Abstract
Heavy alcohol use frequently co-occurs with cigarette smoking and may impede smoking cessation. This clinical trial examined whether smoking cessation treatment that incorporates brief alcohol intervention can improve smoking cessation outcomes (7-day verified point prevalence abstinence) and reduce drinks consumed per week. Heavy drinkers seeking smoking cessation treatment were assigned by urn randomization to receive, along with 8-weeks of nicotine replacement therapy, either a 4-session standard smoking cessation treatment (ST, n = 119) or standard treatment of equal intensity that incorporated brief alcohol intervention (ST-BI, n = 117). Across follow-ups over 26 weeks, participants in ST-BI reported approximately 20% fewer drinks per week (p < .027) and greater smoking abstinence (adjusted odds ratio= 1.56 [95% CI =1.01-2.43]) than those in ST; however, effects on smoking were primarily evident at 2 weeks after quit date and were near zero by 16 weeks. The effect of ST-BI on smoking outcome was most robust among moderately heavy drinkers compared to very heavy drinkers. Integrating brief alcohol intervention into smoking cessation treatment appears feasible, but further development is needed to yield lasting effects on smoking.
Keywords: smoking, alcohol, brief alcohol intervention, smoking cessation treatment
The positive association between cigarette smoking and alcohol use has been well documented (e.g., Chiolero, Wietlisbach, Ruffieux, Paccaud, & Cornuz, 2006; Dawson, 2000; Falk, Yi, & Hiller-Sturmhofel, 2006; Friedman, Tekawa, Klatsky, Sidney, & Armstrong, 1991). Almost 20% of current smokers consume five or more drinks on one occasion at least once per month, compared to about 6.5% of nonsmokers (Dawson, 2000). The combined negative effects of excessive drinking and smoking on health outcomes are substantial. For example, smoking and heavy drinking combine to produce especially negative consequences on brain morphology and function (Durazzo, Cardenas, Studholme, Weiner, & Meyerhoff, 2007; Meyerhoff et al., 2006), and smoking negates the cardioprotective effects of regular drinking (Ebbert, Janney, Sellers, Folsom, & Cerhan, 2005; Schroder, Marrugat, Elosua, & Covas, 2002). Furthermore, a multiplicative effect operates when smoking is combined with heavy drinking, conferring markedly greater risk for oral, pharyngeal, laryngeal, and esophageal cancers relative to just smoking, just drinking, or neither smoking nor drinking (Pelucchi, Gallus, Garavello, Bosetti, & La Vecchia, 2006).
Greater alcohol use is associated with decreased odds of smoking cessation (Hymowitz et al., 1997; Osler, Prescott, Godtfredsen, Hein, & Schnohr, 1999; Sorlie & Kannel, 1990; Zimmerman, Warheit, Ulbrich, & Buhl Auth, 1990) and is a strong prospective predictor of smoking relapse among self-quitters (Garvey, Bliss, Hitchcock, Heinold, & Rosner, 1992; Ockene et al., 2000). Current alcohol use (Humfleet, Munoz, Sees, Reus, & Hall, 1999; Sherman, Wang, & Nguyen, 1996; Smith, Kraemer, Miller, Debusk, & Taylor, 1999) and current binge drinking (Murray, Istvan, Voelker, Rigdon, & Wallace, 1995) at the start of a smoking cessation treatment and use of alcohol after treatment (Humfleet et al., 1999) are negatively associated with abstinence, although there has been an exception (Hughes & Oliveto, 1993). In treatment samples, approximately one quarter of smoking lapses occur in contexts involving alcohol use (Baer & Lichtenstein, 1988; Borland, 1990; Shiffman, 1982).
A number of recent clinical trials have examined smoking cessation interventions initiated during alcoholism treatment. Results have indicated that these interventions do not harm treatment outcome and may even be associated with better drinking outcomes (Prochaska, Delucchi, & Hall, 2004), though there have been exceptions (e.g., Joseph, Willenbring, Nugent, & Nelson, 2004). However, relatively little attention has been devoted to smokers who drink heavily but are not alcohol dependent. The U.S. Department of Health and Human Services guidelines for treating tobacco dependence recommend that smokers reduce or avoid drinking alcohol as much as possible when making a quit attempt (Fiore, Bailey, Cohen, & et al., 2000). However, clinicians currently have little guidance as to how to address heavy drinking in smoking cessation treatment as no empirical studies have been published to date in this area. Brief motivationally-focused behavioral interventions have been shown to reduce drinking (McCrady, 2000; Moyer, Finney, Swearingen, & Vergun, 2002; Whitlock, Polen, Green, Orleans, & Klein, 2004) among drinkers who are not seeking treatment for alcohol problems and especially among those with less severe alcohol problems (Moyer et al., 2002). Incorporating brief interventions into smoking cessation treatment for nondependent heavy drinkers may be efficacious both in reducing drinking and in improving smoking cessation outcomes. Towards this end, the aim of the current study was to test the efficacy of a smoking cessation treatment that incorporated a brief alcohol intervention, which focused on the risks of smoking relapse associated with drinking and the negative effects of continued heavy drinking.
This randomized clinical trial involved 236 non-alcohol dependent, heavy drinking smokers who were recruited from the community and were seeking smoking cessation treatment. Participants were randomized to either standard smoking cessation treatment (ST), which included provision of the nicotine patch, or an equivalent standard treatment that also incorporated a brief alcohol intervention (ST-BI). Treatment conditions were matched on amount of contact time and participants were followed for 26 weeks after their smoking quit date. We hypothesized that ST-BI, compared to ST, would result in higher rates of point prevalence smoking abstinence at 2, 8, 16, and 26 weeks after quit date and a lower number of alcoholic drinks consumed per week. In addition, we hypothesized that ST-BI, relative to ST, would result in lower rates of initial lapses to smoking that involved alcohol use during treatment.
Finally, we examined two potential moderators of treatment effects on smoking: level of drinking prior to treatment and intention to change drinking. These analyses were considered exploratory as there are theoretical reasons to expect either stronger or weaker effects of ST-BI at higher levels of each variable. For pretreatment drinking, the emphasis of ST-BI on alcohol use might be especially relevant for relatively heavier drinkers; on the other hand, asking these very heavy drinkers to change both drinking and smoking simultaneously might prove overly difficult. For intention to change drinking, ST-BI might be relatively more effective for those who intend to change drinking because it reinforces the expectations of such smokers that changing alcohol use may facilitate smoking cessation; on the other hand, ST-BI could be less effective for these smokers because the information in ST-BI is redundant with their intentions.
Method
Participants
Participants were 236 heavy drinking smokers recruited from the community. To be included, participants had to: (a) be at least 18 years of age; (b) have smoked cigarettes regularly for at least one year; (c) currently smoke at least 10 cigarettes a day; (d) currently use no other tobacco products or nicotine replacement therapy; and (e) currently drink heavily according to NIAAA guidelines (National Institute on Alcohol Abuse and Alcoholism, 1995): for men, >14 drinks per week or ≥5 drinks per occasion at least once per month over the past 12 months; for women, >7 drinks per week or ≥4 drinks per occasion at least once per month. Participants were excluded if they: (a) met full DSM-IV criteria for alcohol dependence in the past 12 months; (b) met criteria for other current psychoactive substance abuse or dependence (excluding nicotine dependence and alcohol abuse) in the past 12 months; (c) had a current (past month) affective disorder; (d) were psychotic or suicidal; (e) had an unstable medical condition that would suggest caution in the use of the nicotine patch (e.g., unstable angina, arrhythmia, recent congestive heart failure); (f) were currently pregnant or lactating or intended to become pregnant. Smokers had to agree to be available for 6 months and not to seek other smoking cessation treatment during the active phase of treatment (i.e., during the 8 weeks after their quit date while on the nicotine patch).
Sample size estimation
We determined required sample size based on an effect of ST-BI vs. ST on smoking abstinence that was equivalent to an odds ratio of 2 based on an a priori estimate of abstinence rates in ST ranging from 40% at 2 weeks, to 30% at 8 weeks, 20% at 16 weeks, and 15% at 26 weeks; this represents a small to medium effect size that we believed would be of clinical significance. Because smoking outcomes were analyzed as repeated measures, we followed procedures for sample size determination for such analyses outlined by Rochon (1998). Using Rochon’s GEESIZE program, Version 2.1., we determined that a sample size of 212 participants would be needed. Allowing 10% attrition from follow-up, we chose to recruit a final sample of 236 participants.
Procedure
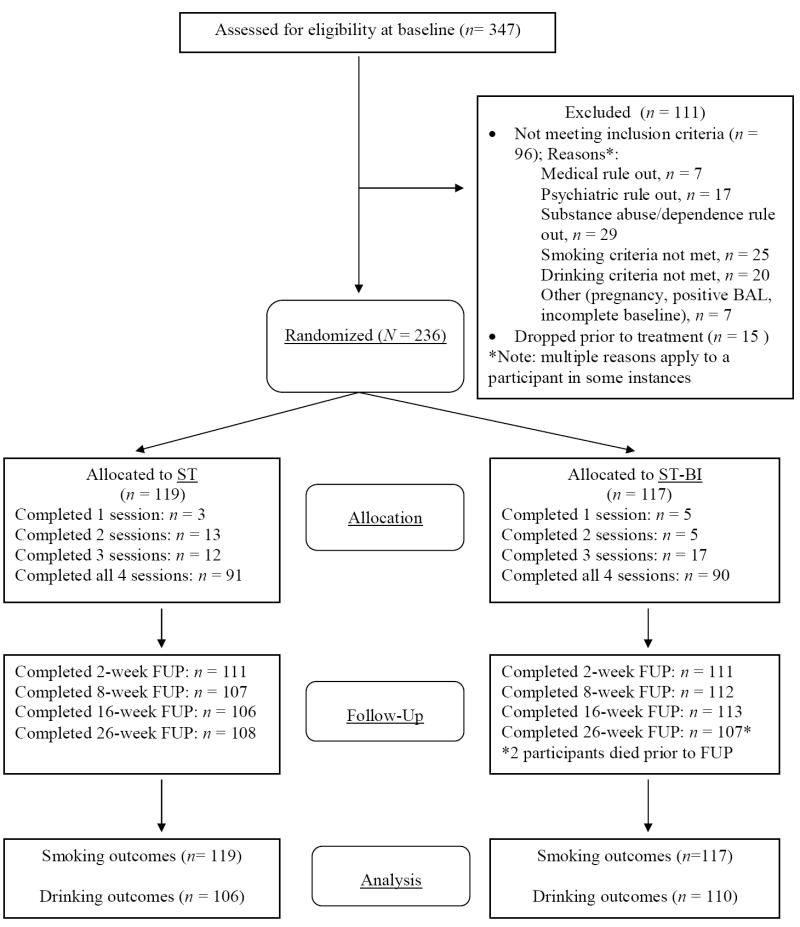
Participants were recruited through postings on community bulletin boards and newspaper and radio advertisements, which asked for social drinkers who wanted to quit smoking. Potential participants were screened by telephone before completing an intake interview, at which they signed a statement of informed consent approved by the Brown University Institutional Review Board. Participants were recruited from October 2003 through July 2006, and follow-ups were conducted from January 2004 through June 2007. Of the 991 individuals screened for the study, 428 did not meet preliminary inclusion/exclusion criteria and 216 were eligible but did not show up for baseline (n = 206) or declined to participate in the study (n = 10). Of the 347 eligible screens, 96 were deemed ineligible at baseline. Fifteen additional individuals dropped out prior to receiving any treatment materials and prior to learning of their treatment condition. They were not followed further and are not included in outcome analyses. Thus, results are based on 236 participants entering treatment rather than a potential intention to treat sample of 251. See Figure 1 for a detailed diagram of participant flow.
Figure 1.
CONSORT flowchart of eligibility, randomization, treatment, follow-up, and inclusion in analyses. ST = standard smoking cessation treatment. ST-BI = standard smoking cessation that integrates a brief alcohol intervention.
Randomization
Participants were assigned to treatment condition using the urn randomization technique (Wei, 1978) to ensure balancing on gender, level of nicotine dependence (FTND), number of drinks consumed per week, and intention to change drinking while quitting smoking. One hundred and nineteen participants were randomized to the ST condition, and 117 were randomized to ST-BI. Data for the randomization were sent by research assistants to the lead author (CWK), who conducted the computer-based urn randomization and informed the treatment provider of treatment assignment.
Assessments
Participants completed brief assessments at each treatment session. In addition, follow-ups were conducted at 8, 16, and 26 weeks after quit date. Prior to assessments, participants provided a breath sample to confirm that they were alcohol-negative. Research assistants who conducted interviews were not informed of treatment condition assignment.
Treatments
Treatment consisted of four individual counseling sessions over three weeks with the quit date occurring at session 2, one week after session 1. Manuals are available upon request to the first author. All participants received treatment with transdermal nicotine patch with the initial dose starting at 21 mg for four weeks, followed by two weeks of 14 mg patch, and then two weeks of 7 mg patch. Standard treatment was based on recent clinical practice guidelines (Fiore et al., 2000) and focused on problem solving regarding high-risk situations for smoking relapse, providing support within the treatment, and encouraging participants to seek support for quitting smoking outside of treatment. Participants in ST were given brief advice to avoid or reduce drinking as much as possible while quitting smoking. In the ST-BI condition, discussion of alcohol use was reserved for the second half of sessions 1 and 2.
Sessions ranged in length from 70 minutes for session 1, 40 minutes for session 2, and 20 minutes for sessions 3 and 4. ST and ST-BI were matched on treatment contact time. In ST, 40 minutes of session 1 and 20 minutes of session 2 were dedicated to teaching progressive muscle relaxation, which has not been shown to improve smoking cessation outcomes (Fiore et al., 2000). Sessions 3 and 4 contained 5-minute check-ins regarding use of relaxation skills. In ST-BI, the same amount of time was dedicated to discussion of the participant’s alcohol use.
The first session of ST-BI included open-ended discussion of current drinking and smoking patterns, normative feedback on drinking level and the risk of smoking relapse associated with drinking, and preliminary goal setting. The module used the potential role of alcohol use in smoking relapse as the entry into discussion of possible short and long-term changes in drinking. Regardless of interest in long-term changes in drinking, participants were encouraged to consider abstaining from alcohol for at least two weeks after quitting smoking and preferably to abstain from drinking entirely while on nicotine patch. They also were provided recommendations for moderate drinking limits and informed of the risks associated with combined heavy drinking and smoking. The second session focused on finalizing drinking goals and supporting self-efficacy for change. A brief, 5-minute, check-in regarding achievement of drinking goals was included in sessions 3, and in session 4 recent benefits of changing drinking and longer-term drinking goals were reviewed. The alcohol module of ST-BI was conducted in the nonconfrontational therapeutic style of Motivational Interviewing (Miller & Rollnick, 2002), which is intended to minimize patient resistance and stresses personal responsibility for deciding to change.
Therapists
Treatments were delivered by 2 male and 4 female therapists; three had doctoral degrees, 2 had masters degrees, and 1 had a bachelors degree, all in psychology or counseling-related fields. Each counselor provided treatment in both conditions, audiotaping all sessions and using detailed therapist manuals to ensure standardization of treatment delivery. Therapists completed a minimum of 20 hours of training in Motivational Interviewing and the specifics of the interventions used in the study. An average of one audiotape per therapist was rated by supervisors (CWK or HRL) on a biweekly basis using a modification of the Yale Adherence and Competence Scale (Carroll et al., 2000). Therapists had to receive at least adequate competence and adherence ratings. After completion of their first 5 cases, during which regular individual supervision was conducted, therapists received one hour of group supervision every other week.
Measures
At the baseline interview, participants provided demographic and background information, such as age, gender, years of education, marital status, number of years of regular smoking, and average number of cigarettes per day. DSM-IV Axis I diagnoses were determined with the Structured Clinical Interview for DSM-IV Non-Patient Edition (SCID; First, Spitzer, Gibbon, & Williams, 1995). Severity of nicotine dependence was assessed using the Fagerstrom Test for Nicotine Dependence (FTND; Heatherton, Kozlowski, Frecker, & Fagerstrom, 1991), a well-validated six-item measure. Participants completed a single item quasi-continuous assessment of whether they intended to cut down on or stop drinking during treatment (0 = definitely not; 1 = possibly; 2 = probably; 3 = definitely). Finally, commitment to quitting smoking was assessed with the eight-item Commitment to Quitting Smoking Scale (Kahler et al., 2007). This variable was used as a covariate in outcomes analyses given its strong predictive validity in this sample (Kahler et al., 2007).
Smoking status
Outcome analyses were based on 7-day point prevalence abstinence (i.e., reported abstinence of at least 7 days prior to the assessment day) as assessed at 2 (end of psychosocial treatment), 8 (end of treatment with the nicotine patch), 16, and 26 weeks after each participant’s quit date. Self-reported abstinence was verified by alveolar carbon monoxide (CO) using a Bedfont Scientific Smokelyzer® breath CO monitor. At 16- and 26-week follow-ups, a saliva sample for cotinine level determination by enzyme immunoassay was collected from those reporting abstinence. Abstinence was confirmed by a combination of CO ≤ 10 ppm and cotinine ≤ 15 ng/ml (SRNT Subcommittee on Biochemical Verification, 2002). Significant other report was used to verify smoking status for those who did not provide self-report data or did not provide biochemical verification of abstinence (a total of 4% of assessments).
Complete smoking data verified either biochemically or by significant other report was obtained from 94.1%, 93.2%, 90.3%, and 94.1% of participants at the 2-, 8-, 16-, and 26-week follow-ups, respectively. Two participants in ST-BI died between the 16- and 26-week follow-ups; these deaths were deemed unrelated to study participation. Their smoking status at the 26-week follow-up was left as missing. The odds of completing follow-ups was not significantly related to treatment condition. For our primary analyses, only individuals who had smoking abstinence confirmed at a given follow-up were considered abstinent; those with missing data were considered non-abstinent. Thus, all 236 participants entering treatment were included in the smoking outcome analyses, and our reported abstinence rates correspond to the reporting of abstinence in most smoking cessation trials. However, we also ran analyses in which no assumptions were made about missing data using only available data for each participant. Those analyses excluded the 12 participants who provided no follow-up data. Results using no assumptions regarding missing data were highly concordant with those using a “worst-case” assumption and are therefore not detailed here. Our secondary smoking outcome was continuous smoking abstinence, which was defined as reporting no smoking from quit date onward and being verified as abstinent at each follow-up. Point prevalence abstinence was chosen as the primary outcome because biochemical verification can only be used for relatively short windows of time and because analyzing point prevalence abstinence in a repeated measures context (a) more appropriately captures the variability in outcomes over time relative to collapsing outcomes, (b) allows examination of differences between treatment conditions over time, and (c) provides greater statistical power compared to single measure outcomes (Hall et al., 2001).
Alcohol Use
The Timeline Followback Interview (TLFB; Sobell & Sobell, 1996), a well-validated daily calendar-assisted assessment of alcohol use, was used at baseline to assess alcohol use in the prior 8 weeks. TLFB also was conducted at sessions 3 and 4, and at each follow-up interval for the period since its last administration. Smoking and nicotine replacement use also were assessed with the TLFB. The primary outcome variable was the number of drinks consumed per week; this variable was chosen as the primary drinking outcome because it most appropriately mirrors the multiple targets of ST-BI that stressed (a) avoiding drinking when possible, (b) limiting the amount consumed when drinking, and (c) drinking at levels that are not considered medically risky, i.e., <15 drinks per week for men and <8 drinks per week for women. Drinks per week after quit date was aggregated into thirteen 2-week blocks. In this way, the first 2-week block reflects drinks consumed per week during the two weeks after quit date when psychosocial treatment was still being delivered. A total of 216 (91.5%; 106 in ST, 110 in ST-BI) participants provided at least some daily drinking data after quit date with 207 (87.7%) providing complete data through 8 weeks after treatment, and 200 (84.7%) providing complete data through 26 weeks. Analyses were conducted using all available data (N = 216).
Relapse Interview
A Relapse Interview was administered to subjects who lapsed to smoking to determine the circumstances surrounding the initial lapse episode, including whether individuals were drinking alcohol at the time. Although the reliability of retrospective recall of smoking lapses is questionable for some variables, retrospective recall for whether alcohol consumption occurred during a lapse episode appears to be fairly accurate when compared to real-time assessment (Shiffman et al., 1997).
Treatment adherence
One audiotape was randomly selected from half of the participants in the sample for adherence coding. Research assistants coded whether or not each topic in the treatment protocol outline was covered, with the number of topics rated ranging from 28 in Session 1 to 14 in Session 4. One out of ten tapes was coded by two research assistants, and any discrepancies in ratings were reviewed to ensure consistency of rating. Specific items were included for elements common to both treatments (e.g., “gives direction on patch use”) as well as behaviors specific to ST (e.g., “describes progressive muscle relaxation”) and ST-BI (e.g., “asks about the pros of drinking”).
Treatment evaluations and quitting processes
To assess the discriminability of the treatment conditions, participants were asked at the 8-week follow-up a series of single item questions regarding the helpfulness of counseling, quality of the interaction with the therapist, and the extent to which the treatments emphasized the importance of learning relaxation skills and reducing drinking while quitting smoking. They were also asked the extent to which they engaged in 8 smoking cessation strategies (e.g., avoiding high-risk situations; α = .78) and the degree to which they avoided drinking alcohol.
Data Analysis Plan
We first used chi-square analyses and t-tests to examine between-groups differences in treatment compliance, treatment fidelity, nicotine patch utilization, participants’ evaluation of treatment, and participants’ reported use of general and alcohol-specific smoking cessation strategies. We expected that each of these indices would be equal across conditions except those that dealt specifically with relaxation or alcohol use.
We next examined point-prevalence smoking outcomes and our primary drinking outcome, drinks per week. To examine the effect of treatment within the context of other covariates that impact outcome, repeated measures analyses were conducted using generalized estimating equations (GEE; Liang & Zeger, 1986) using PROC GENMOD in SAS (SAS Institute Inc., 1997). The inclusion of covariates that are related to outcome but not to treatment condition maximizes statistical power (Cohen, Cohen, West, & Aiken, 2003). These covariates also are informative regarding the key predictors of outcomes in the population. For smoking outcomes, point prevalence abstinence at the four follow-ups was the dependent variable and the binomial distribution and logit link function were specified. Drinks per week followed a count distribution with overdispersion due to positive skewness. We therefore used a negative binomial distribution and the logit link function for analyzing these outcomes. This model is an extension of the Poisson distribution for count data and is appropriate when variability in the counts is greater than expected based on the mean; similar to an odds ratio, the exponent of the model coefficient reflects the ratio of the number of events (drinks) expected to occur in one condition relative to the number of events expected to occur in the other condition.
The following covariates were used in all GEE analyses given their potential influence on outcome: gender, FTND, average number of drinks consumed per week (square-root transformed to correct positive skewness), intention to change drinking, and commitment to quitting smoking. All continuous variables were standardized to facilitate comparison between model coefficients. A variable carrying the linear effect of time was also included. Correlations among the covariates were generally weak with all rs below .15, except for the correlations between male gender and drinks per week, r(236) = .42, p < .0001 and between FTND and drinks per week, r(236) = .15, p = .02. Preliminary tests using effects coding revealed that smoking and drinking outcomes for any one therapist did not differ significantly from the average of the remaining therapists and that there were no therapist by treatment interactions. Therefore, therapist effects were not included in the primary outcome analyses. For both smoking and drinking outcomes, we first entered the main effect of treatment along with the covariates. We then entered interactions between treatment condition and both drinks per week at baseline and intention to change drinking.
Finally, we examined the proportion of participants in ST and ST-BI who experienced an initial lapse to smoking while drinking. Given a treatment effect on this variable, we then examined whether the effect of treatment was reduced by including this variable in the GEE analysis predicting smoking outcome, which would be consistent with a mediational mechanism.
Results
The sample used in these analyses was 45% (n = 106) female and 55% (n = 130) male, with 32.6% (n = 77) married or cohabitating. The mean age of the sample was 41.5 (SD = 12.0) years, and the mean education was 14.0 (SD = 2.6) years. The vast majority of participants (n = 214, 90.7%) identified themselves as non-Hispanic White. The sample was 3.8% African-American, 3.4% Hispanic/Latino, and 0.8% Asian American; 1.2% identified themselves as “other” or of mixed ethnic origin. At baseline, participants smoked an average of 21.3 (SD = 9.4) cigarettes per day and had been smoking for an average of 22.7 years (SD = 11.5) The sample mean on the FTND was 5.0 (SD = 2.2). The majority of participants (90.7%) had made at least one quit attempt that lasted more than 12 hours. The mean Commitment to Quit Smoking Scale score was 3.9 (SD = .62) out of 5. Participants reported that during the eight weeks prior to treatment, they drank on 54.7% (SD = 27.3) of possible days and consumed an average of 16.5 (SD = 11.9) drinks per week. For intentions regarding drinking, 31.4% indicated that they would “possibly”, 33.5% indicated they would “probably”, and 17.8% indicated they would “definitely” reduce or stop drinking while quitting smoking. The remaining 17.4% indicated they would “definitely not” reduce or stop drinking. Based on the SCID, 19.5% of participants met criteria for current alcohol abuse, and 17.4% had past alcohol dependence; exploratory analyses indicated that abuse and dependence diagnoses did not predict either smoking or drinking outcomes or interact with ST-BI in predicting outcomes. For other lifetime diagnoses, 26.5% had past drug abuse, 20.9% had past drug dependence, and 33.6% had past major depressive disorder. Treatment conditions did not differ significantly on any of these baseline variables, all ps > .20.
Adverse Events
As noted above, 2 participants in ST-BI died during follow-up. Two other participants in this condition were hospitalized during the study for reasons deemed unrelated to participation (blocked artery and recurrence of lung cancer). No other serious adverse events were reported.
Treatment Compliance, Fidelity, and Discriminability
Attendance at treatment sessions did not differ significantly by condition (see Figure 1). Patch compliance also did not differ significantly by condition. Participants used patch on 73.7% (SD = 29.7) of days in the 8 weeks after quit date (based on available data from 212 participants).
Counselor adherence to protocol was excellent. In the ST condition, 96.7% of standard treatment protocol topics across sessions sampled were completed, compared to 97.5% in ST-BI. For topics specific to relaxation training in ST, 99.4% were covered in ST vs. 1.8% in ST-BI. For alcohol components specific to ST-BI, 97.6% were covered in ST-BI, compared to 3.5% in ST.
At the 8-week follow-up, 207 participants completed a post-treatment evaluation of the counseling and reported on strategies used to quit smoking. Consistent with expectations, ratings of the overall helpfulness of counseling, therapist concern for the participant, and “getting along with” the counselor did not differ significantly across treatment conditions, ps > .15. Those in ST-BI, compared to those in ST, agreed less strongly that the counselor had “made it clear that relaxation skills might help me in quitting smoking,” (d = -.35, p = .01), whereas they agreed more strongly than those in ST that “my counselor made it very clear to me that changing my alcohol use might help me quit smoking” (d = .59, p < .0001). In regards to strategies used to quit, participants in ST-BI did not differ significantly from those in ST in their reported use of general smoking cessation strategies (p = .75), but did agree significantly more strongly that they had tried to avoid drinking alcohol as much as possible (d = .49, p = .0006) and that they had tried to limit how much alcohol they drank (d = .31, p = .03).
Smoking Outcomes
Seven day point prevalence abstinence rates were 45.4% in ST vs. 57.3% in ST-BI at 2 weeks (odds ratio [OR] = 1.61, 95% CI = 0.96-2.70, p = .07); 31.1% vs. 39.3% at 8 weeks (OR = 1.45, 95% CI = 0.84-2.46, p = .18); 17.7% vs. 18.0% at 16 weeks (OR = 1.02, 95% CI = 0.53-1.99, p = .95); and 17.7% vs. 19.1% at 26 weeks (OR = 1.10, 95% CI = 0.57-2.14, p = .76). Continuous abstinence rates did not differ significantly, 12.6% in ST vs. 13.7% in ST-BI, (OR = 1.09, 95% CI = 0.52-2.34, p = .81).
The initial GEE model with the chosen covariates (gender, FTND, average number of drinks consumed per week, intention to change drinking, and commitment to quitting smoking) and the main effect of treatment is presented in Table 1. There was a significant main effect of ST-BI indicating significantly higher odds of abstinence over time compared to ST. Higher FTND scores were associated with a lower odds of abstinence, whereas greater commitment to quitting was associated with a higher odds of abstinence. Men were significantly more likely than women to be abstinent, whereas the effects of drinks per week and intention to change drinking were nonsignificant.
Table 1.
Generalized Estimation Equations Analyses Predicting 7-day Point Prevalence Smoking Abstinence at 2, 8, 16, and 26 weeks after quit date
| Variable | OR(95% CI) | p |
|---|---|---|
| Main effects | 0.54 (0.46 - 0.62) | .0001 |
| Time | 0.54 (0.46 - 0.62) | .0001 |
| Male gender | 1.71 (1.05 - 2.81) | .033 |
| Fagerström Test for Nicotine Dependence | 0.73 (0.58 - 0.93) | .009 |
| Drinks per week | 1.03 (0.80 - 1.32) | .83 |
| Intention to change drinking | 0.86 (0.69 - 1.08) | .19 |
| Commitment to Quitting Scale | 1.59 (1.24 - 2.02) | .0002 |
| ST-BI compared to ST | 1.56 (1.01 - 2.43) | .047 |
| Interactions with treatment | ||
| ST-BI X Intention to change drinking | 1.39 (0.89 - 2.18) | .15 |
| ST-BI X Drinks per week | 0.57 (0.36 - 0.90) | .016 |
Note. N = 236. ORs < 1 indicate a reduced odds of abstinence; ORs > 1 indicate an increased odds of abstinence. OR = odds ratio; CI= Confidence Interval. All continuous variables were standardized (M = 0; SD = 1), so that their relative effects can be compared directly.
The GEE model weighs all time points equally when assessing the effect of treatment. Therefore, in a next step of the analysis, we added the interaction between ST-BI and time (not shown in Table 1). The ST-BI by time interaction was nonsignificant, AOR = 0.85, 95% CI = 0.64-1.11, p = .23, suggesting no significant differences in treatment effect over time. Nonetheless, the model-based AOR for each time period mirrored the waning effects of treatment seen in the raw data: at 2 weeks - AOR = 1.78, 95% CI = 1.05-3.02, p = .03; 8 weeks - AOR = 1.57, 95% CI = 0.90-2.73, p = .11; 16 weeks - AOR = 1.08, 95% CI = 0.53-2.20, p = .84; 26 weeks - AOR = 1.17, 95% CI = 0.58-2.36, p = .66.
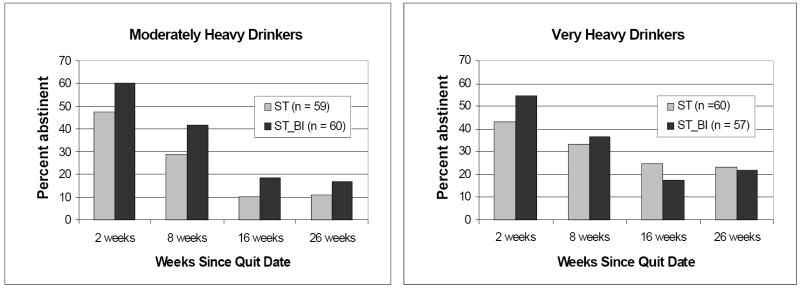
In the final step of the analysis, the time by treatment interaction was removed from the model and the interactions between treatment condition and both drinks per week and intention to change drinking were added (see Table 1, interactions with treatment). Given that two interactions were tested, a Bonferroni-corrected alpha of .025 was used to determine significance. The ST-BI by intention interaction did not reach significance. The ST-BI by drinks per week interaction was significant and indicated that the effect of ST-BI, relative to ST, was attenuated at higher levels of drinking. To illustrate this effect, we divided the sample in half based on the median number of drinks consumed per week for each gender (18.4 drinks per week for men; 8.8 drinks for women). Raw (unadjusted) point prevalence abstinence rates by treatment condition and drinking level are shown in Figure 2. In the moderately heavy drinkers (n = 119), the effect of ST-BI, relative to ST, was relatively robust and significant, AOR = 2.09, 95% CI = 1.15-3.81, p = .015. In the very heavy drinkers (n = 117), the effect was small and nonsignificant, AOR = 1.27, 95% CI = 0.64-2.51, p = .50.
Figure 2.
Raw (undadjusted) 7-day point prevalence smoking abstinence at 2, 8, 16, and 26 weeks after quit date by treatment condition and level of drinking. Participants are classified as moderately heavy or very heavy drinkers using a gender-based median split on mean number of drinks consumed per week at baseline. Median drinks per week for men was 18.4 and for women was 8.8. ST = standard smoking cessation treatment. ST-BI = standard smoking cessation that integrates a brief alcohol intervention.
Drinking Outcomes
Spearman rank-order point biserial correlations between point prevalence smoking abstinence at each follow-up and drinks per week in the two weeks prior to that follow-up were very small and nonsignificant ranging from rs = -.06 to rs = .02, indicating that those who were abstinent from smoking were not drinking significantly less than those who were not abstinent (results did not differ when two other indices of drinking, percent heavy drinking days and percent days abstinent, were examined). Consistent with treatment recommendations, significantly more participants in ST-BI compared to ST abstained entirely from drinking during the first two weeks after quitting (20.2% in ST-BI vs. 9.7% in ST, p = .03) and while on the nicotine patch (i.e., through 8 weeks after quit date, 13.9% vs. 3.9%, p = .01). By the 26 week follow-up, virtually all participants in ST-BI (94.4%) and ST (98.1%) had drunk at least once, and the effect of condition was no longer significant, p = .15. Although fewer participants in ST-BI met NIAAA criteria for heavy drinking over the past 2 weeks compared to those in ST, these differences were not significant at 2 weeks (45.9% in ST-BI vs. 58.7% in ST, p = .06), 8 weeks (51.9% vs. 57.7%, p = .39), or 26 weeks (60.0% vs. 68.0, p = .23).
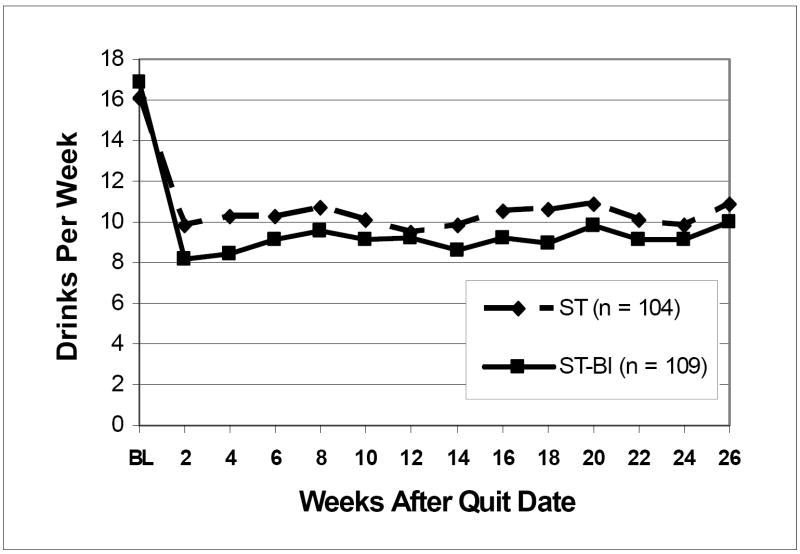
Figure 3 shows the number of drinks consumed per week after quit date in each treatment condition across the 26-week follow-up. In both conditions there was a large reduction in weekly alcohol consumption; overall, 42.3% of drinkers reduced drinking by more than 50% during follow-up, whereas only 1.9% increased their drinking by more than 50%. In the negative binomial GEE model predicting drinks per week after quit date, higher FTND scores, exp(B) = 0.87, 95% CI = 0.79-0.96, p = .005, and greater intentions to change drinking, exp(B) = 0.89, 95% CI = 0.80-0.98, p = .01, were associated with a significantly lower number of drinks consumed per week, whereas greater drinks per week at baseline was associated with a significantly greater number of drinks, exp(B) = 1.84, 95% CI = 1.67- 2.03, p < .0001. Those in ST-BI, compared to those in ST, drank significantly fewer drinks per week, exp(B) = 0.81, 95% CI = 0.67-0.98, p = .027. This represents just under a 20% reduction in drinks per week relative to ST when controlling for baseline drinking. Interactions between treatment condition and time, drinks per week at baseline, and intention to change drinking were all nonsignificant.
Figure 3.
Mean number of drinks consumed per week over the 26 weeks after quit date. Drinks per week are averaged across thirteen 2-week intervals and graphed by treatment condition. Raw (unadjusted) data are shown. ST = standard smoking cessation treatment. ST-BI = standard smoking cessation that integrates a brief alcohol intervention. Those in ST-BI, compared to those in ST, drank significantly fewer drinks per week, exp(B) = 0.81, 95% CI= 0.67-0.98, p = .027.
Alcohol-Involved Lapses
A total of 217 (91.9%) participants provided adequate data to classify whether a slip to smoking while drinking alcohol occurred during the 8 weeks participants were receiving active treatment. In ST, 30.6% (33 of 108) of participants reported having an initial slip to smoking during treatment while drinking alcohol compared to 22.0% (24 of 109) of those in ST-BI, χ2 (1, n = 217) = 2.04, p = .15. We ran a logistic regression model predicting alcohol-involved smoking lapses including the same covariates as in the GEE models. Higher FTND scores were associated with a lower odds of alcohol-involved smoking lapses (AOR = 0.62, 95% CI= 0.45-0.87, p = .005), whereas higher drinks per week was associated with a greater odds of alcohol-involved smoking lapses (AOR = 1.61, 95% CI= 1.11-2.33, p = .01). The effect of ST-BI was nonsignificant (AOR = 0.56, 95% CI= 0.29 - 1.07, p = .08), as were the other covariates. Because the effect of treatment was not significant, we did not examine whether alcohol-related slips mediated the effect of ST-BI on point prevalence smoking outcomes.
As an exploratory analysis, the interaction between treatment condition and drinks per week was entered in the logistic regression model predicting alcohol-involved smoking lapses and was significant, (AOR = 2.64, 95% CI= 1.31-5.32, p = .006). Among those drinking at or below the gender-based median level of drinking in the sample, 31.4% of ST participants reported having an initial slip while drinking compared to only 12.3% in ST-BI, χ2 (1, n = 108) = 5.85, p = .016. Among those drinking above the median, the rates were 29.8% in ST and 32.7% in ST-BI, χ2 (1, n = 109) = 0.10, p = .75. To examine whether this effect might account for the interaction between ST-BI and drinks per week in predicting smoking abstinence, we added the alcohol-involved smoking lapse variable to the GEE model for smoking outcomes. Those having an alcohol-involved smoking lapse during treatment were significantly less likely to be abstinent at each follow-up (AOR = 0.42, 95% CI = 0.24-0.76, p = .004). With this variable included in the model, the interaction between ST-BI and drinks per week was reduced and no longer significant, AOR = 0.70, 95% CI= 0.43-1.15, p = .16, indicating that avoidance of alcohol-involved slips accounted for some of the interaction between treatment and drinks per week.
Discussion
Results of this study suggest that integrating a brief alcohol intervention into smoking cessation treatment for heavy drinkers is feasible, does not detract from smoking outcomes, and leads to somewhat reduced drinking. The results do not appear to differ according to whether smokers meet current criteria for alcohol abuse or have met lifetime criteria for alcohol dependence. The addition of the alcohol module to standard treatment did not affect participants’ willingness to attend counseling sessions, and participants in ST-BI and ST did not differ in their evaluations of the counselor and the smoking treatment and did not report different levels of use of nicotine patch or other smoking cessation strategies. At the same time, ST-BI could be readily distinguished from ST in terms of the session topics covered, participants’ evaluation of the amount of focus on alcohol, and participants’ self-reported efforts to avoid drinking.
In repeated measures analyses of point prevalence abstinence which covaried relevant predictors of outcome and provided maximal statistical power, participants receiving ST-BI were significantly more likely to be abstinent from smoking than those receiving ST. The magnitude of the effect was relatively small, however, with an adjusted OR of 1.56, which is equivalent to an h of 0.25 (Chinn, 2000). Furthermore, the effect of ST-BI on smoking only approached significance during the time in which counseling was ongoing (2 weeks after quit date); effects were diminished by 8 weeks and were essentially absent by 16 weeks. Likewise, the effect of ST-BI on continuous abstinence from smoking was small. Although it has been convincingly argued that effect sizes as small as a difference of 1% in continuous 6-month smoking abstinence rates may be clinically meaningful given the substantial health benefits of stopping smoking (West, 2007), the difference in continuous abstinence rates of 1.1% between ST-BI and ST did not approach statistical significance given the present sample size and can not be considered robust. Enhancements to this treatment approach may be needed to maximize the total effect size for the treatment and to extend its impact over longer periods of time. The maintenance of treatment gains has been an ongoing challenge within the smoking cessation field (e.g., Covey et al., 2007; Lancaster, Hajek, Stead, West, & Jarvis, 2006). In the present study, behavioral treatment was only provided for 2 weeks after quit date. Extending behavioral treatment, perhaps even beyond the time that nicotine replacement is provided, might help maintain the initial gains in smoking abstinence associated with ST-BI. For example, mixing in-person counseling sessions with telephone counseling sessions may provide a practical and cost-effective means of maintaining contact with participants over an extended period of time (Hall, Humfleet, Reus, Munoz, & Cullen, 2004).
The modest main effect of ST-BI on smoking emerged within the context of an interaction between ST-BI and weekly drinking levels prior to treatment. Among the “moderately heavy” drinkers (i.e., the bottom half of the sample in drinks per week within each gender: median = 18.4 for men and 8.8 for women), the effect of ST-BI, compared to ST, was relatively robust, AOR = 2.09, h = 0.41. For the “very heavy” drinkers, the effect of ST-BI was small, AOR = 1.27, h = 0.13, and nonsignificant. Surprisingly, it was the moderately heavy, not the very heavy, drinkers in ST who had particularly poor smoking outcomes.
The effect of ST-BI on drinking outcomes was also small, though significant. This effect occurred within the context of relatively large reductions in drinking in both conditions that averaged 40% or more. Previous studies have yielded equivocal evidence regarding whether quitting smoking is associated with significant reductions in drinking (Carmelli, Swan, & Robinette, 1993; Nothwehr, Lando, & Bobo, 1995), and smoking cessation interventions have not been shown to affect alcohol use (Fox, Sexton, & Hebel, 1987; Murray, Istvan, & Voelker, 1996). The substantial reductions in drinking seen in both conditions in the present study have not been found in prior smoking cessation studies, which could reflect the fact that this was a sample screened specifically for heavy drinking. Therefore, there was more room for drinking to change within these participants. A recent longitudinal community study found that quitting smoking was associated with a reduced odds of heavy drinking in particular (Karlamangla, Zhou, Reuben, Greendale, & Moore, 2006). Participants also were recruited for a study specifically focusing on alcohol drinkers trying to quit smoking. They were informed that the study was examining what types of counseling may be helpful to alcohol drinkers when they try to quit smoking but were not informed of the study hypotheses. Nonetheless, due to the focus of the study, there may have been a particularly large proportion of the sample who believed that changing drinking was important to smoking cessation success. Intentions to change drinking were related to greater reductions in drinking and about half the sample indicated at baseline that they probably or definitely would cut down on drinking while making their quit attempt. Finally, all participants in both conditions completed in-depth assessments of daily drinking behavior at baseline and throughout follow-up, which can cause assessment reactivity effects (Clifford, Maisto, & Davis, 2007).
A second notable finding regarding drinking was that the changes seen in the first few weeks after the quit attempt were largely maintained throughout follow-up. This maintenance occurred even though the vast majority of participants returned to smoking. The beneficial effects of ST-BI in further reducing drinking relative to ST also appeared to be largely maintained, though they were most consistently apparent in the first two weeks after quit date. These data suggest the initiation of smoking cessation provides an opportunity for heavy drinkers to make relatively lasting changes in their drinking and for clinicians to reinforce changes towards moderate drinking levels. We did not conduct diagnostic interviews at follow-up, however, so it is not possible to determine from this study how many participants experienced a reemergence of alcohol dependence during follow-up.
In the aggregate, those participants who were abstinent from smoking at a given follow-up were not necessarily drinking less than those who smoked, nor did the heavier drinkers in the sample have worse smoking outcomes overall. These findings are surprising, as we had expected that drinking and smoking outcomes would correlate at least moderately. Other analyses of this dataset have shown that the odds of lapses to smoking are significantly higher on drinking compared to non-drinking days (Kahler et al., 2005). Also, just over a quarter of participants in the sample experienced an initial lapse to smoking while drinking alcohol during treatment. Alcohol is a relevant risk factor for smoking relapse. However, smoking cessation can occur in the absence of especially large changes in drinking, and relatively large changes in drinking can occur in the absence of successful smoking cessation. Overall, it is not clear that additional interventions to reduce drinking will necessarily impact smoking outcomes in a population of nondependent heavy drinkers.
There was some indication that ST-BI was more effective than ST in reducing the proportion of participants who had an alcohol-involved smoking lapse. However, this effect did not reach significance in the sample as a whole. Among the moderately heavy drinkers, for whom ST-BI was most effective, ST-BI did significantly reduce the odds of alcohol-involved smoking lapses relative to ST. Inclusion of alcohol-involved smoking lapses reduced the effect of the interaction between drinks per day and treatment condition by about 22%, making it nonsignificant. Thus, there was some support for reduced occurrences of alcohol-involved smoking lapses being a potential mechanisms through which ST-BI produced superior smoking cessation outcomes for certain participants. More frequent and extended contacts with participants during the early stages of quitting smoking, potentially through telephone contacts as suggested above, might act to augment the effect of ST-BI on reducing alcohol-involved lapse risk, ultimately leading to more robust effects of ST-BI on overall smoking outcomes.
Limitations and Conclusions
This study was the first of its kind to test the efficacy of a smoking cessation treatment for heavy drinkers that specifically incorporates detailed alcohol-specific intervention components. The treatments were equated on contact time and delivered as intended in a controlled randomized clinical trial with high follow-up rates and adequate sample size. However, the sample recruited lacked racial and ethnic diversity and was limited to smokers who were heavy drinkers but not alcohol dependent. Results can not be generalized to lighter drinkers or those with current alcohol dependence, and replication of these results in a more diverse sample is warranted. In particular, replicating the interaction between drinks per day at baseline and ST-BI would help clarify which smokers are most likely to benefit from ST-BI. Further investigation of potential mechanisms of action of ST-BI also is needed as such investigations can suggest directions for refining the interventions used with this population of smokers. For example, in the present study, assessment of whether drinking was occurring at the time of a smoking lapse was conducted retrospectively, and the number of drinks consumed prior to the smoking lapse was not recorded; ecological data collection techniques using handheld computers could better elucidate these alcohol consumption and smoking relationships. Such mechanistic investigations could help efforts to modify ST-BI to produce larger and more lasting effects on smoking outcomes.
Acknowledgments
This study was supported by grant R01 DA15534 from the National Institute on Drug Abuse to Christopher Kahler. Dr. Monti’s effort was supported in part by a Department of Veterans Affairs Senior Career Research Scientist Award. The authors gratefully acknowledge Andrea Resendes, Jennifer Larence, Dan Belenky, Catherine Costantino, Cheryl Eaton, Timothy Souza, and Kara Szczesny for their assistance on this project, as well as treatment providers John McGeary, Gail Schilke, and James MacKillop.
APPENDIX
The Consort E-Flowchart.
| PAPER SECTION And topic | Item | Description | Reported on Page # |
|---|---|---|---|
| TITLE & ABSTRACT | 1 | How participants were allocated to interventions (e.g., “random allocation”, “randomized”, or “randomly assigned”). | 1 & 2 |
| INTRODUCTION Background | 2 | Scientific background and explanation of rationale. | 3-5 |
| METHODS Participants | 3 | Eligibility criteria for participants and the settings and locations where the data were collected. | 5-6 |
| Interventions | 4 | Precise details of the interventions intended for each group and how and when they were actually administered. | 8-9 |
| Objectives | 5 | Specific objectives and hypotheses. | 5 |
| Outcomes | 6 | Clearly defined primary and secondary outcome measures and, when applicable, any methods used to enhance the quality of measurements (e.g., multiple observations, training of assessors). | 10-13 |
| Sample size | 7 | How sample size was determined and, when applicable, explanation of any interim analyses and stopping rules. | 6 |
| Randomization -- Sequence generation | 8 | Method used to generate the random allocation sequence, including details of any restrictions (e.g., blocking, stratification) | 7 |
| Randomization -- Allocation concealment | 9 | Method used to implement the random allocation sequence (e.g., numbered containers or central telephone), clarifying whether the sequence was concealed until interventions were assigned. | 7 |
| Randomization -- Implementation | 10 | Who generated the allocation sequence, who enrolled participants, and who assigned participants to their groups. | 7 |
| Blinding (masking) | 11 | Whether or not participants, those administering the interventions, and those assessing the outcomes were blinded to group assignment. When relevant, how the success of blinding was evaluated. | 8 |
| Statistical methods | 12 | Statistical methods used to compare groups for primary outcome(s); Methods for additional analyses, such as subgroup analyses and adjusted analyses. | 14-15 |
| RESULTS Participant flow | 13 | Flow of participants through each stage (a diagram is strongly recommended). Specifically, for each group report the numbers of participants randomly assigned, receiving intended treatment, completing the study protocol, and analyzed for the primary outcome. Describe protocol deviations from study as planned, together with reasons. | 7 and 37 |
| Recruitment | 14 | Dates defining the periods of recruitment and follow-up. | 7 |
| Baseline data | 15 | Baseline demographic and clinical characteristics of each group. | 15-16 |
| Numbers analyzed | 16 | Number of participants (denominator) in each group included in each analysis and whether the analysis was by “intention-to-treat”. State the results in absolute numbers when feasible (e.g., 10/20, not 50%). | 11-12 |
| Outcomes and estimation | 17 | For each primary and secondary outcome, a summary of results for each group, and the estimated effect size and its precision (e.g., 95% confidence interval). | 17-20 |
| Ancillary analyses | 18 | Address multiplicity by reporting any other analyses performed, including subgroup analyses and adjusted analyses, indicating those pre-specified and those exploratory. | 20-21 |
| Adverse events | 19 | All important adverse events or side effects in each intervention group. | 16 |
| DISCUSSION Interpretation | 20 | Interpretation of the results, taking into account study hypotheses, sources of potential bias or imprecision and the dangers associated with multiplicity of analyses and outcomes. | 21-26 |
| Generalizability | 21 | Generalizability (external validity) of the trial findings. | 25-26 |
| Overall evidence | 22 | General interpretation of the results in the context of current evidence. | 21 and 25 |
Footnotes
Publisher's Disclaimer: The following manuscript is the final accepted manuscript. It has not been subjected to the final copyediting, fact-checking, and proofreading required for formal publication. It is not the definitive, publisher-authenticated version. The American Psychological Association and its Council of Editors disclaim any responsibility or liabilities for errors or omissions of this manuscript version, any version derived from this manuscript by NIH, or other third parties. The published version is available at http://www.apa.org/journals/ccp/
Contributor Information
Christopher W. Kahler, Center for Alcohol and Addiction Studies, Brown University
Jane Metrik, Center for Alcohol and Addiction Studies, Brown University.
Heather R. LaChance, National Jewish Medical and Research Center
Susan E. Ramsey, Warren Alpert Medical School of Brown University and Rhode Island Hospital
David B. Abrams, Office of Behavioral and Social Sciences Research, National Institutes of Health
Peter M. Monti, Providence VA Medical Center and the Center for Alcohol and Addiction Studies, Brown University
Richard A. Brown, Warren Alpert Medical School of Brown University and Butler Hospital
References
- Baer JS, Lichtenstein E. Classification and prediction of smoking relapse episodes: An exploration of individual differences. Journal of Consulting and Clinical Psychology. 1988;56:104–110. doi: 10.1037//0022-006x.56.1.104. [DOI] [PubMed] [Google Scholar]
- Borland R. Slip-ups and relapse in attempts to quit smoking. Addictive Behaviors. 1990;15:235–245. doi: 10.1016/0306-4603(90)90066-7. [DOI] [PubMed] [Google Scholar]
- Carmelli D, Swan GE, Robinette D. The relationship between quitting smoking and changes in drinking in World War II veteran twins. Journal of Substance Abuse. 1993;5:103–116. doi: 10.1016/0899-3289(93)90055-g. [DOI] [PubMed] [Google Scholar]
- Carroll KM, Nich C, Sifry RL, Nuro KF, Frankforter TL, Ball SA, et al. A general system for evaluating therapist adherence and competence in psychotherapy research in the addictions. Drug and Alcohol Dependence. 2000;57:225–238. doi: 10.1016/s0376-8716(99)00049-6. [DOI] [PubMed] [Google Scholar]
- Chinn S. A simple method for converting an odds ratio to effect size for use in meta-analysis. Statistics in Medicine. 2000;19:3127–3131. doi: 10.1002/1097-0258(20001130)19:22<3127::aid-sim784>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Chiolero A, Wietlisbach V, Ruffieux C, Paccaud F, Cornuz J. Clustering of risk behaviors with cigarette consumption: A population-based survey. Preventive Medicine. 2006;42:348–353. doi: 10.1016/j.ypmed.2006.01.011. [DOI] [PubMed] [Google Scholar]
- Clifford PR, Maisto SA, Davis CM. Alcohol treatment research assessment exposure subject reactivity effects: part I. Alcohol use and related consequences. Journal of Studies on Alcohol and Drugs. 2007;68:519–528. doi: 10.15288/jsad.2007.68.519. [DOI] [PubMed] [Google Scholar]
- Cohen J, Cohen P, West SG, Aiken LS. Applied multiple regression/correlation analysis for the behavioral sciences. 3. Mahwah, NJ: Erlbaum; 2003. [Google Scholar]
- Covey LS, Glassman AH, Jiang H, Fried J, Masmela J, Loduca C, et al. A randomized trial of bupropion and/or nicotine gum as maintenance treatment for preventing smoking relapse. Addiction. 2007;102:1292–1302. doi: 10.1111/j.1360-0443.2007.01887.x. [DOI] [PubMed] [Google Scholar]
- Dawson DA. Drinking as a risk factor for sustained smoking. Drug and Alcohol Dependence. 2000;59:235–249. doi: 10.1016/s0376-8716(99)00130-1. [DOI] [PubMed] [Google Scholar]
- Durazzo TC, Cardenas VA, Studholme C, Weiner MW, Meyerhoff DJ. Non-treatment-seeking heavy drinkers: Effects of chronic cigarette smoking on brain structure. Drug and Alcohol Dependence. 2007;87:76–82. doi: 10.1016/j.drugalcdep.2006.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebbert JO, Janney CA, Sellers TA, Folsom AR, Cerhan JR. The association of alcohol consumption with coronary heart disease mortality and cancer incidence varies by smoking history. Journal of General Internal Medicine. 2005;20:14–20. doi: 10.1111/j.1525-1497.2005.40129.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falk DE, Yi HY, Hiller-Sturmhofel S. An epidemiologic analysis of co-occurring alcohol and tobacco use and disorders: findings from the National Epidemiologic Survey on Alcohol and Related Conditions. Alcohol Research & Health. 2006;29:162–171. [PMC free article] [PubMed] [Google Scholar]
- Fiore MC, Bailey WC, Cohen SJ, et al. Treating Tobacco Use and Dependence: Clinical Practice Guideline. Rockville, MD: U.S. Department of Health and Human Services. Public Health Service; 2000. [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders. New York: New York State Psychiatric Institute; 1995. [Google Scholar]
- Fox NL, Sexton MJ, Hebel JR. Alcohol consumption among pregnant smokers: Effects of a smoking cessation intervention program. American Journal of Public Health. 1987;77:211–213. doi: 10.2105/ajph.77.2.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman GD, Tekawa I, Klatsky AL, Sidney S, Armstrong MA. Alcohol drinking and cigarette smoking: An exploration of the association in middle-aged men and women. Drug and Alcohol Dependence. 1991;27:283–290. doi: 10.1016/0376-8716(91)90011-m. [DOI] [PubMed] [Google Scholar]
- Garvey AJ, Bliss RE, Hitchcock JL, Heinold JW, Rosner B. Predictors of smoking relapse among self-quitters: A report from the normative aging study. Addictive Behaviors. 1992;17:367–377. doi: 10.1016/0306-4603(92)90042-t. [DOI] [PubMed] [Google Scholar]
- Hall SM, Delucchi K, Velicer W, Kahler C, Ranger-Moore J, Hedeker D, Tsoh J, Niaura R. Statistical analysis of randomized trials in tobacco treatment: Longitudinal designs with dichotomous outcome. Nicotine & Tobacco Research. 2001;3:193–202. doi: 10.1080/14622200110050411. [DOI] [PubMed] [Google Scholar]
- Hall SM, Humfleet GL, Reus VI, Munoz RF, Cullen J. Extended nortriptyline and psychological treatment for cigarette smoking. American Journal of Psychiatry. 2004;161:2100–2107. doi: 10.1176/appi.ajp.161.11.2100. [DOI] [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom KO. The Fagerstrom test for nicotine dependence: A revision of the Fagerstrom Tolerance Questionnaire. British Journal of Addiction. 1991;86:1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Hughes JR, Oliveto AH. Coffee and alcohol intake as predictors of smoking cessation and tobacco withdrawal. Journal of Substance Abuse. 1993;5:305–310. doi: 10.1016/0899-3289(93)90072-j. [DOI] [PubMed] [Google Scholar]
- Humfleet G, Munoz R, Sees K, Reus V, Hall S. History of alcohol or drug problems, current use of alcohol or marijuana, and success in quitting smoking. Addictive Behaviors. 1999;24:149–154. doi: 10.1016/s0306-4603(98)00057-4. [DOI] [PubMed] [Google Scholar]
- Hymowitz N, Cummings KM, Hyland A, Lynn WR, Pechacek TF, Hartwell TD. Predictors of smoking cessation in a cohort of adult smokers followed for five years. Tobacco Control. 1997;6(Suppl 2):S57–62. doi: 10.1136/tc.6.suppl_2.s57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph AM, Willenbring ML, Nugent SM, Nelson DB. Randomized trial of concurrent versus delayed smoking intervention for patients in alcohol dependence treatment. Journal of Studies on Alcohol. 2004;65:681–691. doi: 10.15288/jsa.2004.65.681. [DOI] [PubMed] [Google Scholar]
- Kahler CW, LaChance HR, Strong DR, Ramsey SE, Monti PM, Brown RA. The commitment to quitting smoking scale: Initial validation in a smoking cessation trial for heavy social drinkers. Addictive Behaviors. 2007;32:2420–2424. doi: 10.1016/j.addbeh.2007.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahler CW, Resendes A, Monti PM, Ramsey SE, Abrams DA, Brown RA. Alcohol use and smoking outcomes among heavy drinkers in smoking cessation treatment; Paper presented at the Society for Research on Nicotine and Tobacco Annual Meeting; Prague, Czech Republic. 2005. [Google Scholar]
- Karlamangla A, Zhou K, Reuben D, Greendale G, Moore A. Longitudinal trajectories of heavy drinking in adults in the United States of America. Addiction. 2006;101:91–99. doi: 10.1111/j.1360-0443.2005.01299.x. [DOI] [PubMed] [Google Scholar]
- Lancaster T, Hajek P, Stead LF, West R, Jarvis MJ. Prevention of relapse after quitting smoking: a systematic review of trials. Archives of Internal Medicine. 2006;166:828–835. doi: 10.1001/archinte.166.8.828. [DOI] [PubMed] [Google Scholar]
- Liang K-Y, Zeger SL. Longitudinal data analysis using generalized linear models. Biometrika. 1986;73:13–22. [Google Scholar]
- McCrady BS. Alcohol use disorders and the Division 12 Task Force of the American Psychological Association. Psychology of Addictive Behaviors. 2000;14:277–286. [PubMed] [Google Scholar]
- Meyerhoff DJ, Tizabi Y, Staley JK, Durazzo TC, Glass JM, Nixon SJ. Smoking comorbidity in alcoholism: neurobiological and neurocognitive consequences. Alcoholism: Clinical and Experimental Research. 2006;30:253–264. doi: 10.1111/j.1530-0277.2006.00034.x. [DOI] [PubMed] [Google Scholar]
- Miller WR, Rollnick S. Motivational interviewing: Preparing people for change. 2. New York: Guilford Press; 2002. [Google Scholar]
- Moyer A, Finney JW, Swearingen CE, Vergun P. Brief interventions for alcohol problems: a meta-analytic review of controlled investigations in treatment-seeking and non-treatment-seeking populations. Addiction. 2002;97:279–292. doi: 10.1046/j.1360-0443.2002.00018.x. [DOI] [PubMed] [Google Scholar]
- Murray RP, Istvan JA, Voelker HT. Does cessation of smoking cause a change in alcohol consumption? Evidence from the Lung Health Study. Substance Use and Misuse. 1996;31:141–156. doi: 10.3109/10826089609045804. [DOI] [PubMed] [Google Scholar]
- Murray RP, Istvan JA, Voelker HT, Rigdon MA, Wallace MD. Level of involvement with alcohol and success at smoking cessation in the lung health study. Journal of Studies on Alcohol. 1995;56:74–82. doi: 10.15288/jsa.1995.56.74. [DOI] [PubMed] [Google Scholar]
- National Institute on Alcohol Abuse and Alcoholism. Vol. NIH Publication No 95-3769. National Institutes of Health; 1995. The physicians’ guide to helping patients with alcohol problems. [Google Scholar]
- Nothwehr F, Lando HA, Bobo JK. Alcohol and tobacco use in the Minnesota Heart Health Program. Addictive Behaviors. 1995;20:463–470. doi: 10.1016/0306-4603(95)00008-z. [DOI] [PubMed] [Google Scholar]
- Ockene JK, Emmons KM, Mermelstein RJ, Perkins KA, Bonollo DS, Voorhees CC, et al. Relapse and maintenance issues for smoking cessation. Health Psychology. 2000;19:17–31. doi: 10.1037/0278-6133.19.suppl1.17. [DOI] [PubMed] [Google Scholar]
- Osler M, Prescott E, Godtfredsen N, Hein HO, Schnohr P. Gender and determinants of smoking cessation: A longitudinal study. Preventive Medicine. 1999;29:57–62. doi: 10.1006/pmed.1999.0510. [DOI] [PubMed] [Google Scholar]
- Pelucchi C, Gallus S, Garavello W, Bosetti C, La Vecchia C. Cancer risk associated with alcohol and tobacco use: focus on upper aero-digestive tract and liver. Alcohol Research & Health. 2006;29:193–198. [PMC free article] [PubMed] [Google Scholar]
- Prochaska JJ, Delucchi K, Hall SM. A meta-analysis of smoking cessation interventions with individuals in substance abuse treatment or recovery. Journal of Consulting and Clinical Psychology. 2004;72:1144–1156. doi: 10.1037/0022-006X.72.6.1144. [DOI] [PubMed] [Google Scholar]
- Rochon J. Application of GEE procedures for sample size calculations in repeated measures experiments. Statistics in Medicine. 1998;17:1643–1658. doi: 10.1002/(sici)1097-0258(19980730)17:14<1643::aid-sim869>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- SAS Institute Inc. SAS/STAT Software: Changes and Enhancements through Release 6.12. Cary, NC: SAS; 1997. [Google Scholar]
- Schroder H, Marrugat J, Elosua R, Covas MI. Tobacco and alcohol consumption: impact on other cardiovascular and cancer risk factors in a southern European Mediterranean population. British Journal of Nutrition. 2002;88:273–281. doi: 10.1079/BJN2002655. [DOI] [PubMed] [Google Scholar]
- Sherman SE, Wang MM, Nguyen B. Predictors of success in a smoking cessation clinic. Journal of General Internal Medicine. 1996;11:702–704. doi: 10.1007/BF02600163. [DOI] [PubMed] [Google Scholar]
- Shiffman S. Relapse following smoking cessation: A situational analysis. Journal of Consulting and Clinical Psychology. 1982;50:71–86. doi: 10.1037//0022-006x.50.1.71. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Hufford M, Hickcox M, Paty JA, Gnys M, Kassel JD. Remember that? A comparison of real-time versus retrospective recall of smoking lapses. Journal of Consulting and Clinical Psychology. 1997;65:292–300. doi: 10.1037/0022-006x.65.2.292.a. [DOI] [PubMed] [Google Scholar]
- Smith PM, Kraemer HC, Miller NH, Debusk RF, Taylor CB. In-hospital smoking cessation programs: Who responds, who doesn’t? Journal of Consulting and Clinical Psychology. 1999;67:19–27. doi: 10.1037//0022-006x.67.1.19. [DOI] [PubMed] [Google Scholar]
- Sobell LC, Sobell MB. Timeline followback: A calendar method for assessing alcohol and drug use. Toronto, Canada: Addiction Research Foundation; 1996. [Google Scholar]
- Sorlie PD, Kannel WB. A description of cigarette smoking cessation and resumption in the Framingham study. Preventive Medicine. 1990;19:335–345. doi: 10.1016/0091-7435(90)90033-g. [DOI] [PubMed] [Google Scholar]
- SRNT Subcommittee on Biochemical Verification. Biochemical verification of tobacco use and cessation. Nicotine & Tobacco Research. 2002;4:149–159. doi: 10.1080/14622200210123581. [DOI] [PubMed] [Google Scholar]
- Wei I. Application of an urn model to the design of sequential controlled clinical trials. Journal of the American Statistical Association. 1978;73:559–563. [Google Scholar]
- West R. The clinical significance of “small” effect of smoking cessation treatments. Addiction. 2007;102:506–509. doi: 10.1111/j.1360-0443.2007.01750.x. [DOI] [PubMed] [Google Scholar]
- Whitlock EP, Polen MR, Green CA, Orleans T, Klein J. Behavioral counseling interventions in primary care to reduce risky/harmful alcohol use by adults: a summary of the evidence for the U.S. Preventive Services Task Force. Annals of Internal Medicine. 2004;140:557–568. doi: 10.7326/0003-4819-140-7-200404060-00017. [DOI] [PubMed] [Google Scholar]
- Zimmerman RS, Warheit GJ, Ulbrich PM, Buhl Auth J. The relationship between alcohol use and attempts and success at smoking cessation. Addictive Behaviors. 1990;15:197–207. doi: 10.1016/0306-4603(90)90063-4. [DOI] [PubMed] [Google Scholar]