Abstract
Idiotype protein (Id) secreted by myeloma cells is a tumor-specific antigen. Id-based immunotherapy has been explored in patients with myeloma, and results are disappointing. Although previous studies have demonstrated that Id-specific CTLs are able to lyse myeloma cells, it is unclear whether other types of Id-specific T cells, such as type-1 T-helper (Th1) and type-2 T helper (Th2) cells, are also able to suppress or kill myeloma cells. Using a 5T murine myeloma model, we generated T-cell clones of different subsets and examined their function in the context of myeloma cells. Id-specific CTLs specifically lysed myeloma cells via MHC class I, perforin and Fas ligand, and Th1 but not Th2 cells lysed the myeloma cells by Fas ligand-Fas interaction. CTL and Th1 cells also suppressed the growth and function of myeloma cells whereas Th2 cells promoted the proliferation of and enhanced secretion of Id protein and cytokines by myeloma cells. CTL and Th1 but not Th2 cells were able to eradicate established myeloma in vivo after adoptive transfer. These results demonstrate that Id-specific CTL and Th1 are promising effector cells while Th2 provide no protection and may even promote tumor progression in vivo.
Keywords: Multiple myeloma, idiotype, T-cell subsets, immunotherapy, murine model
INTRODUCTION
Multiple myeloma (MM) is a B-cell malignancy, characterized by an accumulation of malignant plasma cells within the bone marrow. Myeloma cells secrete a monoclonal immunoglobulin (idiotype; Id) and induce skeletal destruction and hypercalcemia. Despite the progress in therapy of the disease, MM still remains an incurable malignancy (1, 2). Therefore, there is a great need for new treatments to stabilize or eradicate minimal residual tumors achieved after high-dose chemotherapy supported by autologous stem-cell transplantation.
Id protein secreted by myeloma cells is a tumor-specific antigen because of the unique antigenic structure in its variable regions. Id-based immunotherapy has been explored in patients with MM and other B-cell tumors (3). As Id-based immunotherapies activate different subsets of Id-specific T cells (4), and T cells are potent effectors and critical components of anti-tumor immunity, many investigators have examined the roles of Id-specific T cells in these malignances. Early studies demonstrated not only the presence of low frequencies of naturally occurring Id-specific T cells in patients with monoclonal gammopathy of undetermined significance (MGUS) and MM stage I (5, 6), but also Id-specific type-1 helper T cells (Th1) in MGUS and MM stage I, and type-2 helper T cells (Th2) in MM stage II–III (7). In addition, the presence of major histocompatibility complex (MHC) class II-restricted, Id-specific CD4+ T cells and MHC class I-restricted Id-specific CD8+ T cells has been reported in unimmunized patients with MGUS or MM (8). These results indicate that these naturally occurring T cells are unable to suppress or eradicate myeloma cells in vivo due to inadequate numbers and functional suppression (9).
Although more recent studies have demonstrated that Id-specific CD8+ CTLs, which were generated by using Id-pulsed dendritic cells (DCs) were able to lyse primary myeloma cells from patients (10, 11), it is still unclear whether other types of Id-specific T cells, such as CD4+ Th1 and Th2 cells, are able to suppress or kill myeloma tumor cells. As Id-based immunotherapy may activate all T-cell subsets in patients (4), it is important to understand the functions of these T cells in the context of myeloma cells. In this study, we used the 5TGM1 myeloma murine model originally derived from 5T33 myeloma cells (12–14), to generate Id-specific T-cell subsets in C57BL/KaLwRij mice and explored the functional roles and anti-myeloma immune responses of Id-specific T-cell subsets on the myeloma tumor cells in vitro and in vivo.
MATERIALS AND METHODS
Mice and cell lines
Male C57BL/KaLwRij mice were purchased from Harlan CPB (Zeist, The Netherland). This study was approved by the Institutional Animal Care and Use Committee of the University of Texas, M. D. Anderson Cancer Center. The 5TGM1 murine myeloma cell line was cultured in Iscove’s modified dulbecco’s media (IMDM; Invitrogen, Grand Island, NY) supplemented with 10% heat-inactivated fetal bovine serum (FBS; Atlanta Biologicals, Lawrenceville, GA), 100 U/mL penicillin-streptomycin, and 2 mM L-glutamine (both from Invitrogen). The B16 melanoma cell line, originated from C57BL/6 mice, was purchased from American Type Culture Collection (ATCC; Rockville, MD) and cultured in IMDM.
Preparation of idiotype protein
The 5TGM1 myeloma cells were cultured in AIM-V serum-free medium and mouse IgG2b Id protein, secreted by the 5TGM1 myeloma cells, was purified from cell culture supernatant using Hi-Trap Protein A affinity chromatography (GE Healthcare, Piscataway, NJ) as described previously (15). Id protein and keyhole limpet hemocyanin (KLH; EMD Biosciences, La Jolla, CA) conjugate was made using 0.1% glutaraldehyde (Sigma, St. Louis, MO) as described previously (16) to enhance the immunogenicity of the Id protein.
Generation of dendritic cells
DCs were generated from bone marrow stem cells of mice as described previously (17, 18). Briefly, bone marrow mononuclear cells were cultured at a density of 2 × 105 cells/mL in RPMI-1640 complete medium with 20 ng/mL granulocyte-monocyte colony-stimulating factor (GM-CSF; R&D Systems, Minneapolis, MN) at 37°C in 5% CO2. At day 4, medium was replaced with fresh medium containing GM-CSF (10 ng/mL), and at day 8, immature DCs were pulsed for 8 hours with Id-KLH proteins at a concentration of 50 μg/mL, followed by addition of tumor necrosis factor (TNF)-α (10 ng/mL) and IL-1β (10 ng/mL; both from R&D Systems) for 48 hours to induce DC maturation. Mature DCs were collected and used to immunize mice.
Generation of idiotype-specific T-cell clones
Mice were immunized by three weekly subcutaneous injections of Id-KLH-pulsed mature DCs (106 DCs/injection/mouse). Following each immunization, GM-CSF (200 ng/day) was injected subcutaneously adjacent to the immunization sites for three consecutive days (4). One week after the final immunization, T cells were isolated from the spleens of immunized mice and cultured with 50 μg/mL Id protein in RPMI-1640 complete medium containing recombinant human IL-2 (10 U/mL; Roche Diagnostic, Mannheim, Germany) and IL-15 (5 ng/mL; R&D Systems). At day 7, T cells were collected and seeded into 96-well U-bottom tissues culture plates (Corning Inc., Corning, NY) at a concentration of 1 cell/well by a limiting dilution in RPMI-1640 complete medium containing recombinant human IL-2 (20 U/mL) and IL-15 (5 ng/mL). Irradiated (30 Gy) syngeneic splenocytes from naïve C57BL/KaLwRij mice were added as feeder cells together with Id protein (50 μg/mL). After three to four cycles of stimulation, T-cell clones were established and expanded in RPMI-1640 complete medium containing recombinant human IL-2 (30 U/mL) and IL-15 (10 ng/mL) and subjected to functional tests.
Immunophenotyping
Phycoerythrin- (PE) or fluorescein isothiocyanate- (FITC) conjugated monoclonal antibodies (mAbs) against CD11c, CD40, CD80, CD86, and MHC class II for DCs or CD4, CD8, CD69, and Fas ligand (FasL; all from BD Biosciences, San Diego, CA) for T-cell clones were added to cells, incubated for 30 minutes at 4°C, washed twice, and analyzed using a FACSCalibur flow cytometer (BD Biosciences). Intracellular perforin staining was performed using the Cytofix/Cytoperm kit (BD Biosciences) according to the manufacturer’s instruction.
Detection of cytokine production
Enzyme-linked immunoabsorbent assay (ELISA) was used to measure secreted cytokines such as IFN-γ, TNF-α, IL-2, IL-4, or IL-5 from T-cell clones. T-cell clones were incubated with irradiated (30 Gy) mature DCs pulsed with Id or irrelevant IgG2b protein and unpulsed DCs were used as control. Supernatants were collected, and the amounts of cytokines were quantified using commercially available ELISA kits (R&D Systems).
Proliferation assay
T-cell clones (5 × 104/100 μL/well) were seeded into 96-well U-bottom culture plates. Various numbers of irradiated (30 Gy) mature DCs pulsed with Id or irrelevant mouse IgG2b protein were added and cultured for 4 days at 37°C in 5% CO2. Cells were pulsed with 0.5 μCi/well 3[H]-thymidine (GE Healthcares) and harvested 18 hours later. Radioactivity was measured using a β-liquid scintillation analyzer. Unpulsed DCs were used as control. Results are shown as mean count per minute (CPM).
In some experiments, T-cell clones were pre-labeled with 5(6)-carboxyfluorescein diacetate succinimidyl ester (CFSE; Invitrogen) for 10 minutes at 37°C. After washing, labeled T-cell clones were seeded and co-cultured with irradiated mature DCs pulsed with Id or irrelevant mouse IgG2b protein for 5 days. Unpulsed DCs were used as control. After that, cells were incubated with PE-conjugated CD8 or CD4 mAb for 30 minutes, washed, and ready for analysis.
Cytotoxicity assay
The standard 51Cr-release assay was performed to measure the cytotoxicity of the T-cell clones against 5TGM1 myeloma cells or DCs pulsed with Id protein as described previously (19). B16 melanoma cells or unpulsed DCs were used as control target cells. CD8+ or CD4+ T cells purified from spleens of naïve mice using anti-CD8 or CD4 mAb-coated magnetic beads (Miltenyi Biotec, Auburn, CA) were used as controls for effectors cells. Target cells were labeled with 51Cr-sodium chromate (GE Healthcares) for one hour and incubated with various numbers of T cells in 96-well U-bottom culture plates. After 4 hours, 50% of the supernatants were collected, and radioactivity was measured by a gamma-counter. Results are shown as mean percentage 51Cr release calculated as follows: [(sample counts − spontaneous counts)/(maximum counts − spontaneous counts)] × 100.
In some experiments, 5TGM1 myeloma cells were pre-labeled with PKH26 red fluorescent using cell linker kits (Sigma) and co-cultured with T-cell clones. 5TGM1 myeloma cells cultured without T cells were used as negative control. CD8+ or CD4+ T cells isolated from splenocytes of naïve mice served as controls for effector T cells. After 18 hours, FITC-conjugated Annexin-V (BD Biosciences) was added to the cells, incubated for 15 minutes, and analyzed using a flow cytometer.
Inhibition of T cell-mediated cytotoxicity
To determine whether the cytotoxic activity of the T cells was restricted or mediated by MHC molecules, perforin, or FasL, mAbs against MHC class I, II, or FasL were used to block the cytotoxicity of the T-cell clones against 5TGM1 myeloma cells. CTL or Th1 clones were co-cultured with 5TGM1 myeloma cells in the presence or absence of 20 μg/mL mAbs against MHC class I or II (both from BD Biosciences) or FasL (R&D Systems). Isotypic control IgG (Jackson ImmunoResearch, West Grove, PA) was used as control. In addition, concanamycin A (CMA; Sigma), an inhibitor of vacuolar type H+-ATPase, was used as selective inhibitor of perforin-mediated cytotoxicity (20). Effector T-cell clones were pretreated with 100 nM of CMA for 2 hours and assayed for cytotoxicity in the presence of the reagent.
T-cell inhibition assay
The suppressive activity of T-cell clones on myeloma cell growth and secretion of Id protein or cytokines such as vascular endothelial growth factor (VEGF) was performed by using 3[H]-thymidine incorporation and ELISA assays. 5TGM1 myeloma cells were seeded at a density of 1 × 104/100 μL/well into 96-well U-bottom tissue culture plates. Irradiated (30 Gy) T-cell clones were added at a density of 5 × 104/100 μL/well to the plates and co-cultured with the myeloma cells for 3 days at 37°C in 5% CO2. Cells were pulsed with 0.5 μCi/well 3[H]-thymidine and harvested 18 hours later. Radioactivity was measured using a β-liquid scintillation analyzer.
In some experiments, supernatants from T-cell clones co-cultured with 5TGM1 myeloma cells were collected. Secreted IgG2b Id protein or VEGF from 5TGM1 myeloma cells were measured by ELISA assay as described previously (21).
In vivo adoptive transfer of T-cell clones
Mice were challenged intravenously with 2 × 106 5TGM1 myeloma cells. One week later when myeloma growth was established, 2 × 106 T-cell clones were injected intravenously into tumor-bearing mice. Control mice received an injection of PBS or the same number of splenocytes from naïve mice. Tumor burden was monitored by measuring serum IgG2b Id protein by ELISA assay. Mice were euthanized when moribund or when hind-leg paralysis developed.
Statistical analysis
Student’s t-test was used to compare various experimental groups. P < 0.05 was considered statistically significant. Survival was evaluated from the day of tumor injection until death, and Kaplan-Meier test used to compare mouse survival between the groups. All data are shown as mean and SD.
RESULTS
Generation of idiotype-specific T-cell clones
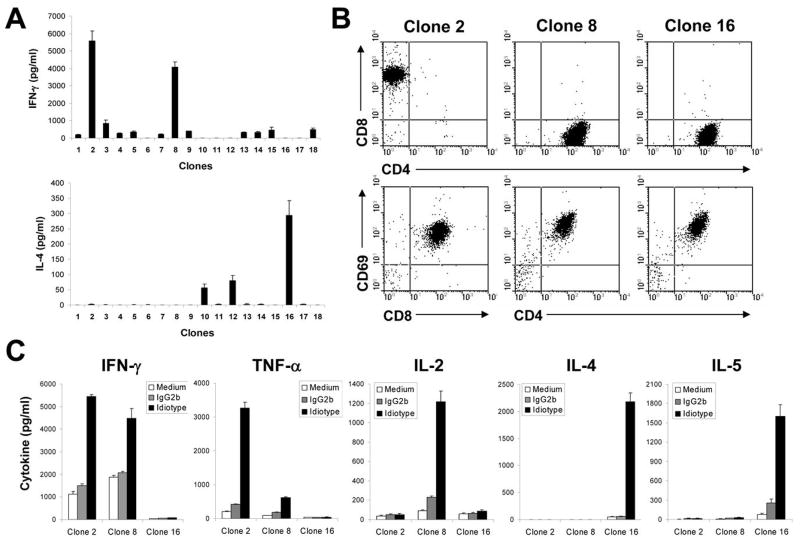
To obtain Id-specific T-cell clones, immature DCs generated from C57BL/KaLwRij mouse bone marrow stem cells were pulsed with purified Id-KLH conjugate, matured with TNF-α and IL-1β, and injected into mice. One week after the third immunization, mice were sacrificed, and splenocytes were restimulated in vitro with Id protein for one week. Using limiting dilution assay, we generated Id-specific T-cell clones that secreted high levels of IFN-γ or IL-4 (Fig. 1A), which were expanded for further functional studies. T-cell clones of different subsets were identified based on their expression of CD8+ or CD4+ T-cell surface markers and secretion of cytokines such as IFN-γ, TNF-α, IL-2, IL-4, or IL-5 in response to Id protein stimulation.
Figure 1. Generation and characterization of Id-specific T-cell clones.
A, Id-specific T-cell clones of different subsets were generated and selected based on Id-induced IFN-γ and IL-4 secretion using ELISA assay. B, flow cytometry analysis showing the expression of CD8+ or CD4+ T-cell surface marker and CD69 activation marker on three T-cell clones. C, cytokine secretion pattern of three T-cell clones. T-cell clones were stimulated with irradiated DC pulsed with Id protein or irrelevant mouse IgG2b protein. Cytokines in cell-culture media were quantified by ELISA. Representative results of three independent experiments are shown.
Fig. 1 shows three representative Id-specific T-cell clones of CD8+ CTL and CD4+ Th1 and Th2 cells. The phenotype of the T cells is shown in Fig. 1B, and pattern of cytokine secretion in Fig. 1C. Clone 2 expressed surface CD8 and CD69 (an activation marker), secreted IFN-γ and TNF-α, but not IL-2, IL-4, or IL-5, and thus may be CTL T-cell clone. Clone 8 expressed CD4 and CD69, secreted high levels of IFN- and IL-2 and a low level of TNF-α but not IL-4 or IL-5, and could be a Th1 T-cell clone. Clone 16 also expressed surface CD4 and CD69, secreted IL-4 and IL-5, but not IFN- γ, TNF- α, or IL-2, and may thus be a Th2 T-cell clone. The same pattern of surface markers and cytokine secretion was also seen with other T-cell clones (data not shown). These results demonstrate that CD8+ Id-specific T-cell clones of the CTLs and Id-specific CD4+ clones of Th1 and Th2 cells were generated.
Proliferative responses of the T-cell clones
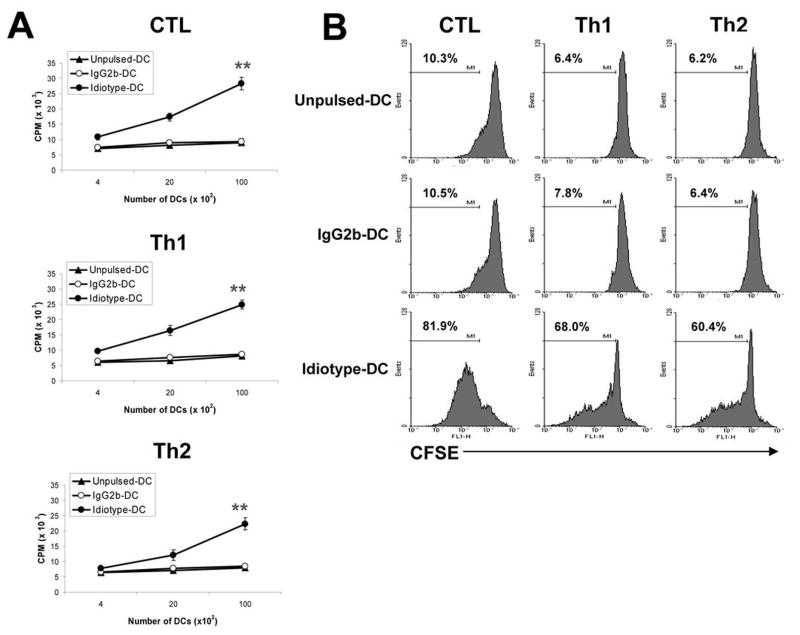
To examine the functional properties of these T-cell clones, we first examined T-cell proliferative response induced by Id protein using 3[H]-thymidine incorporation assay. Findings from clones 2, 8, and 16 are shown as representative results in the following studies. As shown in Fig. 2A, all of the T-cell clones significantly proliferated in response to syngeneic DCs pulsed with Id protein (P < 0.01, compared with unpulsed or irrelevant mouse IgG2b-pulsed DCs). Similar results were also obtained with CFSE dilution assay to measure T-cell proliferation (Fig. 2B). Cultures of the CTL, Th1, and Th2 clones with DCs pulsed with Id protein resulted in high percentages of dividing T cells (60–82%) whereas the percentages of proliferating T cells cultured with unpulsed or irrelevant mouse IgG2b-pulsed DCs were low (6–10%). These T-cell clones did not respond to DCs pulsed with KLH (data not shown). These results were confirmed with other T-cell clones of CTL, Th1 and Th2 cells (data not shown). These results further confirm the specificity of the T cells for Id protein.
Figure 2. Proliferative responses of Id-specific T-cell clones.
Shown are T-cell proliferative responses measured by 3[H]-thymidine incorporation assay (A) or CFSE dilution assay (B), in response to DCs pulsed with Id or mouse IgG2b control. Unpulsed DCs served as a control. T-cell:DC ratio of 5:1 was used. Values above histograms represent the percentages of dividing T cells. Representative results of three independent experiments are shown. ** P < 0.01 compared with unpulsed DCs control.
Cytotoxic activity of the T-cell clones against myeloma cells
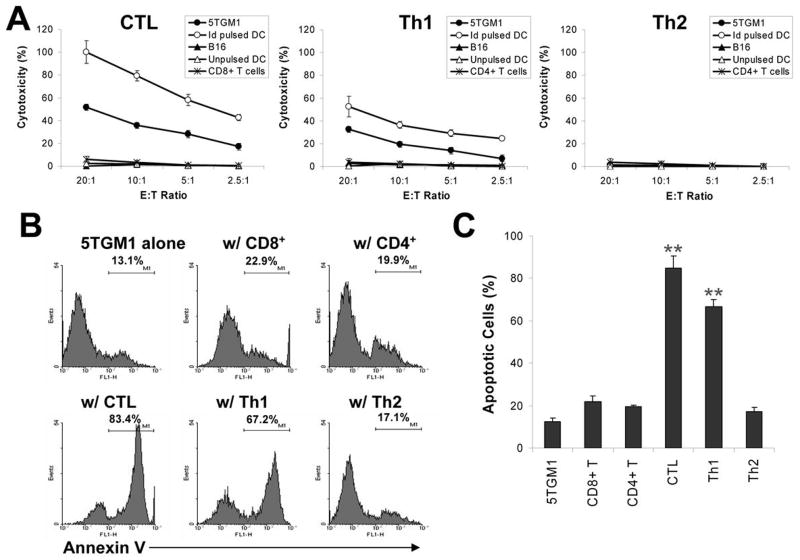
Next we evaluated the cytolytic activity of these T-cell clones against myeloma cells. Both the standard 51Cr-release assay and Annexin V-binding assay were used, and the targets cells were 5TGM1 myeloma cells and Id-pulsed DCs. As shown in Fig. 3A, the CTL and Th1 clones efficiently and specifically lysed 5TGM1 myeloma cells and Id-pulsed DCs, although the CTL clone displayed stronger cytotoxic activity compared with the Th1 cells. No killing was observed against B16 melanoma cells, unpulsed DCs, or DCs pulsed with KLH. In contrast, the Th2 clone was not cytolytic to the target cells, and neither were isolated CD8+ or CD4+ T cells from spleens of naïve mice.
Figure 3. Cytotoxic activity of Id-specific T-cell clones.
A, percentage of cytotoxicity measured by 51Cr-release assay, of T-cell clones against various target cells, including 5TGM1 myeloma cells, unpulsed or Id-pulsed DCs, or B16 tumor cells. B, percentage of apoptotic cells measured by Annexin V-binding assay. T-cell clones were co-cultured with PKH26 red fluorescent pre-labeled 5TGM1 myeloma cells and the cells were collected and analyzed by flow cytometry. A gate was set on the PKH26 positive cell population. 5TGM1 myeloma cells cultured alone were used as control. An effector:target (E:T) ratio of 5:1 was used. Values above histograms represent the percentages of Annexin-V positive myeloma cells. C, pooled data from three experiments showing the percentages of apoptotic 5TGM1 myeloma cells induced by T-cell clones measured by Annexin V-binding assay. CD8+ or CD4+ T cells from splenocytes of naïve mice served as controls for effector cells. Representative results of three independent experiments are shown. ** P < 0.01 compared with 5TGM1 alone.
We obtained similar results by using Annexin V-binding assay. As shown in Fig. 3B, co-culture of the CTL and Th1 clones with PKH26-prelabeled 5TGM1 myeloma cells (to identify and gate on myeloma cells) resulted in high percentages of apoptotic (Annexin-V+) 5TGM1 cells (P < 0.01, compared with 5TGM1 alone or 5TGM1 co-cultured with naïve CD8+ and CD4+ T cells). Co-culture with the Th2 clones or with purified CD4+ or CD8+ T cells from naïve mice did not increase the percentages of apoptotic 5TGM1 cells. Fig. 3C shows the pooled data of T cell-induced apoptosis in the myeloma cells. These results were confirmed with other T-cell clones of CTL, Th1 and Th2 cells (data not shown). These findings indicate that both Id-specific CTL and Th1 but not Th2 cells are efficient killer cells against myeloma cells, and the T cells recognized Id epitopes naturally processed by and presented on 5TGM1 myeloma cells.
MHC restriction of T cell-mediated cytotoxic activity
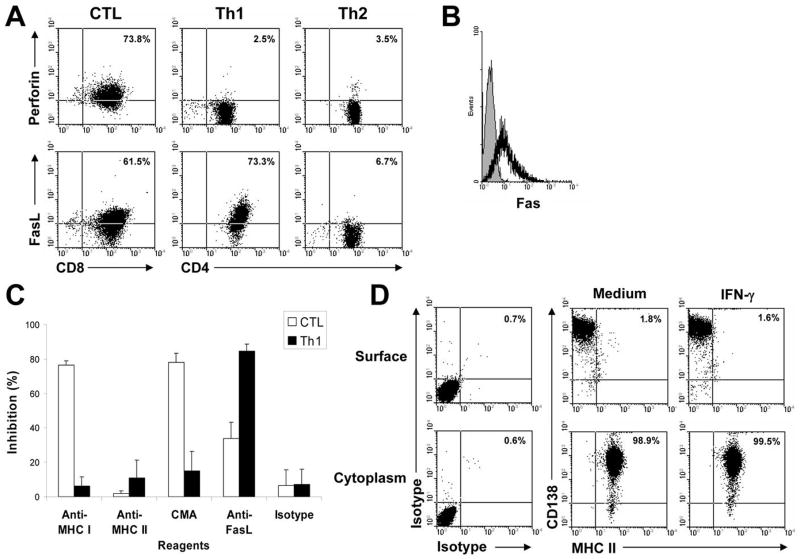
To elucidate the mechanism underlying T cell-mediated cytotoxicity against the tumor cells, flow cytometry analysis was used to examine the expression of perforin and FasL by the T-cell clones. As shown in Fig. 4A, the CTL clone expressed both perforin and FasL, while the Th1 cells expressed FasL but not perforin. In contrast, the Th2 cells did not express either perforin or FasL. Interestingly, 5TGM1 myeloma cells expressed Fas molecules (Fig. 4B).
Figure 4. Restriction of the T-cell clone-mediated cytotoxic activity.
A, flow cytometry analysis showing the expression of perforin and FasL on the T-cell clones. Values in each dot plot represent the percentages of CD8+ CTL or CD4+ Th1 or Th2 cells expressing perforin or FasL. B, expression of Fas molecules (solid line) on 5TGM1 myeloma cells. Shaded area represents staining with isotype control. C, Inhibition of T cell-mediated cytotoxicity against 5TGM1 myeloma cells by mAbs (20 g/mL) against MHC class I (Anti-MHC I), MHC class II (Anti-MHC II), or FasL (Anti-FasL), or by the treatment of CMA. Isotypic control IgG (Isotype) served as control. An effector:target (E:T) ratio of 10:1 was used. D, flow cytometry analysis showing the expression of MHC class II on the cell surface, and in the cytoplasm. 5TGM1 myeloma cells were cultured without (medium) or with recombinant murine IFN-γ for 48 hours. Isotypic IgG staining was used as control. Values in each dot plot represent the percentages of CD138+ 5TGM1 myeloma cells expressing MHC class II. Representative results of three independent experiments are shown.
To determine whether the cytotoxic activity of the CTL or Th1 clones was restricted by MHC molecules and mediated by perforin or FasL, we evaluated the inhibition of cytotoxicity using anti-MHC class I or II mAbs or mAb against FasL, or CMA, an inhibitor of perforin-mediated cytotoxicity. As shown in Fig. 4C, mAbs against MHC class I but not class II, CMA, and mAb against FasL although to a lesser extent, significantly inhibited CTL-mediated cytotoxic activity against 5TGM1 myeloma cells (P < 0.01 and P < 0.05, compared with isotype IgG control). These findings indicate that myeloma cells naturally process and present MHC class I-restricted, Id epitopes to CD8+ T cells.
Surprisingly, our studies showed that mAbs against FasL but not MHC class I or II significantly inhibited Th1-mediated cytotoxic activity (Fig. 4C; P < 0.01, compared with isotype IgG control). We then examined the surface expression of MHC class I and II by 5TGM1 cells and demonstrated that the myeloma cells express MHC class I (data not shown) but not class II molecules (Fig. 4D, surface). However, intracellular MHC class II molecules could be detected in the myeloma cells (Fig. 4D, cytoplasm). Treatment of 5TGM1 cells with IFN-γ (500 U/mL for 48 hours) did not induce surface expression of MHC class II molecules. Taken together, these findings suggest that although myeloma cells do not express MHC class II molecules, Id-specific CD4+ Th1 cells can still recognize and kill the tumor cells by Fas-FasL interaction.
Suppressive activity of the T-cell clones against myeloma cells
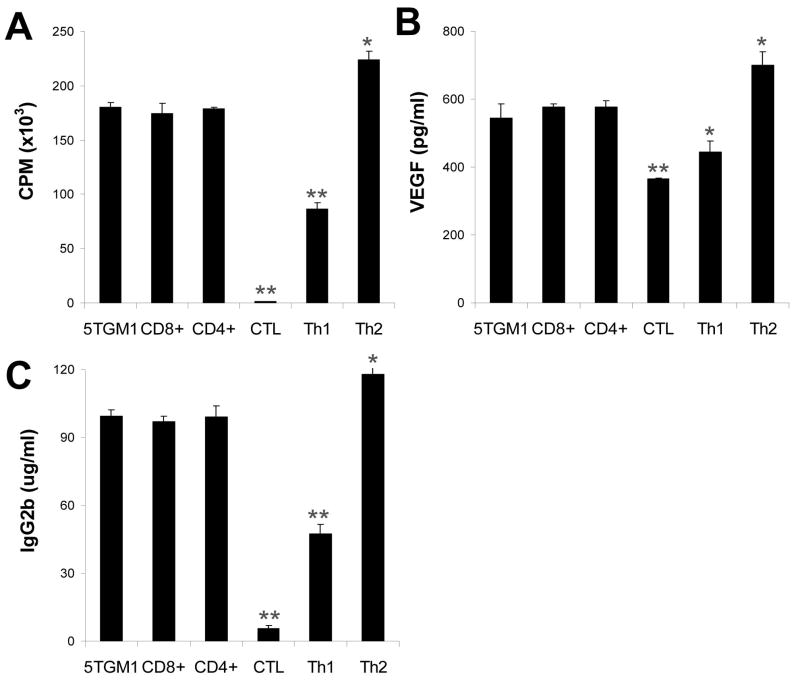
Next we examined the suppressive activity of the T-cell clones in the growth and function of myeloma cells. As shown in Fig. 5A, 5TGM1 myeloma cells co-cultured with irradiated CTL or Th1 cells showed significantly inhibited proliferative response (P < 0.01, compared with 5TGM1 alone). In contrast, 5TGM1 myeloma cells co-cultured with irradiated Th2 cells showed significantly enhanced proliferative response (P < 0.05, compared with 5TGM1 alone). No changes were observed in cell proliferative response of 5TGM1 cells when co-cultured with irradiated CD4+ or CD8+ T cells from naïve mice.
Figure 5. Suppressive activity of the T-cell clones on myeloma cells.
Shown are: A, proliferation of 5TGM1 myeloma cells measured by 3[H]-thymidine incorporation assay; B, concentrations of secreted VEGF by 5TGM1 myeloma cells; and C, concentrations of IgG2b Id protein secreted by 5TGM1 myeloma cells. Irradiated T cells were co-cultured with 5TGM1 myeloma cells for 3 days. 5TGM1 myeloma cells cultured without T-cell clones (5TGM1) or co-cultured with CD8+ and CD4+ T cells from splenocytes of naïve mice were used as controls. T-cell:myeloma cell ratio of 5:1 was used. Representative results of three independent experiments are shown. ** P < 0.01 compared with 5TGM1 cells alone.
We also examined whether the T cells could regulate the secretion of Id protein and cytokine VEGF by the myeloma cells. As shown in Fig. 5B, 5TGM1 myeloma cells co-cultured with irradiated CTL or Th1 cells secreted significantly lower levels of VEGF (P < 0.01 and P < 0.05, compared with 5TGM1 alone), whereas co-culture with irradiated Th2 cells slightly but significantly upregulated the secretion of VEGF by the tumor cells (P < 0.05, compared with 5TGM1 alone). The same results were also observed with the secretion of IgG2b Id protein by the myeloma cells (P < 0.01 and P < 0.05, compared with 5TGM1 alone; Fig. 5C). No changes were observed in cell secretion of these factors when co-cultured with irradiated CD4+ or CD8+ T cells from naïve mice. T cells alone did not produce VEGF or Id protein (data not shown). These results were confirmed with other T-cell clones of CTL, Th1 and Th2 cells (data not shown). These results indicate that Id-specific CTL and Th1 cells could suppress the function of myeloma cells whereas Id-specific Th2 cells promote the proliferation and enhance the function of myeloma cells in vitro.
In vivo effect of the T-cell clones on myeloma cell growth
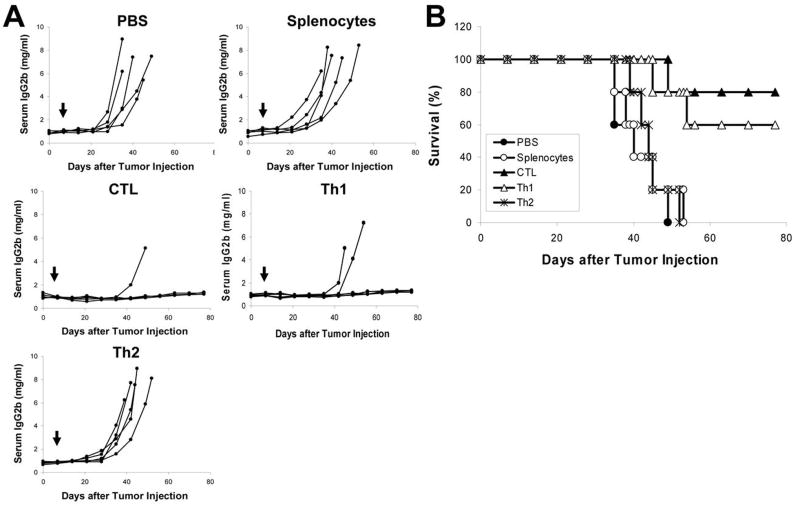
To evaluate the in vivo effects of the T-cell clones on myeloma growth and survival, adoptive transfer experiments were performed. Mice were first injected with 5TGM1 cells to establish myeloma, and one week later, a small number of the T cells, 2 × 106 cells per mouse, were injected intravenously into myeloma-bearing mice. No additional T cells were given and mice were followed for disease progression until death (euthanasia). As shown in Fig. 6A, myeloma-bearing mice receiving an injection of PBS or splenocytes from naïve mice all developed myeloma, while 4 out of 5 mice receiving Id-specific CTLs (P < 0.01, compared with mice receiving PBS or splenocytes) and 3 out of 5 mice receiving Th1 cells (P < 0.05, compared with mice receiving PBS or splenocytes) displayed no increase in serum IgG2b Id protein and showed no sign of myeloma. In contrast, all mice receiving the Th2 cells developed myeloma (P < 0.01, compared with mice receiving CTLs or Th1 cells). Mouse survival data are shown in Fig. 6B; mice receiving PBS, splenocytes of naïve mice, or the Th2 cells all died within 52 days after tumor injection, whereas 80% and 60% of mice receiving CTLs and Th1 cells, respectively, survived without detectable tumors (P < 0.01, compared with mice receiving PBS or splenocytes; Kaplan-Meier test). The difference between mice receiving CTLs and Th1 cells was not statistically significant. These results demonstrate that Id-specific CTLs and Th1 cells but not Th2 cells could efficiently eradicate established myeloma in vivo.
Figure 6. In vivo therapeutic effect of the T-cell clones.
C57BL/KaLwRij mice (5 per group) were challenged intravenously with 2 × 106 5TGM1 myeloma cells, and one week later, 2 × 106 T-cell clones were adoptively transferred into tumor-bearing mice by an intravenous injection. PBS and splenocytes from naïve mice served as controls. Serum samples were collected weekly and tumor burden was monitored by measuring circulating IgG2b Id protein. A, concentrations of serum IgG2b Id protein in mice receiving PBS or different T cells as indicated. B, survival data of mice receiving PBS or different T cells. Arrows indicate injection of T cells. Representative results of one out of two performed are shown.
DISSCUSSION
The 5TGM1 murine myeloma model (22) was used in this study to generate Id-specific T-cell subsets and examine their role in myeloma cells in vitro and in vivo, because this model manifests similarly to human myeloma, including monoclonal gammopathy, marrow replacement, osteolytic bone lesions, and hypercalcemia (23). Mature DCs pulsed with Id proteins were used as immunogen to stimulate induction of Id-specific T-cell responses in mice before spleens were taken to propagate the T cells in vitro. Vaccination with Id-pulsed DCs has been used for immunotherapy in MM (24–26). Recent studies have shown that Id-based DC vaccines were able to protect mice from developing myeloma and were therapeutic against established myeloma in the murine model by inducing Id-specific CTL, Th1 and Th2 cell responses. By using in vivo immunization with Id-pulsed DCs, we successfully generated Id-specific T-cell subsets from the mice. Based on the surface CD4 and CD8 expression and pattern of cytokine secretion, we obtained several clones of three T-cell subsets. We chose three clones corresponding to CTL, Th1 and Th2 cells for functional analyses. These T cells expressed activation marker CD69 and responded by proliferation and secretion of cytokines specifically to Id protein but not to mouse IgG2b or KLH, indicating that indeed these T cells were Id-specific. We anticipate that it may be more difficult to generate functional idiotype-specific CTL and Th1 cells from tumor-bearing mice, since the frequencies of these T cells in the mice may be lower due to the presence of tumor cells and regulatory T cells (4).
We examined in vitro cytolytic activity of the T-cell subsets against target cells including 5TGM1 myeloma cells and Id-pulsed DCs. As expected, Id-specific CTLs effectively lysed these cells but not unpulsed DCs and irrelevant tumor cells, such as B16 melanoma cells. CD8+ T cells from naïve mice could not kill 5TGM1 myeloma cells. In addition, mAbs against MHC class I or FasL, or CMA significantly inhibited CTL-mediated cytotoxic activity against 5TGM1 myeloma cells. Studies have shown that CTLs usually kill their target cells via the mechanisms of the pore-forming perforin and FasL-Fas interaction (27, 28). In this study, our findings confirm that cytotoxic activity of our CTLs was restricted by MHC class I molecules and mediated by the perforin pathway and FasL-Fas interaction. We also examined the ability of the CTLs in inhibiting the growth of myeloma cells and demonstrated that the CTLs significantly suppressed tumor cell proliferation and secretion of Id protein and VEGF. Furthermore, adoptive transfer experiments showed that 80% of mice receiving a small number (2 × 106 per mouse) of CTLs survived of myeloma without increase in serum IgG2b Id protein. Thus, our findings provide strong evidence to support that Id-specific CTLs are potent effectors cells for immunotherapy of MM.
In this study, we also show that Id-specific Th1 cells had strong cytolytic and suppressive activities against myeloma cells in vitro. Furthermore, 60% of mice receiving Th1 cells survived of myeloma without detectable tumor burden after adoptive transfer in our murine myeloma model. CTLs have been considered to be the most important T cells in immunotherapy for tumor due to the fact that the cells can directly kill tumor via MHC class I loaded with tumor-derived peptides. However, the tumor killing effects of Th1 cells are less well defined. A number of studies have reported that CD4+ T cells could directly recognize tumor cells expressing MHC class II molecules through antigen presentation, and kill the tumor cells (29–31). However, myeloma cells express almost no MHC class II molecules (32, 33), which is consistent with our findings with 5TGM1 cells. Hence, our findings suggest that Id-epitope presentation via MHC class II molecules to Id-specific CD4+ T cells was not an important mechanism for Th1-mediated cytotoxicity against MHC class II-negative myeloma cells. Indeed, Id-specific Th1 cell recognition and killing of MHC class II-negative B-cell tumors has been reported previously (34, 35). It has been shown that CD4+ Th1 cells can directly induce tumor apoptosis by FasL-Fas interaction, and also indirectly inhibit tumor growth by destroying tumor angiogenesis through IFN-γ (36, 37). In this study, we show that 5TGM1 myeloma cells expressed Fas molecules, and mAbs against FasL significantly inhibited Th1-mediated cytotoxic activity. Thus, Id-specific Th1 cells are able to recognize and kill the myeloma cells via FasL-Fas interaction. These results indicate that Id-specific Th1 cells are also potent effector cells for immunotherapy in MM.
Th2 cells are known to promote the recruitment of tumoricidal eosinophils and macrophages into the tumor microenvironment and promote anti-tumor immune response (38). However, it is also recognized that Th2 cell-derived cytokines, such as IL-4 and IL-10, inhibit cell-mediated immunity (39), and elimination of CD4+ T cells, especially Th2 cells, enhances anti-tumor effect in mouse melanoma (40). Th2 responses may subvert Th1 cell-mediated immunity, and provide a microenvironment to promote disease progression in patients with renal cell carcinoma or melanoma (41). Nevertheless, the direct effects of tumor-specific Th2 cells on tumor cells such as myeloma cells are not well studied. We show that Id-specific Th2 cells did not have cytolytic activity against the target cells including 5TGM1 myeloma cells. Furthermore, Id-specific Th2 cells promoted the proliferation of and secretion of Id and VEGF by myeloma cells in vitro, and, after adoptive transfer to tumor-bearing mice, displayed no tumor protection in vivo. These results are in a disagreement with an early study showing that murine Id-specific Th2 were suppressive to a B-cell tumor (42). Considering the facts that MM is a B-cell malignancy and T cell:B cell interaction is crucial for regulating B cell function, our findings suggest that Id-specific Th2 cells may positively regulate myeloma cell growth and survival in vivo. Therefore, it will be unbeneficial to induce a Th2 response in patients following Id-based immunotherapy.
In conclusion, our study demonstrates that Id-specific CTLs recognized Id peptides naturally presented by myeloma cells in the context of surface MHC class I molecules, and Id-specific CTL and Th1 but not Th2 cells specifically and effectively lysed myeloma cells and Id-pulsed DCs. In addition, CTL and Th1 cells displayed significantly suppressive activity in the growth and function of myeloma cells, while Th2 cells enhanced the proliferation and cytokine secretion of myeloma cells. To further examine the functional roles of the T-cell subsets on myeloma cells in vivo where tumor cells are protected by the bone marrow microenvironment, adoptive transfer experiments were performed. As shown by the results, a small number of Id-specific CTL and Th1 cells was able to eradicate established myeloma and cured most of myeloma-bearing mice, whereas all mice receiving Th2 cells died of myeloma. Thus, our study indicates that Id-specific CTL and Th1 responses are beneficial and will lead to tumor eradication after immunotherapy in MM. In contrast, a Th2 response provides no protection and may even promote tumor progression in vivo. Although our results were obtained from 5TGM1 myeloma cells in a mouse model, we anticipate that similar functional roles of idiotype-specific T-cell subsets may be observed in other B-cell tumors as well.
Acknowledgments
Grant support: Institutional start up funds from The University of Texas M.D. Anderson Cancer Center, National Cancer Institute (R01 CA96569 and R01 CA103978), Multiple Myeloma Research Foundation, and Commonwealth Foundation for Cancer Research
Footnotes
Conflict-of-interest disclosure: None
References
- 1.Barlogie B, Shaughnessy J, Tricot G, et al. Treatment of multiple myeloma. Blood. 2004;103:20–32. doi: 10.1182/blood-2003-04-1045. [DOI] [PubMed] [Google Scholar]
- 2.Kyle RA, Rajkumar SV. Multiple myeloma. N Engl J Med. 2004;351:1860–73. doi: 10.1056/NEJMra041875. [DOI] [PubMed] [Google Scholar]
- 3.Bogen B, Ruffini PA, Corthay A, et al. Idiotype-specific immunotherapy in multiple myeloma: suggestions for future directions of research. Haematologica. 2006;91:941–8. [PubMed] [Google Scholar]
- 4.Wang S, Hong S, Wezeman M, Qian J, Yang J, Yi Q. Dendritic cell vaccine but not idiotype-KLH protein vaccine primes therapeutic tumor-specific immunity against multiple myeloma. Front Biosci. 2007;12:3566–75. doi: 10.2741/2335. [DOI] [PubMed] [Google Scholar]
- 5.Osterborg A, Yi Q, Bergenbrant S, Holm G, Lefvert AK, Mellstedt H. Idiotype-specific T cells in multiple myeloma stage I: an evaluation by four different functional tests. Br J Haematol. 1995;89:110–6. doi: 10.1111/j.1365-2141.1995.tb08902.x. [DOI] [PubMed] [Google Scholar]
- 6.Yi Q, Bergenbrant S, Osterborg A, et al. T-cell stimulation induced by idiotypes on monoclonal immunoglobulins in patients with monoclonal gammopathies. Scand J Immunol. 1993;38:529–34. doi: 10.1111/j.1365-3083.1993.tb03236.x. [DOI] [PubMed] [Google Scholar]
- 7.Yi Q, Osterborg A, Bergenbrant S, Mellstedt H, Holm G, Lefvert AK. Idiotype-reactive T-cell subsets and tumor load in monoclonal gammopathies. Blood. 1995;86:3043–9. [PubMed] [Google Scholar]
- 8.Yi Q, Eriksson I, He W, Holm G, Mellstedt H, Osterborg A. Idiotype-specific T lymphocytes in monoclonal gammopathies: evidence for the presence of CD4+ and CD8+ subsets. Br J Haematol. 1997;96:338–45. doi: 10.1046/j.1365-2141.1997.d01-2021.x. [DOI] [PubMed] [Google Scholar]
- 9.Yi Q. Dendritic cell-based immunotherapy in multiple myeloma. Leuk Lymphoma. 2003;44:2031–8. doi: 10.1080/1042819031000116599. [DOI] [PubMed] [Google Scholar]
- 10.Li Y, Bendandi M, Deng Y, et al. Tumor-specific recognition of human myeloma cells by idiotype-induced CD8(+) T cells. Blood. 2000;96:2828–33. [PubMed] [Google Scholar]
- 11.Wen YJ, Barlogie B, Yi Q. Idiotype-specific cytotoxic T lymphocytes in multiple myeloma: evidence for their capacity to lyse autologous primary tumor cells. Blood. 2001;97:1750–5. doi: 10.1182/blood.v97.6.1750. [DOI] [PubMed] [Google Scholar]
- 12.Asosingh K, Radl J, Van Riet I, Van Camp B, Vanderkerken K. The 5TMM series: a useful in vivo mouse model of human multiple myeloma. Hematol J. 2000;1:351–6. doi: 10.1038/sj.thj.6200052. [DOI] [PubMed] [Google Scholar]
- 13.Garrett IR, Dallas S, Radl J, Mundy GR. A murine model of human myeloma bone disease. Bone. 1997;20:515–20. doi: 10.1016/s8756-3282(97)00056-2. [DOI] [PubMed] [Google Scholar]
- 14.Mundy G. Preclinical models of bone metastases. Semin Oncol. 2001;28:2–8. doi: 10.1016/s0093-7754(01)90225-8. [DOI] [PubMed] [Google Scholar]
- 15.Liso A, Stockerl-Goldstein KE, Auffermann-Gretzinger S, et al. Idiotype vaccination using dendritic cells after autologous peripheral blood progenitor cell transplantation for multiple myeloma. Biol Blood Marrow Transplant. 2000;6:621–7. doi: 10.1016/s1083-8791(00)70027-9. [DOI] [PubMed] [Google Scholar]
- 16.Kwak LW, Young HA, Pennington RW, Weeks SD. Vaccination with syngeneic, lymphoma-derived immunoglobulin idiotype combined with granulocyte/macrophage colony-stimulating factor primes mice for a protective T-cell response. Proc Natl Acad Sci U S A. 1996;93:10972–7. doi: 10.1073/pnas.93.20.10972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lutz MB, Kukutsch N, Ogilvie AL, et al. An advanced culture method for generating large quantities of highly pure dendritic cells from mouse bone marrow. J Immunol Methods. 1999;223:77–92. doi: 10.1016/s0022-1759(98)00204-x. [DOI] [PubMed] [Google Scholar]
- 18.Qian J, Wang S, Yang J, et al. Targeting heat shock proteins for immunotherapy in multiple myeloma: generation of myeloma-specific CTLs using dendritic cells pulsed with tumor-derived gp96. Clin Cancer Res. 2005;11:8808–15. doi: 10.1158/1078-0432.CCR-05-1553. [DOI] [PubMed] [Google Scholar]
- 19.Wen YJ, Min R, Tricot G, Barlogie B, Yi Q. Tumor lysate-specific cytotoxic T lymphocytes in multiple myeloma: promising effector cells for immunotherapy. Blood. 2002;99:3280–5. doi: 10.1182/blood.v99.9.3280. [DOI] [PubMed] [Google Scholar]
- 20.Kataoka T, Shinohara N, Takayama H, et al. Concanamycin A, a powerful tool for characterization and estimation of contribution of perforin- and Fas-based lytic pathways in cell-mediated cytotoxicity. J Immunol. 1996;156:3678–86. [PubMed] [Google Scholar]
- 21.Alici E, Konstantinidis KV, Aints A, Dilber MS, Abedi-Valugerdi M. Visualization of 5T33 myeloma cells in the C57BL/KaLwRij mouse: establishment of a new syngeneic murine model of multiple myeloma. Exp Hematol. 2004;32:1064–72. doi: 10.1016/j.exphem.2004.07.019. [DOI] [PubMed] [Google Scholar]
- 22.Dallas SL, Garrett IR, Oyajobi BO, et al. Ibandronate reduces osteolytic lesions but not tumor burden in a murine model of myeloma bone disease. Blood. 1999;93:1697–706. [PubMed] [Google Scholar]
- 23.Radl J, De Glopper ED, Schuit HR, Zurcher C. Idiopathic paraproteinemia. II Transplantation of the paraprotein-producing clone from old to young C57BL/KaLwRij mice. J Immunol. 1979;122:609–13. [PubMed] [Google Scholar]
- 24.Cull G, Durrant L, Stainer C, Haynes A, Russell N. Generation of anti-idiotype immune responses following vaccination with idiotype-protein pulsed dendritic cells in myeloma. Br J Haematol. 1999;107:648–55. doi: 10.1046/j.1365-2141.1999.01735.x. [DOI] [PubMed] [Google Scholar]
- 25.Reichardt VL, Okada CY, Liso A, et al. Idiotype vaccination using dendritic cells after autologous peripheral blood stem cell transplantation for multiple myeloma--a feasibility study. Blood. 1999;93:2411–9. [PubMed] [Google Scholar]
- 26.Yi Q, Desikan R, Barlogie B, Munshi N. Optimizing dendritic cell-based immunotherapy in multiple myeloma. Br J Haematol. 2002;117:297–305. doi: 10.1046/j.1365-2141.2002.03411.x. [DOI] [PubMed] [Google Scholar]
- 27.Kagi D, Vignaux F, Ledermann B, et al. Fas and perforin pathways as major mechanisms of T cell-mediated cytotoxicity. Science. 1994;265:528–30. doi: 10.1126/science.7518614. [DOI] [PubMed] [Google Scholar]
- 28.Kojima H, Shinohara N, Hanaoka S, et al. Two distinct pathways of specific killing revealed by perforin mutant cytotoxic T lymphocytes. Immunity. 1994;1:357–64. doi: 10.1016/1074-7613(94)90066-3. [DOI] [PubMed] [Google Scholar]
- 29.Lauritzsen GF, Weiss S, Dembic Z, Bogen B. Naive idiotype-specific CD4+ T cells and immunosurveillance of B-cell tumors. Proc Natl Acad Sci U S A. 1994;91:5700–4. doi: 10.1073/pnas.91.12.5700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lundin KU, Hofgaard PO, Omholt H, Munthe LA, Corthay A, Bogen B. Therapeutic effect of idiotype-specific CD4+ T cells against B-cell lymphoma in the absence of anti-idiotypic antibodies. Blood. 2003;102:605–12. doi: 10.1182/blood-2002-11-3381. [DOI] [PubMed] [Google Scholar]
- 31.Topalian SL, Rivoltini L, Mancini M, Ng J, Hartzman RJ, Rosenberg SA. Melanoma-specific CD4+ T lymphocytes recognize human melanoma antigens processed and presented by Epstein-Barr virus-transformed B cells. Int J Cancer. 1994;58:69–79. doi: 10.1002/ijc.2910580113. [DOI] [PubMed] [Google Scholar]
- 32.Corthay A, Lundin KU, Munthe LA, et al. Immunotherapy in multiple myeloma: Id-specific strategies suggested by studies in animal models. Cancer Immunol Immunother. 2004;53:759–69. doi: 10.1007/s00262-004-0504-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yi Q, Dabadghao S, Osterborg A, Bergenbrant S, Holm G. Myeloma bone marrow plasma cells: evidence for their capacity as antigen-presenting cells. Blood. 1997;90:1960–7. [PubMed] [Google Scholar]
- 34.Lauritzsen GF, Bogen B. The role of idiotype-specific, CD4+ T cells in tumor resistance against major histocompatibility complex class II molecule negative plasmacytoma cells. Cell Immunol. 1993;148:177–88. doi: 10.1006/cimm.1993.1100. [DOI] [PubMed] [Google Scholar]
- 35.Stevenson FK, Anderson KC. Preparing the ground for vaccination against multiple myeloma. Immunol today. 2000;21:170–1. doi: 10.1016/s0167-5699(99)01579-0. [DOI] [PubMed] [Google Scholar]
- 36.Lundin KU, Screpanti V, Omholt H, et al. CD4+ T cells kill Id+ B-lymphoma cells: FasLigand-Fas interaction is dominant in vitro but is redundant in vivo. Cancer Immunol Immunother. 2004;53:1135–45. doi: 10.1007/s00262-004-0538-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Qin Z, Blankenstein T. CD4+ T cell--mediated tumor rejection involves inhibition of angiogenesis that is dependent on IFN gamma receptor expression by nonhematopoietic cells. Immunity. 2000;12:677–86. doi: 10.1016/s1074-7613(00)80218-6. [DOI] [PubMed] [Google Scholar]
- 38.Hung K, Hayashi R, Lafond-Walker A, Lowenstein C, Pardoll D, Levitsky H. The central role of CD4(+) T cells in the antitumor immune response. J Exp Med. 1998;188:2357–68. doi: 10.1084/jem.188.12.2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Powrie F, Menon S, Coffman RL. Interleukin-4 and interleukin-10 synergize to inhibit cell-mediated immunity in vivo. Eur J Immunol. 1993;23:3043–9. doi: 10.1002/eji.1830231147. [DOI] [PubMed] [Google Scholar]
- 40.Nagai H, Hara I, Horikawa T, Oka M, Kamidono S, Ichihashi M. Elimination of CD4(+) T cells enhances anti-tumor effect of locally secreted interleukin-12 on B16 mouse melanoma and induces vitiligo-like coat color alteration. J Invest Dermatol. 2000;115:1059–64. doi: 10.1046/j.1523-1747.2000.00156.x. [DOI] [PubMed] [Google Scholar]
- 41.Tatsumi T, Kierstead LS, Ranieri E, et al. Disease-associated bias in T helper type 1 (Th1)/Th2 CD4(+) T cell responses against MAGE-6 in HLA-DRB10401(+) patients with renal cell carcinoma or melanoma. J Exp Med. 2002;196:619–28. doi: 10.1084/jem.20012142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lauritzsen GF, Weiss S, Bogen B. Anti-tumour activity of idiotype-specific, MHC-restricted Th1 and Th2 clones in vitro and in vivo. Scand J Immunol. 1993;37:77–85. doi: 10.1111/j.1365-3083.1993.tb01668.x. [DOI] [PubMed] [Google Scholar]