Abstract
Previous research has suggested that adolescents with myelomeningocele and shunted hydrocephalus (MMH) have difficulties with aspects of executive functioning and, in turn, with functional independence. There is little research, however, examining patterns of executive functioning across adolescence in this population. The goal of this cross-sectional study was to examine parent ratings of executive function in children with MMH and in typically developing peers across late childhood and adolescence. Parents of 36 individuals with MMH and 35 typically developing peers, ages 10 to 18 years, completed the Behavior Rating Inventory of Executive Function (BRIEF). The BRIEF is organized into eight scales and two primary indices—Metacognition (MCI) and Behavioral Regulation (BRI). As a whole, the children with MMH had significantly higher BRIEF T-scores, as well as a higher prevalence of clinically significant T-scores across subscales, particularly those representing cognitive control. Effects of group, age, and age-by-group interactions on the mean raw scores of the MCI and BRI were examined using regression analyses. There were significant group effects (p < .05) for both the BRI and MCI, with the controls having significantly lower mean ratings than the MMH group. There was also a significant contribution of age-by-group interaction on the BRI (p < .05). Although mean raw scores on the BRI for the MMH group remained stable across ages, mean raw scores in the control group decreased as age increased. Thus, healthy children have age-related improvements in executive control behaviors across adolescence, particularly behavioral control, while children with MMH demonstrate no age-related improvements in parent reported executive behaviors across adolescence. Therefore, children with MMH may continue to require targeted interventions and modifications to address executive dysfunction into young adulthood in order to promote functional independence.
Keywords: BRIEF, Childhood and adolescence, Executive functioning, MMH
INTRODUCTION
Spina bifida, which occurs in approximately 1 in 1000 births (Charney, 1992), is a congenital malformation of the central nervous system associated with incomplete closure of the neural tube early in gestation (Volpe, 2001). Approximately 70% of those with spina bifida have myelomeningocele (Charney, 1992), which is associated with brainstem and cerebellar (i.e., Chiari) malformation and typically results in placement of a ventriculoperitoneal shunt (Fletcher, Dennis, & Northrup, 2000). Other medical and neurological complications associated with myelomeningocele and shunted hydrocephalus (MMH) include callosal agenesis, tethered spinal cord, seizures, bowel and bladder dysfunction, pressure sores and skin breakdown, shunt malfunction, sleep apnea, lower extremity paralysis, and cognitive dysfunction. These complications may require surgical intervention, hospitalization, pharmacological management, use of assistive devices and wheelchairs, and/or modified self-care demands (e.g., catheterization). Therefore, MMH is a dynamic, complex, and potentially unstable medical condition requiring comprehensive care into adolescence and young adulthood (Kennedy et al., 1998).
Children with MMH are at increased risk for neuropsychological dysfunction. Initial and recurrent hydrocephalus can negatively impact cognitive functioning and, in turn, can potentially have deleterious effects on functional independence throughout the lifespan. The pattern of cognitive strengths and weaknesses in MMH are dynamic, and are affected by neurological development as well as associated medical/physiological factors such as lesion level (Fletcher et al., 2005), hydrocephalus (Mataro, Junque, Poca, & Sahuquillo, 2001), Chiari malformation, and surgical procedures (e.g., shunt placement). Given documented associated cognitive sequelae of MMH, developmental and neuropsychological evaluation of these children is an important component of patient care.
Neuropsychologically, children with MMH often demonstrate intellectual abilities in the low average-to-average range, with relatively better performance on tasks assessing verbal skills than nonverbal reasoning skills (Riddle, Morton, Sampson, Vachha, & Adams, 2005; Wills, 1993). However, higher-level language difficulties, including problems with lexical, semantic, and pragmatic language (Vachha & Adams, 2002) and narrative discourse (Barnes & Dennis, 1998) have been described. Deficits have also been noted in aspects of visuospatial functioning (Dennis, Fletcher, Rogers, Hetherington, & Francis, 2002), including visual working memory (Mammarella, Cornoldi, & Donadello, 2003) and math and numeracy skills (Dennis & Barnes, 2002). These and other cognitive impairments, specifically those affecting attention (Brewer, Fletcher, Hiscock, & Davidson, 2001; Loss, Yeates, & Enrile, 1998), motor functioning (Hetherington & Dennis, 1999), memory (Scott et al., 1998), and executive functions (Dennis, Barnes, & Heatherington, 1999; Fletcher et al., 1996; Iddon, Morgan, Loveday, Sahakian, & Pickard, 2004; Snow, 1999), can significantly interfere with adaptive functioning and achievement of independence in this population.
Executive functions involve “developing an approach” to performing a task that is not habitually performed (Mahone et al., 2002a). Measurement of executive functions yields dissociable components that can include initiation, planning, organization, shifting of thought or attention, working memory, inhibitory control, and response preparation (Denckla, 1996; Pennington, 1997), resulting in a broad range of possible behavioral presentations in individuals with executive dysfunction. Ecological validity of neuropsychological tests, including performance-based tests of executive function, remains questionable (Russell, 2001), especially in children with exceptionally high or low intellectual functioning (Mahone et al., 2002a; Mahone et al., 2002b). Although some research suggests that measures of intellectual functioning correlate only modestly with tests of executive functioning in typically developing children and in various clinical groups (Anderson, Anderson, Northam, Jacobs, & Catroppa, 2001; Nigg et al., 2005; Schuck & Crinella, 2005; Snow, 1999; Watkins et al., 2005), others have found that IQ is a significant moderator of performance-based tests of executive function (Mahone et al., 2002b). In addition, executive control skills are thought to mediate the ability of children with deficits in other domains (e.g., language, visuospatial skills, memory) to compensate for such difficulties (Denckla, 1994). Caregiver rating scales such as the Behavior Rating Inventory of Executive Function (BRIEF; Gioia, Isquith, Guy, & Kenworthy, 2000) allow caregivers to rate behaviors seen in daily life functioning. The BRIEF has been shown to capture executive dysfunction in clinical groups when performance-based laboratory tests have not (Anderson, Anderson, Northam, Jacobs, & Mikiewicz, 2002; Cummings, Singer, Krieger, Miller & Mahone, 2002; Mahone et al., 2002a; Vriezen & Pigott, 2002).
Executive functions are thought to support the development of independent, efficient performance of day-to-day functional life skills. For example, in children and adolescents with MMH, parent reports of metacognitive deficits such as problems with working memory and initiation are correlated with their reports of their children's self-care skills (Ries, Zabel, & Mahone, 2003), suggesting that skills such as working memory may mediate the independent implementation of adaptive skills in those with MMH. Conversely, among healthy children, the efficiency of the executive function system is thought to improve parallel to maturational changes in neural structures, including ongoing myelination of neurons, resulting in an increase of white matter volume, a reduction of gray matter volume (i.e., “pruning”), and increased synaptogenesis into young adulthood (Casey, Giedd, & Thomas, 2000; Klingberg, Vaidya, Gabrieli, Moseley, & Hedehus, 1999; Paus, Collins, Evans, Leonard, Pike, & Zijdenbos, 2001; Thompson, Giedd, Woods, MacDonald, Evans, & Toga, 2000; Thompson et al., 2005; Toga, Thompson, & Sowell, 2006).
How these neurological changes may relate to behavioral changes on formal neuropsychological measures is a topic of recent investigation. On formal tests of executive function, significant improvements are achieved across early and middle childhood (Anderson et al., 2001; Brocki & Bohlin, 2004; Hooper, Luciana, Conklin, & Yarger, 2004; Levin et al., 1991; Luciana & Nelson, 1998; Romine & Reynolds, 2005). Although performance often plateaus by late childhood, improvements continue to be observed in complex planning and problem solving, abstraction, attentional control, inhibition, processing speed, motor sequencing, verbal fluency, working memory, and reward-guided decision-making capacity through adolescence and into adulthood (Anderson et al., 2001; Hooper et al., 2004; Levin et al., 1991; Romine & Reynolds, 2005; Welsh, Pennington, & Groisser, 1991).
Children with executive dysfunction may have difficulty developing the requisite skills necessary to interact productively and effectively with the environment. However, children who perform within normal limits on formal tests of executive function in a structured, organized test environment may actually have impairments in their real-world environments. The BRIEF (Gioia et al., 2000) assesses a wide range of executive functions as they relate to day-to-day functioning, with normative data available for those aged 5 to 18 years. Compared to normative data, caregiver ratings of executive function in children and adolescents with MMH suggest specific patterns of deficits, i.e., problems with metacognitive skills, but not behavioral regulation (Mahone, Zabel, Levey, Verda, & Kinsman, 2002c). Parent-reported executive behavior across various stages of adolescence is difficult to determine using standard scores of the BRIEF, since children aged 14 to 18 years are grouped together. This suggests a role for examining raw score data when considering patterns of behavior in adolescents. For example using an assessment of average item ratings, Burmeister and colleagues (2005) found that children with MMH had higher average item ratings on the BRIEF when compared to a control group, with small to moderate effect sizes, although age-related differences in item ratings across age groups were not reported.
Although the presence of executive dysfunction in children with MMH compared to typically developing children has been well documented, there is little known about age-related patterns in executive functioning across childhood and adolescence in this population. In one case study, executive functions were found to be affected by shunt failure, with little evidence of recovery after shunt revision (Matson, Mahone, & Zabel, 2005), suggesting specific vulnerability of this skill area well into childhood. This pattern may reflect the trajectory of neurodevelopment, such that executive functions remain vulnerable to effects of medical complications for a more protracted period during development.
The purpose of this study was to examine group and cross-sectional age effects of parent ratings of executive function on the BRIEF in children with MMH and typically developing peers across late childhood and adolescence. In addition to examining group differences based on standard scores, this study aimed to consider developmental differences in the frequency of reported behaviors by evaluating raw scores across ages. Specifically, paralleling the process of neurological maturation through adolescence, we hypothesized that there would be greater age-related improvements in parent ratings of executive functions among the control group than in the MMH group, which would be reflected by decreases in frequency of reported behaviors (i.e., raw scores) on the BRIEF. Further, based on findings described by Mahone and colleagues (2002c), we hypothesized that age-related group differences would be more pronounced among “metacognitive” behaviors (including task initiation, working memory, and planning) than among behavioral control variables (such as emotional and inhibitory control).
METHOD
Participants
Participants were eligible for the present study if they were between the ages of 10 years, 0 months and 18 years, 11 months, and if they had not been previously diagnosed with Mental Retardation. A total of 36 children and adolescents with MMH (17 males, 19 females) were recruited from the Philip A. Keelty Center for Spina Bifida and Related Conditions at the Kennedy Krieger Institute. Participants with MMH were recruited only from their routine multidisciplinary medical clinic appointment, not after referral for more comprehensive neuropsychological consultation, such that this group was thought to be representative of the population of adolescents with MMH in general. It is important to note, however, that many of these children were seen for neuropsychological evaluation prior or subsequent to participation in this study. Data from 35 typically developing children (14 males, 21 females) were collected as part of their participation as control subjects in one of several research studies being conducted at the Kennedy Krieger Institute. Control participants were excluded if there was a documented history of any psychiatric disorder, mental retardation, learning disability, or neurological disorder.
Measure
The Behavior Rating Inventory of Executive Function, Parent Form (BRIEF; Gioia et al., 2000) is a caregiver report questionnaire designed to assess the behavioral manifestations of problems associated with executive function in children aged 5 to 18 years. The Parent Form contains 86 items on which parents rate each behavior as occurring “never,” “sometimes,” or “often.” Items are organized into eight scales and two primary indices (Metacognition and Behavioral Regulation), and a T-score is derived for each scale and index, with higher T-scores indicating greater impairment. The Metacognition Index is comprised of five subscales (Initiate, Working Memory, Plan/Organize, Organization of Materials, and Monitor) and represents skills that are essential to self-regulation of cognitive processes. The Behavioral Regulation Index is comprised of three subscales (Inhibit, Shift, Emotional Control) and represents skills essential for the self-regulation of behavior. The validity of the BRIEF and the described two-factor structure has been supported in various clinical groups (Gioia, Isquith, Retzlaff, & Espy, 2002; Mahone et al., 2002a, 2002c; Slick, Lautzenhiser, Sherman, & Eyrl, 2006). Previous research has found the BRIEF to be sensitive to changes in executive function even in the absence of changes in performance-based measures (Cummings et al., 2002). Although the BRIEF MCI was found to correlate modestly with VIQ in a sample of children with TBI (Vriezen & Pigott, 2002), others have found little support for a relationship between intellectual functioning and parent report of executive function (Mahone et al., 2002a).
Procedure
Parents of participants were asked to complete the BRIEF during a medical clinic visit (MMH group) or during participation in one of several other approved research studies (control group). If the parent was unable to complete the parent rating scales at the time of the visit, they were provided with a prepaid envelope in which to return the forms. Medical records were reviewed for the MMH group in order to gather data on lesion level and shunt status. Information about ethnicity and cognitive functioning, when available, was gathered from medical records (MMH group) or research data (control group).
Data analyses
For the participants with available neuropsychological data, an estimate of verbal intellectual functioning was generated using the Verbal IQ from the Wechsler Intelligence Scale for Children, Third edition (WISC-III; Wechsler, 1991), Wechsler Adult Intelligence Scale, Third edition (WAIS-III; Wechsler, 1997), or Wechsler Abbreviated Scale of Intelligence (WASI; Wechsler, 1999); the Verbal Comprehension Index of the Wechsler Intelligence Scale for Children, Fourth edition (WISC-IV; Wechsler, 2003); or the standard score of the Peabody Picture Vocabulary Test, Third edition (PPVT-III; Dunn & Dunn, 1997). Groups were compared on age, gender, ethnicity, and Verbal IQ estimate using chi-square (for categorical variables) or t-tests (for continuous variables). One-way ANOVA was used to compare groups on the BRIEF subscale and index T-scores. Chi-square analyses were used to compare the percentage of children in each group with T-scores ≥ 65 across BRIEF scales. In order to account for some possible confounding variables, correlational analyses were used to assess the relationship between IQ and BRIEF scores. For the MMH group only, t-tests were used to compare BRIEF index scores between groups based on presence of Chiari malformation and lesion level.
BRIEF validity indices for all participants were within the expected range. The descriptor for each BRIEF item (never, sometimes, often) was coded with a value of “1,” “2,” or “3” respectively. T-scores for each subscale and index were generated based on normative data. In addition, an average raw score was generated for each index by dividing the total raw score of the items comprising the index by the number of items that were completed. The average raw score for the MCI and BRI ranged from 1.0 to 3.0. Raw scores were used in order to consider frequency of actual behaviors reported by parents across ages. The use of T-scores to examine age and age-by-group interaction effects is limited by the grouping together of children aged 14 to 18 years in the BRIEF manual, since the current sample only included children and adolescents aged 10 to 18 years. In addition, standard scores would not be expected to change over time in typically developing children, such that examination of differences in reported behaviors across age groups would be limited by using standard scores.
Using the methods described by Holmbeck (2002), linear regression analyses were used to examine the effects of age, group, and age-by-group interaction on the MCI and BRI mean raw scores. The continuous predictor variable (age in years) was centered by subtracting the mean age from each participant's age in order to facilitate the interpretation of simple slopes for significant regression results (Holmbeck, 2002). The variables for age, group, and age-by-group interaction were entered simultaneously into the regression. When indicated, post hoc examination of the moderating effects of group on the relationship between age and BRI or MCI mean raw score was performed.
RESULTS
Demographic information
Demographic information for the MMH and control groups is presented in Table 1. The MMH sample included 17 males and 19 females with a mean age of 14.6 years (SD = 2.7; range = 10.4−18.6). Of the 31 patients for which lesion level data were available, 4 had sacral, 3 had lumbar-sacral, 18 had lumbar, and 6 had thoracic lesions. Twenty-five had a Chiari malformation or a probable Chiari malformation, although data regarding the presence or absence of Chiari malformation were missing for 8 participants. A total of 35 of the children were shunted (the unshunted participant had documented hydrocephalus). Of the participants with MMH, 23 had IQ data available, with a mean Verbal IQ/Verbal Comprehension Index of 88.5 (SD = 10.4) and a mean Performance IQ/Perceptual Organization Index of 74.3 (SD = 11.6).
Table 1.
Demographic information
| MMH (n = 36) |
Control (n = 35) |
p | |
|---|---|---|---|
| Age (years) | |||
| M | 14.6 | 14.1 | .351 |
| SD | 2.7 | 2.5 | |
| Gender n (%) | |||
| Male | 17 (47) | 14 (40) | .804 |
| Female | 19 (53) | 21 (60) | |
| Ethnicity n (%) | |||
| Caucasian | 27 (75) | 18 (51) | |
| African Amer. | 7 (19) | 17 (49) | .047 |
| Other | 2 (6) | ||
| Verbal IQ Estimate | (n = 23) | (n = 22) | |
| M | 88.5 | 100.4 | .001 |
| SD | 10.4 | 13.0 |
Verbal IQ estimate was derived from the Verbal IQ of the Wechsler Intelligence Scale for Children, Third Edition (WISC-III), Wechsler Adult Intelligence Scale, Third Edition (WAIS-III), or the Wechsler Abbreviated Scale of Intelligence (WASI); the Verbal Comprehension Index of the Wechsler Intelligence Scale for Children, Fourth Edition (WISC-IV); or the standard score of the Peabody Picture Vocabulary Test, Third Edition (PPVT-III). Age and VIQ compared using t-tests. Gender and Ethnicity compared using chi-square tests.
The control group consisted of 14 males and 21 females with a mean age of 14.1 years (SD = 2.5; range = 10.3−18.9). The estimated Verbal IQ for the 22 control participants with available data was 100.4 (SD = 13.0). There were no significant differences between groups on age or gender ratio; however, the groups differed in regard to ethnicity, with the MMH group having significantly more Caucasian and less African-American participants than the control group (χ2 = 7.95, p = .047). The control group had a significantly higher Verbal IQ estimate than the group with MMH [t(43) = −3.42, p = .001].
BRIEF MCI and BRI scores were not significantly different based on ethnicity. Among the subset of children with IQ data available, Verbal IQ estimate was not significantly correlated with either the BRIEF MCI or BRI. In the MMH group, BRIEF MCI and BRI scores were not significantly related to presence of Chiari malformation or lesion level.
Parent BRIEF ratings
Mean T-scores of the BRIEF subscales and indices for both groups are presented in Table 2. Mean T-scores were significantly higher for children with MMH than controls on six of the eight individual scales. The mean BRI [F(1, 70) = 6.96, p = .010, and MCI, F(1, 70) = 27.40, p < .001] were also significantly higher in children with MMH than the comparison group. Although mean T-scores for the MMH group were below 60 (average) for five of eight subscales, significantly more children with MMH than controls had T-scores in the clinically significant range (T≥65) across all metacognitive scales, as well as the Shift scale (see Table 3).
Table 2.
Group comparisons on BRIEF subscale and index scores
| MMH (n = 36) |
Control (n = 35) |
F(1, 70) | p | |
|---|---|---|---|---|
| Inhibit | 51.7 (10.3) | 49.3 (11.5) | 0.87 | .355 |
| Shift | 57.6 (14.6) | 48.0 (12.3) | 8.87 | .004 |
| Emotional Control | 56.1 (12.8) | 46.9 (11.5) | 10.05 | .002 |
| Behavioral Regulation Index | 55.6 (12.2) | 47.7 (12.9) | 6.96 | .010 |
| Initiate | 63.9 (12.4) | 48.5 (11.8) | 28.35 | < .001 |
| Working Memory | 66.1 (13.4) | 47.6 (11.3) | 39.42 | < .001 |
| Plan/Organize | 62.9 (13.0) | 48.8 (10.6) | 25.12 | < .001 |
| Organization of Materials | 54.0 (12.9) | 49.1 (8.9) | 3.35 | .072 |
| Monitor | 59.8 (12.0) | 48.2 (11.6) | 17.03 | < .001 |
| Metacognition Index | 64.3 (14.5) | 48.3 (10.9) | 27.40 | < .001 |
MMH = myelomeningocele/hydrocephalus. Scores are presented as mean T-scores, with standard deviations in parentheses.
Table 3.
Comparison of proportion of clinically significant T-scores on BRIEF scales and indices
| MMH (n = 36) |
Control (n = 35) |
χ2 | p | |
|---|---|---|---|---|
| Inhibit | 13.9 | 8.6 | 0.50 | .479 |
| Shift | 27.8 | 8.6 | 4.38 | .036 |
| Emotional Control | 27.8 | 11.4 | 3.00 | .083 |
| Behavioral Regulation Index | 19.4 | 11.4 | 0.87 | .351 |
| Initiate | 50.0 | 11.4 | 12.35 | < .001 |
| Working Memory | 52.8 | 5.7 | 18.87 | < .001 |
| Plan/Organize | 47.2 | 8.6 | 13.10 | < .001 |
| Organization of Materials | 27.8 | 5.7 | 6.15 | .013 |
| Monitor | 36.1 | 11.4 | 5.94 | .015 |
| Metacognition Index | 47.2 | 5.7 | 15.60 | < .001 |
MMH = myelomeningocele/hydrocephalus. Scores are presented as percentage of sample with T-scores ≥ 65.
Age effects on BRIEF index scores
The linear relationships between age and mean index raw scores for both groups are presented in Figures 1 and 2. Curve estimation statistics supported the use of a linear model. However, testing of assumptions suggested significant heteroscedasticity, which limits interpretation of regression findings. Other assumptions of regression (i.e., linearity, independence, and normality) were not violated. Multiple regression analyses supported a significant age-by-group interaction for the BRI only. Specifically, results of multiple regression analyses examining the effects of age, group, and age-by-group interaction on MCI mean raw score indicated that the model was significant, R2 = .29, F(3, 70) = 8.97, p < .001. Only group was a significant predictor (β = −.51, p < .001). Age (β = .29, p = .379) and age-by-group interaction (β = −.46, p = .155) did not contribute significantly to the model. Results of multiple regression analyses examining predictors of the BRI mean raw score (age, group, age-by-group interaction) also indicated that the model was significant, R2 = .18, F(3, 70) = 4.85, p = .004. Both group (β = −.32, p = .006) and the age-by-group interaction (β = −.74, p = .037) contributed significantly to the regression model.
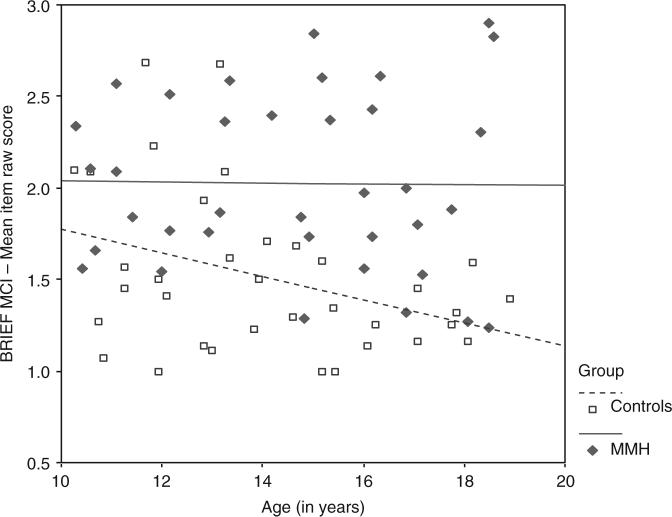
Figure 1.
Relationship between age and BRIEF Metacognition Index (MCI) mean item raw score for children with myelomeningocele/hydrocephalus and controls.
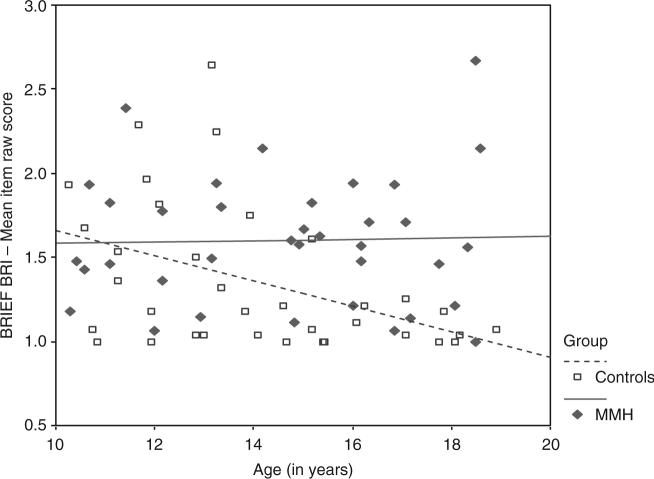
Figure 2.
Relationship between age and BRIEF Behavioral Regulation Index (BRI) mean item raw score for children with myelomeningocele/hydrocephalus and controls.
The moderating effect of group on the relationship between age and BRI raw score was further examined by computing regression equations for each group. For the MMH group, the regression equation is: BRI raw score = (−.0041)(age centered) + 1.60. For the control group, the regression equation is: BRI raw score = (−.0749)(age centered) + 1.33. The simple slope for the control group was significant (t = −2.75, p = .008), but not for the MMH group (t = 0.17, p = .869), suggesting significant age-related reductions in parent reported symptoms in the control group but not in the MMH group.
Post-hoc analyses
Qualitative examination of Figures 1 and 2 suggests that parents of typically developing children report an age-related maturation in executive control behaviors, as suggested by age-related reduction in the mean raw scores of both the BRIEF MCI and the BRIEF BRI, while children with MMH do not display the expected behavioral maturation in executive function.
In order to further examine the effects of group and age on BRIEF index standard scores, age was coded categorically into three groups (10−12-year-olds, 13−15-year-olds, 16−18-year-olds). In children with MMH, 40−60% had MCI T-scores in the clinically significant range in each age group, as compared to 10−30% of children with BRI scores in the clinically significant range across age groups. Chi-square analyses were used to compare the number of participants in the MMH and typically developing groups with BRIEF MCI and BRI T-scores in the clinically significant range across each of the three age groups (see Table 4). For the MCI, there were statistically significant group differences in prevalence of T-scores ≥ 65 for the middle (χ2 = 7.30, p = .007) and oldest (χ2 = 5.93, p = .015) age groups, but not for the youngest age group. For the BRI, there were no significant differences in the prevalence of elevated scores between participant groups within any of the three age groups. BRIEF T-score comparisons across these age groups are confounded by the fact that BRIEF normative data combines children ages 14 to 18 years, such that T-scores do not capture differences within this broad range of adolescence.
Table 4.
Comparison of proportion of clinically significant BRIEF index T-scores in three age groups
| MMH | Control | χ2 | p | |
|---|---|---|---|---|
| Age 10−12 years | (n = 10) | (n = 13) | ||
| Metacognitive Index | 40.0 | 7.7 | 3.47 | .063 |
| Behavioral Regulation Index | 10.0 | 15.4 | 0.14 | .704 |
| Age 13−15 years | (n = 10) | (n = 13) | ||
| Metacognitive Index | 60.0 | 7.7 | 7.30 | .007 |
| Behavioral Regulation Index | 30.0 | 15.4 | 0.71 | .400 |
| Age 16−18 years | (n = 15) | (n = 9) | ||
| Metacognitive Index | 46.7 | 0 | 5.93 | .015 |
| Behavioral Regulation Index | 20.0 | 0 | 2.06 | .151 |
MMH = myelomeningocele/hydrocephalus. Scores are presented as percentage of sample with T-scores ≥ 65.
DISCUSSION
The goal of this cross-sectional study was to compare patterns of parent ratings of executive function across late childhood and adolescence in children with MMH and typically developing peers. The presence of executive dysfunction in children with MMH has been well documented in the literature. On neuropsycho-logical tests, children with MMH have been found to have more severe executive dysfunction than that seen in other pediatric clinical groups, which is not attributable to IQ (Snow, 1999). However, executive dysfunction in adolescence does not necessarily result from the emergence of atypical behaviors (or the presence of clearly pathological behaviors), but rather the lack of developmentally appropriate reduction of certain behaviors that may be considered typical at an earlier stage of development. The unique contribution of the current study lies in the examination of age-related differences in parent-reported behavioral aspects of executive function across different ages. The use of raw scores in the regression analyses, rather than standard scores, allowed for the examination of the pattern of frequencies of reported behaviors.
Results of group comparisons were consistent with previously published literature. Although many of the children with MMH in the current study were referred for neuropsychological evaluation prior or subsequent to participation, the fact that children and adolescents were included in the study regardless of cognitive/ behavioral status increases the generalizability of our findings to the general population of children with MMH. In the current study, comparison of group mean T-scores on the BRIEF indicated that parents of children and adolescents with MMH reported relatively more difficulties with executive functioning compared to parents of typically developing children and adolescents. Despite significant overall group differences, mean T-scores in the MMH group were within normal limits (i.e., T < 60) across all subscales comprising the Behavioral Regulation Index. In contrast, the MMH group as a whole displayed mild elevations (T 60−70) on some subscales comprising the Metacognitive Index (Initiate, Working Memory, Plan/Organize). However, significantly more children with MMH had scores in the clinically significant range across all metacognitive scales as compared to typically developing children. These findings suggest that children with MMH have more relative parent-reported symptoms of executive dysfunction than children with hydrocephalus without spina bifida, although symptom frequency is less severe than that observed in children with localized frontal lesions (Anderson et al., 2002).
Although the mean BRIEF raw scores in the MMH group were higher for the MCI than the BRI, results of regression analyses suggested that the interaction between age and group was only significant for the mean BRI raw score. The results of the regression analyses should be interpreted with caution because of low power resulting from small sample size, as well as heteroscedasticity of the data, reflecting differences in the variability in behaviors that comprise the BRIEF indices. In typically developing children, there is substantial variability among behaviors that make up the MCI through adolescence, especially in males, while variability is more restricted in behaviors comprising the BRI (Gioia et al., 2000). Therefore, adolescents with MMH with even low-frequency behaviors suggesting poor behavioral control may stand out as atypical at this age. This pattern in parent-reported behavior may reflect timing of maturation of neural substrates thought to support these different aspects of executive functioning. That is, executive function behaviors representing cognitive control (MCI) are thought to reflect integrity of the dorsolateral prefrontal cortex and its cortical/subcortical connections, while behavioral control (BRI) is thought to parallel development of the orbitofrontal cortex and its cortical/subcortical connections. The dorsolateral prefrontal cortex has been found to be one of the latest brain regions to develop (Giedd, 2004). In children with MMH, neurological, behavioral, and cognitive abnormalities, likely resulting from hydrocephalus, are not specific to frontal structures and reflect a diffuse, developmental process. Our findings suggest that this developmental neurological process may result in a protracted or truncated behavioral and cognitive development, such that children with MMH appear more different from typically developing children over time as they fail to make age-expected gains in functioning.
Executive functioning skills are age-dependent, such that expectations for independent functioning increases with age. Consistent with normative data (Gioia et al., 2000), our sample of typically developing children demonstrated a reduction in BRIEF mean raw scores (i.e., frequency of reported “inappropriate” behaviors) across late childhood and adolescence. Individuals with MMH may not make the same age-expected gains in the development of behavioral and cognitive control. Despite the lack of a significant age-by-group interaction for the MCI mean raw scores, difficulties with aspects of executive functioning represented by this scale (e.g., initiation, sustaining working memory) appear to be the primary area of concern for children and adolescents with MMH. In fact, when participants with MMH were divided into three age groups (10−12-year-olds, 13−15-year-olds, and 16−18-year-olds), 40−60% of individuals in each age group had MCI scores in the clinically significant range.
Youth with disabilities and cognitive disorders have historically struggled to meet transition expectations, with a recent report finding that 40% of a sample of adult men and women with disabilities who had received special education were single, living at home, and not involved in education or gainful employment (Wells, Sandefur, & Hogan, 2003). Unfortunately, dysfunction in adaptive functioning and difficulty achieving functional independence is commonly reported in adolescents with MMH, even those with intact intellectual ability (Hommet et al., 1999; Mahone et al., 2002c; Sawin, Buran, Brei, & Fastenau, 2003). This is a considerable area of stress for parents of adolescents with MMH, particularly with respect for their children's need for partial or total assistance with activities of daily living (Tsai, Yang, Chan, Huang, & Wong, 2002). Problems with aspects of executive functioning, including initiation, working memory, self-monitoring, and planning, are particularly relevant in the MMH population. Although interpretation of the current results is limited by the cross-sectional nature of the study, our findings suggest that the functional implications of problems with maturation of executive abilities in children with MMH may not be apparent until adolescence. That is, although frequency of symptomatology appears consistent across age groups in children with MMH, we propose that the relative level of dysfunction associated with these behaviors may become more evident as these children do not meet age-appropriate expectations in areas such as independent initiation of goal-directed activity, self-monitoring, planning, and flexibility (Tarazi, Mahone, & Zabel, 2007). This observation is consistent with a qualitative description of youngsters with MMH, in which young children are often described as being hyperactive and distractible, while adolescents reportedly have difficulty with initiation of tasks, skill application, self-monitoring, and flexibility (Mahone et al., 2002c).
Parents of children with MMH often expect to see improvements in initiation and ability to independently complete self-care activities as they enter adolescence and young adulthood. However, in addition to impeded maturation of executive functions, children and adolescents with MMH have additional life skills demands as compared to typically developing children (Tarazi et al., 2007). Due to the nature of the disorder, children and adolescents with MMH are typically required to manage and prevent urological, neurological, orthopedic, skin, and elimination problems, and are responsible for learning how to self-catheterize, reposition, and perform skin checks at specific times (Wolraich & Hesz, 1988). Therefore, they have more to “remember to remember” (i.e., remembering medications, remembering to shift weight in one's wheelchair, remembering to catheterize) than adolescents with less medically complex neurodevelopmental disorders. While evidence suggests that working memory and other metacognitive deficits negatively impact general self-care skills in children/adolescents with MMH, it is thought that executive dysfunction also disrupts the execution of unique MMH-related self-care tasks such as self-catheterization. As children with MMH reach adolescence, issues with spontaneous use of skills, strategic initiation of tasks, and mental flexibility become more salient, resulting in significant trouble integrating these complex skills independently.
In summary, the current findings highlight a pattern suggesting a lack of expected maturation of executive control behaviors in children and adolescents with MMH. Although, as a whole, parents of children and adolescents in the MMH group did not endorse problems in the clinically significant range on the BRIEF, a larger percentage of children and adolescents with MMH had T-scores in the clinically significant range, particularly with aspects of cognitive control, when compared to typically developing children. Examination of frequency of behaviors, as represented by raw scores, suggests a lack of expected decrease in symptom frequency across ages in the MMH group. Poor skill maturation is superimposed on increased demands for organization, integration, and coordination of multiple systems, likely resulting in adaptive dysfunction i.e., problems with the application of skills in one's environment (Mahone & Zabel, 2001).
Although the current study adds to existing literature by examining an age-related pattern of executive function skills in a representative group of children with MMH and by considering the relationship of this developmental pattern to adaptive functioning and independence, there are a number of significant limitations. Consideration of a developmental trajectory of executive function in children with MMH is based on cross-sectional data only. Therefore, cohort effects may affect findings, such that the pattern presented here may not represent a true developmental pattern. Longitudinal data are needed to fully describe executive function development in this population such that a confirmational longitudinal study is warranted. Although we attempted to account for some confounding factors (e.g., IQ, lesion level), interpretation of the current results is limited by the inability to consider other possible confounding variables (e.g., socioeconomic status, other disease factors). IQ data were not available for all participants in either group. The children with MMH for which IQ data were available were referred for neuropsychological evaluation due to concerns about academic or cognitive development. Therefore, average IQ presented for the group with MMH may be a slight underestimate, although our sample presented with the common pattern of higher VIQ than PIQ. A related limitation is the lack of available PIQ for the control group, such that VIQ or a verbal IQ estimate was used to compare groups on intellectual functioning. Our samples were not matched for IQ, and children with MMH had significantly lower estimated Verbal IQ than the group of typically developing children. Although IQ has been found to be a significant moderator of performance-based tests of executive function (Mahone et al., 2002b), consistent with previously published research, verbal IQ scores (actual or estimated) in our sample were not associated with parent-reported behaviors of executive function. The current study also did not assess or control for presence of comorbid ADHD, which has been found to occur at a higher rate in children with MMH than typically developing children (Burmeister et al., 2005). Recent findings indicated that children with MMH and comorbid ADHD are rated more poorly on some BRIEF subscales when compared to children with MMH without ADHD, and that BRIEF scores were better than neuropsychological tests in differentiating these groups (Burmeister et al., 2005). An additional limitation of this study relates to the normative data age distributions available for the BRIEF. Normative data for adolescents ages 14 to 18 are collapsed. Therefore, the group effects based on T-score comparisons observed in the current study may actually be attenuated.
Despite these limitations, the current study provides important information regarding the nature of executive function in children and adolescents with MMH and provides an important first step in considering the developmental aspects and long-term implications of executive dysfunction in this population. Children with MMH are vulnerable to difficulties as they “age into” expectations for independence in daily living, self-care, vocational, social, and community integration skills (Tarazi et al., 2007). Results suggest a role for screening of executive function throughout development in this population, as functional difficulties may arise over time. Examination of specific subscale elevations on the BRIEF may assist in developing individualized intervention programs with the goal of improving independence. Future research directions should include a confirmational longitudinal study examining maturation of executive function skills from early childhood through young adulthood. In addition, future longitudinal studies examining the proposed interaction of decreased executive abilities and increased executive demands are warranted, and could include a comparison group of adolescents who are expected to meet increased executive demands in the context of intact executive function abilities (e.g., SCI without brain involvement).
ACKNOWLEDGMENTS
Supported by HD-24061 (Mental Retardation and Developmental Disabilities Research Center), M01 RR00052 (Johns Hopkins General Clinical Research Center), and R01 NS042851. The authors wish to thank Alena Horska, Ph.D., for assistance with access to control data and Grayson Holmbeck, Ph.D., for his assistance with data analysis.
REFERENCES
- Anderson VA, Anderson P, Northam E, Jacobs R, Catroppa C. Development of executive functions through late childhood and adolescence in an Australian sample. Developmental Neuropsychology. 2001;20:385–406. doi: 10.1207/S15326942DN2001_5. [DOI] [PubMed] [Google Scholar]
- Anderson VA, Anderson P, Northam E, Jacobs R, Mikiewicz O. Relationships between cognitive and behavioral measures of executive function in children with brain disease. Child Neuropsychology. 2002;8:231–240. doi: 10.1076/chin.8.4.231.13509. [DOI] [PubMed] [Google Scholar]
- Barnes M, Dennis M. Discourse after early-onset hydrocephalus: Core deficits in children of average intelligence. Brain and Language. 1998;61:309–334. doi: 10.1006/brln.1998.1843. [DOI] [PubMed] [Google Scholar]
- Brewer VR, Fletcher JM, Hiscock M, Davidson KC. Attention processes in children with shunted hydrocephalus versus attention deficit-hyperactivity disorder. Neuropsychology. 2001;15:185–198. doi: 10.1037//0894-4105.15.2.185. [DOI] [PubMed] [Google Scholar]
- Brocki KC, Bohlin G. Executive functions in children aged 6 to 13: A dimensional and developmental study. Developmental Neuropsychology. 2004;26:571–593. doi: 10.1207/s15326942dn2602_3. [DOI] [PubMed] [Google Scholar]
- Burmeister R, Hannay HJ, Copeland K, Fletcher JM, Boudousquie A, Dennis M. Attention problems and executive functions in children with spina bifida and hydrocephalus. Child Neuropsychology. 2005;11:265–283. doi: 10.1080/092970490911324. [DOI] [PubMed] [Google Scholar]
- Casey BJ, Giedd JN, Thomas KM. Structural and functional brain development and its relation to cognitive development. Biological Psychology. 2000;54:241–257. doi: 10.1016/s0301-0511(00)00058-2. [DOI] [PubMed] [Google Scholar]
- Charney E. Neural tube defects: Spina bifida and myelomeningocele. In: Batshaw M, Perret Y, editors. Children with disabilities: A medical primer. Third edition Brookes Publishing Co.; Baltimore: 1992. pp. 471–488. [Google Scholar]
- Cummings DD, Singer HS, Krieger M, Miller TL, Mahone EM. Neuropsychiatric effects of guanfacine in children with mild Tourette syndrome: A pilot study. Clinical Neuropharmacology. 2002;25:325–332. doi: 10.1097/00002826-200211000-00009. [DOI] [PubMed] [Google Scholar]
- Denckla MB. Measurement of executive function. In: Lyon GR, editor. Frames of reference for the assessment of learning disabilities: New views on measurement issues. Brooks Publishing Co.; Baltimore: 1994. pp. 117–142. [Google Scholar]
- Denckla MB. Research on executive function in a neurodevelopmental context: Application of clinical measures. Developmental Neuropsychology. 1996;12:5–15. [Google Scholar]
- Dennis M, Barnes M. Math and numeracy in young adults with spina bifida and hydrocephalus. Developmental Neuropsychology. 2002;21:141–155. doi: 10.1207/S15326942DN2102_2. [DOI] [PubMed] [Google Scholar]
- Dennis M, Barnes MA, Heatherington CR. Congenital hydrocephalus as a model of neurodevelopmental disorder. In: Tager-Flusberg H, editor. Neurodevelopmental disorders: Contribution to a new perspective from the cognitive neurosciences. MIT Press; Cambridge, MA: 1999. pp. 505–532. [Google Scholar]
- Dennis M, Fletcher JM, Rogers T, Hetherington R, Francis DJ. Object-based and action-based visual perception in children with spina bifida and hydrocephalus. Journal of the International Neuropsychological Society. 2002;8:95–106. [PubMed] [Google Scholar]
- Dunn LM, Dunn LM. Peabody Picture Vocabulary Test. 3rd ed. American Guidance Services, Inc.; Circle Pines, MN: 1997. [Google Scholar]
- Fletcher JM, Brookshire BL, Landry SH, Bohan TP, Davidson KC, Francis DJ, et al. Attentional skills and executive functions in children with early hydrocephalus. Developmental Neuropsychology. 1996;12:53–76. doi: 10.1037//0894-4105.12.4.578. [DOI] [PubMed] [Google Scholar]
- Fletcher JM, Copeland K, Frederick JA, Blaser S, Kramer LA, Northrup H, et al. Spinal lesion level in spina bifida: A source of neural and cognitive heterogeneity. Journal of Neurosurgery: Pediatrics. 2005;102:268–279. doi: 10.3171/ped.2005.102.3.0268. [DOI] [PubMed] [Google Scholar]
- Fletcher JM, Dennis M, Northrup H. Hydrocephalus. In: Yeates KO, Ris MD, Taylor HG, editors. Pediatric neuropsychology: Research, theory, and practice. Guilford Press; New York: 2000. pp. 25–46. [Google Scholar]
- Giedd JN. Structural magnetic resonance imaging of the adolescent brain. Annals of the New York Academy of Sciences. 2004;1021:77–85. doi: 10.1196/annals.1308.009. [DOI] [PubMed] [Google Scholar]
- Gioia GA, Isquith PK, Guy SC, Kenworthy L. Behavior Rating Inventory of Executive Function. Psychological Assessment Resources; Odessa, FL: 2000. [Google Scholar]
- Gioia GA, Isquith PK, Retzlaff PD, Espy KA. Confirmatory factor analysis of the Behavior Rating Inventory of Executive Function (BRIEF) in a clinical sample. Child Neuropsychology. 2002;8:249–257. doi: 10.1076/chin.8.4.249.13513. [DOI] [PubMed] [Google Scholar]
- Hetherington R, Dennis M. Motor function profile in children with early onset hydrocephalus. Developmental Neuropsychology. 1999;15:25–51. [Google Scholar]
- Holmbeck GN. Post-hoc probing of significant moderational and mediational effects in studies of pediatric populations. Journal of Pediatric Psychology. 2002;27:87–96. doi: 10.1093/jpepsy/27.1.87. [DOI] [PubMed] [Google Scholar]
- Hommet C, Billard C, Gillet P, Barthez MA, Lourmiere JM, Santini JJ, et al. Neuropsychologic and adaptive functioning in adolescents and young adults shunted for congenital hydrocephalus. Journal of Child Neurology. 1999;14:144–150. doi: 10.1177/088307389901400302. [DOI] [PubMed] [Google Scholar]
- Hooper CJ, Luciana M, Conklin HM, Yarger RS. Adolescents’ performance on the Iowa Gambling Task: Implications for the development of decision making and ventromedial prefrontal cortex. Developmental Psychology. 2004;40:1148–1158. doi: 10.1037/0012-1649.40.6.1148. [DOI] [PubMed] [Google Scholar]
- Iddon JL, Morgan DJR, Loveday C, Sahakian BJ, Pickard JD. Neuropsychological profile of young adults with spina bifida with or without hydrocephalus. Journal of Neurology, Neurosurgery, and Psychiatry. 2004;75:1112–1118. doi: 10.1136/jnnp.2003.029058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy SE, Martin SDG, Kelley JM, Walton B, Vlcek CK, Hassanein RS, et al. Identification of medical and nonmedical needs of adolescents and young adults with spina bifida and their families: A preliminary study. Children's Health Care. 1998;27:47–61. [Google Scholar]
- Klingberg T, Vaidya CJ, Gabrieli JDE, Moseley ME, Hedehus M. Myelination and organization of the frontal white matter in children: A diffusion tensor MRI study. NeuroReport. 1999;10:2817–2821. doi: 10.1097/00001756-199909090-00022. [DOI] [PubMed] [Google Scholar]
- Levin HS, Culhane KA, Hartmann J, Evankovich K, Mattson AJ, Harward H, et al. Developmental changes in performance on tests of purported frontal lobe functioning. Developmental Neuropsychology. 1991;7:377–395. [Google Scholar]
- Loss N, Yeates KO, Enrile BG. Attention in children with myelomeningocele. Child Neuropsychology. 1998;5:7–20. [Google Scholar]
- Luciana M, Nelson CA. The functional emergence of prefrontallyguided working memory systems in four- to eight-year-old children. Neuropsychologia. 1998;36:273–293. doi: 10.1016/s0028-3932(97)00109-7. [DOI] [PubMed] [Google Scholar]
- Mahone EM, Cirino PT, Cutting LE, Cerrone PM, Hagelthorn KM, Hiemenz JR, et al. Validity of the Behavior Rating Inventory of Executive Function in children with ADHD and/or Tourette syndrome. Archives of Clinical Neuropsychology. 2002a;17:643–662. [PubMed] [Google Scholar]
- Mahone EM, Hagelthorn KM, Cutting LE, Schuerholz LJ, Pelletier SF, Rawlins C, et al. Effects of IQ on executive function measures in children with ADHD. Child Neuropsychology. 2002b;8:41–51. doi: 10.1076/chin.8.1.52.8719. [DOI] [PubMed] [Google Scholar]
- Mahone EM, Zabel TA. Challenges to executive function. Insights into Spina Bifida. 2001;7:1A–8A. [Google Scholar]
- Mahone EM, Zabel TA, Levey E, Verda M, Kinsman S. Parent and self-report ratings of executive function in adolescents with myelomeningocele and hydrocephalus. Child Neuropsychology. 2002c;8:258–270. doi: 10.1076/chin.8.4.258.13510. [DOI] [PubMed] [Google Scholar]
- Mammarella N, Cornoldi C, Donadello E. Visual but not spatial working memory deficit in children with spina bifida. Brain and Cognition. 2003;53:311–314. doi: 10.1016/s0278-2626(03)00132-5. [DOI] [PubMed] [Google Scholar]
- Mataro M, Junque C, Poca MA, Sahuquillo J. Neuropsychological findings in congenital and acquired hydrocephalus. Neuropsychology Review. 2001;11:169–178. doi: 10.1023/a:1012904907249. [DOI] [PubMed] [Google Scholar]
- Matson MA, Mahone EM, Zabel TA. Serial neuropsychological assessment and evidence of shunt malfunction in spina bifida: A longitudinal case study. Child Neuropsychology. 2005;11:1–18. doi: 10.1080/09297040490916910. [DOI] [PubMed] [Google Scholar]
- Nigg JT, Stavro G, Ettenhofer M, Hambrick DZ, Miller T, Henderson JM. Executive functions and ADHD in adults: Evidence for selective effects on ADHD symptom domains. Journal of Abnormal Psychology. 2005;114:706–717. doi: 10.1037/0021-843X.114.3.706. [DOI] [PubMed] [Google Scholar]
- Paus T, Collins DL, Evans AC, Leonard G, Pike B, Zijdenbos A. Maturation of white matter in the human brain: A review of magnetic resonance studies. Brain Research Bulletin. 2001;54:255–266. doi: 10.1016/s0361-9230(00)00434-2. [DOI] [PubMed] [Google Scholar]
- Pennington BF. Dimensions of executive function in normal and abnormal development. In: Krasnegor NA, Lyon GR, Goldman-Rakic PS, editors. Development of the prefrontal cortex: Evolution, neurobiology, and behavior. Brookes Publishing Co.; Baltimore: 1997. pp. 265–281. [Google Scholar]
- Riddle R, Morton A, Sampson JD, Vachha B, Adams R. Performance on the NEPSY among children with spina bifida. Archives of Clinical Neuropsychology. 2005;20:243–248. doi: 10.1016/j.acn.2004.07.004. [DOI] [PubMed] [Google Scholar]
- Ries J, Zabel TA, Mahone EM. Parent report of adaptive abilities and executive functions in children and adolescents with myelomeningocele and hydrocephalus [Abstract]. Archives of Clinical Neuropsychology. 2003;18:762. [Google Scholar]
- Romine CB, Reynolds CR. A model of the development of frontal lobe functioning: Findings from a meta-analysis. Applied Neuropsychology. 2005;12:190–201. doi: 10.1207/s15324826an1204_2. [DOI] [PubMed] [Google Scholar]
- Russell EW. Toward an explanation of Dodrill's observation: High neuropsychological test performance does not accompany high IQs. The Clinical Neuropsychologist. 2001;15:423–428. doi: 10.1076/clin.15.3.423.10267. [DOI] [PubMed] [Google Scholar]
- Sawin KJ, Buran CF, Brei TJ, Fastenau PS. Correlates of functional status, self-management, and developmental competence outcomes in adolescents with spina bifida. SCI Nursing. 2003;20:72–85. [PubMed] [Google Scholar]
- Schuck SEB, Crinella FM. Why children with ADHD do not have low IQs. Journal of Learning Disabilities. 2005;38:262–280. doi: 10.1177/00222194050380030701. [DOI] [PubMed] [Google Scholar]
- Scott MA, Fletcher JM, Brookshire BL, Davidson KC, Landry SH, Bohan TC, et al. Memory functions in children with early hydrocephalus. Neuropsychology. 1998;12:578–589. doi: 10.1037//0894-4105.12.4.578. [DOI] [PubMed] [Google Scholar]
- Slick DJ, Lautzenhiser A, Sherman EM, Eyrl K. Frequency of scale elevations and factor structure of the Behavior Rating Inventory of Executive Function (BRIEF) in children and adolescents with intractable epilepsy. Child Neuropsychology. 2006;12:181–189. doi: 10.1080/09297040600611320. [DOI] [PubMed] [Google Scholar]
- Snow JH. Executive processes for children with spina bifida. Children's Health Care. 1999;28:241–253. [Google Scholar]
- Tarazi RA, Mahone EM, Zabel TA. Self-care independence in children with neurological disorders: An interactional model of adaptive demands and executive dysfunction. Rehabilitation Psychology. 2007;52:196–205. [Google Scholar]
- Thompson PM, Giedd JN, Woods RP, MacDonald D, Evans AC, Toga AW. Growth patterns in the developing brain detected by using continuum mechanical tensor maps. Nature. 2000;404:190–193. doi: 10.1038/35004593. [DOI] [PubMed] [Google Scholar]
- Thompson PM, Sowell ER, Gogtay N, Giedd JN, Vidal CN, Hayashi KM, et al. Structural MRI and brain development. International Review of Neurobiology. 2005;67:285–323. doi: 10.1016/S0074-7742(05)67009-2. [DOI] [PubMed] [Google Scholar]
- Toga AW, Thompson PM, Sowell ER. Mapping brain maturation. Trends in Neurosciences. 2006;29:148–159. doi: 10.1016/j.tins.2006.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai PY, Yang TF, Chan RC, Huang PH, Wong TT. Functional investigation in children with spina bifida – measured by the Pediatric Evaluation of Disability Inventory (PEDI). Child's Nervous System. 2002;18:48–53. doi: 10.1007/s00381-001-0531-6. [DOI] [PubMed] [Google Scholar]
- Vachha B, Adams RC. Application of the Token Test to children with myelomeningocele and shunted hydrocephalus. Short Reports. European Journal of Pediatric Surgery. 2002;12:S33–S34. [PubMed] [Google Scholar]
- Volpe J. Neurology of the newborn. 4th ed. W.B. Saunders, Co.; Philadelphia: 2001. [Google Scholar]
- Vriezen ER, Pigott SE. The relationship between parental report on the BRIEF and performance-based measures of executive function in children with moderate to severe traumatic brain injury. Child Neuropsychology. 2002;8:296–303. doi: 10.1076/chin.8.4.296.13505. [DOI] [PubMed] [Google Scholar]
- Watkins LH, Sahakian BJ, Robertson MM, Veale DM, Rogers RD, Pickard KM, et al. Executive function in Tourette's syndrome and obsessive-compulsive disorder. Psychological Medicine. 2005;35:571–582. doi: 10.1017/s0033291704003691. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Intelligence Scale for Children. Third edition The Psychological Corporation; San Antonio, TX: 1991. [Google Scholar]
- Wechsler D. Wechsler Adult Intelligence Scale. Third edition The Psychological Corporation; San Antonio, TX: 1997. [Google Scholar]
- Wechsler D. Wechsler Abbreviated Scale of Intelligence. The Psychological Corporation; San Antonio, TX: 1999. [Google Scholar]
- Wechsler D. Wechsler Intelligence Scale for Children. Fourth edition The Psychological Corporation; San Antonio, TX: 2003. [Google Scholar]
- Wells T, Sandefur GD, Hogan DP. What happens after the high school years among young persons with disabilities? Social Forces. 2003;82:803–832. [Google Scholar]
- Welsh MC, Pennington BF, Groisser DB. A normative-developmental study of executive function: A window on prefrontal function in children. Developmental Neuropsychology. 1991;7:131–149. [Google Scholar]
- Wills KE. Neuropsychological functioning in children with spina bifida and/or hydrocephalus. Journal of Clinical Child Psychology. 1993;22:247–265. [Google Scholar]
- Wolraich ML, Hesz N. Meningocele: Assessment and management. Pediatrician. 1988;15:21–28. [PubMed] [Google Scholar]