Abstract
Background
Treatment of sex partners by patient-delivered partner therapy (PDPT) may prove to be an effective strategy in reducing reinfection and preventing the sequelae of sexually transmitted infections (STIs). However, limited data exists regarding STIs within sexual partnerships (dyads).
Objective
The objective of this study was to determine the prevalence of Chlamydia trachomatis (CT), Neisseria gonorrhoeae (GC), and Trichomonas vaginalis (TV) in sexual dyads to estimate the potential yield and limitations of PDPT.
Methods
Male and female STI clinic attendees were invited to participate. Index subjects and partners were interviewed and tested for CT, GC, and TV. All partners were sought regardless of infection status of the index subject.
Results
Of 210 dyads, the prevalence in index subjects was CT, 46%; GC, 18%; and TV, 14%. Considering the partners of 72 CT-only-infected index subjects, 57% had CT, 6% had GC, and 11% had TV. Considering the partners of 35 index subjects with GC or GC–CT coinfection, 57% had GC and/or CT; however, in 20% of partners, unsuspected TV was present. Among 74 dyads with uninfected index subjects, 26% of partners had an STI. Among the partners of 19 index subjects with TV only, 11% had CT, 5% had GC, and 37% had TV.
Conclusion
In our clinic population, a substantial number of partners had infections different from or in addition to those infections in the index. Many of these infected partners would not be diagnosed and treated using PDPT. Partners of index attendees without detected infection were at high risk (26%) for STI, mostly CT.
Reinfection of treated index subjects by untreated partners accounts for 14% to 30% of incident bacterial sexually transmitted infections (STIs).1-3 Treatment of sex partners through partner notification is a cornerstone of STI prevention efforts and reduces the risk of reinfection in index subjects.2 Partner notification is the process whereby the index patients (i.e., patient referral) or healthcare provider (i.e., provider referral) notify the sex partner of potential exposure and encourage them to seek treatment. The goals of partner notification are to ensure timely treatment of infected sex partners, to reduce potential for sequelae, and to prevent reinfection of the treated index patient and infection of other sexual contacts. Major shortcomings of partner notification are low rates of partner contact and treatment, partner noncompliance in seeking care after notification, and difficulties in identifying and contacting nonregular partners.4-8
An alternative to traditional partner notification is patient-delivered partner treatment (PDPT) in which infected index subjects deliver appropriate medication to partners. Several studies have shown that PDPT is well accepted by index subjects and effective in preventing repeated chlamydia and gonorrhea infections.9-12 PDPT is often prescribed by individual clinicians13 and is routinely used in some public health settings.9,14 Although PDPT appears to be a useful clinical strategy for partner treatment, some important limitations are the absence of diagnostic testing of the partner for other STIs before receiving treatment and missed opportunities for partner counseling and education.15
Conceptually, PDPT is an extension of “epidemiologic” partner treatment. The Centers of Disease Control and Prevention recommends initial presumptive treatment (ie, without confirmation of infection) of sexual contacts of infected index patients followed by diagnostic testing. Possible coinfections can also be treated presumptively. 16 Studies conducted in a number of high and low sexually transmitted disease (STD) prevalence settings have documented high rates of coinfection with Neisseria gonorrhoeae (GC) and Chlamydia trachomatis (CT) (range, 9–67%)17-21 and with Trichomonas vaginalis (TV).22-24 Alternatively, diagnostic testing can be used with subsequent treatment of any additional infections. Epidemiologic partner treatment is generally safe and cost-effective because sexual partners have a high likelihood of infection.25,26 In common with other forms of epidemiologic treatment, PDPT is likely associated with overtreatment of a substantial number of uninfected partners.
This study was designed to examine the prevalence of CT, GC, and TV in partners of index subjects infected with CT, GC and/or CT, and TV. We also examined the prevalence of infections in partners of uninfected STI clinic clients with the hypothesis that these partners may be a part of sexual network with high STI prevalence, and therefore at high risk for infection even if the index patient is uninfected.
Methods
Study Design and Population
Sexually active heterosexual males and females between the ages of 15 and 25 years (regardless of genital symptoms or infection status) visiting an urban STD clinic in Indianapolis, IN, were invited to participate in a study of STD in sexual partnerships (dyads). Eligibility criteria included sexual activity in the last 30 days, willingness to identify all sex partners in the last 30 days, and a working knowledge of English. Exclusion criteria were antibiotic use in the last 30 days, known HIV infection or any other immunesuppressive condition, clinic visit necessitated by sexual assault, and emotional or mental health conditions that would preclude partner enrollment. The most common reasons for nonparticipation were lack of time and lack of interest. Eligibility criteria applied to index participants. Sex partners were enrolled on the basis of willingness to participate.
Written informed consent was obtained from all eligible participants. The study protocols were reviewed and approved by the Indiana University–Purdue University Indianapolis Institutional Review Board.
Enrollment
Both index subjects and partners completed an enrollment questionnaire to provide demographic information, including age, race, gender, education, reasons for seeking care, age at first sexual intercourse, prior self-reported STIs, and total number of partners in the last 30 days. Trained research staff conducted face-to-face interviews using a 30-day calendar recall for coitus and the presence of genital symptoms. Participants were asked to identify each day on which coitus occurred during the previous 30 days. For each day with coital activity, participants were asked about the number of sex partners, the number of coital events with each partner, and whether a condom was used during each identified coital event.
The first enrolled subject was designated the index subject. In cases in which both presented together, the distinction between index and partner was arbitrary. In our study, 83 (40%) of the dyad members came together to the clinic. Research disease intervention specialists offered study participation to all partners of infected and uninfected index subjects whom they could locate. Each index subject could provide information for up to 4 sex partners in the last 30 days. A partner could also enroll as an index for additional partners. Dyads with multiple partners were analyzed as separate dyads.
Laboratory Data
All index subjects and enrolled partners were tested for chlamydia, gonorrhea, and trichomonas. N. gonorrhoeae and C. trachomatis were sought by culture on cervical (women) and urethral (men) specimens. From women, rectal and urethral swabs were obtained for N. gonorrhoeae and C. trachomatis cultures, respectively. Nucleic acid amplification testing (NAAT) for N. gonorrhoeae and C. trachomatis was done on cervical, vaginal, urethral, and urine specimens for women and on urethral and urine specimens for the men using COBAS AMPLICOR CT/NG (Roche Diagnostic Systems, Indianapolis, IN). NAAT testing for T. vaginalis was done on vaginal (women) and urine (women and men) specimens using a T. vaginalis polymerase chain reaction based on the Amplicor platform with primers specific for T. vaginalis as previously described.27,28 Specimens were collected by experienced clinical research staff and processed at Indiana University research laboratory facilities. Infection for the target organism was defined as any positive test from any specimen in a given subject.
Statistical Methods
Demographic, behavioral, and clinical characteristics of index subjects and their partners in the study were compared using descriptive statistics. The main outcomes examined were the difference in infection status between CT-only- and GC and/or CT-coinfected index subjects and their partners. In addition, infection status of partners of uninfected index subjects and infection status of index subjects infected only with TV were also examined. SAS version 8 was used for the analysis.
To estimate potential yield and missed infections, we assumed a theoretical PDPT program in which 1) partners of index subjects infected only with CT would be treated for CT only; and 2) partners of index subjects infected with GC with or without CT coinfection would be treated for both GC and CT. Yield is expressed as the percent of partners that harbor the target organism(s), and missed infections are expressed as the percent of partners that harbor an infection not present in the index subject that would therefore go untreated.
Results
Between April 2000 and October 2003, 210 heterosexual sexual dyads were enrolled, consisting of 101 males and 109 females among the index subjects (Table 1). The majority of participants were black. Although 55% self-reported a history of STI, 60% named only 1 sexual partner in the preceding 30 days. The median number of coital events was 5 (range, 1–73) in the last 30 days and 37% of these events were condom-protected. Index subjects were more likely than partners to report symptoms preceding the clinic visit. Approximately 40% of the dyad members presented to the clinic together.
TABLE 1.
Dyad Characteristics
| Variable | Index | Partner |
|---|---|---|
| Age, years (median) | 21 | 21 |
| Race | ||
| Black, no. (%) | 151 (72) | 142 (68) |
| White, no. (%) | 47 (22) | 59 (28) |
| Other, no. (%) | 12 (6) | 9 (4) |
| Age at sexual debut, years (median) | 14 | 14 |
| Prior sexually transmitted infection, no. (%) | 116 (55) | 103 (49) |
| Number of partners, past 30 d (median) | 1 | 1 |
| Coital events, past 30 d (mean) | 9.8 | 10 |
| Condom-protected coital events, past 30 d (%) | 37 | 29 |
| Genital symptoms, past 30 d, no. (%)* | 126 (60) | 82 (39) |
Statistically significant P <0.05.
Sexually Transmitted Infection Prevalence in Index Subjects
The overall prevalence in the entire study population (index and partners) of CT (41%), GC (15%), and TV(14%) was high, as would be expected in an STD clinic sample (Table 2). Among 210 index subjects, 96 (46%) had CT, of whom 72 had CT only and 24 had GC or TV coinfection. In 38 (18%) index subjects with GC, 19 had only GC, 16 had GC–CT, and 3 had GC–TV coinfections. TV was present in 29 (14%) index subjects, including 19 with only TV; 7 had TV–CT and 2 had TV–GC coinfections. One index subject was infected with all 3 organisms.
TABLE 2.
Prevalence of Sexually Transmitted Infection Among Index Subjects and Partner
| Infection | Index (N = 210)
No. (%) |
Partner (N = 210)
No. (%) |
|---|---|---|
| CT only | 72 (34) | 53 (25) |
| GC only | 19 (9) | 7 (3) |
| TV only | 19 (9) | 12 (6) |
| CT–GC | 16 (8) | 9 (4) |
| CT–TV | 7 (3) | 9 (4) |
| GC–TV | 2 (1) | 2 (1) |
| CT–GC–TV | 1(<1) | 5 (2) |
| None | 74 (35) | 113 (54) |
CT = Chlamydia trachomatis; GC = Neisseria gonorrhoeae; TV = Trichomonas vaginalis.
Sexually Transmitted Infection Prevalence in Sexual Dyads
In the 210 dyads studied, 55 (26%) dyads were uninfected (i.e., the 3 target organisms were not detected at any site in either partner) and 155 (74%) had 1 or both dyad members infected with CT, GC, or TV (Fig. 1). Among the 155 infected dyads, 77 (50%) dyads had only 1 infected person and in 78 (50%) dyads, both members were infected. In dyads having only 1 infected member, the index subject only was positive in 58 dyads (75%). In 19 dyads (26%), the partner only was subsequently found to be positive. Thus, 19 index subjects who themselves were uninfected had partners who were infected with an STI.
Fig. 1.
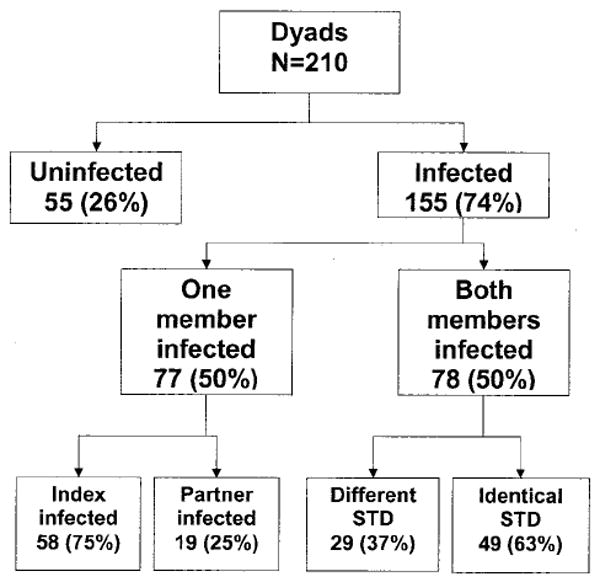
Sexually transmitted infections in dyads.
Among 78 dyads with both members infected, 63% had identical infections (Fig. 1). In these dyads, the same organism infected both members, and no additional infections were identified. However, in 29 dyads (37%), there were either single but different infections or additional infections between the index subject and partner.
Potential Yield of Theoretical Patient-Delivered Partner Therapy Approach
To estimate the potential yield and missed infections, we assumed a theoretical PDPT program in which 1) partners of index subjects infected only with CT would be treated for CT only; and 2) partners of index subjects infected with GC with or without CT coinfection would be treated for both GC and CT.
Partners of Chlamydia trachomatis-Only Infected Index Subjects
The partner infection status of index subjects with CT only is shown in Table 3. The overall yield of a theoretical PDPT program for CT is 41 CT-infected partners (57%) of 72 CT-infected index subjects. Missed infections include 4 partners (6%) with GC and 8 partners (11%) with TV. Twenty-nine (40%) partners were not infected and would receive CT treatment unnecessarily.
TABLE 3.
Partner Infection Status
| Infection Status
| ||||
|---|---|---|---|---|
| Partner, No.
(%)* |
Index CT Only | GC or GC–CT | TV Only | Uninfected |
| (N = 72) | (N = 35) | (N = 19) | (N = 74) | |
| CT | 41 (57) | 12 (34) | 2 (11) | 16 (22) |
| GC | 4 (6) | 16 (46) | 1 (5) | — |
| TV | 8 (11) | 7 (20) | 7 (37) | 3 (4) |
| Uninfected | 29 (40) | 15 (43) | 10 (53) | 55 (74) |
The sum total is greater than 100% because there were partners with multiple infections present.
CT = Chlamydia trachomatis; GC = Neisseria gonorrhoeae; TV = Trichomonas vaginalis.
Partners of Neisseria gonorrhoeae or Neisseria gonorrhoeae–Chlamydia trachomatis-Coinfected Index Subjects
The partner infection status of index subjects infected with GC only or with GC–CT coinfections is shown in Table 3. The overall yield of a theoretical PDPT program for GC or GC–CT coinfections was 20 GC and/or CT-infected partners (57%) of 35 index subjects. Missed infections included 7 (20%) partners with TV. All 7 partners with TV infections had additional GC, CT, or both. Fifteen partners (43%) did not have either GC or CT and would receive dual treatment unnecessarily.
Partners of Uninfected Index Subjects
Most (55 of 74 [74%]) partners of uninfected index subjects seeking care were uninfected. However, 19 partners (26%) were infected, most frequently with CT (84%) (Table 3)
Partners of Trichomonas vaginalis-Only-infected Index Subjects
The partner infection status of index subjects with TV only is shown in Table 3. Among 19 TV-infected index subjects, 6 (32%) partners had TV only, 1 (5%) partner had CT, 1 (5%) partner had GC, and 1 partner had TV–CT coinfection. In 10 partners (53%), no infection was found.
Discussion
Our study allows us to compare infections found in extensively sampled sexual partnerships recruited from a high-risk urban STI clinic. We do not have either directly evaluated PDPT or strategies pertaining to PDPT. However, the detailed information on STIs in sexual partnerships can be used to illustrate the potential yields and limitations of PDPT were it to be used in a high-risk setting such as ours.
In addition, our data provides evidence that the partners of uninfected index clients of an STI clinic are at a substantial risk of infection, mostly CT. Although understanding the limitation that there currently does not exist any public health consensus regarding screening for TV with sensitive amplification tests, we provide evidence of relatively frequent unsuspected TV infection in partners.
These results demonstrate the potential yield of PDPT and the risk of missed and untreated infections in sex partners. Consistent with other studies,25,26,30 our results demonstrate that dyad members have a high likelihood of similar STD, thus providing a significant opportunity for PDPT to reduce the risk of reinfection and transmission.
We found CT in 57% of partners of index subjects infected with CT only and GC and/or CT in 57% of partners of GC and GC–CT-coinfected index subjects. Current epidemiologic treatment protocols and PDPT are both based on the implicit assumption that sex partners of infected index patients are likely to have the same organism, either by transmitting the infection or having been exposed themselves through sexual contact. Thus, empiric treatment of partners before availability of test results is a feasible and cost-effective strategy in reducing repeated infections.2,17,29,30 Based on our results, we estimated that PDPT for chlamydia only or gonorrhea– chlamydia coinfection, depending on the index subject’s diagnosed infection, would effectively treat 57% (41 of 72) of partners of CT-only index subjects and 57%(20 of 35) of the partners of GC or GC–CT-coinfected index subjects.
It should be noted that our study does not measure the actual efficacy or outcome of PDPT. The success of which would invariably depend on a number of factors such as reliable delivery of medications by the index patient to the partner, partner compliance with the treatment regimen, and the presence and degree of antibiotic resistance present in the community. Previous studies have shown reduced rates of reinfection among index subjects receiving PDPT,9-12 providing indirect evidence of acceptability of PDPT by both the index subjects and the partner and partner compliance with the given medications.31,32 However, no studies have directly assessed partner infection status and compliance with PDPT, including the effect of different treatment regimens on efficacy and outcomes.
The design of our study allows us to look at the magnitude of untreated or missed infections in partners if PDPT were implemented based on the organism identified in the index subjects. For example, if, according to Centers for Disease Control and Prevention guidelines, partners of index subjects infected only with CT were treated only for CT, then 4 of 72 (6%) partners with unsuspected GC would remain untreated. Lacking the availability of diagnostic testing and therefore follow up, these infections would remain undiagnosed and untreated unless the partners independently chose to seek care. Research of traditional partner notification programs has demonstrated that a significant number of partners do not seek care in a timely manner.4,33,34 We suggest that, when possible within STI clinic settings using PDPT, partners be screened for other infections regardless of the treatment received. Strategies that would enhance partner testing such as self-collected specimens (eg, urine or vaginal/urethral swabs) that could be mailed or dropped in at the STI clinic need to be examined.
In our study, partners of index subjects infected with CT only (8 of 72 [11%]) and index subjects infected with GC or GC–CT (7 of 35 [20%]) had a significant likelihood of unsuspected TV infection (overall, 15 of 107 [14%]). Although no public health consensus currently exists for screening for TV with sensitive tests in any population, these partners with unsuspected TV would go undiagnosed and untreated and comprise a significant group of missed infections in our population. A considerable literature suggests that untreated trichomoniasis leads to adverse health outcomes in women and men, including increased human immunodeficiency virus (HIV) transmission.23,24,35-37 Among women, sequelae of untreated trichomonas include premature delivery, low birth weight, atypical pelvic inflammatory disease, and cervical intra-epithelial neoplasia (CIN).24 TV has also been associated with nongonococcal urethritis and infertility in men.23,24 Thus, the substantial frequency of unanticipated TV infections found in the partners in our study may take on greater significance as ongoing research solidifies and expands appreciation of the health consequences of trichomoniasis.
Potential harms of PDPT are unnecessary treatment of uninfected partners and risk of fostering antibiotic resistance. However, these risks are no greater than if the partners had presented for evaluation and received the recommended epidemiologic treatment. Our results showed that PDPT directed toward CT and GC would result in treatment of 40% and 43% uninfected partners, respectively. From a public health perspective, if PDPT is effective in reducing the transmission of CT and GC, the decreased prevalence of these infections in the population could result in decreased use of antimicrobials in the long-term and prevent the development of resistance.
In our study, infections were present in one fourth of partners of uninfected STI clinic clients. Because STI clinics generally serve a population of high-risk individuals frequently involved in sexual networks with a high prevalence of STD, our findings support the concept that seeking care in STI clinic settings, in of itself, may be a predictor of infection risk within a network.3 Although generally not done, we suggest that posttest counseling for uninfected STI clients should emphasize the importance of routine screening and testing for their partners.
Available data suggest that PDPT may be an effective and expeditious strategy in partner treatment and should decrease the risk of reinfections in index patients. Further prospective studies are needed to examine the impact of partner treatment, reinfection rates, and actual partner compliance with PDPT. Additional work needs to be done looking at the cost of missed infections and unnecessary treatments along with comparing cost-effectiveness of PDPT to self-referral outcomes.
The limitations of this study should be noted. The study population was recruited from an STI clinic and may be different from sexual dyads seeking care in non-STD settings or those not seeking care at all. Our dyads had a very high prevalence of infection, and our results may not be generalizable to other STI clinics with lower infection rates or different population characteristics. Overall, 40% of our dyad members presented together to seek care, for whom PDPT is not applicable. The majority of the dyads members reported only 1 partner (61%) in the last 30 days, although given the high rates of infection, this may represent underreporting of partners. Additionally, our sample included only the partners that we were able to recruit, and in cases in which there were index subjects with multiple partners, the partners who either refused participation or were unable to be contacted may have increased the risk of infection in the index subject. In our study, women had more biologic sites/samples checked for infection as compared with men, increasing the likelihood of overestimating infection rates in women and underestimating rates in men. Finally, our study was not designed to address the actual efficacy of, adherence to, or outcome of PDPT; therefore, our results are at best an estimation of the potential yield and limitations of such a strategy.
In conclusion, sexual dyad members frequently share similar infections, and PDPT based on index subjects’ testing results could be an effective approach in similar STI clinics. However, a substantial number of partners have different infections than found in index subjects. Thus, a theoretical PDPT program focused on CT and GC would miss a relatively small number of GC infections in partners. A larger number of TV infections would be missed in partners, which may assume greater significance as the health consequences of TV infections are better understood.
Acknowledgments
The authors would like to thank Tim Breen for his excellent assistance in preparing the data for analysis. This work was supported by Public Health Service grant AI31494 from the National Institute of Allergy and Infectious Diseases and by grant UR3/CCU5516481 from the Centers for Disease Control and Prevention. The work of Wanzhu Tu was supported in part by grant R01 HD042402 from the National Institute of Child Health and Human Development.
References
- 1.Rietmeijer CAVBR, Judson FN, Douglas JM., Jr Incidence and repeat infection rates of Chlamydia trachomatis among male and female patients in an STD clinic: Implications for screening and rescreening. Sex Transm Dis. 2002;29:65–72. doi: 10.1097/00007435-200202000-00001. [DOI] [PubMed] [Google Scholar]
- 2.Whittington WL, Kent C, Kissinger P, et al. Determinants of persistent and recurrent Chlamydia trachomatis infection in young women: Results of a multicenter cohort study. Sex Transm Dis. 2001;28:117–123. doi: 10.1097/00007435-200102000-00011. [DOI] [PubMed] [Google Scholar]
- 3.Gunn R, Fitzgerald S, Aral S. Sexually transmitted disease clinic clients at risk for subsequent gonorrhea and chlamydia infections: Possible ‘core’ transmitters. Sex Transm Dis. 2000;27:343–349. doi: 10.1097/00007435-200007000-00008. [DOI] [PubMed] [Google Scholar]
- 4.Katz BP, Danos CS, Quinn TS, Caine V, Jones RB. Efficiency and cost-effectiveness of field follow-up for patients with Chlamydia trachomatis infection in a sexually transmitted diseases clinic. Sex Transm Dis. 1988;15:11–16. doi: 10.1097/00007435-198801000-00003. [DOI] [PubMed] [Google Scholar]
- 5.Oxman AD, Scott EAE, Sellors JW, et al. Partner notification for sexually transmitted diseases: An overview of the evidence. Can J Public Health. 1994;85:S41–S47. [PubMed] [Google Scholar]
- 6.Cowan FM, French R, Johnson AM. The role and effectiveness of partner notification in STD control: A review. Genitourin Med. 1996;72:247–252. doi: 10.1136/sti.72.4.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Katz BP, Caine VA, Jones RB. Evaluation of field follow-up in a sexually transmitted disease clinic for patients at risk for infection with Neisseria gonorrhoeae and Chlamydia trachomatis. Sex Transm Dis. 1992;19:99–104. [PubMed] [Google Scholar]
- 8.Steen R, Soliman C, Bucyana S, Dallabetta G. Partner referral as a component of integrated sexually transmitted disease services in two Rwandan towns. Genitourin Med. 1996;72:56–59. doi: 10.1136/sti.72.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kissinger P, Brown R, Reed K, et al. Effectiveness of patient delivered partner medication for preventing recurrent Chlamydia trachomatis. Sex Transm Infect. 1998;74:331–333. doi: 10.1136/sti.74.5.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ramstedt K, Forssman L, Johannisson G. Contact tracing in the control of genital Chlamydia trachomatis infection. Int J STD AIDS. 1991;2:116–118. doi: 10.1177/095646249100200208. [DOI] [PubMed] [Google Scholar]
- 11.Schillinger JA, Kissinger P, Calvet H, et al. Patient-delivered partner treatment with azithromycin to prevent repeated Chlamydia trachomatis infection among women: A randomized, controlled trial. Sex Transm Dis. 2003;30:49–56. doi: 10.1097/00007435-200301000-00011. [DOI] [PubMed] [Google Scholar]
- 12.Golden MR, Whittington WHL, Handsfield HH, et al. Impact of sex partner treatment without mandatory prior clinical evaluation on recurrent/persistent infection in patients with gonorrhea or chlamydia infection: A randomized trial. Abstract ISSTDR; 2003. Unpublished data. [Google Scholar]
- 13.Golden MR, Whittington WL, Gorbach PM, et al. Partner notification for chlamydial infections among private sector clinicians in Seattle–King County: A clinician and patient survey. Sex Transm Dis. 1999;26:543–547. doi: 10.1097/00007435-199910000-00011. [DOI] [PubMed] [Google Scholar]
- 14.Hammer G, Kent C, Courtney J, Kohn R, Klausner J. Does patient-delivered partner therapy decrease sexually transmitted disease reinfection?. Presented at the National STD Prevention Conference; Milwaukee, WI. 2000. [Abstract] [Google Scholar]
- 15.Klausner JD, Chaw JK. Patient delivered therapy for chlamydia: Putting research into practice. Sex Transm Dis. 2003;30:509–511. doi: 10.1097/00007435-200306000-00007. [DOI] [PubMed] [Google Scholar]
- 16.Centers for Disease Control and Prevention. Sexually transmitted disease treatment guidelines 2002. MMWR Morb Mortal Wkly Rep. 2002;51:32–34. [Google Scholar]
- 17.Lyss SB, Kamb ML, Peterman TA, et al. Chlamydia trachomatis among patients infected with and treated for Neisseria gonorrhoeae in sexually transmitted disease clinics in the United States. Ann Intern Med. 139:178–185. doi: 10.7326/0003-4819-139-3-200308050-00007. [DOI] [PubMed] [Google Scholar]
- 18.Dicker L, Mosure D, Berman S, et al. Gonorrhea prevalence and coinfection with chlamydia in women in the United States, 2000. Sex Transm Dis. 2003;30:472–476. doi: 10.1097/00007435-200305000-00016. [DOI] [PubMed] [Google Scholar]
- 19.Chow JM, Kang M-S, Dunn T, et al. Co-infection with Chlamydia trachomatis in a population with low gonorrhea prevalence: Implications for dual therapy recommendations. Abstract ISSTDR; 2001. [Google Scholar]
- 20.Creighton S, Tenant-Flowers M, Taylor CB, et al. Co-infection with gonorrhea and chlamydia: How much is there and what does it mean? Int J STD AIDS. 2003;14:109–113. doi: 10.1258/095646203321156872. [DOI] [PubMed] [Google Scholar]
- 21.Mehta SD, Rothman RE, Kelen GD, Quinn TC, Zenilman JM. Unsuspected gonorrhea and chlamydia in patients of an urban adult emergency department: A critical population for STD control intervention. Sex Transm Dis. 2001;28:33–39. doi: 10.1097/00007435-200101000-00008. [DOI] [PubMed] [Google Scholar]
- 22.Hardy PH, Hardy JB, Nell EE, et al. Prevalence of six sexually transmitted disease agents among pregnant inner-city adolescents and pregnancy outcome. Lancet. 1984;2:333–337. doi: 10.1016/s0140-6736(84)92698-9. [DOI] [PubMed] [Google Scholar]
- 23.Heine P, McGregor J. Trichomonas vaginalis: A reemerging pathogen. Clin Obstet Gynecol. 1993;36:137–144. doi: 10.1097/00003081-199303000-00019. [DOI] [PubMed] [Google Scholar]
- 24.Soper D. Trichomoniasis: Under control or undercontrolled. Am J Obstet Gynecol. 2004;190:281–290. doi: 10.1016/j.ajog.2003.08.023. [DOI] [PubMed] [Google Scholar]
- 25.Quinn TC, Gaydos C, Shepherd M, et al. Epidemiologic and microbiologic correlates of Chlamydia trachomatis infection in sexual partnerships. JAMA. 1996;276:1737–1742. [PubMed] [Google Scholar]
- 26.van de Laar MJ, Termorshuizen F, van den Hoek A. Partner referral by patients with gonorrhea and chlamydial infection. Case-finding observations. Sex Transm Dis. 1997;24:334–342. doi: 10.1097/00007435-199707000-00005. [DOI] [PubMed] [Google Scholar]
- 27.Kengne P, Veas F, Vidal N, et al. Trichomonas vaginalis: Repeated DNA target for highly sensitive and specific polymerase chain reaction diagnosis. Cell Mol Biol. 1994;40:813–831. [PubMed] [Google Scholar]
- 28.Madico G, Quinn TC, Rampalo A, et al. Diagnosis of Trichomonas vaginalis infection by PCR using vaginal swab samples. J Clin Microbiol. 36:3205–3210. doi: 10.1128/jcm.36.11.3205-3210.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Blythe MJ, Katz BP, Batteiger BE, Ganser JA, Jones RB. Recurrent genitourinary chlamydial infections in sexually active female adolescents. J Pediatr. 1992;121:487–493. doi: 10.1016/s0022-3476(05)81812-8. [DOI] [PubMed] [Google Scholar]
- 30.Hook EW, III, Reichart CA, Upchurch DM, et al. Comparative behavioral epidemiology of gonococcal and chlamydial infections among patients attending a Baltimore, Maryland, sexually transmitted disease clinic. Am J Epidemiol. 1992;136:662–672. doi: 10.1093/oxfordjournals.aje.a116546. [DOI] [PubMed] [Google Scholar]
- 31.Klausner JD, Kimball S, Zolt I, et al. Patient delivered partner therapy at San Francisco city clinic, 1998–1999. Abstract ISSTDR. 1999 [Google Scholar]
- 32.Golden MR, Whittington WLH, Handsfield HH, et al. Partner management for gonococcal and chlamydia infection: Expansion of public health services to a private sector and expedited sex partner treatment through a partnership with commercial pharmacies. Sex Transm Dis. 2001;28:658–665. doi: 10.1097/00007435-200111000-00009. [DOI] [PubMed] [Google Scholar]
- 33.David LM, Wade AA, Natin D, Radcliffe KW. Gonorrhoea in Coventry 1991–1994: Epidemiology, coinfection and evaluation of partner notification in the STD clinic. Int J STD AIDS. 1997;8:311–316. doi: 10.1258/0956462971920154. [DOI] [PubMed] [Google Scholar]
- 34.Mathews C, Coetzee N, Zwarenstein M, et al. A systematic review of strategies for partner notification for sexually transmitted diseases, including HIV/AIDS. Int J STD AIDS. 2002;13:285–300. doi: 10.1258/0956462021925081. [DOI] [PubMed] [Google Scholar]
- 35.Sorvillo F, Smith C, Kerndt P, Ash L. Trichomonas vaginalis, HIV, and African-Americans. Emerg Infect Dis. 2001;7:927–932. doi: 10.3201/eid0706.010603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang C, McClelland S, Reilly M, et al. The effect of treatment of vaginal infections on shedding of HIV-type I. J Infect Dis. 2001;183:1017–1022. doi: 10.1086/319287. [DOI] [PubMed] [Google Scholar]
- 37.Moodley P, Wilkinson D, Connolly C, et al. Trichomonas vaginalis is associated with pelvic inflammatory disease in women infected with HIV. Clin Infect Dis. 2002;34:519–522. doi: 10.1086/338399. [DOI] [PubMed] [Google Scholar]


