Abstract
Interaction between angiogenesis and axonal remodeling after stroke was dynamically investigated by MRI in rats with or without sildenafil treatments. Male Wistar rats were subjected to embolic stroke and treated daily with saline (n = 10) or with sildenafil (n = 11) initiated at 24 h and subsequently for 7 days after onset of ischemia. -weighted imaging, cerebral blood flow (CBF), and diffusion tensor imaging (DTI) measurements were performed from 24 h to 6 weeks after embolization. and fractional anisotropy (FA) maps detected angiogenesis and axonal remodeling after stroke, respectively, starting from 1 week in sildenafil-treated rats. Areas demarcated by MRI with enhanced angiogenesis, elevated local CBF, and augmented axonal remodeling were spatially and temporally matched, and FA values were significantly correlated with the corresponding CBF values (R = 0.66, P <4×10−5) at 6 weeks after stroke. Axonal projections were reorganized along the ischemic boundary after stroke. These MRI measurements, confirmed by histology, showed that sildenafil treatment simultaneously enhanced angiogenesis and axonal remodeling, which were MRI detectable starting from 1 week after stroke in rats. The spatial and temporal consistency of MRI metrics and the correlation between FA and local CBF suggest that angiogenesis, by elevating local CBF, promotes axonal remodeling after stroke.
Keywords: angiogenesis, axonal remodeling, cerebral blood flow, diffusion tensor imaging, embolic stroke, magnetic resonance imaging
Introduction
The critical step for the treatment of ischemic stroke is early recanalization (Broderick and Hacke, 2002). The recanalization of occluded arteries leads to the recovery of cerebral blood flow (CBF), which helps to prevent further damage of cerebral tissue and to promote brain remodeling after stroke.
The majority of patients with ischemic stroke fail to receive thrombolytic treatment with recombinant tissue plasminogen activator because of its narrow therapeutic window, which is limited to 3 h after the onset of ischemic stroke (Grotta et al, 2001; Zweifler et al, 1998). Neurorestorative therapy, thus, is an alternative treatment for all these patients to maximize neural function of the surviving brain. Neurorestorative treatments of either cell-based or pharmacological therapies promote angiogenesis and neurogenesis in ischemic cerebral tissue after stroke (Chen and Chopp, 2006; Jiang et al, 2006, 2005; Zhang et al, 2005, 2003).
Angiogenesis in ischemic cerebral tissue elevates regional CBF, which may benefit functional outcome. Patients with higher cerebral blood vessel density appear to make better progress and survive longer than patients with lower vascular density (Senior, 2001; Slevin et al, 2000). The elevated CBF, caused by either recanalization of occluded arteries or angiogenesis, improves regional cerebral tissue microenvironments to promote axonal outgrowth, remyelination, and white matter reorganization, which may improve neurologic function recovery (Jiang et al, 2006; Zhang et al, 2005). Therefore, neurorestorative treatments may integrate angiogenesis and brain plasticity after stroke. However, few studies to date have provided evidence to show the association between angiogenesis and brain plasticity via the improved CBF after stroke.
Treatment of experimental stroke with sildenafil, a phosphodiesterase type 5 (PDE5) enzyme inhibitor, initiated at 24 h after onset of ischemic stroke, enhanced angiogenesis, neurogenesis, and synapto-genesis in ischemic rat brain (Zhang et al, 2005, 2002), all of which are associated with improvement of functional recovery compared with saline-treated rats (Li et al, 2007; Zhang et al, 2005). Magnetic resonance imaging (MRI) measurements showed that the cerebrovascular changes caused by angio-genesis in rats treated with sildenafil can be detected by -weighted imaging (T2*WI) and susceptibility-weighted imaging (SWI) starting from 1 week after stroke (Ding et al, 2008). Cerebral blood flow values of the ischemic tissue along the ischemic lesion boundary area measured by MRI were significantly elevated in sildenafil treated rats compared with control rats and were associated with the induction of angiogenesis after stroke (Li et al, 2007).
Diffusion tensor imaging (DTI) of MRI provides a means for delineating the anatomic connectivity of white matter pathways and can be used to detect pathologic tract disruption based on the movement of water. Water in white matter moves more freely in the direction parallel to the tract than perpendicular to it, as axonal membranes and myelin restrict motion transverse to the tract. This diffusional directionality is known as fractional anisotropy (FA) and is directly correlated with histologic markers of myelination (Beaulieu, 2002; Mori and van Zijl, 2002; Sotak, 2002; Watanabe et al, 2001). Furthermore, increased FA appears to correlate with white matter tract integrity, whereas reduced FA is correlated with functional deficits (Beaulieu, 2002; Sotak, 2002; Watanabe et al, 2001).
Thus, MRI techniques were used in this study. Axonal changes of ischemic brain in rat after stroke were detected by DTI and FA, and angiogenic cerebral tissue was identified by T2*WI. We tested the hypothesis, with macroscopic MRI measurements, that angiogenesis in cerebral tissue locally benefits axonal reorganization after stroke in rat.
Materials and methods
Animals and Experimental Protocol
All studies were performed in accordance with institutional guidelines for animal research under a protocol approved by the Institutional Animal Care and Use Committee of Henry Ford Hospital.
Adult male Wistar rats at ages of 8 to 12 weeks, weighing 300 to 350 g, were subjected to embolic stroke by placement of an aged white clot, that is femoral arterial blood from a donor rat was withdrawn into 20 cm of PE-50 tubing and retained in the tube for 24 h (2 h to clot at room temperature and subsequently for 22 h at 4°C), at the origin of the middle cerebral artery (Zhang et al, 1997) and were randomly assigned to treatment (n = 11) and control (n = 10) groups. Sildenafil (Viagras®, Pfizer Inc., New York, NY, USA) at a dose of 10 mg/kg was administered subcutaneously to rats in the treated group at 24 h after middle cerebral artery occlusion and was administered daily for a total of 7 days. The selected dose has been previously shown to be effective for this model (Zhang et al, 2005). Rats in the control group were treated with the same volume of saline as in the treated group. Functional tests, MRI, and histologic data analyses were performed on all animals by investigators who were blinded to the experimental groups. Data, including behavioral outcomes from these animals, have been published (Li et al, 2007). Tests were performed preischemia, and once a week starting from 24 h to 6 weeks after the onset of stroke. Neurologic function of animals was graded as a modified Neurological Severity Score (Chen et al, 2001). All rats were euthanized 6 weeks after stroke.
Magnetic Resonance Imaging Measurements
Magnetic resonance imaging measurements were performed using a 7 Tesla system with a Bruker console (Bruker Biospin Inc., Billerica, MA, USA). A radio frequency saddle coil was used as the transmitter and an actively radio frequency decoupled surface coil as the receiver. Stereotaxic ear bars were used to minimize movement during the imaging procedure. During MRI measurements, anesthesia was maintained using a gas mixture of nitrous oxide (69%), oxygen (30%), and halothane (0.75% to 1.00%). Rectal temperature was kept at 37°C±1.0°C using a feedback-controlled water bath.
A tri-pilot imaging sequence was used for reproducible positioning of the animal in the magnet at each MRI session. A complete set of MRI images was performed before ischemia, repeatedly at 24 h, and weekly up to 6 weeks after embolization for all animals.
T2-weighted imaging and T2*WI were performed by multiple slices (13 slices, 1 mm slice thickness), multiple spin, or gradient echoes sequence, respectively, using a 32 × 32 mm field of view (FOV) and 128 × 64 image matrix, where all six echoes were acquired with equal interval of echo time (TE) and under the same readout gradient polarity in T2*WI. The TE was 3.6 or 15 ms for gradient or spin-echo sequence, respectively. The repetition time (TR) was 8 secs. The total time for the each sequence was approximately 9 mins.
The arterial spin labeling technique (Williams et al, 1992) was used for quantifying CBF in cerebral tissue. The adiabatic inversion of arterial water protons (Dixon et al, 1986) was accomplished via an axial gradient of 0.3 kHz/mm, and a continuous radio frequency power wave of approximately 0.3 kHz at a frequency offset of 6 kHz. This was followed by a spin-echo imaging sequence with TR/TE = 1,000/20 ms. The labeled slice was 2 cm distal from the imaging slice with 1 mm thickness. To remove the asymmetry of gradients in the axial direction, an image average was applied by switching around the gradient polarities. Field of view is 32 × 32 mm, matrix is 64 × 64. The scan time was 18 mins.
The DTI uses a two-dimensional multislice spin-echo diffusion-weighted imaging sequence. Seven diffusion-weighted imaging images were acquired for each slice, including one baseline with b = 0 and six images with b = 850 secs/mm2 at independent directions of diffusion gradients along XZ, –XZ,YZ Y–Z, XY and –XY. Two 10 ms (δ) gradient pulses separated 18 ms (Δ) on either side of the refocusing 180° radio frequency pulse were in sequence with 32 × 32 mm FOV, 128 × 128 matrix, 15 slices, and 1 mm slice thickness. Repetition time was 1500 ms and TE was 40 ms. The total time needed for DTI images with two averages was approximately 45 mins. Complete DTI scans from 1 day to 6 weeks after stroke were applied to seven treated and five control animals in the study.
Histology
Animals were anesthetized with ketamine (44 mg/kg, intraperitoneally) and xylazine (13 mg/kg, intraperitoneally), and were transcardially perfused with heparinized saline followed by 10% neutral-buffered formalin. The brain was removed and immersed in formalin solution for 1 h, after which a total of seven 2-mm-thick blocks of brain tissue were cut, processed, and embedded in paraffin.
MCID image analysis system (Imaging Research Inc., St Catherines, ON, Canada) with a × 40 objective (Olympus BX40) and a 3-CCD color video camera (Sony DXC-970MD) was used for histologic immunohistochemical measurements. Coronal sections (6 μm thick) were cut from each block and stained with hematoxylin and eosin for the evaluation of cerebral infarction. Immunostaining with antibodies against the endothelial barrier antigen was performed for quantification of cerebral vessels (Rosenstein et al, 1992). To measure myelinated axons, double Bielschowsky's silver stain for axons (modified) and Luxol fast blue (B&LFB) staining was performed (Wakefield et al, 1994). Three coronal sections and four locations of each coronal section were used for quantification. The immunoreactive areas in each location (percentage to FOV) were measured under a × 40 objective of optical microscope in the ischemic lesion boundary area and the homologous area in the contralateral side of the rat brain, and area sizes were digitized for assessing axonal density using an average of two FOVs for each location.
Data Analysis and Statistics
Image analysis of MRI was performed with homemade software, ‘Eigentool,’ in Sun workstations (Jacobs et al, 1999; Peck et al, 1992; Windham et al, 1988). All images were reconstructed using a 128 × 128 matrix. The different MRI maps and histologic section images were coregistered and analyzed using the Eigentool package. T2-weighted imaging images are referred to as reference images in coregistration, warping, and lesion size detection. Ischemic lesion size was determined by T2 maps acquired after stroke with values above mean plus two standard deviations (s.d.) of contralateral measurements (Ding et al, 2004; Hoehn-Berlage et al, 1995). The difference of the ischemic lesion sizes in T2 maps acquired at 24 h (Figure 1A) and 6 weeks (Figure 1B) after stroke is referred to as the recovery area (Figure 1C).
Figure 1.
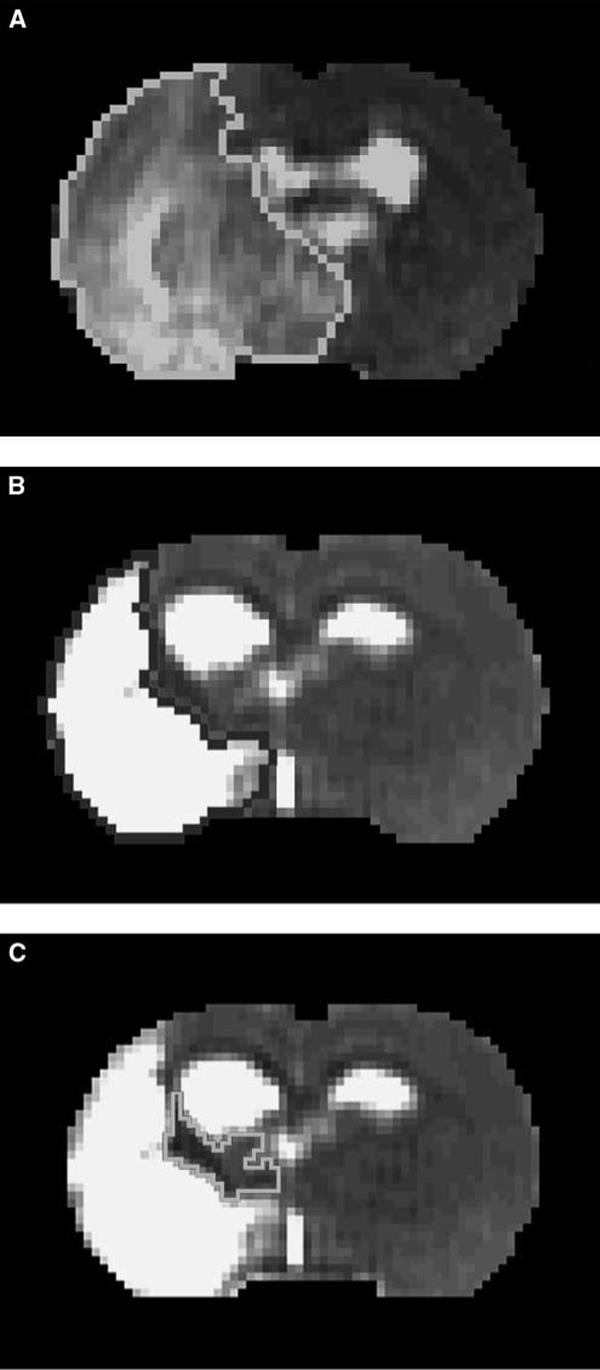
T2 map acquired at 24 h after stroke (A) demarcated acute ischemic lesion area with values above mean plus two s.d. of contralateral measurements. (B) Final infarction area was determined by T2 map acquired at 6 weeks after stroke. (C) The difference was referred as recovery area after stroke.
To quantitatively analyze MRI data, a region of interest (ROI) for recovery cerebral tissue was determined as follows: first, we measured FA values of homologous tissue in the mirror image of the recovery area on FA maps acquired at 6 weeks after stroke. Then, we acquired the ROI composed of pixels in the recovery area whose FA values are above the mean plus two s.d. This ROI was animal dependent and used to measure all MRI metrics for all time points in each animal. Using the ROI and its mirror image, MRI values of recovery ischemic tissue and homologous tissue in contralateral hemisphere were measured and used to obtain ratios. In the same way, ratios for irreversibly damaged tissue were obtained, where the ipsilateral ROI of the ischemic core was a small area located in the center of the infarct area based on a T2 map acquired at 6 weeks after stroke.
Diffusion tensor imaging data were analyzed using DTI studio software (Mori and van Zijl, 2002). Fiber tracking using DTI data started with an FA value > 0.15 and stopped with an FA < 0.15 or the turning angle greater than 651. Fractional anisotropy maps were also produced from the conventional diffusion-weighted imaging data in three perpendicular directions (van Gelderen et al, 1994).
The area with elevated CBF at 6 weeks after stroke was identified as that above the threshold of 50 mL/100 g per min (Ding et al, 2005) within the original low CBF area measured at 24 h after stroke, because mean CBF value of recovered cerebral tissue was usually lower than that of homologous tissue in the contralateral side.
Magnetic resonance imaging observations and histo-logic measurements are summarized as mean value with s.d., but the group average data for MRI measurements are presented as mean value with standard error (s.e.). The differences of MRI and histologic measurements between two groups were analyzed by t-test. The t-test was performed on MRI data obtained at all time points between the treated and control groups, assuming equal variances. For data with apparent differences in variances, the t-test was performed again, with the assumption of unequal variances. All t-tests were two-tailed. A linear regression was performed to compute the correlation between FA and CBF values. The significance level (α) was set at 5%.
Results
The quantitative MRI data of group averaged measurements (ratios of ipsilateral ROI with higher FA values to its contralateral ROI), as shown in Figures 2A to C, showed the temporal features of (Figure 2A), CBF (Figure 2B), and FA (Figure 2C) for cerebral tissue with axonal changes, that is higher FA values, in stroke rats with or without sildenafil treatments. Compared with the control group, tissue with axonal changes in the sildenafil-treated group had relatively lower values, and higher CBF and FA values at 1 to 6 weeks after stroke. The differences were significant (P < 0.05) during 1 to 4 weeks for , at 6 weeks for CBF, or at 2, 5, and 6 weeks for FA measurements. These data indicate that sildenafil treatment promoted angiogenesis, elevated CBF, and enhanced axonal changes after stroke in rats compared with the saline treatment. The relative CBF and FA values, which were individually measured at 6 weeks after stroke within both ROIs in areas with remarkable axonal changes along the boundary and in ischemic core, exhibited a significant linear correlation (P <4 × 10−5) with a correlation coefficient of R = 0.66 (Figure 2D).
Figure 2.
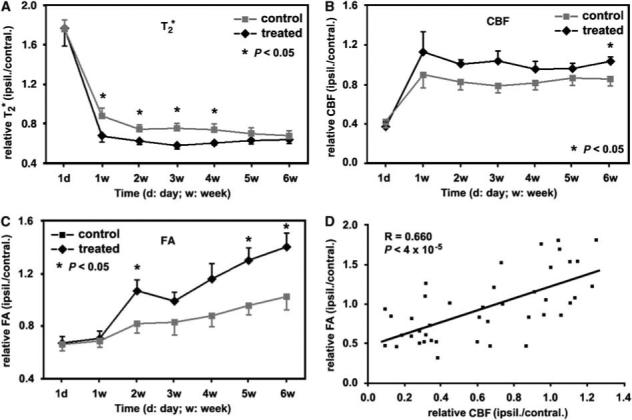
With the ROI identified hyperintensity in FA map acquired at 6 weeks after stroke, the group averaged ratios, measurements from the ROI to its contralateral ROI, showed the temporal features of (A), CBF (B), and FA (C) in stroke rats with or without treatments. Compared with control rats, sildenafil-treated rats had relative lower values, higher CBF and FA values within the 6 weeks after stroke, although similar values at 1 day after stroke. The differences were significant (P < 0.05) during 1 to 4 weeks for , at 6 weeks for CBF, or at 2, 5, and 6 weeks for FA measurements. Error bars in panels A to C are presented as standard errors. The relative CBF and FA values, individually measured at 6 weeks after stroke in both ischemic boundary and core, exhibited a linear correlation (P <4 × 10−5) with a correlation coefficient of R = 0.66 (D).
In a representative rat treated with sildenafil after stroke, areas with lower values, high CBF, and FA values (indicated by red arrows in A2, B2 and C2 of Figure 3) appeared in , CBF, and FA maps starting from 1 week after stroke, respectively. These areas, located in the recovery region, exhibited individual temporal profiles (red arrows in Figures 3A to C) up to 6 weeks after stroke. These temporal maps showed that areas with lower values, elevated CBF, and higher FA values synchronously developed from 1 to 6 weeks after stroke. As areas destined for angiogenesis or axonal changes can be characterized by lower values or higher FA values in or FA maps, respectively, Figures 3A to C indicate that the cerebrovascular changes caused by angiogenesis and axonal changes were synchronous at 1 week and were MRI detectable during 1 to 6 weeks after embolic stroke in the sildenafil-treated rat, whereas the CBF value of the area was elevated at 1 week and remained so to 6 weeks after stroke. In each map acquired at 6 weeks after stroke, areas with lower values, elevated CBF, and increased FA values were outlined in individual maps, as shown in Figures 3D to 3F, and all projected onto a T2 map (Figure 3G). This overlapped image showed that the locations of the areas with lower values, elevated CBF and higher FA values were highly spatially correlated each other. Fiber tracking of this animal using DTI showed that the directions of axonal projections in the fiber tract at 6 weeks after stroke were highly consistent with each other and present along the ischemic boundary (Figure 3H), which was coincident with the result of hyperintensity area in the FA map (Figure 3,C7). In this case, the DTI analysis gave means of fiber length () and density (numbers per voxel, ) in the area with higher FA values along the ischemic boundary as = 88.17 mm and = 10.36, whereas the numbers for whole brain were = 29.15 mm and = 7.02.
Figure 3.

In a representative sildenafil-treated rat, (A), CBF (B), and FA (C) maps tracked angiogenesis via lower values, recovered blood supply with hyperintensity in CBF, and axonal remodeling by higher FA values after stroke, respectively. Red arrows in A2, B2, and C2 indicated that angiogenesis, CBF recovery, and axonal remodeling simultaneously occurred starting from 1 week after stroke, respectively. At 6 weeks after stroke, the low value area (D), elevated CBF area (E), and increased FA value area (F) were outlined in individual maps and projected onto a T2 map (G). Fiber tracking by DTI in the treated (H) and in a control (I) rats indicated that the axons were reorganized along ischemic boundary, which were coincident with FA maps of the treated (C7) and the control (J) rats.
In control rats treated with saline after stroke, areas with lower value, high CBF, and FA values in , CBF, and FA maps along ischemic boundary, respectively, developed slower than sildenafil-treated rats, as showed with quantitative data in Figures 2A to C. The fiber tracking at 6 weeks after stroke of a control rat showed that the directionality of fibers paralleled the ischemic boundary (Figure 3I). However, the length and density of fibers tracked by the DTI data acquired and analyzed with same parameters were shorter ( = 26.20 mm) and lower ( = 3.62) than sildenafil-treated rats. An area with weakly increased values in FA map acquired at 6 weeks after stroke, as indicated by a red arrow in Figure 3J, can be identified for this animal in the FA map, and was coincident with the DTI result (Figure 3I).
Histologic measurements were consistent with MRI results. Using the endothelial barrier antigen-stained section (Figure 4A) of the representative rat treated with sildenafil, the microvascular density in the red box, which matched the area demarcated with low value along ischemic boundary in the map (Figure 3D) at 6 weeks after stroke, was higher (Figure 4B) than that of the homologous tissue (Figure 4C) in the contralateral hemisphere. Using the section with B&LFB staining (Figure 4D), the axonal density (Figure 4D) in the red box, which was at the same location as the red box in endothelial barrier antigen section and the area characterized by hyperintensity in FA map along the ischemic boundary (Figure 3F) at 6 weeks after stroke, was higher than that of the homologous tissue in contralateral hemisphere (Figure 4F). The 2 × enlarged image of B&LFB-stained section (Figure 4G) also showed a high density of axonal projections that paralleled the rim of the ischemic boundary in the treated rat. For the control rat, the enlarged B&LFB-stained section (Figure 4H) showed a line along the lesion border with shorter and lower density of axonal projections (Figure 4I) than the sildenafil-treated rat, and was consistent with DTI results (Figure 3I). Quantitatively, histologic data of B&LFB staining showed that axonal density increased in the ischemic boundary area compared with the contralateral side for both control and treated rats at 6 weeks after stroke. However, the increase is 46.8%±18.8% for treated animals and 24.3%±14.7% for control rats, and the difference was significant (P < 0.04) between the sildenafil and saline treatment of embolic stroke rats. However, current histology of B&LFB staining cannot provide quantitative data to delineate the directionality of axonal projection.
Figure 4.
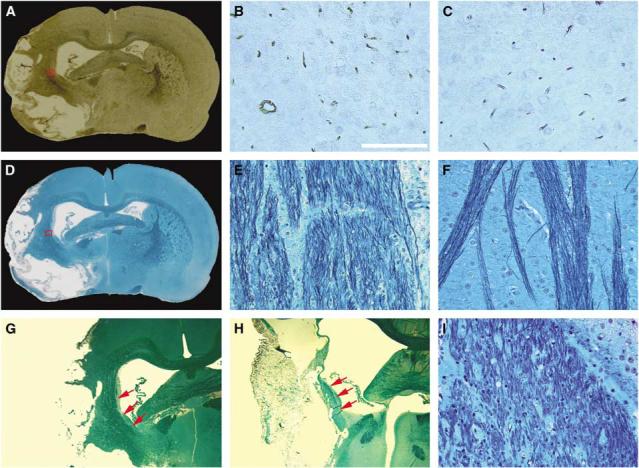
With EBA (A) and B&LFB (D) sections stained at 6 weeks after stroke, microvascular (B) and axonal (E) density at 6 weeks after stroke in the angiogenic area demarcated with lower values and axonal remodeling area characterized by higher FA values along ischemic boundary were higher than those in the homologous tissue of contralateral hemisphere (C and F), respectively. The nerve fibers after stroke were reorganized along the rim of ischemic boundary in both treated (G) and control (H) rats, as indicated by red arrows. Axonal density along ischemic boundary in the control rat (I) was increased compared with normal tissue, but less than in the treated rat. Scale bar = 100 μm (B).
Performed with threshold in FA map acquired at 6 weeks after stroke and then histologically confirmed by B&LFB-stained section under a light microscope, pixels with remarkably increased FA values in the recovery area along the ischemic boundary were found in 3 out of 10 control rats and 9 out of 11 sildenafil-treated rats. The difference of axonal changes identified by higher values in FA maps between the control and treated groups of animals was significant (Fisher's test; P < 0.02, or P ≈ 0.05 after correction).
Neurologic functional test indicated that the treatment of stroke with sildenafil significantly (P < 0.05) improved the modified Neurological Severity Score and performance on foot-fault test in rats from 2 or 3 weeks up to 6 weeks after stroke compared with control animals treated with saline (Li et al, 2007).
Discussion
On the basis of the MRI data of DTI fiber tracking at 6 weeks after stroke, orientation of axonal projections in rat brain was reorganized to parallel the rim of the ischemic boundary, and FA values were increased after stroke along the ischemic boundary, which may result from the increase of both density and directionality of axonal projections. In corresponding areas, histologic data of B&LFB staining in the study confirmed that the axonal density was increased, and the orientation of axonal projections was reorganized along the ischemic boundary after stroke. Therefore, we refer to such changes of axonal density and directionality after stroke as axonal remodeling.
We recently reported that sildenafil treatment of embolic stroke in rats enhances angiogenesis in ischemic cerebral tissue after stroke and that sildenafil affects CBF differences between the ischemic core and boundary, which may contribute to the neurologic function improvements after stroke in treated rats (Li et al, 2007). To show the associations among angiogenesis, CBF, and axonal remodeling after stroke in rats, we used the same cohort of animals. Here, we show novel findings that sildenafil treatment of embolic stroke in rats amplifies axonal remodeling along the ischemic boundary after stroke, and angiogenesis, CBF, and axonal remodeling are significantly correlated with spatial and temporal consistencies.
By using MRI to simultaneously monitor angio-genesis and axonal remodeling after embolic stroke in rats, we found in this investigation that the temporal profiles of angiogenic area and axonal remodeling along the ischemic boundary were temporally and spatially coincident after stroke. Treatment of stroke with sildenafil in rats augmented angiogenesis and axonal remodeling in ischemic rat brains after embolization, as measured by and FA maps, respectively, and confirmed by histology. The tractography using DTI or immunohistochemical staining of axon and myelin showed that reorganized nerve fibers after stroke was monotonically orientated along the ischemic boundary. Because CBF correlated with FA at 6 weeks after stroke, this study indicated that angiogenesis, via elevating CBF, may coordinate axonal remodeling later after stroke.
Previous studies have shown that treatment of embolic stroke in rats with sildenafil, administered subcutaneously at a dose of 10 mg/kg and initiated 24 h after stroke and continuing daily for 7 days, significantly increased the cortical cGMP (cyclic guanosine monophosphate) level by 60% in adult rats compared with saline-treated ones (Zhang et al, 2005). The elevated cGMP concentration evokes and enhances neurogenesis, angiogenesis, and synapto-genesis (Zhang et al, 2005, 2002). Administration of sildenafil 24 h after stroke also increases synthesis of vascular endothelia growth factor and enhances angiogenesis in ischemic brain via the nitric oxide-/cGMP-dependent pathway (Zhang et al, 2003). Vascular endothelia growth factor also regulates neurogenesis (Greenberg and Jin 2005). Angiogenesis and neurogenesis are linked in the adult brain via vascular endothelia growth factor and brain-derived neurotrophic factor (Palmer et al, 2000; Leventhal et al, 1999). Experiments with adult female canarys suggested a causal interaction between the testosterone-induced angiogenesis and neurogenesis in forebrain of the songbird (Louissaint et al, 2002).
In this study, and FA maps detected angiogenic areas with low values in the ischemic lesion (Ding et al, 2008) and axonal remodeling with high FA values along ischemic boundary (Jiang et al, 2006) starting from 1 week (Figure 2A1 and 2C1) after stroke, respectively. These MRI data indicate that angiogenesis and axonal remodeling after ischemia may be simultaneously promoted and amplified by the sildenafil treatment in rats in an early stage of recovery from stroke. Thus, in an angiogenic area where growth factors (e.g., vascular endothelia growth factor) were enhanced by sildenafil treatment (Zhang et al, 2002, 2003, 2005), axonal remodeling was also amplified. As a result, the areas with angiogenesis and axonal remodeling enhanced by sildenafil treatment spatially matched each other in later stages of stroke, for example, at 6 weeks after stroke.
With the significant development of angiogenesis (Figure 2A) that increases the density of vessels by sprouting from existing vessels and by expanding the volume of existing vessels (Figure 4B), local CBF around the ischemic boundary was significantly increased in sildenafil-treated rats compared with control animals at 6 weeks after stroke (Figure 2B). The angiogenic area (Figure 3A7) identified by the map matched the area with elevated CBF values around the ischemic border (Figure 3B7) at 6 weeks after stroke. The CBF of the treated group was consistently higher than the control group at all time points. The P-values at 2 and 3 weeks were 0.066 and 0.063, respectively, showing marginally significant differences between the two groups. We have also previously shown that angiogenesis is prominent at 6 weeks after stroke in sildenafil-treated animals (Ding et al, 2008). Thus, the significant difference of CBF between control and treatment groups at 6-week time point is not accidental. In addition to growth factors that promoted axonal remodeling in angiogenic area, the elevated CBF may provide a permissive microenvironment for axonal remodeling, as evident from the significant differences of axonal remodeling characterized by FA values between the treated and control animals at 5 and 6 weeks after stroke (Figure 2C). Statistical analysis showed that the FA correlated with the local CBF of the same area with R = 0.66 and P < 4 × 10−5 (Figure 2D) at 6 weeks after stroke.
Preclinical studies indicate that angiogenesis enhanced by pharmacological treatments or cell therapy of stroke promotes neurologic improvement after stroke (Jiang et al, 2005; Wei et al, 2001). In the clinic, patients with higher cerebral blood vessel density appear to make better progress and survive longer than patients with lower vascular density (Senior, 2001; Slevin et al, 2000). In this study, sildenafil treatment of embolic stroke showed that it can enhance angiogenesis to improve CBF, and then benefit axonal remodeling after stroke in the ischemic boundary area. All of these changes may contribute to the significant improvement of functional recovery in stroke rats treated with sildenafil compared with control animals up to 6 weeks after ischemia.
Axonal density (percentage of FOV) in the ischemic boundary measured by histology at 6 weeks after stroke was significantly increased (P < 0.04) in sildenafil-treated rats compared with control rats treated with saline. Fractional anisotropy measurements exhibited significant differences between treated and control groups of rats from 5 weeks after stroke (Figure 2C). Hyperintensity areas in the FA map along the ischemic boundary after stroke primarily result from increases of axonal density and directionality, because a random orientation of fibers in a voxel likely reduces FA values due to the average effect of diffusion of water in tissue. The increase of axonal density in rat brain after stroke could be caused by axonal outgrowth and remyelination. Diffusion tensor imaging can dynamically track the orientation of axonal projections and provide the means of density and length of fiber tracts in rats after stroke. However, DTI or FA maps do not provide information for analyzing the changes of axonal density and directionality. This limitation of MRI restricts access to the details of axonal remodeling. The presence of axonal remodeling in the boundary region induced by sildenafil treatment does not necessarily imply anything about cell death in this region. They are likely disconnected events. Thus, there is no discrepancy between the significant enhancement of axonal remodeling and nonsignificant reduction of lesion volume (Li et al, 2007) in the sildenafil-treated group, compared with the control group.
In summary, our data in this study showed that daily treatment of embolic stroke in rats with sildenafil initiated 1 day after embolism and continued 7 days significantly augmented axonal remodeling in the ischemic boundary zone. Dynamic MRI measurements indicated that the cerebrovascular changes caused by angiogenesis and axonal remodeling were synchronously promoted and amplified by sildenafil treatment and were detectable from 1 week after stroke by and FA maps. Because areas demarcated by MRI as angiogenic, with elevated CBF and axonal remodeling, were histologically confirmed, and spatially and temporally matched each other, sildenafil may independently augment angiogenesis, axonal remodeling, and CBF. In addition to the significant correlation between the FA values and the local CBF at 6 weeks after stroke (R = 0.66, P < 4 × 10−5), we therefore may conclude that angiogenesis, local CBF, and axonal remodeling significantly correlate after stroke in rat.
Acknowledgments
This work was supported by NINDS Grants PO1 NS23393, PO1 NS42345, RO1 NS38292, RO1 NS48349, RO1 NS43324, and HL64766.
Footnotes
Conflict of interest The authors state no conflict of interest.
References
- Beaulieu C. The basis of anisotropic water diffusion in the nerve system—a technical review. NMR Biomed. 2002;15:435–55. doi: 10.1002/nbm.782. [DOI] [PubMed] [Google Scholar]
- Broderick JP, Hacke W. Treatment of acute ischemic stroke Part I: recanalization strategies. Circulation. 2002;106:1563–9. doi: 10.1161/01.cir.0000030406.47365.26. [DOI] [PubMed] [Google Scholar]
- Chen J, Chopp M. Neurorestorative treatment of stroke: cell and pharmacological approaches. NeuroRx. 2006;3:466–73. doi: 10.1016/j.nurx.2006.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Li Y, Wang L, Zhang ZG, Lu D, Lu M, Chopp M. Therapeutic benefit of intravenous administration of bone marrow stromal cells after cerebral ischemia in rats. Stroke. 2001;32:1005–11. doi: 10.1161/01.str.32.4.1005. [DOI] [PubMed] [Google Scholar]
- Ding G, Jiang Q, Li L, Zhang L, Zhang ZG, Ledbetter KA, Gollapalli L, Panda S, Li Q, Ewing JR, Chopp M. Angiogenesis detected post embolic stroke in rat brain using magnetic resonance T2*WI. Stroke. 2008 doi: 10.1161/STROKEAHA.107.502146. PM ID: 18356548 (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding G, Jiang Q, Zhang L, Zhang ZG, Knight RA, Soltanian-Zadeh H, Lu M, Ewing JR, Li Q, Whitton PA, Chopp M. Multiparametric ISODATA analysis of embolic stroke and rt-PA intervention in rat. J Neurol Sci. 2004;223:135–43. doi: 10.1016/j.jns.2004.05.017. [DOI] [PubMed] [Google Scholar]
- Ding G, Jiang Q, Zhang L, Zhang ZG, Li L, Knight RA, Ewing JR, Wang Y, Chopp M. Analysis of combined treatment of embolic stroke in rat with r-tPA and a GPIIb/IIIa inhibitor. J Cereb Blood Flow Metab. 2005;25:87–97. doi: 10.1038/sj.jcbfm.9600010. [DOI] [PubMed] [Google Scholar]
- Dixon WT, Du LN, Faul DD, Gado M, Rossnick S. Projection angiograms of blood labeled by adiabatic fast passage. Magn Reson Med. 1986;3:454–62. doi: 10.1002/mrm.1910030311. [DOI] [PubMed] [Google Scholar]
- Greenberg DA, Jin K. From angiogenesis to neuro-pathology. Nature. 2005;438:954–9. doi: 10.1038/nature04481. [DOI] [PubMed] [Google Scholar]
- Grotta JC, Burgin WS, El-Mitwalli A, Long M, Campbell M, Morgenstern LB, Malkoff M, Alexanderov AV. Intravenous tissue-type plasminogen activator therapy for ischemic stroke: Houston experience 1996 to 2000. Arch Neurol. 2001;58:2009–13. doi: 10.1001/archneur.58.12.2009. [DOI] [PubMed] [Google Scholar]
- Hoehn-Berlage M, Eis M, Back T, Kohno K, Yamashita K. Changes of relaxation times (T1, T2) and apparent diffusion coefficient after permanent middle cerebral artery occlusion in the rat: temporal evolution, regional extent, and comparison with histology. Magn Reson Med. 1995;34:824–34. doi: 10.1002/mrm.1910340607. [DOI] [PubMed] [Google Scholar]
- Jacobs MA, Windham JP, Soltanian-Zadeh H, Peck DJ, Knight RA. Registration and warping of magnetic resonance images to histological sections. Med Phys. 1999;26:1568–78. doi: 10.1118/1.598671. [DOI] [PubMed] [Google Scholar]
- Jiang Q, Zhang ZG, Ding G, Zhang L, Ewing JR, Wang L, Zhang RL, Li L, Lu M, Meng H, Arbab AS, Hu J, Li Q, Pourabdillah-Nejed-D S, Athiiraman H, Chopp M. Investigation of neural progenitor cell induced angiogenesis after embolic stroke in rat using MRI. Neuroimage. 2005;28:698–707. doi: 10.1016/j.neuroimage.2005.06.063. [DOI] [PubMed] [Google Scholar]
- Jiang Q, Zhang ZG, Ding GL, Silver B, Zhang L, Meng H, Lu M, Pourabdillah-Nejed-D S, Wang L, Savant-Bhonsale S, Li L, Bagher-Ebadian H, Hu J, Arbab AS, Vanguri P, Ewing JR, Ledbetter KA, Chopp M. MRI detects white matter reorganization after neural progenitor cell treatment of stroke. Neuroimage. 2006;32:1080–9. doi: 10.1016/j.neuroimage.2006.05.025. [DOI] [PubMed] [Google Scholar]
- Leventhal C, Rafii S, Rafii D, Shahar A, Goldman SA. Endothelial trophic support of neuronal production and recruitment from the adult mammalian subependyma. Mol Cell Neurosci. 1999;13:450–64. doi: 10.1006/mcne.1999.0762. [DOI] [PubMed] [Google Scholar]
- Li L, Jiang Q, Zhang L, Ding G, Zhang ZG, Li Q, Ewing JR, Lu M, Panda S, Ledbetter KA, Whitton PA, Chopp M. Angiogenesis and improved cerebral blood flow in the ischemic boundary area detected by mri after administration of sildenafil to rats with embolic stroke. Brain Res. 2007;1132:185–92. doi: 10.1016/j.brainres.2006.10.098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louissaint A, Jr, Rao S, Leventhal C, Goldman SA. Coordinated interaction of neurogenesis and angiogenesis in the adult songbird brain. Neuron. 2002;34:856–8. doi: 10.1016/s0896-6273(02)00722-5. [DOI] [PubMed] [Google Scholar]
- Mori S, van Zijl PC. Fiber tracking: principles and strategies—a technical review. NMR Biomed. 2002;15:468–80. doi: 10.1002/nbm.781. [DOI] [PubMed] [Google Scholar]
- Palmer TD, Willhoite AR, Gage FH. Vascular niche for adult hippocampal neurogenesis. J Comp Neurol. 2000;425:479–94. doi: 10.1002/1096-9861(20001002)425:4<479::aid-cne2>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Peck DJ, Spickler EM, Knight RA, Hearshen DO, Windham JP. Analysis of the evolution of focal cerebral ischemia in the rat using the eigenimage filter. Magn Reson Med. 1992;26:259–73. doi: 10.1002/mrm.1910260207. [DOI] [PubMed] [Google Scholar]
- Rosenstein JM, Krum JM, Sternberger LA, Pulley MT, Sternberger NH. Immunocytochemical expression of the endothelial barrier antigen (EBA) during brain angiogenesis. Dev Brain Res. 1992;66:47–54. doi: 10.1016/0165-3806(92)90138-m. [DOI] [PubMed] [Google Scholar]
- Senior K. Angiogenesis and functional recovery demonstrated after minor stroke. Lancet. 2001;358:817. doi: 10.1016/S0140-6736(01)06014-7. [DOI] [PubMed] [Google Scholar]
- Slevin M, Krupinski J, Slowik A, Kumar P, Szczudlik A, Gaffney J. Serial measurement of vascular endothelial growth factor and transforming growth factor-beta 1 in serum of patients with acute ischemic stroke. Stroke. 2000;31:1863–70. doi: 10.1161/01.str.31.8.1863. [DOI] [PubMed] [Google Scholar]
- Sotak CH. The role of diffusion tensor imaging 2 in the evaluation of ischemic brain injury—a review. NMR Biomed. 2002;15:561–9. doi: 10.1002/nbm.786. [DOI] [PubMed] [Google Scholar]
- van Gelderen P, de Vleeschouwer MH, DesPres D, Pekar J, van Zijl PC, Moonen CT. Water diffusion and acute stroke. Magn Reson Med. 1994;31:154–63. doi: 10.1002/mrm.1910310209. [DOI] [PubMed] [Google Scholar]
- Wakefield AJ, More LJ, Difford J, McLaughlin JE. Immunohistochemical study of vascular injury in acute multiple sclerosis. J Clin Pathol. 1994;47:129–33. doi: 10.1136/jcp.47.2.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe T, Honda Y, Fujii Y, Koyama M, Matsuzawa H, Tanaka R. Three-dimensional anisotropy contrast magnetic resonance axonography to predict the prognosis for motor function in patients suffering from stroke. J Neurosurg. 2001;94:955–60. doi: 10.3171/jns.2001.94.6.0955. [DOI] [PubMed] [Google Scholar]
- Wei L, Erinjeri JP, Rovainen CM, Woolsey TA. Collateral growth and angiogenesis around cortical stroke. Stroke. 2001;32:2179–84. doi: 10.1161/hs0901.094282. [DOI] [PubMed] [Google Scholar]
- Williams DS, Detre JA, Leigh JS, Koretsky AP. Magnetic resonance imaging of perfusion using spin inversion of artery water. Proc Natl Acad Sci USA. 1992;89:212–6. doi: 10.1073/pnas.89.1.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Windham JP, Abd-Allah MA, Eimann DA, Froelich JW, Haggar AM. Eigenimage filtering in MR imaging. J Comput Assist Tomogr. 1988;12:1–9. doi: 10.1097/00004728-198801000-00001. [DOI] [PubMed] [Google Scholar]
- Zhang L, Zhang RL, Wang Y, Zhang CL, Zhang ZG, Meng H, Chopp M. Functional recovery in aged and young rats after embolic stroke: treatment with a phosphodiesterase type 5 inhibitor. Stroke. 2005;36:847–52. doi: 10.1161/01.STR.0000158923.19956.73. [DOI] [PubMed] [Google Scholar]
- Zhang RL, Chopp M, Zhang ZG, Jiang Q, Ewing JR. A rat model of focal embolic cerebral ischemia. Brain Res. 1997;766:83–92. doi: 10.1016/s0006-8993(97)00580-5. [DOI] [PubMed] [Google Scholar]
- Zhang RL, Wang L, Zhang L, Chen J, Zhu Z, Zhang Z, Chopp M. Nitric oxide enhances angiogenesis via the synthesis of vascular endothelial growth Factor and cGMP after stroke in the rat. Circ Res. 2003;92:308–13. doi: 10.1161/01.res.0000056757.93432.8c. [DOI] [PubMed] [Google Scholar]
- Zhang RL, Wang Y, Zhang L, Zhang ZG, Tsang W, Lu M, Zhang LJ, Chopp M. Sildenafil (Viagra) induces neurogenesis and promotes functional recovery after stroke in rats. Stroke. 2002;33:2675–80. doi: 10.1161/01.str.0000034399.95249.59. [DOI] [PubMed] [Google Scholar]
- Zweifler RM, Brody ML, Graves GC, U TT, Drinkard R, Cunningham S, Rothrock JF. Intravenous t-PA for acute ischemic stroke: therapeutic yield of a stroke code system. Neurology. 1998;50:501–3. doi: 10.1212/wnl.50.2.501. [DOI] [PubMed] [Google Scholar]


