Abstract
To better understand the role of progestins in the C1 area of the rostral ventrolateral medulla (RVLM), immunocytochemical localization of progestin receptors (PRs) was combined with tyrosine hydroxylase (TH) in single sections of RVLM from proestrus rat brains prepared for light and electron microscopy. By light microscopy, PR-immunoreactivity (-ir) was detected in a few nuclei that were interspersed between TH-labeled perikarya and dendrites. Electron microscopy revealed that PR-ir was in several extranuclear locations. The majority of PR-labeling was in non-TH immunoreactive axons (51 ± 9%) near the plasma membrane. Additional dual labeling studies revealed that PR-immunoreactive axons could give rise to terminals containing the GABAergic marker GAD65. PR-ir also was found in non-neuronal processes (29 ± 9%), some resembling astrocytes. Occasionally, PR-ir was in non-TH-labeled terminals (10 ± 3%) affiliated with clusters of small synaptic vesicles, or in patches contained in the cytoplasm of dendrites (10 ± 1%). These findings suggest that progestins can primarily modulate neurons in the C1 area of the RVLM by presynaptic mechanisms involving GABAergic transmission. Moreover, they suggest that PR activation may contribute to progestin’s effects on arterial blood pressure during pregnancy as well as to sex differences in central cardiovascular regulation.
Keywords: Baroreceptor, Electron microscopy, Bulbospinal neurons, Presynaptic profiles, GABA, Glia
Ovarian hormones, especially estrogens and progestins, regulate arterial blood pressure (ABP) and cardiovascular tone. Within the CNS, estrogens are known to act within the well-established medullary sites that regulate sympathetic outflow (for review see [32]). In particular, local injections of 17β-estradiol into the rostroventrolateral medulla (RVLM), which contains sympathoexcitatory bulbospinal neurons and is critical for tonic (resting) and reflex control of systemic arterial pressure [1,7], decreases sympathetic tone [30,31]. Our recent study revealed that estrogens modulate calcium currents in C1 catecholaminergic bulbospinal neurons in the RVLM and that estrogen receptor (ER) alpha and beta have unique distributions relative to C1 neurons [39].
The role of progestins in the RVLM has been studied largely in the context of pregnancy, where elevated progestins have been associated with decreased ABP likely due to increased GABAergic inhibition of the RVLM [11,17]. Other studies have reported that many genomic and non-genomic effects of progestins are attributable to progestin metabolites; however, a role for progestin activation of progestin receptors (PRs) remains [26,29]. PR expression in many brain regions can be directly regulated by estrogens [9,27]. We have shown that few nuclear PRs in non-catecholaminergic cells are found in the RVLM (unpublished observations). However, whether extranuclear PRs are also present in the RVLM has not been studied.
To better understand the potential role of progestins in modulating neurons in the C1 area of the RVLM, the present study examined the relationship of PR-immunoreactivity (-ir) relative to tyrosine hydroxylase (TH) in the RVLM of female rats by light and electron microscopy (EM). To further characterize the cellular identity of PR in presynaptic profiles, some sections were processed for PR and a GABAergic marker.
Female (N = 8; 275–325 g) adult Sprague–Dawley rats (Charles River Laboratories (Wilmington, MA) or Taconic Laboratories (Chatham, NY)) were used. All rats were housed with 12:12 h light/dark cycles. Estrous cycle stage was determined using vaginal smear cytology [37], and females were assessed for two full cycles. The proestrus (high estrogen) stage was examined since high levels of gonadal steroids are known to produce maximal expression of nuclear PRs in other brainstem regions [10]. Estrous stages were confirmed by uterine weights and by determining levels of estrogens and progestins from blood samples collected during the perfusion procedure using radioimmunoassay. Uterine weights were approximately 21% heavier in proestrus rats (0.8 ± 0.07 g) compared to diestrus rats (0.63 ± 0.04 g). On average the blood levels for estrogens were 10 pg/ml higher at proestrus than diestrus. All experiments were conducted in accordance with the NIH Guide for the Care and Use of Laboratory Animals and were approved by the Weill Cornell Medical College Institutional Animal Care and Use Committee.
PR-labeling was examined using a well-characterized antiserum. A rabbit polyclonal antiserum to a peptide corresponding to amino acids 533–547 (the N-terminal region present in the A and B isoforms) of the human PR was purchased from Dako (Carpinteria, CA). Specificity has been demonstrated by sucrose density gradients and absence of labeling either in absorption controls by immunoprecipitation methods [36] or in thymus and uterus of PR knock-out mice [16,35]. As an additional control, the PR antibody was preadsorbed with 150 mg/ml of the cognate peptide overnight at 4oC before incubating the tissue. After preadsorption of the PR antibody (diluted 1:500) in acrolein/paraformaldehye fixed tissue, no nuclear immunoreactivity was detected in the arcuate nucleus by light microscopy (not shown) and no extranuclear immunoreactivity was detected in a 6050-μm2 field sampled from the hippocampal CA1 region or the RVLM (colabeled with TH) by EM.
Catecholaminergic labeling was identified using a monoclonal mouse antibody to TH (Incstar (Stillwater, MN)). This antibody has been extensively characterized and localized in fixed rat brain [24].
GABAergic labeling was identified using a monoclonal antibody (MAB 351) to glutamic acid decarboxylase (GAD) 65 (Chemicon (Temecula, CA)). This antibody has been tested previously for specificity [6].
Rats were deeply anesthetized with sodium pentobarbital (150 mg/kg, i.p.) and perfused through the ascending aorta with solutions of (1) 10–15 ml saline (0.9%) containing 1000 units of heparin; (2) 50 ml of 3.75% acrolein (Polysciences, Washington, PA) and 2% paraformaldehyde in 0.1 M phosphate buffer (PB; pH 7.4); (3) 200 ml of 2% paraformaldehyde in PB. During the perfusion, aortic blood was collected for radioimmunoassays of circulating hormone levels. Each brain was removed from the skull, cut into 5 mm coronal blocks and post-fixed with 2% paraformaldehyde in PB for 30 min. Sections (40 μm thick) through the RVLM were cut on a Vibratome (Leica, Wien, Austria) and collected into PB. Sections were stored in cryoprotectant (30% sucrose, 30% ethylene glycol in PB) until immunocytochemical processing.
Free-floating sections were dual-labeled for PR-immunoperoxidase and TH- (or GAD-) immunogold using methods described previously [39]. Sections were treated with 1% sodium borohydride in PB for 30 min, incubated in 0.5% bovine serum albumin (BSA) in 0.1 M Tris-saline (TS; pH 7.6) for 30 min and rinsed in TS. Sections were placed in PR antiserum (1:1000) in Triton X 100 (0.25% for light microscopy; 0.025% for EM) and 0.1% BSA in TS for 1 day at room temperature followed by an additional 4–5 days at 4 °C. One day prior to processing, TH antiserum (1:2000) or GAD antiserum (1:1000) was added to the primary antibody diluent.
To visualize PR-labeling, the sections were incubated in (1) biotinylated goat anti-rabbit IgG in 0.1% BSA (1:400, Vector Labs, Burlingame, CA), 30 min; (2) avidin–biotin complex (ABC), 30 min; (3) diaminobenzidine (Aldrich, Milwaukee, WI) and H2O2, 6–8 min. All incubations were separated by TS washes. TH- (or GAD-) labeling was visualized using silver-enhanced immunogold. For this, sections were rinsed in TS and incubated in donkey anti-mouse IgG conjugated to 1 nm gold particles (1:50, Electron Microscopy Sciences, Fort Washington, PA) in 0.08% BSA and 0.01% gelatin in phosphate-buffered saline (PBS; pH 7.4) for 2 h at room temperature. Sections were rinsed in PBS, post-fixed in 2% glutaraldehyde in PBS for 10 min, and rinsed in PBS followed by 0.2 M sodium citrate buffer (pH 7.4). The conjugated gold particles were enhanced by treatment with silver solution (EMS) for 5–7 min.
Sections for light microscopy were mounted on gelatin-coated slides, dehydrated through alcohols and xylene and coverslipped with DPX (Aldrich, Milwaukee, WI). Sections for EM were post-fixed for 1 h in 2% osmium tetroxide, dehydrated through alcohols and propylene oxide, and embedded between two sheets of plastic in EMbed 812 (EMS). Ultrathin sections (70 nm thick) were collected from the RVLM (Fig. 1; approximate levels 61–63 of Swanson [33]) as previously described [24]. Sections were counter-stained with uranyl acetate and Reynold’s lead citrate.
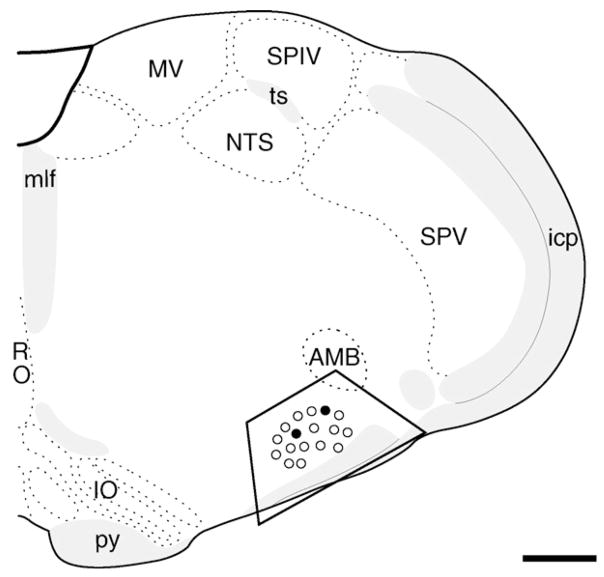
Fig. 1.
Nuclei with PR-ir are intermixed with TH-labeled neurons in the RVLM. Schematic drawing through the medulla showing the level of the RVLM (trapezoid) analyzed light and EM (adapted from Swanson plate 61 [33], about 1.2 mm caudal to bregma) and the relative distribution of PR-labeled (solid circles) and TH-labeled (open circles) nuclei. Abbreviations: AMB, nucleus ambiguus; icp, inferior cerebellar peduncle; IO, inferior olivary complex; mlf, medial longitudinal fascicle; MV, medial vestibular nucleus; NTS, nucleus of the solitary tract; py, pyramidal tract; RO, nucleus raphe obscurus; SPV, spinal nucleus of trigeminal; ts, tractus solitarius; XII, hypoglossal nucleus. Bar, 1 mm.
Light microscopic sections were photographed with a Nikon E800 light microscope using a Micropublisher digital camera (Q-imaging, Barnaby, British Columbia). Electron microscopic preparations were analyzed on a FEI Tecnai Biotwin transmission electron microscope and a digital camera system (Advanced Microscopy Techniques, software version 3.2). Levels, sharpness, brightness and contrast in figures were adjusted in Adobe Photoshop 7.0 and assembled in Quark Xpress 6.1 using an Apple Power Macintosh G5 computer. The subcellular location of PR-labeling relative to TH-labeled profiles was examined by EM from one RVLM block from five rats each. From these rats, one block (approximately 1.2 × 103 μm2 per block) from each of the three rats with the highest estrogen levels as determined from the radioimmunoassays were selected for semiquantitative analysis. Profiles were sampled near (within 1.5 μm) the plastic–tissue interface to ensure equivalent antibody penetration [18]. In each field, all profiles containing PR-ir were photographed, and categorized as catecholaminergic (TH-containing), non-catecholaminergic, or glial. To correct for differences in areas sampled in each block, the percentage of each type of profile was determined per block. To determine the relative distribution of PR immunoreactive profiles, the mean percentage and standard error was determined by averaging the percentages from one block from each of three rats. PR-labeled profiles were classified according to the nomenclature of Peters et al. [28]. Dendrite profiles contained regular microtubule arrays and usually were postsynaptic to axon terminal profiles. Unmyelinated axon profiles had a diameter less than 0.2 μm, had a few small synaptic vesicles and lacked synaptic junctions in the plane of section. Terminal profiles had minimal diameters greater than 0.2 μm, contained numerous small synaptic vesicles, sometimes contained large dense-core vesicles and often contacted other neuronal profiles. Glial profiles tended to conform to the boundaries of surrounding profiles and/or lacked microtubules and, if astrocytic, were identified by the presence of glial filaments.
Since previous studies showed that nuclear PR-ir in the rat brainstem was highest at proestrus [10], tissue from this stage was chosen for the analysis. Consistent with our previous observations using two fluorescent markers (unpublished), PR-ir was detected in a few nuclei within the RVLM (Fig. 1). Usually one or two PR-labeled nuclei were detected per section and almost all were in the anterior portion of the RVLM and intermingled with TH-labeled perikarya and dendrites (not shown). No PR-labeled nuclei were found in neurons with TH-ir.
The electron microscopic analysis was focused on the anterior portion of the RVLM since it contains the bulk of C1 and non-C1 sympathoexcitatory bulbospinal neurons [4,25]. The morphological characteristics of catecholaminergic profiles in the C1 area of the RVLM have been described in detail previously [23,39]. PR immunoperoxidase labeling in RVLM tissue prepared for EM was primarily detected at extranuclear sites. In an experiment identifying PR-labeling with the immunogold–silver method, the subcellular localization was similar but less prominent due to the reduced sensitivity of this method for small amounts of antigen (not shown).
Semiquantitative analysis revealed that axonal profiles with PR-labeling were most numerous (51 ± 9% of 91 processes from three blocks from three separate rats). The majority (40 of 42) of PR-labeled axons were small (<0.2 μm in diameter), unmyelinated and lacked TH-ir (Fig. 2A). Rarely (2 of 42), PR-ir was detected in non-TH-labeled myelinated axons (not shown).
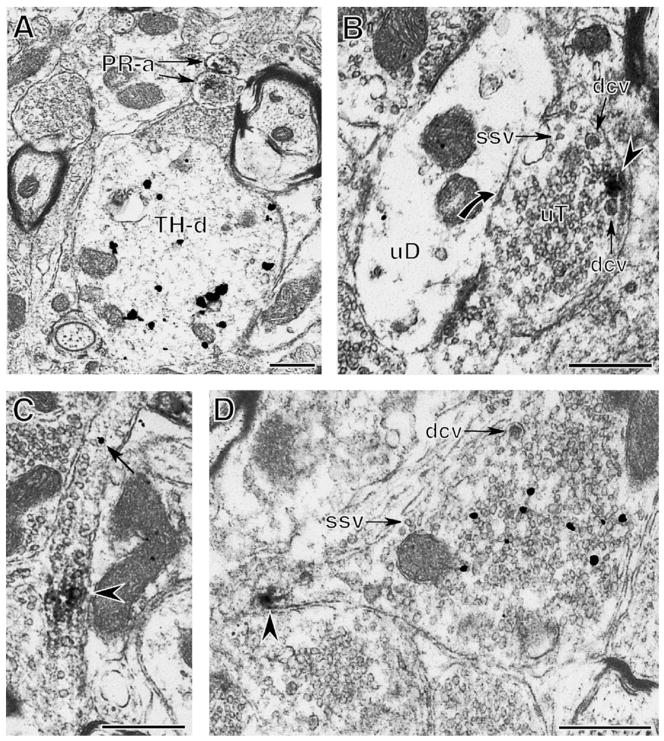
Fig. 2.
Extranuclear PR-ir is found in presynaptic profiles. (A) Two axons with PR-ir (PR-a) are near a TH-immunoreactive dendrite (TH-d). (B) A cluster of PR-ir (arrowhead) is near the plasma membrane of an unlabeled terminal (uT) that contains small synaptic vesicles (ssv) and dense-core vesicles (dcv). The PR-labeled terminal contacts (curved arrow) an unlabeled dendrite (uD). (C) A patch of PR-ir (arrowhead) and immunogold for GAD65 (arrow) are in a longitudinal section of an axon. (D) PR-ir (arrowhead) is found in a preterminal axon of a terminal with GAD65-ir (black particles). The GAD65-labeled terminal has numerous small synaptic vesicles (ssv) and a dense core vesicle (dcv). Bar, 500 nm.
Extranuclear neuronal PR-labeling also was detected in axon terminals (9.7 ± 3% out of three blocks). Terminals with PR-ir (n = 9) had an average diameter of 0.95 ± 0.05 μm, contained numerous small synaptic vesicles and 1–3 dense-core vesicles (Fig. 2B). PR-ir in terminals was found in clusters affiliated with the plasma membrane (Fig. 2B). Of the PR-labeled terminals observed, one formed a symmetric contact on the shaft of a TH-labeled dendrite (not shown) and two formed symmetric synapses or closely apposed unlabeled dendritic shafts (Fig. 2B).
The observation that some terminals with PR-labeling formed symmetric synapses suggested that these terminals contained the inhibitory transmitter GABA. Thus, to further characterize the phenotype of PR-labeled axons, sections through the RVLM were dually labeled with PR and the GABAergic marker, GAD65. This marker was selected since our previous studies [21] showed that presynaptic GABAergic profiles are prominent in the RVLM and that they form numerous contacts with bulbospinal neurons. In fortuitous planes of section, PR-ir was detected in axons with GAD65-ir (Fig. 2C). Moreover, PR-ir was detected in preterminal axons of GAD65-immunoreactive terminals (Fig. 2D). Terminals with GAD65-ir were large (about 1.3 μm in diameter), contained numerous small synaptic vesicles and an occasional dense-core vesicle (Fig. 2D).
Extranuclear neuronal PR-labeling also was detected in dendrites (9.7 ± 1% out of three blocks). PR-labeled dendrites (n = 9) had an average diameter of 0.88 ± 0.09 μm and lacked TH-ir (Fig. 3A). Within dendrites, PR-ir was found in clusters in the cytoplasm and not affiliated with the plasma membrane.
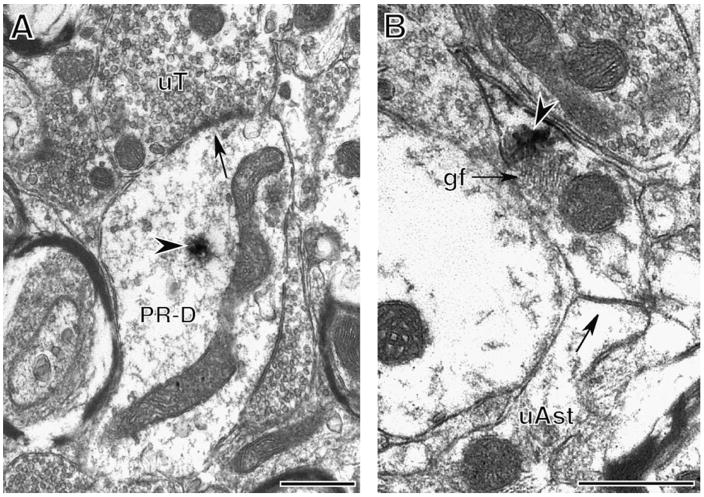
Fig. 3.
PR-ir is found in dendrites and glia. (A) A cluster of PR-ir (arrowhead) is in the cytoplasm of a dendrite (PR-D). An unlabeled terminal (uT) contacts (arrow) the PR-containing dendrite. (B) PR-ir (arrowhead) is in an astrocytic process identified by glial filaments (gf) that forms a gap junction (arrow) with an unlabeled astrocytic profile (uAst). Bar, 500 nm.
In addition to neuronal profiles, extranuclear PR-ir was detected in glial profiles (29 ± 9% out of three blocks). Glial profiles were interspersed in the neuropil and occasionally contained glial filaments identifying them as astrocytes (Fig. 3B). Sometimes, PR-labeled astrocytic profiles formed gap junctions with unlabeled glial profiles (Fig. 3B).
The present study demonstrates that in addition to nuclei, PR-ir is found at extranuclear sites in the RVLM C1 area. Most PR-ir is in presynaptic profiles, some of which are GABAergic, while the remainder is in non-catecholaminergic dendrites and glia.
The demonstration of PR in nuclei in the RVLM of the female Sprague–Dawley rat is consistent with previous reports describing PR nuclei in ventral brainstem regions [10,15]. However, in contrast to other catecholaminergic brain stem regions (e.g., nucleus of the solitary tract and A1 area), C1 adrenergic neurons in the RVLM did not contain detectable levels of nuclear PR. Whether the little nuclear PR that was observed is contained in non-C1 bulbospinal neurons or intrinsic RVLM cells remains to be determined.
The subcellular localization of PRs in the RVLM is similar to the distribution of extranuclear ERα and ERβ [39]. However, PRs were relatively less abundant and were allocated to fewer and different cellular compartments. In particular, unlike PRs, extranuclear ERs were detected in C1 neuronal perikarya and dendrites. Moreover, ERα-ir was prominent in large terminals containing numerous dense-core vesicles similar to afferents from the nucleus of the solitary tract and hypothalamus. PR-containing terminals more closely resembled ERβ-containing terminals in size but, unlike ERβ-labeled terminals, contained a few dense-core vesicles suggesting that they had different cellular origins.
PR-ir was primarily in unmyelinated axons as well as a few myelinated axons and axon terminals. Within axons and axon terminals, PR-ir was usually associated with the plasma membrane. Although likely underestimated due to their different subcellular locations, at least some of the PR-labeled axons contained GAD65-ir. These findings suggest that progestins may rapidly modulate neurotransmitter release, particularly GABA. In support, progestins are modulators of GABAA receptors as well as glutamatergic NMDA receptors and others [3]. Moreover, activation of PRs in GABAergic axons may affect transduction of electrical signals to the terminal [38].
Although the source of presynaptic PR-containing profiles is unknown, the presence of dense-core vesicles suggests that they colocalize peptides [34]. At least a portion of PR-labeled terminals are GABAergic which arise in part from the caudal ventrolateral medulla as well as the nucleus of the solitary tract [2,5,12]. Previous studies have shown that GABAergic terminals form synapses on and tonically inhibit bulbospinal neurons in the RVLM [7,21]. Thus, these findings suggest that progestins could directly modulate baroreceptor function by acting directly on PRs in presynaptic GABA inputs.
The demonstration that PR-ir is in dendritic and glial profiles is consistent with our other reports examining the cellular distribution of extranuclear gonadal steroid receptors in the RVLM as well as the hippocampal formation [20,22,39]. Within dendritic profiles, PR-ir was primarily detected in the cytoplasm, distal from the plasma membrane suggesting that PRs are primarily in transit and/or stored for future use. Since PR-containing dendrites lacked detectable TH-ir they probably do not arise from C1 neurons. However, whether they arise from non-C1 bulbospinal neurons or interneurons remains to be determined.
The finding that PR-ir is in glial cells is consistent with previous studies demonstrating PR protein in glial cells in vitro [14]. The presence of PRs on glial cells could be important in controlling ionic balance and synaptic transmission [13,19]. Moreover, since some PR-ir was found on astrocytic profiles that formed gap junctions, activation of PRs could be involved in calcium signaling between adjacent cells [8].
In conclusion, these findings suggest that progestins can primarily modulate neurons in the C1 area of the RVLM by presynaptic mechanisms involving GABAergic transmission. The presence of extranuclear PRs in the RVLM suggests another mechanism through which progestins may regulate ABP during pregnancy and may contribute to sex differences in central cardiovascular regulation in this critical sympathoexcitatory region.
Acknowledgments
We thank Dr. Annelyn Torres-Reveron, Ms. Nora Tabori and Mr. Scott Herrick for technical assistance.
Footnotes
Grant support: NIH grants HL18974, DA08259 (TAM), NS07080 and T32 DK07313 (E.M.W.).
References
- 1.Aicher SA, Milner TA, Pickel VM, Reis DJ. Anatomical substrates for baroreflex sympathoinhibition in the rat. Brain Res Bull. 2000;51:107–110. doi: 10.1016/s0361-9230(99)00233-6. [DOI] [PubMed] [Google Scholar]
- 2.Aicher SA, Saravay RH, Cravo S, Jeske I, Morrison SF, Reis DJ, Milner TA. Monosynaptic projections from the nucleus tractus solitarii to C1 adrenergic neurons in the rostral ventrolateral medulla: comparison with input from the caudal ventrolateral medulla. J Comp Neurol. 1996;373:62–75. doi: 10.1002/(SICI)1096-9861(19960909)373:1<62::AID-CNE6>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 3.Baulieu EE. Neurosteroids: a novel function of the brain. Psychoneuroendocrinology. 1998;23:963–987. doi: 10.1016/s0306-4530(98)00071-7. [DOI] [PubMed] [Google Scholar]
- 4.Card JP, Sved JC, Craig B, Raizada M, Vazquez J, Sved AF. Efferent projections of rat rostroventrolateral medulla C1 catecholamine neurons: implications for the central control of cardiovascular regulation. J Comp Neurol. 2006;499:840–859. doi: 10.1002/cne.21140. [DOI] [PubMed] [Google Scholar]
- 5.Chan RKW, Sawchenko PE. Organization and transmitter specificity of medullary neurons activated by sustained hypertension: implications for understanding baroreceptor reflex circuitry. J Neurosci. 1998;18:371–387. doi: 10.1523/JNEUROSCI.18-01-00371.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chang YC, Gottlieb DI. Characterization of the proteins purified with monoclonal antibodies to glutamic acid decarboxylase. J Neurosci. 1988;8:2123–2130. doi: 10.1523/JNEUROSCI.08-06-02123.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dampney RAL, Tagawa T, Horiuchi J, Potts PD, Fontes M, Polson JW. What drives the tonic activity of presympathetic neurons in the rostral ventrolateral medulla? Clin Exp Pharmacol Physiol. 2000;27:1049–1053. doi: 10.1046/j.1440-1681.2000.03375.x. [DOI] [PubMed] [Google Scholar]
- 8.Giaume C, Venance L. Intercellular calcium signaling and gap junctional communication in astrocytes. Glia. 1998;24:50–64. [PubMed] [Google Scholar]
- 9.Guerra-Araiza C, Cerbón MA, Morimoto S, Camacho-Arroyo I. Progesterone receptor isoforms expression pattern in the rat brain during the estrous cycle. Life Sci. 2000;66:1743–1752. doi: 10.1016/s0024-3205(00)00497-5. [DOI] [PubMed] [Google Scholar]
- 10.Haywood SA, Simonian SX, Van der Beek EM, Bicknell RJ, Herbison AE. Fluctuating estrogen and progesterone receptor expression in brainstem norepinephrine neurons through the rat estrous cycle. Endocrinology. 1999;140:3255–3263. doi: 10.1210/endo.140.7.6869. [DOI] [PubMed] [Google Scholar]
- 11.Heesch CM, Rogers RC. Effects of pregnancy and progesterone metabolites on regulation of sympathetic outflow. Clin Exp Pharmacol Physiol. 1995;22:136–142. doi: 10.1111/j.1440-1681.1995.tb01970.x. [DOI] [PubMed] [Google Scholar]
- 12.Jeske I, Reis DJ, Milner TA. Neurons in the barosensory area of the caudal ventrolateral medulla project monosynaptically on to sympathoexcitatory bulbospinal neurons in the rostral ventrolateral medulla. Neuroscience. 1995;65:343–353. doi: 10.1016/0306-4522(94)00470-p. [DOI] [PubMed] [Google Scholar]
- 13.Jordan CL. Glia as mediators of steroid hormone action on the nervous system: an overview. J Neurobiol. 1999;40:434–445. doi: 10.1002/(sici)1097-4695(19990915)40:4<434::aid-neu2>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 14.Jung-Testas I, Renoir JM, Gasc JM, Baulieu EE. Estrogen-inducible progesterone receptor in primary cultures of rat glial cells. Exp Cell Res. 1991;193:12–19. doi: 10.1016/0014-4827(91)90532-y. [DOI] [PubMed] [Google Scholar]
- 15.Kastrup Y, Hallbeck M, Amandusson Å, Hirata S, Hermanson O, Blomqvist A. Progesterone receptor expression in the brainstem of the female rat. Neurosci Lett. 1999;275:85–88. doi: 10.1016/s0304-3940(99)00753-3. [DOI] [PubMed] [Google Scholar]
- 16.Kurita T, Young P, Brody JR, Lydon JP, O’Malley BW, Cunha GR. Stromal progesterone receptors mediate the inhibitory effects of progesterone on estrogen-induced uterine epithelial cell deoxyribonucleic acid synthesis. Endocrinology. 1998;139:4708–4713. doi: 10.1210/endo.139.11.6317. [DOI] [PubMed] [Google Scholar]
- 17.Laiprasert JD, Rogers RC, Heesch CM. Neurosteroid modulation of arterial baroreflex-sensitive neurons in rat rostral ventrolateral medulla. Am J Physiol Regul Integr Comp Physiol. 1998;274:R903–R911. doi: 10.1152/ajpregu.1998.274.4.R903. [DOI] [PubMed] [Google Scholar]
- 18.Leranth C, Pickel VM. Electron microscopic preembedding double-labeling methods. In: Heimer L, Zaborszky L, editors. Neuroanatomical Tract-Tracing Methods. Vol. 2. Plenum Press; New York: 1989. pp. 129–172. [Google Scholar]
- 19.Mhyre AJ, Dorsa DM. Estrogen activates rapid signaling in the brain: role of estrogen receptor alpha and estrogen receptor beta in neurons and glia. Neuroscience. 2006;138:851–858. doi: 10.1016/j.neuroscience.2005.10.019. [DOI] [PubMed] [Google Scholar]
- 20.Milner TA, Ayoola K, Drake CT, Herrick SP, Tabori NE, McEwen BS, Warrier S, Alves SE. Ultrastructural localization of estrogen receptor beta immunoreactivity in the rat hippocampal formation. J Comp Neurol. 2005;491:81–95. doi: 10.1002/cne.20724. [DOI] [PubMed] [Google Scholar]
- 21.Milner TA, Drake CT, Aicher SA. Cellular relations between μ-opioid receptive, GABAergic and reticulospinal neurons in the rostral ventrolateral medulla. Brain Res. 2001;917:1–14. doi: 10.1016/s0006-8993(01)02827-x. [DOI] [PubMed] [Google Scholar]
- 22.Milner TA, McEwen BS, Hayashi S, Li CJ, Reagan LP, Alves SE. Ultrastructural evidence that hippocampal alpha estrogen receptors are located at extranuclear sites. J Comp Neurol. 2001;429:355–371. [PubMed] [Google Scholar]
- 23.Milner TA, Morrison SF, Abate C, Reis DJ. Phenylethanolamine N-methyl transferase containing neurons in the rostral ventrolateral medulla of the rat. I. Normal ultrastructure. Brain Res. 1987;411:28–45. doi: 10.1016/0006-8993(87)90678-0. [DOI] [PubMed] [Google Scholar]
- 24.Milner TA, Rosin DL, Lee A, Aicher SA. Alpha2A-adrenergic receptors are primarily presynaptic heteroreceptors in the C1 area of the rat rostral ventrolateral medulla. Brain Res. 1999;821:200–211. doi: 10.1016/s0006-8993(98)00725-2. [DOI] [PubMed] [Google Scholar]
- 25.Morrison S, Milner TA, Reis DJ. Reticulospinal vasomotor neurons of the rat rostral ventrolateral medulla: relationship to sympathetic nerve activity and the C1 adrenergic cell group. J Neurosci. 1988;8:1286–1302. doi: 10.1523/JNEUROSCI.08-04-01286.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mostallino MC, Mura ML, Maciocco E, Murru L, Sanna E, Biggio G. Changes in expression of the delta subunit of the GABA (A) receptor and in receptor function induced by progesterone exposure and withdrawal. J Neurochem. 2006;99:321–332. doi: 10.1111/j.1471-4159.2006.04055.x. [DOI] [PubMed] [Google Scholar]
- 27.Parsons B, Rainbow TC, MacLusky NJ, McEwen BS. Progestin receptor levels in rat hypothalamic and limbic nuclei. J Neurosci. 1982;2:1446–1452. doi: 10.1523/JNEUROSCI.02-10-01446.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Peters A, Palay SL, Webster Hd. The fine structure of the nervous system. 3. Oxford University Press; New York: 1991. [Google Scholar]
- 29.Rupprecht R, Reul JM, Trapp T, Van Steensel B, Wetzel C, Damm K, Zieglgansberger W, Holsboer F. Progesterone receptor-mediated effects of neuroactive steroids. Neuron. 1993;11:523–530. doi: 10.1016/0896-6273(93)90156-l. [DOI] [PubMed] [Google Scholar]
- 30.Saleh MC, Connell BJ, Saleh TM. Autonomic and cardiovascular reflex responses to central estrogen injection in ovariectomized female rats. Brain Res. 2000;879:105–114. doi: 10.1016/s0006-8993(00)02757-8. [DOI] [PubMed] [Google Scholar]
- 31.Saleh MC, Connell BJ, Saleh TM. Medullary and intrathecal injections of 17β-estradiol in male rats. Brain Res. 2000;867:200–209. doi: 10.1016/s0006-8993(00)02313-1. [DOI] [PubMed] [Google Scholar]
- 32.Saleh TM, Connell BJ. Role of oestrogen in the central regulation of autonomic function. Clin Exp Pharmacol Physiol. 2007;34:1–6. doi: 10.1111/j.1440-1681.2007.04663.x. [DOI] [PubMed] [Google Scholar]
- 33.Swanson LW. Brain Maps: Structure of the Rat Brain. Elsevier; Amsterdam: 1992. [Google Scholar]
- 34.Thureson-Klein AK, Klein RL, Zhu PC. Exocytosis from large dense cored vesicles as a mechanism for neuropeptide release in the peripheral and central nervous system. Scan Electron Microsc. 1986;1:179–187. [PubMed] [Google Scholar]
- 35.Tibbetts TA, DeMayo F, Rich S, Conneely OM, O’Malley BW. Progesterone receptors in the thymus are required for thymic involution during pregnancy and for normal fertility. Proc Natl Acad Sci USA. 1999;96:12021–12026. doi: 10.1073/pnas.96.21.12021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Traish AM, Wotiz HH. Monoclonal and polyclonal antibodies to human progesterone receptor peptide-(533–547) recognize a specific site in unactivated (8S) and activated (4S) progesterone receptor and distinguish between intact and proteolyzed receptors. Endocrinology. 1990;127:1167–1175. doi: 10.1210/endo-127-3-1167. [DOI] [PubMed] [Google Scholar]
- 37.Turner CD, Bagnara JT. General Endocrinology. WB Saunders; Philadelphia: 1971. [Google Scholar]
- 38.Verdier D, Lund JP, Kolta A. GABAergic control of action potential propagation along axonal branches of mammalian sensory neurons. J Neurosci. 2003;23:2002–2007. doi: 10.1523/JNEUROSCI.23-06-02002.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang G, Drake CT, Rozenblit M, Zhou P, Alves SE, Herrick SP, Hayashi S, Warrier S, Iadecola C, Milner TA. Evidence that estrogen directly and indirectly modulates C1 adrenergic bulbospinal neurons in the rostral ventrolateral medulla. Brain Res. 2006;1094:163–178. doi: 10.1016/j.brainres.2006.03.089. [DOI] [PubMed] [Google Scholar]