Khayat D, Spano JP. Novel therapeutic strategies combining antihormonal and biological targeted therapies in breast cancer: focus o clinical trials and perspectives. Crit Rev Oncol Hematol. 2007;64:115–128. doi: 10.1016/j.critrevonc.2007.06.010. [DOI] [PubMed] [Google Scholar]
- Luo Y, Zhou H, Krueger J, Kaplan C, Lee SH, Dolman C, Markowitz D, Wu W, Liu C, Reisfeld RA, Xiang R. Targeting tumor-associated macrophages as a novel strategy against breast cancer. J Clin Invest. 2006;116:2132–2141. doi: 10.1172/JCI27648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim KJ, Li B, Winer J, Armanini M, Gillett N, Phillips HS, Ferrara N. Inhibition of vascular endothelial growth factor-induced angiogenesis suppresses tumour growth in vivo. Nature. 1993;362:841–844. doi: 10.1038/362841a0. [DOI] [PubMed] [Google Scholar]
- Zhu Z, Bohlen P, Witte L. Clinical development of angiogenesis inhibitors to vascular endothelial growth factors and its receptor as cancer therapeutics. Curr Cancer Drug Targets. 2002;2:135–156. doi: 10.2174/1568009023333881. [DOI] [PubMed] [Google Scholar]
- Cobleigh MA, Langmuir VK, Sledge GW, Miller KD, Haney L, Novotny WF, Reimann JD, Vassel A. A phase I/II dose-escalation trial of bevacizumab in previously treated metastatic breast cancer. Semin Oncol. 2003;30:117–124. doi: 10.1053/j.seminoncol.2003.08.013. [DOI] [PubMed] [Google Scholar]
- Sledge G, Miller K, Novotny W, Gaudreault J, Ash M, Cobleigh M. A phase II trial of single-agent Rhumab VEGF (recombinant humanizerd monoclonal antibody to vascular endothelial cell growth factor) in patients with relapsed metastatic breast cancer [abstract 5C] Proc Am Soc Clin Oncol. 2000;19:3a. [Google Scholar]
- Rugo HS. Bevacizumab in the treatment of breast cancer: rationale and current data. Oncologist. 2004;9(suppl 1):43–49. doi: 10.1634/theoncologist.9-suppl_1-43. [DOI] [PubMed] [Google Scholar]
- Burstein H, Parker L, Savoie J, Younger J, Kuter I, Ryan PD, Garber JE, Campos SM, Shulman LN, Harris LN, Gelman R, Winer EP. Phase II trial of the anti-VEGF antibody beva-cizumab in combination with vinorelbine for refractory advanced breast cancer [abstract 446] Breast Cancer Res Treat. 2002;76:S115. [Google Scholar]
- Ramaswamy B, Elias AD, Kelbick NT, Dodley A, Morrow M, Hauger M, Allen J, Rhoades C, Kendra K, Chen HX, Eckhardt SG, Shapiro CL. Phase II trial of bevacizumab in combination with weekly docetaxel in metastatic breast cancer patients. Clin Cancer Res. 2006;12:3124–3129. doi: 10.1158/1078-0432.CCR-05-2603. [DOI] [PubMed] [Google Scholar]
- Bernstein JA, Schubbert T, Kong K, Mehta RS. Weekly carboplatin and nab-paclitaxel plus trastuzumab, or plus or minus bevacizumab: clinical response in patients with breast cancer [abstract 10699] Proc Am Soc Clin Oncol. 2006;24:584s. [Google Scholar]
- Miller KD, Chap LI, Holmes FA, Cobleigh MA, Marcom PK, Fehrenbacher L, Dickler M, Overmoyer BA, Reimann JD, Sing AP, Langmuir V, Rugo HS. Randomized phase III trial of capecitabine with bevacizumab plus capecitabine in patients with previously treated metastatic breast cancer. J Clin Oncol. 2005;23:792–799. doi: 10.1200/JCO.2005.05.098. [DOI] [PubMed] [Google Scholar]
- Overmoyer B, Silverman P, Leeming R, Shenk R, Lyons J, Jesberger J, Hartman P, Dumadag L, Chen H. Phase II trial of neoadjuvant docetaxel with or without bevacizumab in patients with locally advanced breast cancer [abstract 2088] Breast Cancer Res Treat. 2004;88:S106. [Google Scholar]
- Wedam SB, Low JA, Yang SX, Chow CK, Choyke P, Danforth D, Hewitt SM, Berman A, Steinberg SM, Liewehr DJ, Plehn J, Doshi A, Thomasson D, McCarthy N, Koeppen H, Sherman M, Zujewski J, Camphausen K, Chen H, Swain SM. Antiangiogenic and antitumor effects of bevacizumab in patients with inflammatory and locally advanced breast cancer. J Clin Oncol. 2006;24:769–777. doi: 10.1200/JCO.2005.03.4645. [DOI] [PubMed] [Google Scholar]
- Konecny GE, Meng YG, Untch M, Wang HJ, Bauerfeind I, Epstein M, Stieber P, Vernes JM, Gutierrez J, Hong K, Beryt M, Hepp H, Slamon DJ, Pegram MD. Association between HER-2/neu and vascular endothelial growth factor expression predicts clinical outcome in primary breast cancer patients. Clin Cancer Res. 2004;10:1706–1716. doi: 10.1158/1078-0432.CCR-0951-3. [DOI] [PubMed] [Google Scholar]
- Pegram M, Yeon C, Ku NC. Phase I combined biological therapy of breast cancer using two humanized monoclonal antibodies directed against HER2 proto-oncogene and vascular endothelial growth factor (VEGF) [abstract 3039] Breast Cancer Res Treat. 2004;88(suppl 1):S124. [Google Scholar]
- Pegram M, Chan D, Dichmann RA. Phase II combined biological therapy targeting the HER2 proto-oncogene and the vascular endothelial growth factor using trastuzumab (T) and bevacizumab (B) as first line therapy of HER2 amplified breast cancer [abstract 301] Breast Cancer Res Treat. 2006;100(suppl 1):S28. [Google Scholar]
- Randomized, Open-Label Study to Compare the Effect of First-Line Treatment With Avastin in Combination with Herceptin/Docetaxel Alone on Progression-Free Survival in Patients With HER2 positive Locally Recurrent or Metastatic Breast Cancer http://clinicaltrials.gov/ct/show/ct/show/NCT00391092?order=6
- Rugo HS, Dickler MN, Scott JH, Moore DH, Park JW. Circulating endothelial cell (CEC) and tumor cell (CTC) analysis in patients receiving bevacizumab and erlotinib for metastatic breast cancer (MBC) [abstract 3088] Breast Cancer Res Treat. 2004;88:S142. [Google Scholar]
- Zafar Y, Bendell J, Lager J, Yu D, George D, Nixon A, Petros W, Beci R, Arrowood C, Hurwitz H. Preliminary results of a phase I study of bevacizumab in combination with everolimus in patients with advanced solid tumors [abstract 3097] Proc Am Soc Clin Oncol. 2006;24:145s. [Google Scholar]
- Schuetz F, Sohn C, Schneeweiss A. Bevacizumab in the treatment of metastatic breast cancer. Breast Care. 2007;2:82–88. doi: 10.1159/000100559. [DOI] [Google Scholar]
- Brandt B. Gene Expression Analyses. In: Schlotter CM, Bonk U, Brandt B Bremen, editor. Individualized concepts of neo-adjuvant and adjuvant therapy of breast cancergene and gene expressions [in German] Vol. 4. London, Boston: UNI-MED Verlag; 2007. pp. 55–77. [Google Scholar]
- Garman KS, Nevins JR, Potti A. Genomic strategies for personalized cancer therapy. Hum Mol Genet. 2007;16(Spec No 2):R226–R232. doi: 10.1093/hmg/ddm184. [DOI] [PubMed] [Google Scholar]
- Pruthi S, Boughey JC, Brandt KR, Degnim AC, Dy GK, Goetz MP, Perez EA, Reynolds CA, Schomberg PJ, Ingle JN. A multidisciplinary approach to the management of breast cancer, part 2: therapeutic considerations. Mayo Clin Proc. 2007;82:1131–1140. doi: 10.4065/82.9.1131. [DOI] [PubMed] [Google Scholar]
- Eniu A. Integrating biological agents into systemic therapy of breast cancer: trastuzumab, lapatinib, bevacizumab. J BUON. 2007;12(suppl 1):S119–S126. [PubMed] [Google Scholar]
- Gepar Quinto http://www.germanbreastgroup.de/geparquinto
- RADAR http://www.germanbreastgroup.de/radar
- http://www.germanbreastgroup.de/herceptin/GBG
- Neo-ALTTO http://www.germanbreastgroup.de/neo-altto
- ALTTO Trial http://alttotrials.com
- REACT http://www.germanbreastgroup.de/react
- German Breast Group http://www.germanbreastgroup.de