Summary
Background
Laboratory testing for heparin-induced thrombocytopenia (HIT) includes the highly sensitive, though less specific, heparin/platelet factor 4 (PF4) ELISA. A confirmatory test with excess heparin is routinely performed on positive ELISA results to improve test specificity; the significance of a negative confirmatory result is unknown.
Objectives
1. Evaluate the clinical utility of the PF4 ELISA confirmatory assay. 2. Examine the relationship between ELISA OD value and clinical diagnosis of HIT. 3. Assess current practice at a tertiary care medical center regarding patients with anti-heparin/PF4 antibodies.
Patients/Methods
Patients with anti-heparin/PF4 antibodies detected by commercial ELISA during 2005 were identified. A confirmatory test was performed on positive ELISA results. Patients were labeled confirmatory positive (confirm+) or confirmatory negative (confirm-). Patients were classified as HIT+ (met criteria for HIT), HIT? (HIT possible), and HIT- (did not meet criteria for HIT) utilizing ACCP guidelines.
Results
115 patients with anti-heparin/PF4 antibodies were identified. 98 patients were confirm+; 17 were confirm-. The majority of confirm+ patients were HIT+ or HIT?(72%); the majority of confirm- patients were HIT-(81%). Patients who were HIT+/confirm+ had higher ELISA OD values than patients who were HIT?/confirm+ or HIT-/confirm+ (p=0.031, p=0.001). Two confirm- patients were HIT+, one was HIT?; all had high ELISA OD values.
Conclusions
Although confirm+ status correlated with clinical HIT, the confirmatory procedure misclassified some patients by yielding a confirm- result despite clinical HIT with high ELISA OD values. Future studies should compare higher ELISA OD values with the confirmatory procedure as strategies to improve ELISA diagnostic specificity for HIT.
Introduction
Heparin-induced thrombocytopenia (HIT) is a clinicopathologic syndrome of immune-mediated thrombocytopenia associated with an increased thrombotic risk in patients exposed to heparin [1]. Diagnosis requires that patients fulfill certain clinical criteria as well as demonstrate the presence of platelet activating antibodies induced by heparin interaction with platelet factor 4 (PF4). Clinical criteria for HIT include thrombocytopenia that develops typically after 5-10 days of heparin exposure, in the absence of other, predominant causes of thrombocytopenia, with or without thrombosis [2, 3]. Thrombotic complications have been reported to develop in up to 20 to 50% of patients with HIT, and can be life-threatening events [4], necessitating swift and accurate diagnosis of this disorder.
Laboratory testing for antibodies to heparin/PF4 complexes includes the commercially available enzyme-linked immunoabsorbent assay (ELISA) which detects IgG, IgA, and IgM antibodies. At Duke University Medical Center, over 1,000 heparin/PF4 ELISA tests are performed annually. This test is very sensitive to the presence of anti-heparin/PF4 antibodies (greater than 97%) [5], but it is less specific for the clinical syndrome of HIT (74% in post-operative orthopedic patients), and is limited by the fact that it can detect non-pathologic antibodies [6, 7]. This is particularly a problem in patients undergoing cardiac bypass surgery, a patient population in which antibodies to heparin/PF4 appear to frequently develop in the absence of clinical manifestations of HIT, resulting in a much lower specificity of the ELISA for the syndrome [8]. A strategy recommended by the manufacturer to improve specificity of the heparin/PF4 ELISA is the confirmatory procedure, whereby inhibition of a positive ELISA result by 50% or more in the presence of excess heparin is considered confirmatory of heparin-dependent antibodies. The significance of a negative confirmatory result is unknown, however, and there are data that suggests in the post-cardiac bypass surgery setting, the confirmatory result does not improve the diagnostic specificity of the heparin/PF4 ELISA [9].
Our primary objective in performing this study was to evaluate whether the heparin/PF4 ELISA confirmatory test is of clinical utility in determining which patients with anti-heparin/PF4 antibodies have HIT. We also sought to determine if higher anti-heparin/PF4 antibody optical density (OD) values correlate with a clinical diagnosis of HIT, as previous single-institution studies have found an association between higher OD values and diagnosis of HIT [7, 10]. Lastly, we sought to assess current practice at a tertiary care medical center related to patients with heparin/PF4 antibodies, investigating diagnostic criteria for HIT, therapeutic interventions, and clinical outcomes in these patients.
Patients and methods
This retrospective study was approved by the Institutional Review Board at Duke University Medical Center. A coagulation laboratory database was utilized to identify patients who tested positive for anti-heparin/PF4 antibodies by commercial ELISA (GTI Inc., Brookfield, WI, USA) during a single year, using a threshold OD measurement of 0.40. A confirmatory step was performed on all positive ELISA results per manufacturer guidelines, with a positive confirmatory result defined as >50% decrease in absorbance in the presence of added heparin. Testing for anti-heparin/PF4 antibodies was performed at the discretion of each patient's treating physician. The PF4 ELISA and confirmatory test were performed simultaneously to avoid delays in getting positive results back to the clinicians managing the patients. The confirmatory test was only reported if the PF4 ELISA test was positive. For patients who had more than one PF4 ELISA test performed, the confirmatory test result of the initial positive PF4 ELISA (OD ≥ 0.4) designated the patient as confirm+ or confirm-, and the maximal OD of the positive test results was utilized for data analysis.
All Duke University Medical Center records were reviewed from the patient's hospitalization and for up to a 30-day period following an initial positive assay including review of outpatient notes when available, but patients were not contacted. Data collected for each patient included: age, race, medical service, dates of platelet count decline, platelet count nadir, platelet count increase and normalization, dates of anti-heparin/PF4 antibody testing, anti-heparin/PF4 antibody values, dates and types of heparin administration, dates and types of thromboembolic events, whether or not the patient was evaluated by the hematology consult service, whether or not a diagnosis of HIT was stated in the medical chart, whether or not the patient received a direct thrombin inhibitor (DTI) or fondaparinux, the type of DTI utilized, major bleeding events (considered major if required transfusion of one or more units of packed red blood cells), patient death and cause of death, and anticoagulation upon hospital discharge.
All venous thrombotic events documented in patient records were confirmed by review of radiographic reports. Arterial thrombotic events, including stroke and MI, were documented radiographically (MRI, CT or cardiac catheterization reports) but also included intra-operative assessment of bowel infarction, autopsy findings, and several cases of digital or limb ischemia based upon physical examination performed by vascular surgeons.
Data Analysis
Patients with positive anti-heparin/PF4 antibodies were sub-divided into confirmatory positive (confirm+) and confirmatory negative (confirm-) groups for analysis. Extensive chart review was undertaken to classify patients clinically as: (1) positive for HIT (HIT+), (2) possible HIT (HIT?), and (3) negative for HIT (HIT-). To be HIT+, patients had to meet the following clinical criteria, consistent with American College of Chest Physicians (ACCP) guidelines for diagnosis of HIT: (1) thrombocytopenia defined as at least a 30-50% decline in the platelet count, with platelet count increase upon heparin cessation, (2) timing of platelet count fall between 4 and 14 days after heparin exposure or within 24 to 48 hours if recent (within last 100 days) heparin exposure, and (3) lack of other, predominant causes of thrombocytopenia. For patients after cardiac bypass surgery, a platelet count drop of 50% or more from the highest post-operative value occurring between postoperative days 4-14 or persisting for six or more days after surgery was considered consistent with a diagnosis of HIT [2, 11, 12]. Patients who met all three criteria but who had no clinical evidence for thromboembolism were referred to as isolated HIT; those who sustained a thromboembolic complication were referred to as having HIT with thrombosis (HITT). A diagnosis of HIT? occurred when patients developed thrombocytopenia in the setting of heparin exposure with the correct timing, with or without thrombosis, but had other, possible alternative diagnoses to explain a decrease in platelet count. The chart reviews were systematically carried out using the diagnostic criteria above by the first and second authors. The senior author independently reviewed all clinical assessments (HIT+, HIT?, HIT-), and confirmed the assignment of patients into these categories. Since all of the patients had positive heparin/PF4 ELISA results, the authors were not blinded to assay results.
Statistical analysis
Fisher's exact test was used to evaluate categorical data for statistical significance. The Mann-Whitney test was used to evaluate differences in the anti-heparin/PF4 antibody levels between the confirm+/HIT+ patients and the other confirm+ groups (HIT? and HIT-) and the confirm- patients as a group (since this was a smaller group). The Mann-Whitney test was used to evaluate differences in the platelet count nadirs between the same groups of patients, and to evaluate differences in the continuous variables between the confirm+ and confirm- patients. A p-value of <0.05 was considered statistically significant.
Results
Patients with elevated anti-heparin/PF4 antibody levels
Retrospective analysis from 2005 revealed that a total of 1,194 heparin/PF4 ELISA tests were performed, and of these, 244 were positive (20%), representing 115 unique patients with positive test results (many patients had the test ordered more than once). Of these 115 patients, 98 were confirm+ and 17 were confirm-. Only two patients with more than one test performed had discordant confirmatory assay results, and both of these were included in the confirm+ group. There was a wide distribution of patients across medical and surgical services, with about half of the patients in both confirm+ and confirm- groups being on medical services and the other half being on surgical services (Table 1). Among medical patients, 17 confirm+ patients (17%) and 4 confirm- patients (24%) were on cardiology services. Among surgical patients, 35 confirm+ patients (36%) and 4 confirm- patients (24%) were from the cardiothoracic surgery service. Only one patient with anti-heparin/PF4 antibodies was from the orthopedic surgery service, in the confirm- group. Age and gender were similar between the two groups, but there was some variation in race, with more Caucasians in the confirm+ and more African Americans in the confirm- group (Table 1). By laboratory criteria, the groups were similar, with comparable platelet count nadirs and anti-heparin/PF4 titers.
Table 1. Confirmatory Positive compared to Confirmatory Negative Patients.
Comparison of demographics and laboratory values.
| Outcome Variable | Confirm+
N= 98 |
Confirm-
N=17 |
P-value |
|---|---|---|---|
| Age* | 65 ± 15 | 63 ± 17 | 0.148 |
| Female | 55 (56%) | 9 (53%) | 1.000 |
| African American | 32 (32%) | 9 (53%) | 0.168 |
| Caucasian | 67 (68%) | 8 (47%) | 0.166 |
| Platelet Count Nadir* | 55,500 ± 44,000/μL | 46,000 ± 31,000/μL | 0.489 |
| % Decrease Platelet Count from Baseline* | 70% ± 23% | 69% ± 20% | 0.543 |
| Anti-Heparin/PF4 Titer* | 0.77 ± 0.84 | 0.82 ± 0.78 | 0.552 |
| Cardiothoracic surgery service | 35 (35%) | 4 (24%) | 0.413 |
| Medicine service | 30 (30%) | 6 (34%) | 0.779 |
median.
Patients with HIT
Although all patients in this study had positive anti-heparin/PF4 antibody titers, not all of these patients met clinical criteria for HIT. In the confirm+ group, 56 of 98 patients (57%) clearly met clinical criteria for HIT (HIT+; Table 2). Sixteen of the HIT+ patients had other potential causes for thrombocytopenia, but these were not felt to be the primary cause for platelet count decline. Specifically, the majority of these patients had been exposed to antibiotics associated with a low incidence (<1%) of thrombocytopenia. Further, the nadir platelet count of patients exposed to antibiotics was >30,000 μL in 80% of these patients, supporting the view that these patients did not truly have antibiotic-associated thrombocytopenia, which is typically associated with a nadir platelet count of less than 30,000 μL [13]. There were 15 HIT? patients, all of whom had other likely causes for thrombocytopenia. These included: history of pancytopenia, history of thrombocytopenia, antiphospholipid antibody syndrome, sepsis, disseminated intravascular coagulation (DIC), intraaortic balloon pump, left ventricular assist device, shock liver, idiopathic thrombocytopenia, and linezolid administration (associated with up to a 10% incidence of thrombocytopenia). Of the cardiothoracic surgery patients who had HIT, the majority had a drop in the platelet count of 50% or more between postoperative days 4-14 (13 of 19 patients or 68%), with 4 patients experiencing a persistence of thrombocytopenia beyond postoperative day 6, without another cause of thrombocytopenia identified. The remaining 2 patients met criteria for HIT pre-operatively. A total of 27 patients in the confirm+ group did not meet clinical criteria for HIT (28% HIT-).
Table 2. Demographics for Confirmatory Positive Patients.
Patients are distributed according to clinical criteria for HIT.
| Demographics | HIT +
(n=56) |
HIT ?
(n=15) |
HIT −
(n=27) |
|---|---|---|---|
| Age* | 66 | 66 | 63 |
| Female | 32 (57%) | 9 (64%) | 13 (48%) |
| African American | 19 (34%) | 6 (43%) | 6 (22%) |
| Caucasian | 38 (68%) | 8 (57%) | 21 (78%) |
| Cardiothoracic surgery service | 19 (34%) | 5 (36%) | 11 (41%) |
| Medicine service | 14 (25%) | 5 (36%) | 10 (37%) |
median.
Of the 17 confirm- patients, two patients met clinical criteria for HIT (HIT+) and one patient met criteria for possible HIT (HIT?). These three patients had relatively high anti-heparin/PF4 antibody OD values (Absorbance at 405 nm: 1.1, 2.2, and 3.3). The two patients with the highest OD values were on the cardiothoracic surgery service. The majority of patients in the confirm-group (14 of 17 or 82% HIT-) did not meet clinical criteria for HIT.
Antibody OD value and clinical diagnosis of HIT
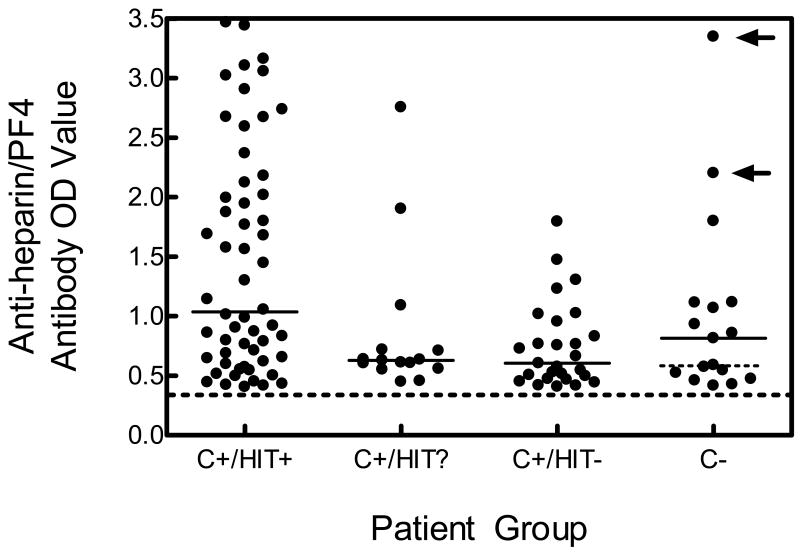
To examine the relationship between anti-heparin/PF4 antibody OD value and the clinical diagnosis of HIT, we compared antibody OD values among the different patient sub-groups (Fig.1). Among confirm+ patients, we found that higher anti-heparin/PF4 antibody OD values were present in patients who clearly met clinical criteria for HIT (HIT+; median OD 1.04) versus those who were HIT? (median OD 0.63; p= 0.031) and those who were HIT- (median OD 0.60; p=0.001). Comparing antibody OD values between patients who were confirm+/HIT+ and all confirm- patients, we did not find a statistically significant difference in the anti-heparin/PF4 antibody OD value (median OD value 0.82, p=0.104). However, when we removed the anti-heparin/PF4 antibody OD value of the two patients in the confirm- group who met clinical criteria for HIT, we found a statistically significant difference between the anti-heparin/PF4 antibody OD values in the confirm+/HIT+ group and the remaining patients in the confirm-group (median OD 0.59; p=0.019). There was no significant difference between platelet count nadirs and the patient groups (data not shown).
Figure 1. Distribution of Anti-heparin/PF4 Antibody OD Values for the Different Patient Groups.
The four groups includes patients who were confirm positive (C+) and (1) fulfilled clinical criteria for HIT (C+/HIT+); (2) possibly had HIT (C+/HIT?); and (3) who did not meet criteria for HIT (C+/HIT-); and those patients who were confirm negative (C-). The cut-off for an elevated anti-heparin/PF4 antibody level is shown by the dashed line (absorbance of 0.40 or greater), and the median values for each group are shown by the solid horizontal lines. In the confirm negative (C-) group, the two arrows identify the two patients in this group who clinically fulfilled criteria for HIT, and the dotted horizontal line represents the median of the confirm negative group excluding the two patients who did meet clinical criteria for HIT.
Clinical outcomes
Among the confirm+ patients, the incidence of thromboembolism (at any time point) was 46% in patients who were HIT+ (26 of 56); 40% in patients who were HIT? (6 of 15; p=0.774 compared to HIT+ patients); and 19% in patients who were HIT− (5 of 27; p=0.016 compared to HIT+, and p=0.158 compared to HIT?). Of the 32 patients with HITT, there were 20 arterial thrombotic events and 14 venous thrombotic events. Two of these patients had both an arterial event and a venous event. Regarding treatment with alternative anticoagulants, 41 HIT+ patients (73%) received a DTI. More than half of these patients had HITT (Table 3). Fifteen HIT+ patients did not receive a DTI for the following reasons: 7 patients were felt to have too high of a hemorrhagic risk, 5 patients had positive anti-heparin/PF4 ELISA results return after discharge or death, and 1 patient was treated with fondaparinux instead of a DTI. The reason for lack of treatment was not clear in two patients. In the HIT? group, only 4 patients received a DTI, all of whom had a thromboembolic event (Table 3). Reasons for lack of treatment with a DTI in the HIT? group included: 1 patient had active bleeding, 2 patients were felt to have too high of a hemorrhagic risk, one positive anti-heparin/PF4 ELISA result returned after patient discharge, and there was significant uncertainty regarding the diagnosis of HIT in 5 patients. The remaining 2 patients had no clear reason for lack of treatment.
Table 3. Clinical Outcomes.
Results are presented according to clinical laboratory testing status (Confirm+ or Confirm-) and according to clinical diagnosis of HIT (HIT+, HIT?, and HIT-). Abbreviations: HIT, heparin-induced thrombocytopenia; HITT, heparin-induced thrombocytopenia with thrombosis; DTI, direct thrombin inhibitor.
| Outcome | Confirm+ (n=98) | Confirm- (n=17) | |||
|---|---|---|---|---|---|
| HIT+
(n=56) |
HIT?
(n=15) |
HIT-
(n=27) |
HIT+/HIT?
(n=3) |
HIT-
(n=14) |
|
| Thromboembolic event (HITT; n=32) | 26 (46%) | 6 (40%) | 0 | 0 | 0 |
| Treated with a DTI (n=50) | 41/56 (73%) | 4/15 (27%) | 1/27 (4%) | 3/3 (100%) | 1/14 (7%) |
| Isolated HIT patients | 18/30 (60%) | 0 | - | 3/3 (100%) | 0 |
| HIT with thrombosis patients | 23/26 (88%) | 4/6 (67%) | - | 0 | 0 |
| Hemorrhagic complication * | 11/41 (27%) | 2/4 (50%) | 0 | 1/3 (33%) | 0 |
| Thromboembolic event * | 3/41 (7%) | 0 | 1/27 (4%) | 2/3 (66%) | 1/14 (7%) |
| Death * | 9/41 (22%) | 2/4 (50%) | 1/27 (4%) | 1 (33%) | 0 |
| Not treated with a DTI (n=65) | 15/56 (27%) | 11/15 (73%) | 26/27 (96%) | 0 | 13/14 (93%) |
| Hemorrhagic complications | 2/15 (13%) | 1/11 (9%) | 8/26 (31%) | - | 3/14 (21%) |
| Thromboembolic complications | 2/15 (13%) | 2/11 (18%) | 4/26 (15%) | - | 7/14 (50%) |
| Death | 3/15 (20%) | 2/11 (18%) | 4/26 (15%) | - | 2/14 (14%) |
Clinical events occurring after diagnosis of HIT initially made. These events may have occurred while the patient was on a DTI, or after the DTI had been discontinued.
Among the HIT+ and HIT? patients who received a DTI, there was a high incidence of hemorrhage (Table 3). Eleven deaths occurred in patients who were treated with a DTI; 5 died while on therapy and 6 died soon after DTI cessation. No patient died as a result of hemorrhage. One patient died of ischemic stroke hours after DTI cessation. Interestingly, only 4 of 26 HIT+ and HIT? patients (15%) who did not receive a DTI developed a new thromboembolic event. However, the rates of new thromboembolism in the untreated groups (HIT+ and HIT?) were higher than in the treated group (13% versus 7% in the HIT+ group; 18% vs. 0% in the HIT? group). The incidence of death was higher in patients who were treated with a DTI (24% versus 19%). Cause of death was sepsis and/or multisystem organ failure in the majority of these cases. In the untreated group, two deaths were related to thrombotic events.
Overall, 12 patients who clearly met clinical criteria for HIT died (21%), versus 4 patients with HIT? (27%), and 5 patients who did not meet clinical criteria for HIT (19%). Hemorrhage (at any time point) occurred in 23% of HIT+, 20% of HIT? and 30% of HIT- patients.
In the confirm- group, the two HIT+ patients received treatment with a DTI. The HIT? patient also received a DTI for 72 hours, but it was discontinued given concerns for hemorrhage. Thromboembolism occurred in both patients who were HIT+ in the setting of being treated with a DTI (Table 3). Among the HIT- patients, one received a DTI transiently, until it was concluded that the patient did not meet clinical criteria for HIT, and heparin was resumed. Of the HIT- patients, 7 (50%) developed thromboembolic events. These events included: DVT in a patient with cancer, acute coronary syndrome upon presentation in one patient, thrombosed digits in a patient with DIC and sepsis, two line-associated thrombi, and DVT in two patients with a history of recurrent thromboembolic events. There were two deaths in the HIT- group. Three HIT- patients (21%) had a hemorrhagic event (unrelated to DTI therapy), and one HIT+ patient had a hemorrhagic event prior to initiation of a DTI. No patient in the confirm- group bled while on DTI therapy.
Discussion
Anti-heparin/PF4 antibodies were detected in a diverse patient population, including medical and surgical patients. Although all were thrombocytopenic to some degree, and up to half developed thromboembolism, more than a third (36%) of these patients did not meet strict clinical criteria for the diagnosis of HIT. Patients undergoing cardiothoracic surgery represented more than one third of the total patient population diagnosed with HIT at our institution during 2005 (21 of 58 patients; 36%). This is particularly interesting as this is a patient population in which the incidence of anti-heparin/PF4 antibodies is known to be high, but the actual incidence of HIT is thought to be low [8, 14, 15]. It may be that there is a higher incidence of HIT in cardiothoracic surgery patients than previously thought. Conversely, there was only one orthopedic patient with positive anti-heparin/PF4 antibodies, and this patient did not meet clinical criteria for HIT. This is also somewhat surprising given traditionally, orthopedic patients have the highest incidence of HIT amongst the surgical patients [8, 16]. However, it is now standard practice among the orthopedic surgeons at our institution to use enoxaparin perioperatively for DVT prophylaxis, which is likely to reduce the incidence of HIT in this patient population [8].
Part of our objective in performing this analysis was to look at diagnostic strategies for HIT in our patient population. We found that patients with confirm+ test results were more likely to meet clinical criteria for HIT than patients who had confirm- results (72% versus 18% respectively; p<0.001). This study is the first to demonstrate that the confirmatory step in the anti-heparin/PF4 antibody ELISA may be of clinical utility in the diagnosis of HIT, although this needs to be prospectively confirmed. In terms of diagnosis of HIT, we also sought to evaluate how the anti-heparin/PF4 antibody OD value relates to a clinical diagnosis of HIT in our patient population. Other single institution studies have shown that higher anti-heparin/PF4 antibody OD values correlate with both diagnosis of HIT and the development of HITT [10, 16]. Similarly, we found that patients who met clinical criteria for HIT had significantly higher antibody OD values.
When we initially compared the confirm+/HIT+ patients to the confirm- group as a whole, we did not see a significant difference in the anti-heparin/PF4 antibody OD values. However, when we removed the two patients who met clinical criteria for HIT in the confirm- group, the difference between anti-heparin/PF4 antibody OD values became statistically significant (Fig. 1). This raises the question of how the anti-heparin/PF4 antibody OD value relates to the confirmatory step. The two patients in the confirm- group who had clinical scenarios consistent with HIT had high OD values (2.2 and 3.3 respectively). It may be that higher OD values have more clinical significance than a negative confirmatory assay result. The other question that these data raise is the reliability of the heparin/PF4 ELISA confirmatory test across different patient populations. Both patients who were confirm-/HIT+ were cardiothoracic surgery patients. To our knowledge, there has only been one prior study that had addressed the impact of the confirmatory procedure upon heparin/PF4 ELISA test specificity, and this was in cardiac surgery patients [9]. Warkentin and Sheppard looked at archived plasma from 98 cardiac surgery patients, and found that 78 (79%) of these patients tested positive for anti-heparin/PF4 antibodies by commercial ELISA. Of these 78 samples, 76 (97%) had positive confirmatory test results. However, only one of these patients met clinical criteria for HIT by ACCP guidelines, which led them to conclude that in the postcardiac surgery setting, the confirmatory procedure does little to improve diagnostic specificity of the heparin/PF4 ELISA. Further, of the two samples that had negative confirmatory test results, they report that one tested strongly positive by serotonin release assay, leading them to conclude that in cardiac surgery patients, failure to exhibit inhibition in the confirmatory procedure may not exclude the presence of platelet-activating antibodies. However, by their description, the patient with platelet activating antibodies and a negative confirmatory test result did not meet clinical criteria for HIT, which questions the significance of this particular finding.
Our clinical coagulation laboratory does not offer a sensitive, highly specific functional assay, such as the serotonin release assay, for the diagnosis of HIT. This assay is available through a reference laboratory, but it is seldom requested by our clinical services. Furthermore, we believe that using the heparin/PF4 ELISA as the primary laboratory component for the diagnosis of HIT is more reflective of current practice patterns at most hospitals in the United States, which is what we wished to assess in this study.
Thromboembolic and hemorrhagic complications occurred frequently in patients being evaluated for HIT, indicating that the decision to use a direct thrombin inhibitor must be individualized, as antithrombotic therapy in these patients may exacerbate the bleeding risk [17]. Some patients with anti-heparin/PF4 antibodies and isolated HIT may not require treatment with a direct thrombin inhibitor, but this observation needs to be confirmed in a prospective analysis. Specifically, we found that of 26 patients who met criteria for HIT (HIT+/HIT?) and did not receive a DTI, 4 experienced a thromboembolic event (85% did not develop thrombosis). However, this observation is limited by the fact that this was a retrospective study, and most of these patients were not followed beyond their hospital discharge. Furthermore, as a functional study for HIT was not included in the diagnostic criteria, some of these patients may not have truly had HIT, which would certainly effect thrombotic rates. Lastly, our findings could be underestimating the true incidence of thrombosis, since patients were not being systematically evaluated for occult thrombotic events.
Future studies aimed at refining diagnostic criteria to avoid misdiagnosis of HIT are critical, including the role of the heparin/PF4 ELISA confirmatory test in the diagnosis of HIT. The heparin/PF4 ELISA has a high negative predictive value, but only a moderate positive predictive value. Therefore, a negative heparin/PF4 ELISA result essentially rules out a diagnosis of HIT, but if positive, the antibodies present may be nonpathogenic [2]. The data presented here suggests that the heparin/PF4 ELISA confirmatory assay may be of clinical utility in determining which antibodies are pathogenic, as patients who met clinical criteria for HIT generally had positive confirmatory results. However, in settings where the heparin/PF4 antibody OD value is high (e.g., OD ≥ 2.00), as in the two confirmatory negative patients who met clinical criteria for HIT, the confirmatory test may have limited clinical utility and could even result in misclassifying a patient. It may be that the confirmatory test is most useful to eliminate low positive heparin/PF4 antibody OD values in cases where the clinical significance is uncertain (i.e. cases where patients do not clearly meet clinical criteria for HIT, but antibodies are detected by ELISA). Future studies aimed at further defining the role of the confirmatory test are warranted. It would be ideal if a predictive algorithm for HIT could be developed, utilizing anti-heparin/PF4 antibody OD value and confirmatory assay result, as this would assist in the clinical management of these patients.
Acknowledgments
The authors wish to thank Dr. Richard Becker and Dr.Gowthami Arepally for reviewing the manuscript prior to submission and providing critical feedback and comments.
Grant support: N.L.W.: NIH Training grant 5T32 HL07057-32; S.L.P: K23-HL084233; T.L.O.: CDC (UO1-DD000014); NIH (UO1-HL072289; U54-HL077878)
References
- 1.Warkentin TE. Heparin-induced thrombocytopenia: a clinicopathologic syndrome. Thromb Haemost. 1999;82:439–447. [PubMed] [Google Scholar]
- 2.Warkentin TE, Greinacher A. Heparin-induced thrombocytopenia: recognition, treatment, and prevention: the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest. 2004;126:311S–337S. doi: 10.1378/chest.126.3_suppl.311S. [DOI] [PubMed] [Google Scholar]
- 3.Lo GK, Juhl D, Warkentin TE, et al. Evaluation of pretest clinical score (4 T's) for the diagnosis of heparin-induced thrombocytopenia in two clinical settings. J Thromb Haemost. 2006;4:759–765. doi: 10.1111/j.1538-7836.2006.01787.x. [DOI] [PubMed] [Google Scholar]
- 4.Warkentin TE, Kelton JG. A 14-year study of heparin-induced thrombocytopenia. Am J Med. 1996;101:502–507. doi: 10.1016/s0002-9343(96)00258-6. [DOI] [PubMed] [Google Scholar]
- 5.Greinacher A, Juhl D, Strobel U, et al. Heparin-induced thrombocytopenia: a prospective study on the incidence, platelet-activating capacity and clinical significance of antiplatelet factor 4/heparin antibodies of the IgG, IgM, and IgA classes. J Thromb Haemost. 2007;5:1666–1673. doi: 10.1111/j.1538-7836.2007.02617.x. [DOI] [PubMed] [Google Scholar]
- 6.Arepally GM, Ortel TL. Clinical practice. Heparin-induced thrombocytopenia. N Engl J Med. 2006;355:809–817. doi: 10.1056/NEJMcp052967. [DOI] [PubMed] [Google Scholar]
- 7.Warkentin TE, Sheppard JA, Moore JC, et al. Laboratory testing for the antibodies that cause heparin-induced thrombocytopenia: how much class do we need? J Lab Clin Med. 2005;146:341–346. doi: 10.1016/j.lab.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 8.Warkentin TE, Sheppard JA, Horsewood P, et al. Impact of the patient population on the risk for heparin-induced thrombocytopenia. Blood. 2000;96:1703–1708. [PubMed] [Google Scholar]
- 9.Warkentin TE, Sheppard JI. No significant improvement in diagnostic specificity of an anti-PF4/polyanion immunoassay with use of high heparin confirmatory procedure. J Thromb Haemost. 2006;4:281–282. doi: 10.1111/j.1538-7836.2005.01698.x. [DOI] [PubMed] [Google Scholar]
- 10.Zwicker JI, Uhl L, Huang WY, et al. Thrombosis and ELISA optical density values in hospitalized patients with heparin-induced thrombocytopenia. J Thromb Haemost. 2004;2:2133–2137. doi: 10.1111/j.1538-7836.2004.01039.x. [DOI] [PubMed] [Google Scholar]
- 11.Pouplard C, May MA, Regina S, et al. Changes in platelet count after cardiac surgery can effectively predict the development of pathogenic heparin-dependent antibodies. Br J Haematol. 2005;128:837–841. doi: 10.1111/j.1365-2141.2005.05381.x. [DOI] [PubMed] [Google Scholar]
- 12.Lillo-Le Louet A, Boutouyrie P, Alhenc-Gelas M, et al. Diagnostic score for heparin-induced thrombocytopenia after cardiopulmonary bypass. J Thromb Haemost. 2004;2:1882–1888. doi: 10.1111/j.1538-7836.2004.00949.x. [DOI] [PubMed] [Google Scholar]
- 13.Pedersen-Bjergaard U, Andersen M, Hansen PB. Drug-induced thrombocytopenia: clinical data on 309 cases and the effect of corticosteroid therapy. European journal of clinical pharmacology. 1997;52:183–189. doi: 10.1007/s002280050272. [DOI] [PubMed] [Google Scholar]
- 14.Singer RL, Mannion JD, Bauer TL, et al. Complications from heparin-induced thrombocytopenia in patients undergoing cardiopulmonary bypass. Chest. 1993;104:1436–1440. doi: 10.1378/chest.104.5.1436. [DOI] [PubMed] [Google Scholar]
- 15.Warkentin TE, Greinacher A. Heparin-induced thrombocytopenia and cardiac surgery. Ann Thorac Surg. 2003;76:2121–2131. doi: 10.1016/j.athoracsur.2003.09.034. [DOI] [PubMed] [Google Scholar]
- 16.Fabris F, Luzzatto G, Soini B, et al. Risk factors for thrombosis in patients with immune mediated heparin-induced thrombocytopenia. J Intern Med. 2002;252:149–154. doi: 10.1046/j.1365-2796.2002.01021.x. [DOI] [PubMed] [Google Scholar]
- 17.Lubenow N, Eichler P, Lietz T, et al. Lepirudin in patients with heparin-induced thrombocytopenia - results of the third prospective study (HAT-3) and a combined analysis of HAT-1, HAT-2, and HAT-3. J Thromb Haemost. 2005;3:2428–2436. doi: 10.1111/j.1538-7836.2005.01623.x. [DOI] [PubMed] [Google Scholar]