Abstract
Context
Little is known about the population-based prevalence of neuropsychiatric symptoms in mild cognitive impairment (MCI).
Objective
To estimate the prevalence of neuropsychiatric symptoms in MCI and normal cognitive aging in a defined population.
Design
Cross-sectional study derived from an ongoing population-based prospective cohort study.
Setting
The Mayo Clinic Study of Aging.
Participants
We studied a random sample of 1969 non-demented participants out of the target population of 9965 elderly persons residing in Olmsted County on the prevalence date (October 1, 2004). Neuropsychiatric data were available on 319 of the 329 MCI subjects (97.0%) and on 1590 of the 1640 cognitively normal subjects (97.0%).
Method
Neurological, cognitive, and neuropsychiatric data were gathered from the study participants. A classification of normal cognitive aging, MCI, and dementia was adjudicated by an expert consensus panel. Accordingly, 329 subjects were classified as having MCI and the remaining 1640 subjects were classified as cognitively normal.
Main Outcome Measure
The Neuropsychiatric Inventory Questionnaire (NPI-Q).
Results
Multi-variable logistic regression analyses were conducted, after adjusting for age, sex, and education. By taking into consideration both the odds ratio and the frequency of a symptom, the most distinguishing features between the 2 groups were apathy (odds ratio [OR], 4.53; 95% confidence interval [95% CI], 3.11–6.60; P<.001), agitation (OR, 3.60; 95% CI, 2.18–5.92; P<.001), anxiety (OR, 3.00; 95% CI, 2.01–4.48; P<.001), irritability (OR, 2.99; 95% CI, 2.11–4.22; P<.001), and depression (OR, 2.78; 95% CI, 2.06–3.76; P<.001). Delusion had the highest OR (8.12; 95% CI, 2.92–22.60; P<.001); however, it was rare in both cognitively normal subjects (6/1590=0.4%) and MCI (11/319=3.4%). Thus, the population attributable risk for delusion was only 2.62% as compared to 14.60% for apathy.
Conclusions
Non-psychotic symptoms affected approximately 50% of subjects with MCI and 25% of cognitively normal subjects. By contrast, psychotic symptoms were rare.
Mild cognitive impairment (MCI) is the transitional state between normal cognitive aging and dementia.1–3 However, various other terms have been proposed to describe this transitional state.4–9 Subjects with MCI constitute a high risk group because they develop dementia at a rate of 10 to 15% per year as compared to 1 to 2% per year in the general population.10
The original operational definition of MCI focused on amnestic MCI: 1) a memory complaint preferably corroborated by an informant; 2) impaired memory for age on psychometric testing; 3) normal general cognitive function; 4) intact activities of daily living; 5) absence of dementia.1 Even though amnestic MCI is the most widely investigated and empirically validated construct, the first international consensus panel on MCI has also endorsed the non-amnestic construct proposed by Petersen et al.11 The reader is referred elsewhere for a detailed definition and classification of MCI.11,12
We and others have reported the frequency of neuropsychiatric symptoms in MCI clinical settings, but little is known about these estimates in a population-based setting. The Cardiovascular Health Study (CHS) group reported the first prevalence estimate of neuropsychiatric symptoms in MCI.13 However, the study did not include normal controls from the same population; therefore, the investigators compared the prevalence of neuropsychiatric symptoms in patients with MCI recruited in one population with the prevalence in normal controls from a study conducted in a different population by other investigators.14 By contrast, we measured the prevalence of neuropsychiatric symptoms in subjects with MCI as well as in cognitively normal subjects from the same population as part of an ongoing population-based study in Olmsted County, Minnesota.15
METHODS
STUDY DESIGN
We conducted a cross-sectional case-control study comparing 319 subjects with MCI to 1590 cognitively normal elderly persons. Both cases with MCI and cognitively normal controls were identified as part of the Mayo Clinic Study of Aging. The Mayo Clinic Study of Aging is a population-based investigation designed to estimate the prevalence and incidence of MCI in Olmsted County. Extensive details about the design and conduct of the study were reported elsewhere.15 Here, we briefly describe the study design and methodology directly pertinent to the neuropsychiatric study. October 1, 2004, was selected as the prevalence date and elderly individuals were recruited by using a stratified random sampling from the target population of nearly 10 000 elderly individuals in Olmsted County. We used equal allocation of men and women in 2 age strata, i.e., 70 to 79 and 80 to 89 years old.15
The study was approved by the Institutional Review Boards (IRB) of the Mayo Clinic and Olmsted Medical Center. All participants underwent neurological, neuropsychiatric, and psychometric evaluations. An expert consensus panel of physicians, nurses, and psychologists determined the classification of subjects as having normal cognitive aging, MCI, or dementia based on published criteria.16,17,1 Subjects with dementia were excluded from the case-control comparison.
NEUROPSYCHIATRIC ASSESSMENT
The Neuropsychiatric Inventory Questionnaire (NPI-Q) was administered to a spouse or another informant for all study participants.18 The NPI-Q is a shorter version of the Neuropsychiatric Inventory (NPI) which is a structured interview with established reliability and validity.19 Both NPI and NPI-Q measure 12 emotional behavioral domains. We chose to use NPI-Q because it was selected by the Uniform Data Set initiative of the National Institute on Aging.20
Data regarding observed emotional behavior in the NPI-Q were gathered from a spouse or an informant knowledgeable about the study participant. The structured interview addressed 12 neuropsychiatric domains, i.e., agitation, delusion, hallucination, depression, anxiety, euphoria, apathy, disinhibition, irritability, aberrant motor behavior, sleep, and eating/appetite. There was a “yes” or “no” screening question for each domain. If the respondent answered affirmatively, then further questions were asked in order to rate the symptom in terms of severity (1=mild, 2=moderate, 3=severe). Thus, the total score for symptom severity would add up to a maximum score of 36.
CRITERIA FOR MCI
Subjects who were neither demented nor cognitively normal were classified as having MCI according to published criteria.11 The criteria were the following: 1) cognitive concern expressed by a physician, informant, participant, or nurse; 2) cognitive impairment in 1 or more domains (executive function, memory, language, or visuospatial); 3) normal functional activities; and 4) not demented. Subjects with MCI could have a CDR of 0 or 0.5; however, the final diagnosis of MCI was not based exclusively on the CDR, but rather on all available data. Subjects were further classified as having amnestic or non-amnestic MCI, as having single or multiple domain MCI, and according to the presumed etiology of MCI (e.g., degenerative, vascular, trauma, or psychiatric). The diagnosis of normal cognition, MCI, dementia, or AD was made by consensus, taking into account all the data collected. If the information obtained by 1 of the 3 evaluators (nurse, physician, or psychometrist) was not consistent with the final diagnosis, this was noted as discordance.15
STATISTICAL ANALYSIS
We compared the prevalence of neuropsychiatric symptoms in subjects with normal cognitive aging and in subjects with MCI using multi-variable logistic regression analysis to adjust for age (continuous variable), sex, and education (years of education as a continuous variable). We quantified the magnitude of the association between a specific neuropsychiatric symptom and MCI by computing the odds ratios (OR) and the corresponding 95% confidence intervals (95% CI). We also computed a population attributable risk (expressed as percent) using the formula [P(OR-1)] ÷ [1 + P(OR-1)] × 100, where P is the prevalence of the neuropsychiatric symptom in cognitively normal subjects.21 The population attributable risk takes into account both the frequency of a particular neuropsychiatric domain and the magnitude of the corresponding OR. Thus, we used the attributable risk to order the symptoms by overall importance. We also conducted stratified analyses by MCI types (amnestic MCI vs. non-amnestic MCI). Statistical testing was done at the conventional 2-tailed alpha level of 0.05. All analyses were performed using SAS® version 8.2 (SAS Institute, Cary, NC).
Additionally, we conducted 2 sets of sensitivity analyses to examine potential sources of bias. In particular, we computed propensity scores to investigate bias in 2 scenarios. First, we considered missing data as a potential source of bias. Understandably, some neuropsychiatric symptoms such as night-time behavior were prone to missing data. This resulted either from absence of an informant or from the informant being unable to recognize the symptom. This happened even though nearly 90% of the informants were spouses. Second, we considered refusal to participate in the study as a potential source of bias. It is possible that refusers might be systematically different from participants. Details on the calculation of propensity scores were published elsewhere.22–24 Our propensity scores were based on age (continuous variable), sex, and education (continuous variable).
RESULTS
Between October 1, 2004, and September 1, 2007, a total of 1969 non-demented participants were randomly selected and gave their consent for the study. There were 329 subjects with MCI and 1940 cognitively normal subjects. Neuropsychiatric data were available on 319 of the 329 participants with MCI (97.0%) and 1590 of the 1640 cognitively normal persons (97.0%). Table 1 summarizes the demographic data. There were almost equal number of men and women among cognitively normal subjects; however, there were more men in the MCI group. As expected, subjects with MCI were older than cognitively normal subjects. Hence, we controlled for age (as a continuous variable) by entering it as a covariate in the multivariate analysis. Within the MCI group, there were more subjects with amnestic MCI (232/319 = 72.7%) than with non-amnestic MCI (87/319 = 27.3%). Within the amnestic MCI group, 61.2% were men whereas within the non-amnestic MCI group, 47.1% were men.
Table 1.
Demographics Characteristics of Study Participants
| Normal (n=1590) | MCI (n=319) | P Value | |
|---|---|---|---|
| Men, n (%) | 791 (49.7) | 183 (57.4) | .013 |
| Age, y, median (range) | 79 (70–91) | 82 (70–91) | <.001 |
| 70–79, n (%) | 816 (51.3) | 100 (31.3) | |
| 80–91, n (%) | 774 (48.7) | 219 (68.7) | |
| Education, y, median (range) | 13 (5–25) | 12 (2–25) | <.001 |
| >12 y, n (%) | 869 (54.7) | 135 (42.3) |
The median education of the cognitively normal group was 13 years and that of the MCI group was 12 years (P< 001). The difference between the 2 groups remained significant when education was dichotomized at 12 years of education (P< 001). All the analyses that compared the OR of neuropsychiatric symptoms between the MCI and cognitively normal group were adjusted by age, sex, and education.
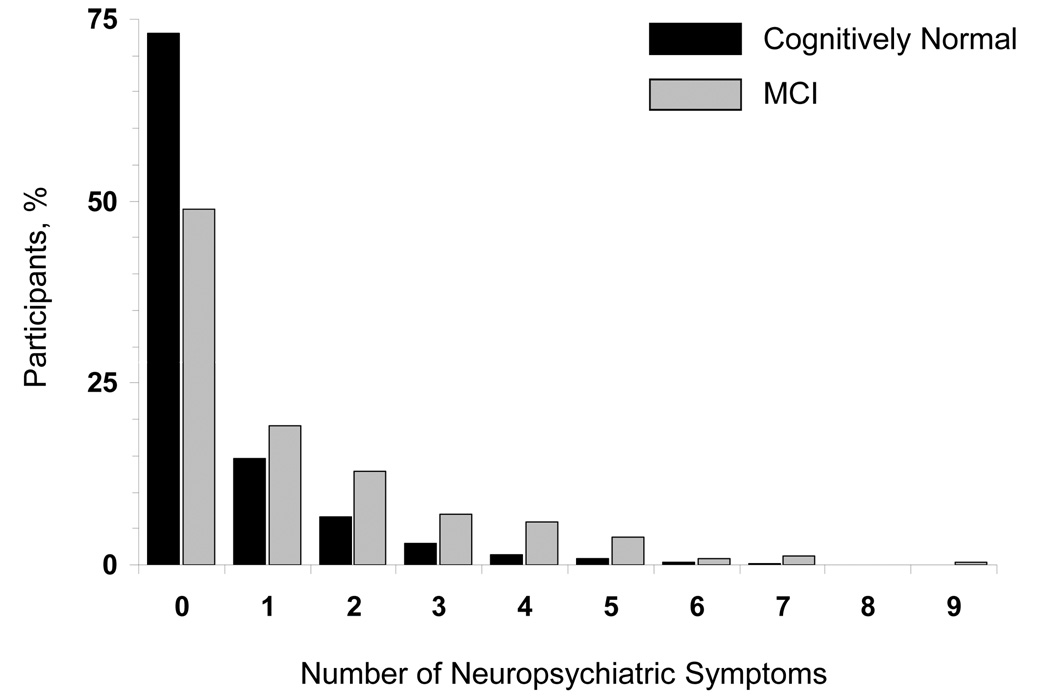
Table 2 displays the frequency of neuropsychiatric symptoms in MCI and cognitively normal elderly participants, along with odds ratios, associated 95% CI, and P values. About 51% of MCI subjects and 27% of cognitively normal subjects had 1 or more neuropsychiatric symptoms (Figure). The prevalence of neuropsychiatric symptoms in MCI subjects was significantly higher than in cognitively normal persons; however, there was no difference between the 2 groups regarding hallucination and aberrant motor behavior. Symptoms were ordered by descending magnitude of the population attributable risk which takes into consideration both the frequency of a symptom and the magnitude of the OR. The most distinguishing neuropsychiatric features between MCI and cognitively normal controls were apathy (OR, 4.53; 95% CI, 3.11–6.60; P<.001), followed by agitation (OR, 3.60; 95% CI, 2.18–5.92; P<.001), anxiety OR, 3.00; 95% CI, 2.01–4.48; P<.001), irritability (OR, 2.98; 95% CI, 2.11–4.22; P<.001), and depression (OR, 2.78; 95% CI, 2.06–3.76; P<.001). Delusions, hallucinations, and euphoria were rare events in MCI and virtually absent in the cognitively normal group. For instance, delusion was present in 11 out of 319 MCI subjects (3.4%), and in 6 out of 1590 cognitively normal subjects (0.4%). Thus, the OR of delusion was large and the corresponding CI was wide (OR, 8.12; 95% CI, 2.92–22.6; P<.001). However, the population attributable risk for delusion was only 2.62% as compared to 14.60% for apathy. There were relatively more events for disinhibition, with 15/319 (4.7%) in MCI and 26/1590 (1.6%) in the cognitively normal group. There was no difference between the 2 groups regarding hallucinations (P=.693).
Table 2.
Prevalence of Neuropsychiatric Symptoms in Cognitively Normal Subjects and Subjects with MCI
| NPI Domain | Normal (n=1590) n (%) | MCI (n=319) n (%) | OR (95% CI)* | P Value | Population Attributable Risk† (%) |
|---|---|---|---|---|---|
| Depression/Dysphoria | 182 (11.4) | 86 (27.0) | 2.78 (2.06–3.76) | <.001 | 16.96 |
| Apathy/Indifference | 77 (4.8) | 59 (18.5) | 4.53 (3.11–6.60) | <.001 | 14.60 |
| Irritability/Lability | 121 (7.6) | 62 (19.4) | 2.99 (2.11–4.22) | <.001 | 13.13 |
| Anxiety | 80 (5.0) | 45 (14.1) | 3.00 (2.01–4.48) | <.001 | 9.13 |
| Night-time behavior‡ | 141 (10.9) | 49 (18.3) | 1.80 (1.25–2.60) | .002 | 8.07 |
| Agitation | 45 (2.8) | 29 (9.1) | 3.60 (2.18–5.92) | <.001 | 6.84 |
| Appetite/Eating change | 84 (5.3) | 34 (10.7) | 2.02 (1.31–3.10) | .001 | 5.11 |
| Disinhibition | 26 (1.6) | 15 (4.7) | 2.74 (1.42–5.32) | .003 | 2.77 |
| Delusions | 6 (0.4) | 11 (3.4) | 8.12 (2.92–22.6) | <.001 | 2.62 |
| Euphoria/Elation | 7 (0.4) | 4 (1.3) | 3.58 (1.02–12.6) | .047 | 1.12 |
| Aberrant motor behavior | 9 (0.6) | 4 (1.3) | 2.30 (0.70–7.61) | .171 | 0.73 |
| Hallucinations | 6 (0.4) | 2 (0.6) | 1.39 (0.27–7.05) | .693 | 0.15 |
OR and 95% CI adjusted for age (continuous variable) sex, and education (continuous variable).
Population attributable risk (expressed as percent) was calculated by using the formula [P(OR-1)] ÷ [1 + P(OR-1)] × 100, where P is the prevalence of the neuropsychiatric symptom in cognitively normal subjects.21
353 subjects did not have night-time behavior data because their informant was unable to assess. The proportion without data was similar in both groups: 19.0% of cognitively normal patients and 16.0% of patients with MCI, P=.21. Sensitivity analyses adjusted for missing data using propensity scores yielded similar results (OR, 1.79; 95% CI, 1.24–2.58).
Figure.
Distribution by number of neuropsychiatric symptoms in cognitively normal subjects and subjects with MCI.
Table 3 displays stratified analyses by MCI subtypes. These analyses were conducted to explore whether the prevalence of neuropsychiatric symptoms varied by MCI subtype. There were 232 subjects with amnestic and 87 subjects with non-amnestic MCI. The comparison between amnestic and non-amnestic MCI was made by computing ORs and the corresponding 95% CIs for each neuropsychiatric domain. The OR was computed by comparing subjects with a specific MCI subtype with all the cognitively normal subjects. Hallucinations were not significantly associated in either group. The OR and 95% CI for euphoria approached significance in the amnestic MCI group (OR, 2.44; 95% CI, 0.49–12.2; P=.276) whereas it was significant in the non-amnestic MCI group (OR, 6.64; 95% CI, 1.33–33.1; P=.021). However, one should interpret this finding with caution because euphoria was a rare event (2 persons with euphoria out of 232 individuals with amnestic MCI and 2 persons with euphoria out of 87 individuals with non-amnestic MCI).
Table 3.
Prevalence of Neuropsychiatric Symptoms in Subjects with Amnestic MCI or with Non-Amnestic MCI
| Amnestic MCI |
Non-Amnestic MCI |
|||||
|---|---|---|---|---|---|---|
| NPI Domain | (n=232) n (%) | OR (95% CI)* | P Value | (n=87) n (%) | OR (95% CI)* | P Value |
| Apathy/Indifference | 48 (20.7) | 5.17 (3.44–7.77) | <.001 | 11 (12.6) | 2.82 (1.42–5.58) | .003 |
| Irritability/Lability | 46 (19.8) | 2.96 (2.01–4.36) | <.001 | 16 (18.4) | 2.89 (1.61–5.20) | <.001 |
| Agitation | 22 (9.5) | 3.79 (2.20–6.54) | <.001 | 7 (8.0) | 3.30 (1.42–7.69) | .006 |
| Depression/Dysphoria | 60 (25.9) | 2.66 (1.88–3.74) | <.001 | 26 (29.9) | 3.14 (1.92–5.12) | <.001 |
| Anxiety | 32 (13.8) | 2.97 (1.89–4.66) | <.001 | 13 (14.9) | 3.05 (1.61–5.79) | <.001 |
| Night-time behavior | 34 (17.3) | 1.65 (1.08–2.51) | .020 | 15 (20.8) | 2.13 (1.16–3.89) | .014 |
| Disinhibition | 10 (4.3) | 2.42 (1.13–5.16) | .023 | 5 (5.7) | 3.53 (1.30–9.57) | .013 |
| Delusions | 6 (2.6) | 6.65 (2.07–21.4) | .001 | 5 (5.7) | 12.7 (3.70–43.6) | <.001 |
| Euphoria/Elation | 2 (0.9) | 2.44 (0.49–12.2) | .276 | 2 (2.3) | 6.64 (1.33–33.1) | .021 |
| Appetite/Eating change | 25 (10.8) | 1.98 (1.23–3.21) | .005 | 9 (10.3) | 1.97 (0.95–4.11) | .070 |
| Aberrant motor behavior | 2 (0.9) | 1.66 (0.35–7.80) | .522 | 2 (2.3) | 4.43 (0.92–21.2) | .063 |
| Hallucinations | 1 (0.4) | 0.85 (0.10–7.23) | .883 | 1 (1.1) | 2.72 (0.32–23.3) | .362 |
OR and 95% CI adjusted for age (continuous variable) sex, and education (continuous variable). The whole group of cognitively normal subjects (n=1590) was used for comparison (see Table 2 for frequencies).
The OR and 95% CI for apathy were higher in amnestic MCI (OR, 5.17; 95% CI, 3.44–7.77; P<.001) than in non-amnestic MCI (OR, 2.82; 95% CI, 1.42–5.58; P=.003). Similarly, the OR for agitation and irritability were slightly higher for amnestic MCI than for non-amnestic MCI. On the other hand, the OR for depression, anxiety, disinhibition, and delusion were higher in non-amnestic than in amnestic MCI. The OR of delusion for the non-amnestic group (OR, 12.7; 95% CI, 3.70–43.6; P<.001) was almost twice that for the amnestic MCI type (OR, 6.65; 95% CI, 2.07–21.4; P=.001); however, the symptom was rare in both groups. The OR for appetite was comparable between the 2 groups.
SENSITIVITY ANALYSIS
We used the demographic data reported in this manuscript (age, sex, and education) to compute propensity scores for each subject. We then used these scores in analyses that weighted the data more heavily towards subjects with higher propensity for missing data or for refusal to participate in the study. We performed 2 sets of sensitivity analyses. In the first set of analyses, we adjusted the observed results back to the complete dataset of 1969 subjects who participated in the study. In this analysis, the propensity score reflected the propensity of missing data on a per-variable basis. In the second set of analyses, we adjusted the observed results back to all subjects who were eligible for the study (1969 participants plus 1657 subjects who refused and 669 with partial participation).15 In neither of these assessments did we observe markedly different results before and after propensity adjustment.
We illustrate our findings using the missing data on night-time behavior. The primary analyses showed an odds ratio of 1.80 (95% CI, 1.25–2.60). The propensity-weighted analysis adjusted for missing data yielded an almost identical odds ratio of 1.79 (95% CI, 1.24–2.58). The other adjustments for missing data were even smaller because far fewer observations were missing for the other neuropsychiatric symptoms. In the analyses for refusal to participate, the adjustments back to all the individuals who were eligible for the study produced relatively minor differences in odds ratios. For only 1 of the symptoms (aberrant motor behavior), the adjustment resulted in a qualitatively different conclusion because the odds ratio increased from 2.30 (95% CI, 0.70–7.61; P=.171) to 3.17 (95% CI, 1.04–9.66; P=.042). However, even this difference was relatively small and statistically not significant.
COMMENT
We are reporting the prevalence of neuropsychiatric symptoms in 1590 cognitively normal elderly and 319 subjects with MCI randomly sampled from the elderly population residing in Olmsted County on the prevalence date (October 1, 2004). About 50% of subjects with MCI and approximately 25% of cognitively normal persons had 1 or more neuropsychiatric symptoms. After adjusting for age, sex, and education, and taking into consideration both the frequency of a symptom and its corresponding OR, the most distinguishing features between persons with MCI and cognitively normal persons were apathy, agitation, anxiety, irritability, and depression. Delusion had the highest OR but with a wide 95% CI because it was rare in both cognitively normal subjects (0.4%) and in MCI subjects (3.4%). The population attributable risk, which takes into consideration both the OR and the frequency of a symptom, was only 2.62% for delusion as compared to 14.60% for apathy.21
We also observed that the prevalence of apathy, agitation, and irritability were slightly higher in persons with amnestic MCI than in persons with non-amnestic MCI. On the other hand, depression and anxiety were slightly higher in persons with non-amnestic than in persons with amnestic MCI. Although delusion was a relatively rare event, the OR for delusion in non-amnestic MCI was almost twice that of amnestic MCI. Similarly, the OR for disinhibition was higher in non-amnestic MCI than in amnestic MCI. We hypothesize that apathy, agitation, and irritability may be neuropsychiatric markers of amnestic MCI that is likely to progress to AD, whereas symptoms such as delusion and disinhibition may be neuropsychiatric markers for progression of non-amnestic MCI to a non-AD dementia. This hypothesis needs to be tested in studies involving longitudinal follow-up of subjects over many years.
There have been a few studies on the frequency of neuropsychiatric symptoms in MCI conducted in clinical settings.25,26 For three reasons stated below, our study can be directly compared with the population-based study reported by Lyketsos and colleagues of the Cardiovascular Health Study (CHS) group.13 Between 1989 and 1994, the CHS collected data on cognition and neuropsychiatric symptoms from 3 counties in the East Coast and 1 county in the West Coast (East Coast: Washington County, Maryland; Allegheny County, Pennsylvania; and Forsyth County, North Carolina. West Coast: Sacramento County, California). The CHS group reported the first population-based estimate of the prevalence of neuropsychiatric symptoms in MCI.13 There are 3 grounds that permit the comparison of our findings with those of the CHS group. First, both studies were population-based. Second, both studies used essentially identical instruments to measure the 12 behavioral domains. CHS used the NPI19 to measure 12 emotional behaviors and we used the NPI-Q which measures exactly the same 12 behavioral domains.18 NPI-Q is a shorter version of NPI and has been selected by the Uniform Data Set (UDS) initiative of the National Institute on Aging (NIA).20 Third, both studies used similar criteria to measure MCI and also had comparable numbers of MCI subjects, i.e., 320 in CHS and 319 in our study. One major difference pertains to the cognitively normal persons. In our study, we were able to compare the MCI group with 1590 cognitively normal subjects from the same Olmsted County population whereas the CHS study did not have cognitively normal subjects from the same population. Thus, the CHS investigators compared the prevalence of neuropsychiatric symptoms in patients with MCI from the CHS study with the published data on the prevalence of neuropsychiatric symptoms among normal controls from Cache County, Utah.14
The CHS reported that the 3 most common neuropsychiatric symptoms in MCI were depression (20%), apathy (15%), and irritability (15%). Similarly, we found that the 3 most frequent neuropsychiatric symptoms in MCI were depression (27%), apathy 18.5%), and irritability (19.4%). Furthermore, the CHS group suggested that selection bias might have led to an underestimation of their prevalence estimates. This bias may account for the differences in crude frequency rates across the 2 studies. We could not make similar comparisons regarding neuropsychiatric prevalence estimates in cognitively normal persons, because as stated earlier, the CHS study did not have cognitively normal participants. The Cache County study reported prevalence figures for depression (7.2 %), apathy (3.2 %), and irritability of (4.6%) in cognitively normal persons.14 We did observe slightly higher figures for depression (11.5 %), apathy 4.8%), and irritability (7.6%). Some of these differences may be due to differences in age and sex distributions in the 2 samples.
There are several strengths to our study. First, we used a population-based sample involving a large number of study participants. Second, we measured MCI using a face-to-face evaluation adjudicated by an expert consensus panel at a clinical center that has a well established reputation for measuring MCI. Third, the neuropsychiatric symptoms were measured by an instrument similar to the one used by the CHS study, thus enabling us to make comparisons. Fourth, we have measured the prevalence of neuropsychiatric symptoms in both amnestic and non-amnestic MCI.
The study also has limitations. The NPI-Q gathered information from an informant who was knowledgeable about the participant. In our sample, 90% of the informants were spouses; nevertheless, it is still possible that an informant may over or under report neuropsychiatric symptoms. However, it is reassuring to know that despite its smaller sample size (number of MCI subjects = 47), a Swedish population-based study that used a structured face-to-face interview to measure neuropsychiatric symptoms, reported comparable frequencies of symptoms.27 In addition, our sensitivity analyses did not reveal bias emanating from either missing data (non-response) or non-participation in the study.
Our findings may have implications for future studies. A prospective follow-up of our patients will clarify whether MCI patients with neuropsychiatric symptoms are at greater risk of developing AD or other dementias than the neuropsychiatrically asymptomatic MCI subjects.27 Recent publications indicate that MCI is a heterogeneous entity that can evolve into different types of dementia.12 The most empirically validated type, amnestic MCI, evolves to AD at a higher rate than the general population. However, non-amnestic MCI could also evolve to AD or other types of dementias. We hypothesize that MCI subjects with disinhibition and/or delusion may be at increased risk of developing dementia including frontotemporal dementia or dementia with Lewy bodies. In addition, a prospective follow-up of the 1590 cognitively normal persons will allow us to investigate whether baseline neuropsychiatric symptoms are predictive of an increased risk of incident MCI.28
ACKNOWLEDGEMENT
Funding/Support: This study was supported by grants K01 MH68351, U01 AG-06786, K01 AG028573, R01 AR30582, and R01NS33978 from the National Institutes of Health, Bethesda, MD; and Robert H. and Clarice Smith and Abigail Van Buren Alzheimer’s Disease Research Program. The sponsors of this study had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
Footnotes
Financial Disclosures: No potential conflicts of interest relevant to this article were reported.
Data Access and Responsibility: Dr. Geda (the Principal Investigator who is a full-time employee of the College of Medicine, Mayo Clinic) had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
REFERENCES
- 1.Petersen RC, Smith GE, Waring SC, Ivnik RJ, Tangalos EG, Kokmen E. Mild cognitive impairment: clinical characterization and outcome. Arch Neurol. 1999;56:303–308. doi: 10.1001/archneur.56.3.303. [DOI] [PubMed] [Google Scholar]
- 2.Grundman M, Petersen RC, Ferris SH, Thomas RG, Aisen PS, Bennett DA, Foster NL, Jack CR, Jr, Galasko DR, Doody R, Kaye J, Sano M, Mohs R, Gauthier S, Kim HT, Jin S, Schultz AN, Schafer K, Mulnard R, van Dyck CH, Mintzer J, Zamrini EY, Cahn-Weiner D, Thal LJ. Mild cognitive impairment can be distinguished from Alzheimer disease and normal aging for clinical trials. Arch Neurol. 2004;61:59–66. doi: 10.1001/archneur.61.1.59. [DOI] [PubMed] [Google Scholar]
- 3.Petersen RC, Thomas RG, Grundman M, Bennett D, Doody R, Ferris S, Galasko D, Jin S, Kaye J, Levey A, Pfeiffer E, Sano M, van Dyck CH, Thal LJ. Vitamin E and donepezil for the treatment of mild cognitive impairment. N Engl J Med. 2005;352:2379–2388. doi: 10.1056/NEJMoa050151. [DOI] [PubMed] [Google Scholar]
- 4.Crook T, Bartus R, Ferris S, Whitehouse P, Cohen G, Gershon S. Age-associated memory impairment: proposed diagnostic criteria and measures of clinical change-Report of a National Institute of Mental Health Work Group. Dev Neuropsychol. 1986;2:261–276. [Google Scholar]
- 5.Reisberg B, Ferris SH, de Leon MJ, Sinaiko E, Franssen E, Kluger A, Mir P, Borenstein J, George AE, Shulman E, Steinberg G, Cohen J. Stage-specific behavioral, cognitive, and in vivo changes in community residing subjects with age-associated memory impairment and primary degenerative dementia of the Alzheimer type. Drug Dev Res. 1988;15:101–114. [Google Scholar]
- 6.Blackford R, LaRue A. Criteria for diagnosing age associated memory impairment. Dev Neuropsychol. 1989;5:295–306. [Google Scholar]
- 7.Flicker C, Ferris SH, Reisberg B. Mild cognitive impairment in the elderly: predictors of dementia. Neurology. 1991;41:1006–1009. doi: 10.1212/wnl.41.7.1006. [DOI] [PubMed] [Google Scholar]
- 8.Devanand DP, Folz M, Gorlyn M, Moeller JR, Stern Y. Questionable dementia: clinical course and predictors of outcome. J Am Geriatr Soc. 1997;45:321–328. doi: 10.1111/j.1532-5415.1997.tb00947.x. [DOI] [PubMed] [Google Scholar]
- 9.Graham JE, Rockwood K, Beattie BL, Eastwood R, Gauthier S, Tuokko H, McDowell I. Prevalence and severity of cognitive impairment with and without dementia in an elderly population. Lancet. 1997;349:1793–1796. doi: 10.1016/S0140-6736(97)01007-6. [DOI] [PubMed] [Google Scholar]
- 10.Petersen RC, Stevens JC, Ganguli M, Tangalos EG, Cummings JL, DeKosky ST. Practice parameter: early detection of dementia: mild cognitive impairment (an evidence-based review). Report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2001;56:1133–1142. doi: 10.1212/wnl.56.9.1133. [DOI] [PubMed] [Google Scholar]
- 11.Petersen RC. Mild cognitive impairment as a diagnostic entity. J Intern Med. 2004;256:183–194. doi: 10.1111/j.1365-2796.2004.01388.x. [DOI] [PubMed] [Google Scholar]
- 12.Winblad B, Palmer K, Kivipelto M, Jelic V, Fratiglioni L, Wahlund LO, Nordberg A, Backman L, Albert M, Almkvist O, Arai H, Basun H, Blennow K, de Leon M, DeCarli C, Erkinjuntti T, Giacobini E, Graff C, Hardy J, Jack C, Jorm A, Ritchie K, van Duijn C, Visser P, Petersen RC. Mild cognitive impairment--beyond controversies, towards a consensus: report of the International Working Group on Mild Cognitive Impairment. J Intern Med. 2004;256:240–246. doi: 10.1111/j.1365-2796.2004.01380.x. [DOI] [PubMed] [Google Scholar]
- 13.Lyketsos CG, Lopez O, Jones B, Fitzpatrick AL, Breitner J, DeKosky S. Prevalence of neuropsychiatric symptoms in dementia and mild cognitive impairment: results from the cardiovascular health study. JAMA. 2002;288:1475–1483. doi: 10.1001/jama.288.12.1475. [DOI] [PubMed] [Google Scholar]
- 14.Lyketsos CG, Steinberg M, Tschanz JT, Norton MC, Steffens DC, Breitner JC. Mental and behavioral disturbances in dementia: findings from the Cache County Study on Memory in Aging. Am J Psychiatry. 2000;157:708–714. doi: 10.1176/appi.ajp.157.5.708. [DOI] [PubMed] [Google Scholar]
- 15.Roberts RO, Geda YE, Knopman DS, Cha RH, Pankratz VS, Boeve BF, Ivnik RJ, Tangalos EG, Petersen RC, Rocca WA. The Mayo Clinic Study of Aging: design and sampling, participation, baseline measures and sample characteristics. Neuroepidemiology. 2008;30:58–69. doi: 10.1159/000115751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ivnik RJ, Malec JF, Smith GE, Tangalos EG, Petersen RC, Kokmen E, Kurland LT. Mayo's Older Americans Normative Studies: WAIS-R Norms for Ages 56 to 97. Clin Neuropsychol. 1992;65:1–30. [Google Scholar]
- 17.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders: DSM-IV. 4th ed. Washington, DC: American Psychiatric Association; 1994. Task Force on DSM-IV. [Google Scholar]
- 18.Kaufer DI, Cummings JL, Ketchel P, Smith V, MacMillan A, Shelley T, Lopez OL, DeKosky ST. Validation of the NPI-Q, a brief clinical form of the Neuropsychiatric Inventory. J Neuropsychiatry Clin Neurosci. 2000;12:233–239. doi: 10.1176/jnp.12.2.233. [DOI] [PubMed] [Google Scholar]
- 19.Cummings JL, Mega M, Gray K, Rosenberg-Thompson S, Carusi DA, Gornbein J. The Neuropsychiatric Inventory: comprehensive assessment of psychopathology in dementia. Neurology. 1994;44:2308–2314. doi: 10.1212/wnl.44.12.2308. [DOI] [PubMed] [Google Scholar]
- 20.Morris JC, Weintraub S, Chui HC, Cummings J, Decarli C, Ferris S, Foster NL, Galasko D, Graff-Radford N, Peskind ER, Beekly D, Ramos EM, Kukull WA. The Uniform Data Set (UDS): clinical and cognitive variables and descriptive data from Alzheimer Disease Centers. Alzheimer Dis Assoc Disord. 2006;20:210–216. doi: 10.1097/01.wad.0000213865.09806.92. [DOI] [PubMed] [Google Scholar]
- 21.Last JM International Epidemiological Association. A Dictionary of Epidemiology. 4th ed. New York: Oxford University Press; 2001. [Google Scholar]
- 22.Little RJ. Survey nonresponse adjustments for estimates of means. Int Stat Rev. 1986;54:139–157. [Google Scholar]
- 23.Kessler RC, Little RJ, Groves RM. Advances in strategies for minimizing and adjusting for survey nonresponse. Epidemiol Rev. 1995;17:192–204. doi: 10.1093/oxfordjournals.epirev.a036176. [DOI] [PubMed] [Google Scholar]
- 24.D'Agostino RB, Jr, Rubin DB. Estimating and using propensity scores with partially missing data. J Am Stat Assoc. 2000;95:749–759. [Google Scholar]
- 25.Geda YE, Smith GE, Knopman DS, Boeve BF, Tangalos EG, Ivnik RJ, Mrazek DA, Edland SD, Petersen RC. De novo genesis of neuropsychiatric symptoms in mild cognitive impairment (MCI) Int Psychogeriatr. 2004;16:51–60. doi: 10.1017/s1041610204000067. [DOI] [PubMed] [Google Scholar]
- 26.Hwang TJ, Masterman DL, Ortiz F, Fairbanks LA, Cummings JL. Mild cognitive impairment is associated with characteristic neuropsychiatric symptoms. Alzheimer Dis Assoc Disord. 2004;18:17–21. doi: 10.1097/00002093-200401000-00004. [DOI] [PubMed] [Google Scholar]
- 27.Palmer K, Berger AK, Monastero R, Winblad B, Backman L, Fratiglioni L. Predictors of progression from mild cognitive impairment to Alzheimer disease. Neurology. 2007;68:1596–1602. doi: 10.1212/01.wnl.0000260968.92345.3f. [DOI] [PubMed] [Google Scholar]
- 28.Wilson RS, Barnes LL, Mendes de Leon CF, Aggarwal NT, Schneider JS, Bach J, Pilat J, Beckett LA, Arnold SE, Evans DA, Bennett DA. Depressive symptoms, cognitive decline, and risk of AD in older persons. Neurology. 2002;59:364–370. doi: 10.1212/wnl.59.3.364. [DOI] [PubMed] [Google Scholar]