Abstract
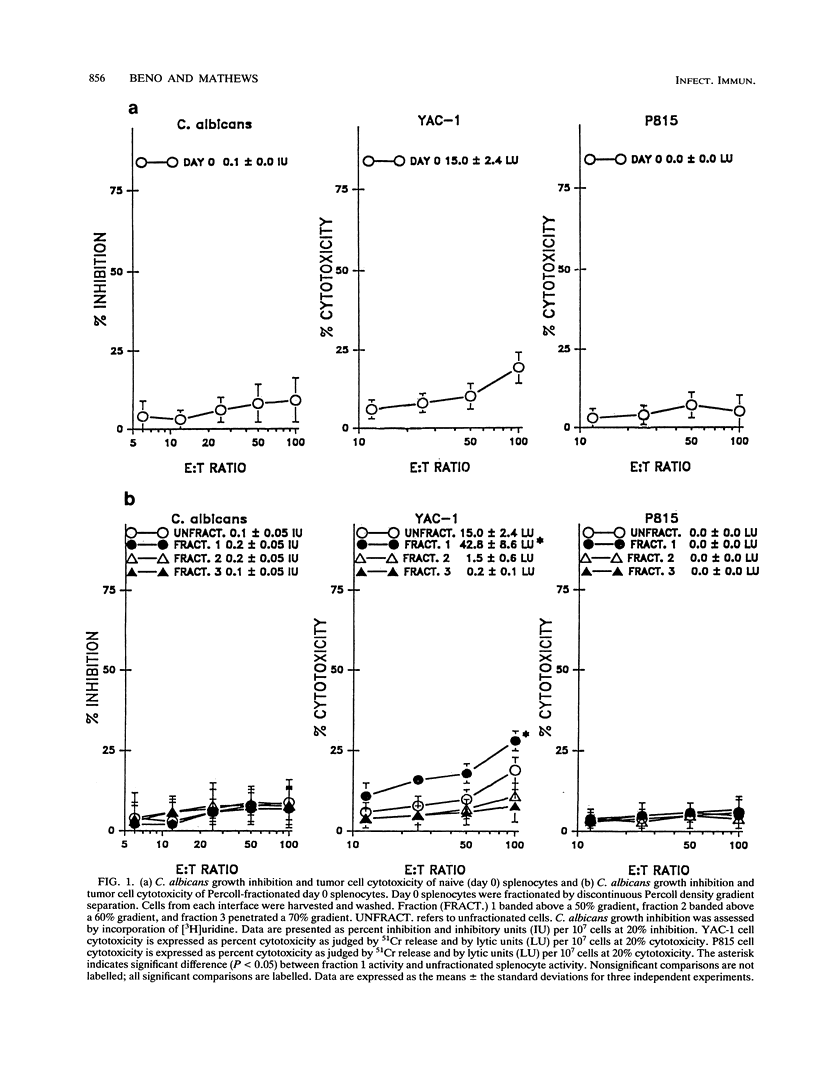
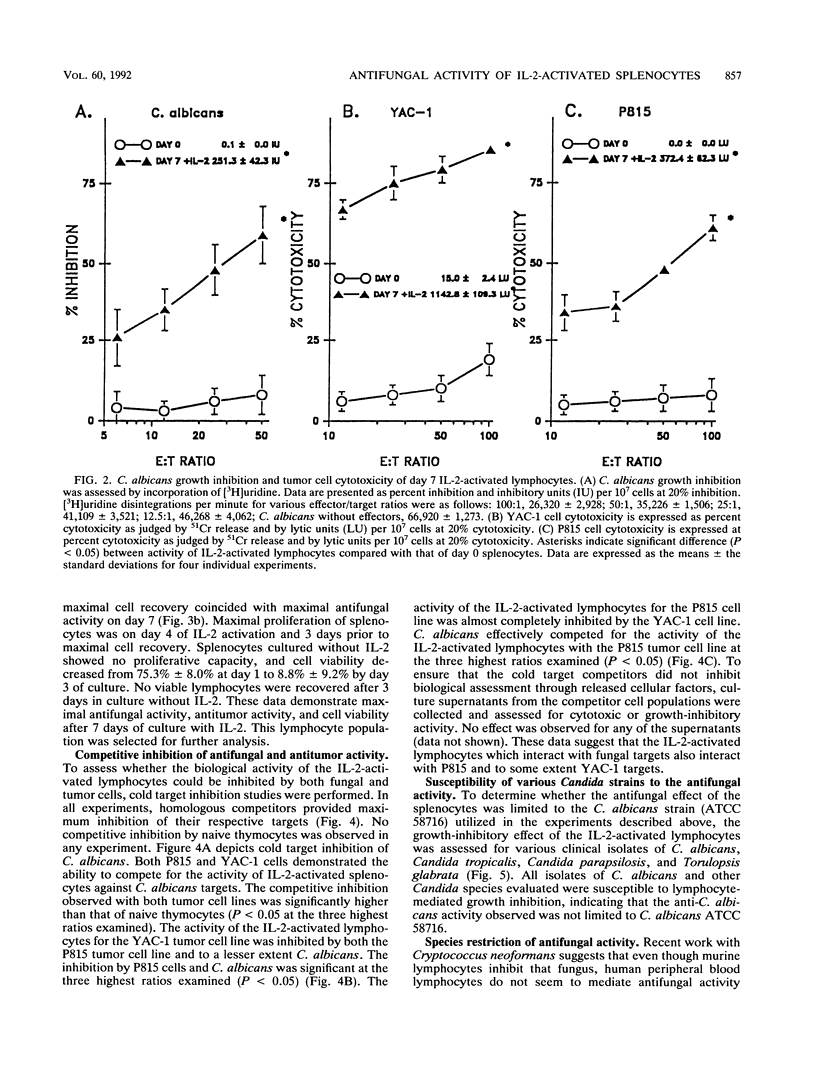
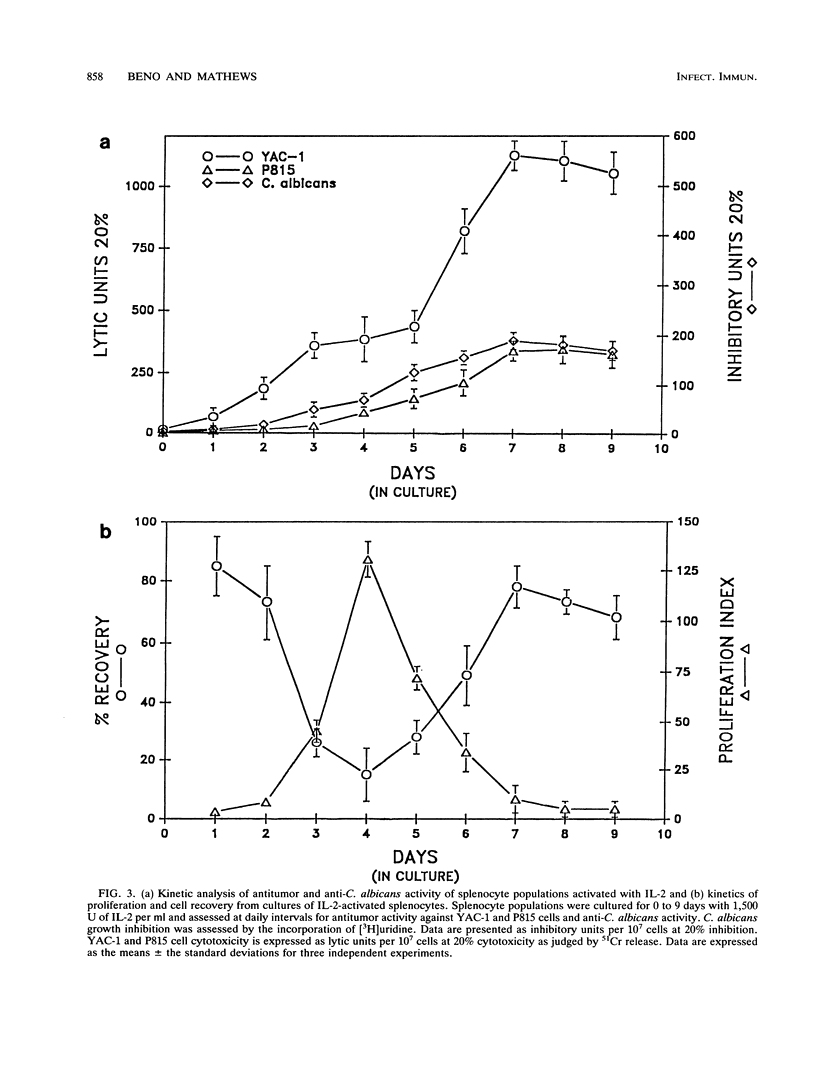
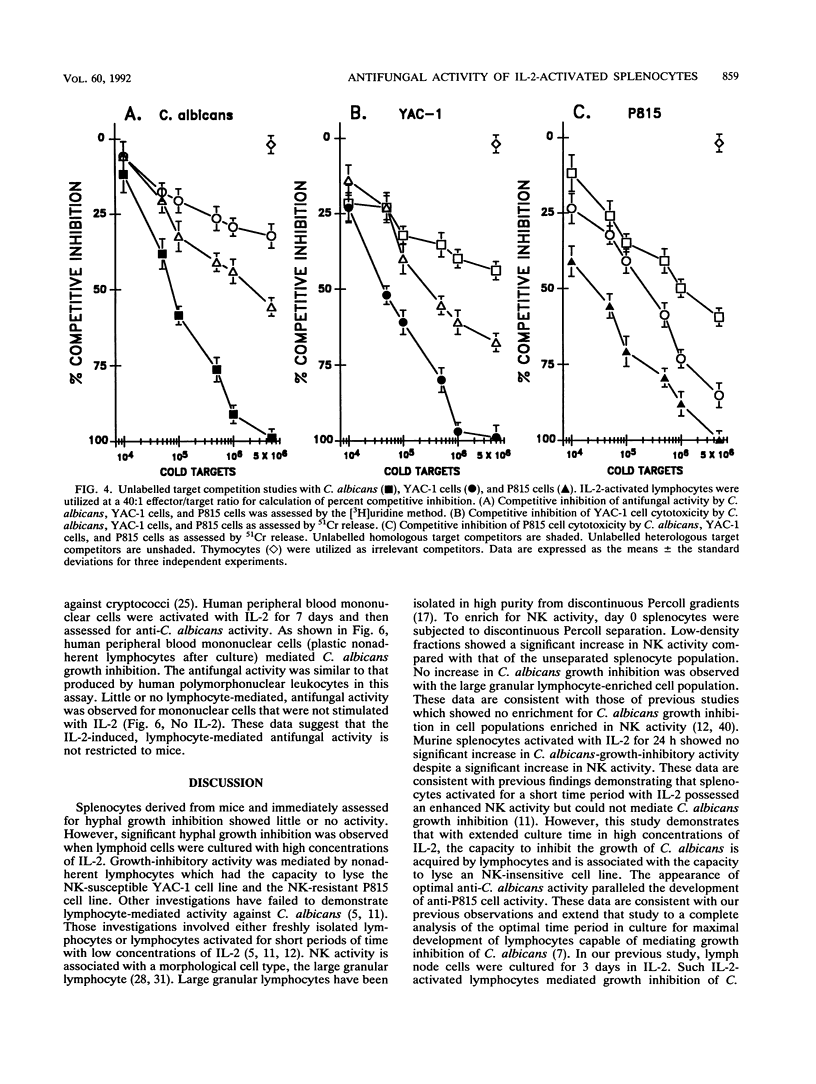
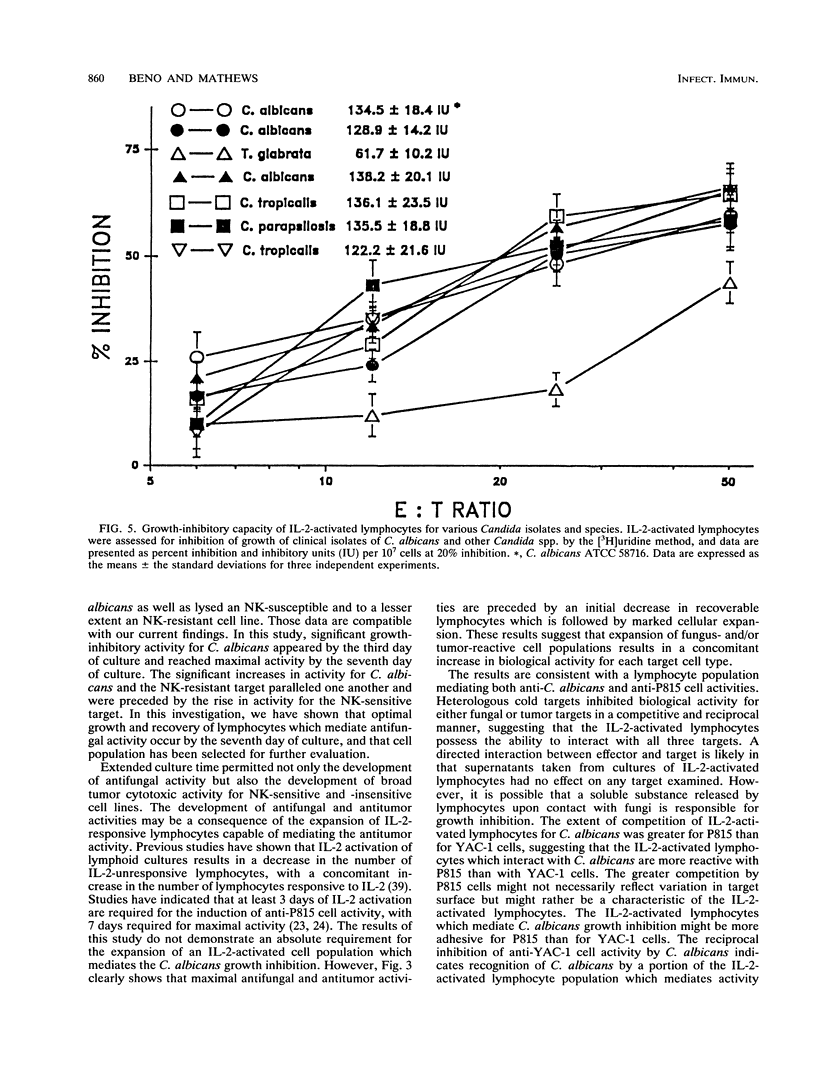
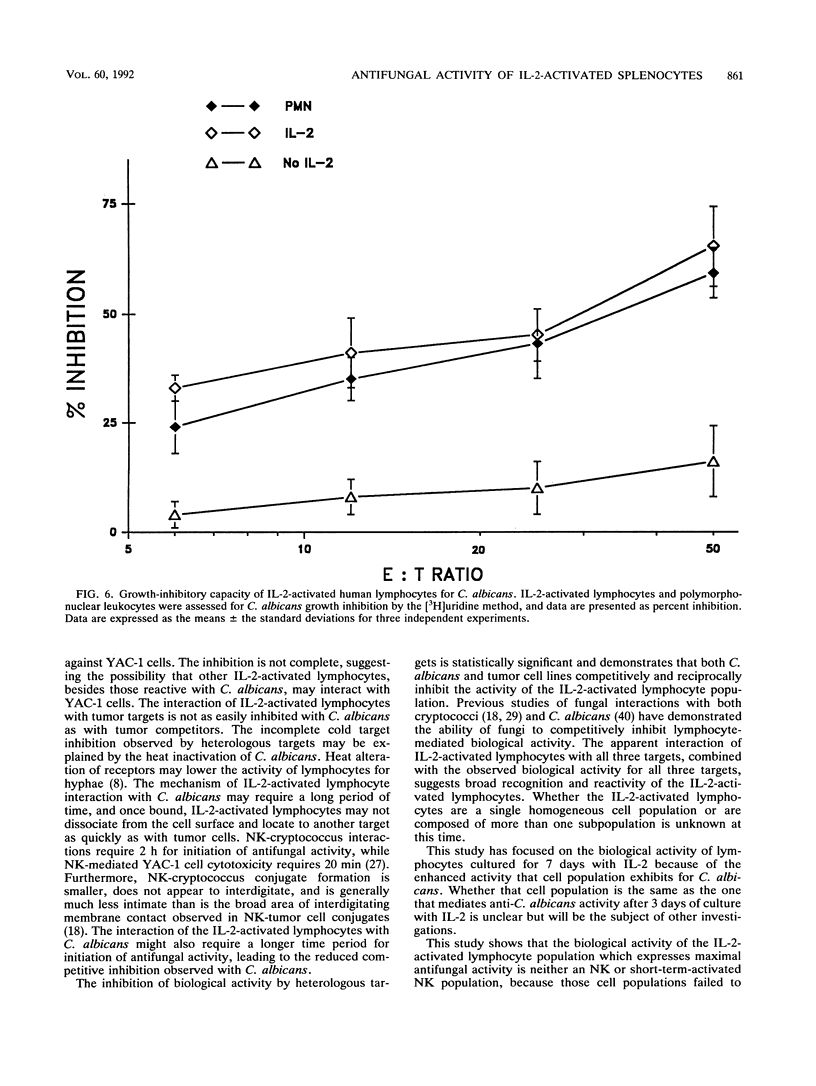
Murine splenocytes, Percoll-enriched low-density lymphocytes, and interleukin-2 (IL-2)-activated lymphocytes were assessed for the capacity to limit the growth of the hyphal form of Candida albicans. No fungal-growth-inhibitory activity was exhibited for C. albicans by either splenocytes or Percoll-enriched lymphocytes. These cells were capable of cytotoxic activity for a natural killer cell-sensitive cell line. However, when cultured for several days with IL-2, splenocytes acquired the capacity to inhibit the growth of the fungus. The appearance of the antifungal activity coincided with the development of cytotoxic activity for the natural killer cell-insensitive cell line. Anti-C. albicans and antitumor activities of IL-2-activated lymphocytes were competitively and reciprocally inhibited by C. albicans and the natural killer cell-sensitive and -insensitive cell lines. The antifungal activity of the IL-2-activated lymphocytes was exhibited against a number of clinical isolates of C. albicans and related fungal species. IL-2-activated human peripheral blood lymphocytes also acquired the capacity to inhibit the growth of C. albicans. These data show that in vitro growth inhibition can be mediated by IL-2-stimulated lymphocytes which are neither fungal strain nor mammalian species restricted in their biological activity.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashman R. B. Murine candidiasis: cell-mediated immune responses correlate directly with susceptibility and resistance to infection. Immunol Cell Biol. 1990 Feb;68(Pt 1):15–20. doi: 10.1038/icb.1990.2. [DOI] [PubMed] [Google Scholar]
- Ashman R. B., Papadimitriou J. M. Murine candidiasis. Pathogenesis and host responses in genetically distinct inbred mice. Immunol Cell Biol. 1987 Apr;65(Pt 2):163–171. doi: 10.1038/icb.1987.18. [DOI] [PubMed] [Google Scholar]
- Ausiello C., Maleci A., Spagnoli G. C., Antonelli G., Cassone A. Cell-mediated cytotoxicity in glioma-bearing patients: differential responses of peripheral blood mononuclear cells to stimulation with interleukin-2 and microbial antigen. J Neurooncol. 1988 Dec;6(4):329–338. doi: 10.1007/BF00177428. [DOI] [PubMed] [Google Scholar]
- Baccarini M., Bistoni F., Puccetti P., Garaci E. Natural cell-mediated cytotoxicity against Candida albicans induced by cyclophosphamide: nature of the in vitro cytotoxic effector. Infect Immun. 1983 Oct;42(1):1–9. doi: 10.1128/iai.42.1.1-9.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baccarini M., Vecchiarelli A., Cassone A., Bistoni F. Killing of yeast, germ-tube and mycelial forms of Candida albicans by murine effectors as measured by a radiolabel release microassay. J Gen Microbiol. 1985 Mar;131(3):505–513. doi: 10.1099/00221287-131-3-505. [DOI] [PubMed] [Google Scholar]
- Beno D. W., Mathews H. L. Growth inhibition of Candida albicans by interleukin-2-induced lymph node cells. Cell Immunol. 1990 Jun;128(1):89–100. doi: 10.1016/0008-8749(90)90009-g. [DOI] [PubMed] [Google Scholar]
- Bouchara J. P., Tronchin G., Annaix V., Robert R., Senet J. M. Laminin receptors on Candida albicans germ tubes. Infect Immun. 1990 Jan;58(1):48–54. doi: 10.1128/iai.58.1.48-54.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daimond R. D., Krzesicki R. Mechanisms of attachment of neutrophils to Candida albicans pseudohyphae in the absence of serum, and of subsequent damage to pseudohyphae by microbicidal processes of neutrophils in vitro. J Clin Invest. 1978 Feb;61(2):360–369. doi: 10.1172/JCI108946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond R. D., Haudenschild C. C. Monocyte-mediated serum-independent damage to hyphal and pseudohyphal forms of Candida albicans in vitro. J Clin Invest. 1981 Jan;67(1):173–182. doi: 10.1172/JCI110010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djeu J. Y., Blanchard D. K. Regulation of human polymorphonuclear neutrophil (PMN) activity against Candida albicans by large granular lymphocytes via release of a PMN-activating factor. J Immunol. 1987 Oct 15;139(8):2761–2767. [PubMed] [Google Scholar]
- Djeu J. Y., Blanchard D. K., Richards A. L., Friedman H. Tumor necrosis factor induction by Candida albicans from human natural killer cells and monocytes. J Immunol. 1988 Dec 1;141(11):4047–4052. [PubMed] [Google Scholar]
- Goto M., Zvaifler N. J. Characterization of the natural killer-like lymphocytes in rheumatoid synovial fluid. J Immunol. 1985 Mar;134(3):1483–1486. [PubMed] [Google Scholar]
- Grimm E. A., Mazumder A., Zhang H. Z., Rosenberg S. A. Lymphokine-activated killer cell phenomenon. Lysis of natural killer-resistant fresh solid tumor cells by interleukin 2-activated autologous human peripheral blood lymphocytes. J Exp Med. 1982 Jun 1;155(6):1823–1841. doi: 10.1084/jem.155.6.1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herberman R. B., Holden H. T. Natural cell-mediated immunity. Adv Cancer Res. 1978;27:305–377. doi: 10.1016/s0065-230x(08)60936-7. [DOI] [PubMed] [Google Scholar]
- Hidore M. R., Murphy J. W. Correlation of natural killer cell activity and clearance of Cryptococcus neoformans from mice after adoptive transfer of splenic nylon wool-nonadherent cells. Infect Immun. 1986 Feb;51(2):547–555. doi: 10.1128/iai.51.2.547-555.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hidore M. R., Nabavi N., Reynolds C. W., Henkart P. A., Murphy J. W. Cytoplasmic components of natural killer cells limit the growth of Cryptococcus neoformans. J Leukoc Biol. 1990 Jul;48(1):15–26. doi: 10.1002/jlb.48.1.15. [DOI] [PubMed] [Google Scholar]
- Lafreniere R., Rosenstein M. S., Rosenberg S. A. Optimal methods for generating expanded lymphokine activated killer cells capable of reducing established murine tumors in vivo. J Immunol Methods. 1986 Nov 20;94(1-2):37–49. doi: 10.1016/0022-1759(86)90213-9. [DOI] [PubMed] [Google Scholar]
- Lanier L. L., Le A. M., Civin C. I., Loken M. R., Phillips J. H. The relationship of CD16 (Leu-11) and Leu-19 (NKH-1) antigen expression on human peripheral blood NK cells and cytotoxic T lymphocytes. J Immunol. 1986 Jun 15;136(12):4480–4486. [PubMed] [Google Scholar]
- Mahanty S., Greenfield R. A., Joyce W. A., Kincade P. W. Inoculation candidiasis in a murine model of severe combined immunodeficiency syndrome. Infect Immun. 1988 Dec;56(12):3162–3166. doi: 10.1128/iai.56.12.3162-3166.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marquis G., Montplaisir S., Pelletier M., Mousseau S., Auger P. Strain-dependent differences in susceptibility of mice to experimental candidosis. J Infect Dis. 1986 Nov;154(5):906–909. doi: 10.1093/infdis/154.5.906. [DOI] [PubMed] [Google Scholar]
- Merluzzi V. J. Comparison of murine lymphokine-activated killer cells, natural killer cells, and cytotoxic T lymphocytes. Cell Immunol. 1985 Oct 1;95(1):95–104. doi: 10.1016/0008-8749(85)90298-9. [DOI] [PubMed] [Google Scholar]
- Merluzzi V. J., Smith M. D., Last-Barney K. Similarities and distinctions between murine natural killer cells and lymphokine-activated killer cells. Cell Immunol. 1986 Jul;100(2):563–569. doi: 10.1016/0008-8749(86)90054-7. [DOI] [PubMed] [Google Scholar]
- Miller M. F., Mitchell T. G., Storkus W. J., Dawson J. R. Human natural killer cells do not inhibit growth of Cryptococcus neoformans in the absence of antibody. Infect Immun. 1990 Mar;58(3):639–645. doi: 10.1128/iai.58.3.639-645.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulé J. J., Krosnick J. A., Rosenberg S. A. IL-4 regulation of murine lymphokine-activated killer activity in vitro. Effects on the IL-2-induced expansion, cytotoxicity, and phenotype of lymphokine-activated killer effectors. J Immunol. 1989 Jan 15;142(2):726–733. [PubMed] [Google Scholar]
- Murphy J. W., McDaniel D. O. In vitro reactivity of natural killer (NK) cells against Cryptococcus neoformans. J Immunol. 1982 Apr;128(4):1577–1583. [PubMed] [Google Scholar]
- Nabavi N., Murphy J. W. In vitro binding of natural killer cells to Cryptococcus neoformans targets. Infect Immun. 1985 Oct;50(1):50–57. doi: 10.1128/iai.50.1.50-57.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neta R., Salvin S. B. Resistance and susceptibility to infection in inbred murine strains. II. Variations in the effect of treatment with thymosin fraction 5 on the release of lymphokines in vivo. Cell Immunol. 1983 Jan;75(1):173–180. doi: 10.1016/0008-8749(83)90316-7. [DOI] [PubMed] [Google Scholar]
- Ortaldo J. R., Longo D. L. Human natural lymphocyte effector cells: definition, analysis of activity, and clinical effectiveness. J Natl Cancer Inst. 1988 Sep 7;80(13):999–1010. doi: 10.1093/jnci/80.13.999. [DOI] [PubMed] [Google Scholar]
- Peace D. J., Kern D. E., Schultz K. R., Greenberg P. D., Cheever M. A. IL-4-induced lymphokine-activated killer cells. Lytic activity is mediated by phenotypically distinct natural killer-like and T cell-like large granular lymphocytes. J Immunol. 1988 May 15;140(10):3679–3685. [PubMed] [Google Scholar]
- Pross H. F., Baines M. G., Rubin P., Shragge P., Patterson M. S. Spontaneous human lymphocyte-mediated cytotoxicity against tumor target cells. IX. The quantitation of natural killer cell activity. J Clin Immunol. 1981 Jan;1(1):51–63. doi: 10.1007/BF00915477. [DOI] [PubMed] [Google Scholar]
- Vecchiarelli A., Bistoni F., Cenci E., Perito S., Cassone A. In-vitro killing of Candida species by murine immunoeffectors and its relationship to the experimental pathogenicity. Sabouraudia. 1985 Oct;23(5):377–387. doi: 10.1080/00362178585380541. [DOI] [PubMed] [Google Scholar]
- Yamamura M., Boler J., Valdimarsson H. Phagocytosis measured as inhibition of uridine uptake by Candida albicans. J Immunol Methods. 1977;14(1):19–24. doi: 10.1016/s0022-1759(97)90016-8. [DOI] [PubMed] [Google Scholar]
- Yang J. C., Mulé J. J., Rosenberg S. A. Murine lymphokine-activated killer (LAK) cells: phenotypic characterization of the precursor and effector cells. J Immunol. 1986 Jul 15;137(2):715–722. [PubMed] [Google Scholar]
- Zunino S. J., Hudig D. Interactions between human natural killer (NK) lymphocytes and yeast cells: human NK cells do not kill Candida albicans, although C. albicans blocks NK lysis of K562 cells. Infect Immun. 1988 Mar;56(3):564–569. doi: 10.1128/iai.56.3.564-569.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]



