I would like to give a warning, from a theoretical point of view, about all the carbonium ion mechanisms. They are all probably concerted reactions with a beginning and an end, and the reason you write down your carbonium mechanisms in the way you do is chiefly because in writing down on paper you are bound to write something first and then something else afterwards, and the human mind is not quick and clever as the enzyme.
Sir Robert Robinson, 1959 (ref 44c)
1. Introduction
Without exception, the most complex organic architectures have been produced by natural organisms. The finding that many of these can be both selective and potent disruptors of disease mechanisms has largely driven the development of organic synthesis as a science. Nearly a century ago, machinations about nature’s pathway to organic compounds, such as those of Robinson concerning tropinone (1917), were beginning to be used as a virtual blueprint to possible synthetic. Today, the use of catalysts having no biological relevance can create nonnatural organic structures with levels of complexity that approximate those found naturally. Despite the considerable wealth of methods at the disposal of today’s chemist, it is arguable that nature still creates the most selective catalysts (e.g. enzymes, antibodies1) with efficiencies that can be equally impressive. More to the point, enzymes have at their disposal only a peptidic structure in many cases (largely limited by naturally occurring amino acids), whereas the chemists’ traditional arsenal extends to metal and main group catalysts, solvents, and temperature range. Even in cases where metal-peptide-cofactor combinations are used to effect unlikely organic reactions (e.g. methane monooxygenase and the photosystems), we generally stand at the stage of mimicry, and not at the precipice of utilizing this knowledge to develop practical analogs. It is the promise of identifying the minimal functional unit, and the assumption that one will always exist, that drives this enterprise.
The earliest days of targeted organic synthesis (total synthesis) combined the available synthetic methods with a contemporary understanding of biosynthesis.2 Biosynthetic considerations are largely (if not completely) inspiration for a synthesis route to target.3 An interesting feature of the interplay between biosynthesis and total synthesis is the increasing tendency for the latter to inspire discrete hypotheses about a target’s biosynthesis, particularly where the latter has not even been studied. A reading of the total synthesis literature as it pertains to biosynthesis hypotheses must therefore be done with a critical eye. This review provides a case study in polyolefin cyclizations in which the biosynthesis-total synthesis interplay presents an incontrovertible synergism. Moreover, there is now a clearer understanding of the mutual benefits. An unexpected outcome of this appraoch is the development of synthetic methods that converge upon the efficient techniques of nature. Despite this evolution, intriguing new questions remain about these transformations in either venue to further stimulate questions of biosynthesis mechanisms and synthesis possibilities, thereby maintaining an excitement about the potential of both fields. Biosynthesis hypotheses have also guided experiments to establish natural product structure, and this practical benefit cannot be overstated.4
An enzyme’s ability to activate relatively unreactive substrates, such as simple olefins, is only part of the phenomenon. Perhaps more impressive is the selectivity with which a multiple bond-forming reaction can occur. In contrast to synthetic systems from which numerous pathways compete with carbocyclization, enzymes exert an amazing level of control to produce just a few of these possibilities, resulting in high selectivity for the overall transformation. The case of polyolefin carbocyclization provides both an enzyme model for remarkable selectivity,5 as well as a driver for investigations that ask provocative fundamental questions in this area. Selectivity in these cases is multifaceted: ring size, number of rings formed, stereochemistry of fused ring formation, and the degree of atom/group rearrangement.
The interplay between biomimicry and total synthesis has been discussed as part of several reviews, including those that provide an overview,6 a focus on domino reactions,7 elucidate enzymology,8,9 treat the discovery of enzyme inhibitors,10 and explore non-enzymatic cyclizations.11,12 In part to mark the 50th anniversary of the Stork-Eschenmoser hypothesis, we review here a subgroup of polycarbocyclizations while attempting to define the relationship that ultimately evolved between the purely synthetic work and its biological counterpart. Substantial progress has been made in the understanding of how nature converts squalene to hopene (in bacteria), or the corresponding conversion of oxidosqualene to steroids (in eukaryotes).
One caveat to a review of this type is that the literature rarely permits a measurement of relative efficiency and selectivity in these polycyclizations. For example, isolated yields for the desired polycycle provide only a maximum for a particular diastereoselective cyclization. Premature termination of the polycyclization resulting in mono-, di-, and tricycles in a tetracyclization go largely unreported, if not unmeasured. Moreover, the diastereoselection accompanying each cyclization is also typically unreported/unanalyzed. We are therefore left only with an understanding of accessible pathways to products.
1.1 Steroid biosynthesis from squalene: the fundamentals
Steroid biosynthesis from squalene and its derivatives is generally treated in two segments. The first is an enzyme catalyzed series of carbocyclizations, followed by a termination step such as proton loss or a series of atom/group rearrangments prior to proton loss. Subsequent modification of the ‘side chain’ is performed by a distinct set of enzymes and provides an impressive list of transformations itself. In the biosynthesis of steroidal terpenes, this second segment can be treated separately and has been reviewed as late as 1993 by Giner.13
The first committed step in the cholesterol biosynthesis is the squalene synthase-catalyzed homocoupling of farnesyl diphosphate (1) to squalene (3) via PSPP (2) (Scheme 1).14 This is followed by an enantioselective epoxidation using molecular oxygen catalyzed by squalene epoxidase to afford (3S)-2,3-oxidosqualene (5).15 There are numerous oxidosqualene cyclases and each is capable of generating a unique, naturally occurring product such as lanosterol (6) (animals and fungi) and cycloartenol (7) (plants). Among these studies, the efforts of Bloch16,17,18 on the biosynthesis of triterpenes (including lanosterol) are notable, as are those of Cornforth (squalene and cholesterol biosynthesis).19 Furthermore, the structural work of Ruzicka profoundly influenced the experimental studies of terpene biosynthesis.20
Scheme 1.
General outline of the biosynthesis pathways involving squalene and oxidosqualene cyclases
Bacteria do not epoxidize squalene prior to the π-cation cyclization.22,23 Rather, squalene-hopene cyclase catalyzes an enantioselective, diastereoselective polycyclization initiated by proton transfer to the 2,3-alkene. It is believed that this bacterial cyclase is the ‘primitive’ cyclase from which all other oxidosqualene cyclases have evolved.22 Squalene-hopene cyclase has been found to be more substrate permissive, accepting squalene, (3R)-oxidosqualene, (3S)-oxidosqualene, and even 2,3-dihydroxysqualene.24,25 In contrast, oxidosqualene cyclase is selective for a relatively small number of substrates, e.g. (3S)-oxidosqualene, but not (3R)-oxidosqualene.26 Moreover, the specificity of sterol biosynthesis has been used to classify organisms since all sterol producing organisms can be divided into two groups depending on whether they cyclize squalene oxide to lanosterol or cycloartenol.27
1.2 Sterol biosynthesis: the acetic acid-squalene-lanosterol-cholesterol connection
The topic of this review involves facets of chemistry that span a tremendous period of time for the development of any scientific enterprise - more than seventy years. In the present case, it is fair to say that even the basic principles governing the reactions of acyclic and cyclic organic compounds were still being formulated circa 1950, but the synthesis and biosynthesis of terpenes and sterols were topics of broad and intense interest. It is purely for clarity that work prior to 1950 is depicted in conformational perspective in the following Schemes, despite the fact that Barton’s pivotal Experientia paper describing the manner in which steroid conformation determines their diastereoselective transformations was not published until 1950.28 The reader is reminded, therefore, to follow a discussion of these events with an appreciation for what the investigators of the period could not take for granted. Our decision to depict work prior to 1950 in conformational perspective is motivated by the desire for conceptual clarity and is admittedly ahistorical.
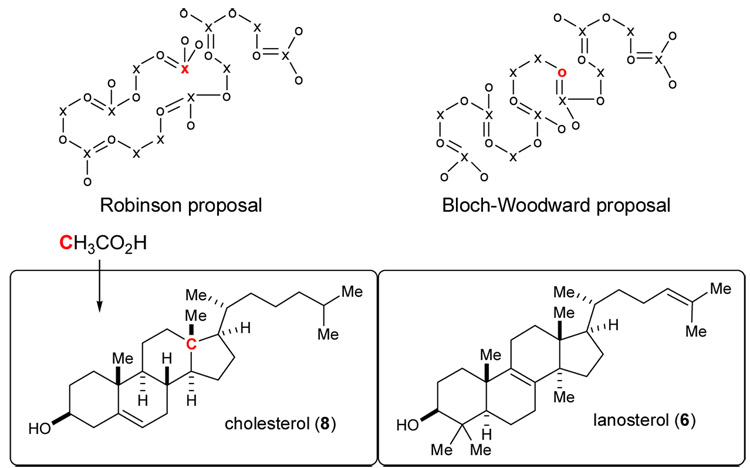
As of 1950, the biosynthetic precursor to the sterols had not been established as squalene, and the essential fatty acids (e.g. arachidonic acid) were believed by some to be a more viable precursor than other polyolefins known at the time. For cholesterol to be formed from squalene, significant structural reorganization via methyl shifts, and degradation (demethylation) reactions would be necessary. A proposal for the conversion of squalene to cholesterol that minimized reliance on these mechanisms, at least conceptually, was advanced by Robinson in 1934 (Figure 1).29 Bloch and Rittenberg used isotopic labeling experiments in 1945 to outline the pathway from acetic acid to cholesterol, and again hypothesized that squalene was an intermediate.30 The 1952 determination of lanosterol’s structure20 made the connection between squalene and cholesterol31 more evident, as well as the connection between squalene and most pentacyclic triterpenes. The visible structural similarities between lanosterol and both squalene and cholesterol substantially increased the resolution of this highly studied biosynthetic pathway. For example, Bloch was able to revisit the proposed conversion of squalene to cholesterol by interrogating the carbon skeletal arrangement using labeled acetic acid. Bloch found that 14CH3− labeled acetate is incorporated at the C13 quaternary carbon in cholesterol. This finding led him to exclude Robinson’s 1934 proposal (Figure 1) which embodied multiple transannular carbon-carbon bond formations from a circular squalene conformation, in preference to the serpentine conformation we now know to be operative.
Figure 1.
Robinson and Bloch proposals for pre-cholesterol squalene organization (x=acetate carboxyl, o=acetate methyl), and Bloch labeling outcome
Clarification of squalene’s role in sterol (and terpene) biosynthesis was also addressed by several investigators initially focused on the role of π-cation carbocyclizations in alkaloid and terpene natural product synthesis. Not surprisingly, these studies were concurrent with the biosynthetic and structural work described above, and the exact origins of several likely pre-date 1950 as well. In some respects, it is quite remarkable that the thinking of Stork,32 Bloch and Woodward, and the Züich school33 (Eschenmoser, Ruzicka, Jeger, and Arigoni) coalesced in the early 1950s to a unified theme of squalene-terpene/sterol biosynthesis, yet each with a unique origin, perspective, and ultimately contribution. Not only did their work establish a complete picture of the conversion of squalene to lanosterol (and its congeners), including the mechanism (stereocontrol) of carbon-carbon bond formation, but they also provided a powerful predictive tool. Many of these predictions have since been validated.
2. Stereochemistry of the squalene-sterol cyclization
2.1 Context
The constitutional relationship between squalene and the sterols could be elucidated, at least in part, by biosynthetic pathways interrogated by labeling experiments. The earliest documented suggestion40 that 1,5-polyolefin cyclizations to decalin ring systems might serve as the chemical model for the constitutional relationship was made by Stork. The stereocontrol attendant to the squalene-cyclic triterpene transformations, however, could only be addressed through independent chemical synthesis using substrates that might model the π-olefin cyclization, but serve as more tractable tools than squalene. The work of the Züich school and Stork began from only their respective interest in the stereocontrol of polyolefin cyclizations, and arrived later at the hypothesis that these findings might be relevant to more sophisticated polyolefin cyclizations in biosynthesis pathways. The term ‘Stork-Eschenmoser’ hypothesis, first propounded by W. S. Johnson, has its origins in their work of this time, but is perhaps best equated with stereospecific antiparallel-addition of carbenium ion and olefin to an alkene. Use of this term and more restricted definition, however, should not lead one to forget Stork’s first suggestion of the constitutional relationship between 1,5-diene cyclizations and terpene biosynthesis. Nor should one neglect the far-reaching contributions made by the Züich school during the 1950s to the broader subject of squalene-triterpene biosynthesis.
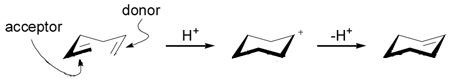
The fundamental biosynthesis question to be answered is the extent to which a polyolefin cyclization, such as the conversion of squalene to the tetracycle lanosterol or the pentacycle lupeol, is stereospecific or stereoselective. These possibilities reflect mechanisms in which the addition of a carbenium ion and alkene pair to an olefin (Figure 2) is either concerted (no intermediate), or proceeds through a carbenium ion intermediate, respectively. A second stereospecific pathway involves a ‘nonclassical’ carbenium ion intermediate that would also result in antiparallel addition. Juxtaposed with the limiting possibility of a stereospecific mechanism is the question how the parent squalene might produce cyclic triterpene progeny with such stereochemical diversity?
Figure 2.
Carbenium ion additions to olefins: mechanistic possibilities and their stereochemical outcomes
2.2 1,5-Diene cationic cyclization
Substantial attention was directed toward the fundamental process in which a 1,5-diene is cyclized to a cyclohexyl carbenium ion (Scheme 2) prior to 1950. The basic relationship between terpenes and acid-promoted cyclizations of olefins had been recognized as early as 1893 (cyclization of pseudo-ionone to ionone).35 By 1936, constitutional constraints for the acid-catalyzed cyclization of various substituted 1,5-dienes to cyclohexenes had been elucidated, driven not only by interest in terpene synthesis but also by structural elucidation efforts in the steroid area. Regioselectivity for six-membered ring formation could be increased through a balance of higher substitution and greater nucleophilicity of the donor olefin.36 Within the subclass of cyclohexannulations, Linstead37 was the first to treat the issue of diastereoselection attendant with formation of a fused bicyclic ring system. Specifically, ‘mild’ Brønsted acid cyclization conditions (H2SO4, AcOH, Ac2O, 25 °C or H3PO4, 140 °C) converted tertiary alcohol 9 to cis-decalin product 10 in 89% yield (Scheme 3).38 The intermediacy of a tetrasubstituted olefin cannot be excluded given Linstead’s finding that the regioisomeric tertiary alcohol of 9 behaves similarly to 9, providing a cis-decalol product (not shown).39
Scheme 2.
Brønsted Acid catalyzed 1,5-diene cyclization
Scheme 3.
Acid catalyzed cyclohexannulation: Linstead's stereoselective decalin formation
Stork’s logic concerning the stereochemistry of carbenium ion additions to olefins, discussed publicly as early as 1950,40 used Linstead’s findings as a foundation. He surmised that isolation of cis-decalins 12 from the cyclization of cyclohexene 1141 might indicate stereospecific anti-addition of proton and electron rich donor olefin to the alkene acceptor (not unlike the stereospecific addition of bromine to an olefin). This transformation could involve a single transition state to a carbenium ion that would then accept acetate. Conversely, the intermediacy of a discrete cyclohexane carbenium ion would be expected to produce measurable quantitites of the trans-decalin stereoisomer (Figure 4).
Figure 4.
Acid catalyzed cyclohexannulation: stereochemistry and mechanism
As an extension of this interpretation, Stork hypothesized that the anti-addition mechanism might be conserved when a proton is replaced by a carbenium ion (A, Figure 3). The cyclization of a carbenium ion to an E-olefin would thereby generate the trans-decalin through a transition state reflecting the anti-arrangement of carbenium ion and olefin donor. Confirmation of this hypothesis was sought through cyclizations of farnesic acid (13) and farnesylacetic acid (22).
Figure 3.
Construct relating stereospecific π-cation biscyclization to decalin configuration
Schinz had previously reported the Brønsted acid-promoted cyclization of farnesic acid to decalin carboxylic acid 15 in 10% yield using a combination of formic and sulfuric acids (Scheme 4). Stork found that boron trifluoride etherate produced the same acid in 35% yield at slightly elevated temperature, yet effected only monocyclization to 14 when the reaction was cooled to 5 °C. Comparison of the IR spectra of these products with the degradation product of ambrein (a trans-decalin) led to the assignment of the cis-decalin stereochemistry. Isolation of the monocycle enabled the key experiment in which both Brønsted and Lewis acid were applied and found to result in the same solid (mp 131 °C) as Schinz. Stork interpreted the cyclization of (E)-14 to cis-decalin 15 as a concerted anti-addition of proton and donor olefin, consistent with his hypothesis derived from the Linstead work. However, since a different cis-decalin was formed from Brønsted acid cyclization of farnesic acid and (E)-14, and the latter reacted more slowly, he concluded that a discrete carbenium ion exists as an intermediate to cis-decalin 15, and the tetrasubstituted olefin 14 is not an intermediate in the farnesic acid cyclization. The nonconcerted nature of this cyclization could be rationalized as a consequence of the diminished nucleophilicity of the donor olefin which is conjugated to the carboxylic acid. These experimental findings were later revised, however, in a joint publication with Eschenmoser in which the fused decalin stereochemical assignment of cis-15 and cis-16 was corrected to trans-15 and trans-16 (Scheme 4).44 Although the conversion of 13 to trans-16 may itself be reinterpreted as concerted anti-addition to the central trisubstituted olefin, the Zürich collaboration demonstated with apofarnesic acid (18) that a high level of stereoselectivity is possible in the cyclization of the electron deficient olefin to the intermediate cyclohexyl carbenium ion. Moreover, under these conditions the intermediacy of various olefin isomers (17, 18) would be otherwise undetected since they are competent precursors to trans-21 in independent experiments (Scheme 5). Further demonstration of the reversibility of cationic cyclizations follows from their conversion of (+)−20 to the common product trans-21 with complete racemization, thereby indicating the intermediacy of the endocyclic cyclohexene and that the seemingly diastereospecific carbocyclization (19 to 21) is under thermodynamic control. These experiments certainly do not preclude the possibility of stereospecific cyclization under other conditions (c.f. Section 4.3 (Yamamoto)), but clearly outline the importance of substrate and conditions to the interpretation of the cyclization stereochemical outcome.
Scheme 4.
Schinz and Stork cyclization of farnesic acid, and correction of product stereochemical assignment
Scheme 5.
Stork's cyclization of farnesyl acetic acid
The hypothesis that a weakly nucleophilic donor olefin favors a nonconcerted addition, and therefore a carbenium ion intermediate, was examined by studying the behavior of farnesyl acetic acid under similar cyclization conditions. The cyclization of farnesyl acetic acid was also an opportunity to merge the Linstead work with a polycyclization involving two electron rich trisubstituted olefins. Stork found that farnesyl acetic acid produced ambreinolide, presumably as a single diastereomer, in 3% yield using stannic bromide (Scheme 6). In contrast, exposure of cyclohexene 25, analogous to farnesic acid congener 14, produced isoambreinolide (26) in 7% yield. The contrasting diastereoselection of these two reactions, the latter one clearly proceeding first to a carbenium ion (c.f. 27), indicated that the cyclization of farnesyl acetic acid might be concerted. That is, formation of ambreinolide must involve a single transition state 24 involving three concerted anti-additions to the trisubstituted olefins. Isoambreinolide then serves as an indicator of carbenium ion intermediate 27 which cyclizes to the trans-decalin intermediate. The cis-stereochemistry of the subsequent lactonization then follows from the trapping of the carbenium ion by the carboxylic acid.
Scheme 6.
Stork's cyclization of farnesyl acetic acid
Stork and Burgstahler’s studies of the stereochemistry of intramolecular carbenium ion additions to olefins described above was concurrent with the stereochemical determination of many triterpenes and steroids. Their findings were published in 1955 as support for a proposed link between electrophilic polyolefin cyclization and the polycyclohexane fused ring structure germane to triterpenes and steroids (Figure 5),40 even when other structural features (e.g. the positions of angular methyls) were not readily explained. They identified the seemingly stereospecific polycarbocyclization of squalene to lupeol, but explained the stereochemical dichotomy between lupeol and lanosterol by hypothesizing a ‘concerted formation of the first two rings, followed by concerted closure of the last two’ that reflected their interpretation of experiment at the time. While this suggestion was not ultimately an accurate explanation for the stereochemical phenomenon in question, it foreshadowed the potential complexity of some cyclase-mediated biosynthesis that has since unfolded.
Figure 5.
Stork and Burgstahler hypothesis.
Reprinted with permission from ACS publications.
The definitive experimental support for diene cyclization via antiparallel addition and chair-like folding came ultimately from complementing experiments in the hands of Schinz and Eschenmoser.45 The choice of substrate and cyclization conditions are clearly critical (vide supra), and the Zürich school’s 1,5-diene substrates require equatorial addition of the electrophile/nucleophile pair (Scheme 7). The use of an equatorial addition minimizes the potential for stereocontrol by steric influences (e.g. 1,3-diaxial interactions), and the ability to correlate olefin geometry with the direction and degree of diasteroselection serves as the needed indirect mechanistic probe. In the event, geometric isomers of norgeranic acid were subjected to cyclization in formic acid. Subsequent saponification of the formate ester produced a single diastereomer in each case, indicating a stereospecific reaction that led the Zürich team to minimally propose the intermediacy of a nonclassical cyclic carbonium ion (Scheme 7), if not a concerted anti-addition of the elements of formic acid to the 1,5-diene passing through a single transition state.
Scheme 7.
Schinz-Eschenmoser evidence for anti-carbenium ion addition to an olefin ('nonclassical carbonium ion intermediate')
A corollary to this demonstration of stereospecific addition is that the stereochemical outcome of an addition to a 1,5-diene might also be used as an indication of the 6-membered ring transition state conformation (‘Scheme 2’in Figure 6). For example, concerted anti-addition to a 1,5-diene would lead to a trans-anti-trans tetrasubstituted cyclohexane via a chair transition state (VI→VIII, Figure 6). Addition to the same diene, but in a boat conformation, would result in a trans-syn-trans tetrasubstituted cyclohexane (VII→IX, Figure 6). This aspect placed the Zürich school in position to comment on the squalene precyclization conformation and suggest a relationship between the conformation of 1,5-diene segments and cyclization stereochemistry.
Figure 6.
Eschenmoser, Ruzicka, Jeger, and Arigoni hypothesis correlating diene conformation to cyclization diastereoselection, and its application to chair-chair-chair-boat (‘Scheme 4’) and chair-boat-chair-boat (‘Scheme 7’) squalene conformations in the biosynthesis of triterpenes. Reprinted with permission from Verlag Helvetica Chimica Acta AG.
2.3 1,5-Diene cationic cyclization: the Zürich school’s biosynthesis insights
Few will dispute the importance of Wallach’s isoprene rule (1887) and Ruzicka’s variation now known as the biogenetic isoprene rule.20 The biogenetic isoprene rule maintained a connection between isoprene and the cyclic terpenes via oxidative or protonative carbocyclization.46 In contrast to the isoprene rule which recognized the ability to represent terpenes as conglomerates of isoprene, the biogenetic isoprene rule provided a guide, perhaps a pseudo-mechanistic guide, to the process of constructing terpenes from isoprene. As testament to its importance, the application of the biogenetic isoprene rule led Ruzicka and his students to revise the structures of numerous terpenes whose proposed structures defied the rule.47 Similarly, to establish the intermediacy of squalene requires that 1) the isoprene fragments polymerize in linear fashion first to squalene, and 2) squalene cyclize through a series of regioselective cyclohexannulations. Prior to the 1950s, one could envision the construction of, for example, β-amyrin from the connection of pairwise isoprene couplings, or suggest arachidonic acid (vide supra) as an intermediate. These mutually exclusive biosynthesis possibilities illustrate the importance of considering and confirming the intermediacy of squalene in lanosterol synthesis.
Deconvolution of the stereochemical complexity of these terpenes has similar implications, and the Eschenmoser 1954 Helvetica paper made this suggestion in its concluding paragraph, notably in advance of their highly cited 1955 work: ‘the cyclization of aliphatic all-trans-isoprenologs would demand that the configuration of the polycyclic ring systems in corresponding cyclization products would have to be trans-anti-trans’48
The Zürich school’s 1955 contribution elaborated this concept in great detail. A key aspect of this proposal, one uniquely elaborated by the Zürich team, is the importance of squalene conformation (conserving the anti-addition noted also by Stork)40 to the stereochemical outcome of the cyclization.49 In order to rationalize the stereochemical dichotomy provided by the triterpenes (e.g. lupeol vs. lanosterol), the importance of squalene’s precyclization fold was delineated (Figure 6). Namely, a chair-chair-chair squalene conformation would lead to the lupeol configuration (‘Scheme 4’ in Figure 6), whereas a chair-boat-chair conformation would lead to lanosterol (‘Scheme 6’ in Figure 6). Moreover, this mechanistic hypothesis also suggested the hydride and methyl shifts necessary to connect the protosterol carbenium ion to lanosterol.
It is interesting to note that, not unlike many proposals that constitute major intellectual paradigm shifts, not all of the findings of the 1950s were immediately assimilated into the general texts of the time.50 Nevertheless, the concurrent studies of Barton, Stork, the Zurich school, Bloch-Woodward, and Cornforth-Popják during these years truly changed the way in which terpene studies, a topic studied for over half a century by that time, were executed. And the reader is reminded that the purification and analytical tools available at that time were a small fraction of the repertoire now available.
3. Enzymology: Squalene-Hopene Cyclase
3.1 Site-directed mutagenesis and crystallography
The first site-directed mutagenesis experiments conducted on squalene-hopene cyclase (SHC) were reported by Poralla in 1996.55 He examined the conserved aspartate-containing domain in SHC, specifically the sequence Asp-Asp-Thr-Ala, which is analogous to the Asp-Cys-Thr-Ala domain in oxidosqualene cyclase. Replacement of Asp376 with Glu significantly diminished the relative activity of the cyclase to just 10% of the wild-type, while replacement of Asp376 with Gln or Arg resulted in complete loss of all enzyme activity. Although replacement with Gly showed some activity, it was 0.1% that of wild-type. Furthermore, Asp377 was also exchanged with Glu, Gln, Gly, and Arg, dropping activities to less than 1% in all cases.
A three-dimensional picture of squalene-hopene cyclase emerged in 1997 with the first X-ray crystal structure of SHC refined to 2.9 Å resolution by Schulz56 and later refined to 2.0 Å resolution in 1999.57 Among the key active site residues the importance of Asp376, as defined by Poralla, was supported by its position near C3 of a hopene molecule modeled into the active site. On one side of the active site cavity are Asp374 and Asp377 which are important to catalysis and conserved throughout the cyclases. These residues are believed to bear a negative charge to balance the positive charge of Asp376 and His451. Moreover, the role of the protonated histidine is to activate the aspartic acid residue by increasing its acidity, and therefore the electrophilicity of the proton for C3 of squalene. The resulting positive charge at squalene C2 is in turn stabilized by the squalene 9,10-olefin that has already arranged conformationally for the cascade reaction.
Rohmer and Poralla also determined the importance of the His451 residue using site-directed mutagenesis in 1999.58 Replacement of His451 with Ala provided the same product pattern as the wild-type, but at a much slower rate. This presumably follows from the acid-strengthening effect of His451 on Asp376 but its otherwise innocuous steric influence. It is significant to note that His451 is not a conserved residue among SHCs, so complete inactivation was not expected by this mutation. It is often replaced by Arg in other cyclases and believed to function analogously.58
Hoshino used site-directed mutagenesis to clarify the existence of a secondary support mechanism that provides activation of residues along the front line. For example, substitution of Tyr495 by Phe resulted in complete loss of activity due simply to removal of the hydroxyl group. However, when Tyr495 is replaced with Ala, the mutant activity was attenuated by 52% relative to native enzyme, despite the lack of a hydrogen-bond donor.60 While reasoning for the latter remains unclear, the suggestion then followed that Tyr495 activates Asp376 prior to the initial protonation through hydrogen bonding between the phenolic hydroxyl group, a water molecule, and the aspartic acid residue.9,61
Schulz successfully cocrystallized squalene-hopene cyclase with the known inhibitor 2-azasqualene in the active site just last year (2004).62 This enzyme-substrate complex was consistent with their 1997 picture of the enzyme complexed to a modeled squalene. The nitrogen of 2-azasqualene, which corresponds to C2 of squalene, lies in close proximity to Asp376 which was hypothesized to initiate the carbocyclization by proton transfer. Figure 8 shows the X-ray structure obtained by Schulz centered on the more polar ceiling of the active site, with only the residues believed to be important in the intial protonation shown. Figure 7 also contains a chart correlating these residues to their function.
Figure 8.
X-Ray crystal structure of 2-azasqualene-bound SHC illustrating residues necessary for activation (protonation)
Figure 7.
Hoshino's mnemonic illustrating primary and secondary residues known to play a role in squalene-hopene cyclase (Reproduced by permission of The Royal Society of Chemistry)
Although the existence of discrete carbenium ion intermediates has yet to be unequivocally confirmed or eliminated from consideration, the large number of aromatic residues resident in the active site has led to the suggestion that they may stabilize any positive charge(s) that develops (Figure 9). Indeed the role of these aromatic residues was examined by Poralla58 as well as Hoshino.63 Phe365 is highly conserved among both prokaryotic and eukaryotic species of cyclase enzymes. When Phe365 is mutated to Ala, bicyclic products are obtained, presumably from loss of stabilization of the bicyclic carbocation. In order to test this hypothesis, Hoshino replaced Phe365 with the more electron rich Tyr and observed a 41-fold acceleration in rate compared to the wild-type.64
Figure 9.
X-Ray crystal structure of 2-azasqualene-bound SHC illustrating residues necessary for charge stabilization during polycyclization
Tyr609 is also positioned in the active site to stabilize the bicyclic carbenium ion. Mutagenesis experiments that replaced Tyr609 with Ala produced bicyclic compounds, suggesting that Tyr609 may in fact also serve to stabilize the bicyclic carbocation. However, mutation of Tyr609 with Ala only produced these bicyclic products in 50% yield, whereas Phe365 replacement by Ala formed them in 96% yield. Furthermore, replacement of Tyr609 with Phe does not stop the cascade process at the bicyclic carbocation. This suggests that the aromatic π-electrons, rather than the hydroxyl group, are necessary for stabilization of the bicyclic carbenium ion by Phe365.
Phe601 is also a highly conserved residue in both species of squalene cyclases (Figure 10). This residue is believed to stabilize the C18 carbenium ion that results from a 5-exo Markovnikov D-ring closure. This stabilization allows for ring expansion, followed by E-ring closure. Loss of this stabilization might lead to truncated polycycles that result from premature termination of the carbenium ion. Poralla58 and Hoshino65 replaced Phe601 by Ala, and the resulting product distribution was significantly different from that of the native enzyme. There was a significant increase in the formation of the 5-exo D-ring closure product (see 34 in Scheme 8), supporting the theory that the D-ring closure is initially a 5-exo process follwed by ring expansion and E-ring closure.
Figure 10.
X-Ray crystal structure of 2-azasqualene-bound SHC illustrating residues necessary for charge stabilization during polycyclization
Scheme 8.
Mechanism of polycyclization supported by AM1* calculations (Gao)
Phe605 is present in all prokaryotic squalene-hopene cyclases that form the pentacyclic hopene skeleton, but it is not conserved in lanosterol synthase in which a tetracyclic skeleton is formed. When Phe605 was mutated to Ala, the activity decreased by 67% relative to the native enzyme.66 However, when Phe605 was mutated to either of the more electron rich Tyr and Trp residues, the relative activity increased by 165% and 256%, respectively.63 This increase in rate was interpreted as Phe605’s facilitation of the 5-membered D-ring expansion to the 6-membered D-ring. Moreover, Phe605 may be involved in stabilization of the hopanyl cation prior to loss of proton and formation of the neutral product.
Recognition that the enzyme may play a critical role in the stabilization of an intermediate carbenium ion, perhaps to prevent an alternative reaction pathway short of the complete series of cyclizations, was made well before site-directed mutagenesis became a technical possibility. Based on his synthetic work in steroid total synthesis, Johnson advanced in 1987 the notion of point charge stabilization for the enzymatic cyclization of oxidosqualene.67 Johnson explained that as positive charge accumulates on each carbon during the cascade cyclization, there might be a proximal negative point charge that stabilizes the transition state (Figure 11).68 That ion pairs between substrate and enzyme might be an enzyme control element was well documented for lysozyme by Phillips where Asp52, a negative point charge, interacts with a carbenium ion intermediate.69
Figure 11.
Johnson's theory of point-charge stabilization by the oxidosqualene cyclase to control regio- and stereoselection
Johnson’s theory provides a stereoelectronic aspect to the polycyclization mechanism, and is therefore complementary to the picture of purely steric conformational control offered by the active site cavity. Moreover, the negative point charges can also be used to explain the closure of the B-ring in a boat conformation, as well as the anti-Markovnikov C-ring closure. The delivery of a point charge to the α-face at pro-C8 could lower the activation energy of the B-ring boat conformation relative to the chair alternative. Similarly, an additional point charge near pro-C13 (but not pro-C14) could direct the cyclization of the C-ring in the observed anti-Markovnikov manner. Johnson concludes with the possibility that the enzyme may not, in fact, provide a ‘steric relief’ of the cyclized product, but instead only “provide an electronic environment which guides and sustains processes that are already inherent in it, i.e., the Stork-Eschenmoser principle.”9
3.2 Molecular modeling
The question of concertedness (versus stepwise via carbenium ion intermediates) in olefin electrophilic polycyclizations was discussed in the experimental beginnings in the work of Stork (vide supra) who acknowledged the possibility of mixed single-transition state polycyclizations separated by discrete carbenium ion intermediates. Similarly, the Zürich school focused much of their interpretation upon a ‘nonclassical’ carbenium ion. Indeed, these synthetic studies revealed the spectrum of possibilities throughout the 1950, and similar investigational lines offer the ability to identify products accessible only through carbenium ion intermediates.70 Site-directed mutagenesis can bridge these studies to the enzyme catalyzed cyclization environment. And X-ray crystallography, when available, can quantitatively determine proximity of enzyme residues to specific positions within substrate mimics in their ground state. Advances in computational power have been combined with theory in recent years to address the mechanism of the enzyme catalyzed polycyclization as well. 71,72,73,74,75
Gao recently used a combined QM/MM approach to model the squalene cyclization cascade.76 His method used AM1 parameters, but included force fields appropriate for enzyme and water. Gao proposed a mechanism whereby 3 is converted to the monocyclic intermediate 30 (Scheme 8). Cyclization of the B-ring then provides carbocation 31. If 31 were to form the five-membered C-ring product 32 (Markovnikov addition), it could equilibrate back to 31 before eventually forming intermediates 33 or 34. Once 33 is formed, hopene (80%) and diplopterol (20%) are produced. Gao noted that a highly concerted polycyclization is normally concluded from site-directed mutagenesis studies, but he defends his findings by noting that the formation of new byproducts from mutation experiments does not necessarily indicate the presence of the corresponding cationic intermediate. Rather, mutagenesis lends more credibility to an individual residue’s role in controlling both the regio- and stereochemistry of the cyclized products.
The work of Hess supports the concerted formation of the first two rings (A–B) from squalene using a more sophisticated gas-phase DFT calculation, albeit with only a fragment of squalene.77 Notably, Hess’ calculations appear to be in agreement with Schulz’ high resolution crystal structure of 2-azasqualene bound to squalene-hopene cyclase. A comparison of the experimental conformation derived from Hess’ DFT calculations revealed remarkably similar bond lengths (Figure 12).
Figure 12.
Comparison of Schulz (X-ray) and Hess (calculation) distances for AB-ring formation from squalene. Reprinted with permission from ACS publications.
4. Biomimetic Total Synthesis Based on Squalene Cyclase
The earliest work in the area of terpene biomimetic synthesis dates to the early part of the twentieth century, and was highlighted in a previous section. While these Lewis and Brønsted acid-promoted olefin cyclizations made advances to control the number of olefin cyclizations and their attendant diastereoselectivity, no basis for enantioselection in vitro evolved until Yamamoto’s recent development of Lewis acid-assisted chiral Brønsted acids (LBA’s). To be successful, a catalyst must replicate the enzyme’s ability to transfer a proton from an active site residue to the 2-alkene, as well as control the addition of the proton to a single face of the prochiral π-bond. As described above, an enzyme clearly benefits from its size and evolutionary complexity to arrive at a proton that is sufficiently acidic and stereoselectively encased with the large squalene substrate in a chiral peptidic cavity.
4.1 (±)-Sophoradiol
Johnson provided a comprehensive picture of his contributions in monograph form,78 but it is important to provide here a short summary of his achievements. Johnson developed initiators for the cyclization (most notably acetals), terminators for the cyclization (alkynes and allyl silanes), and cation-stabilizing groups (fluorine).79 He was the first to report a nonenzymatic, biomimetic polyene pentacyclization (Scheme 9).80 Using fluorine as a cation-stabilizing group, the cyclization of five rings was effected in one chemical transformation. The Lewis acid SnCl4 was effective but resulted in an undesired dehydrofluorination in moderate yield. Notwithstanding, trifluoroacetic acid afforded the pentacyclic product (37) in 31% yield without elimination of HF. This pentacyclic product (37) was converted to (±)-sophoradiol (40) in 3 steps. When olefin 36 was used, the experimental procedures required to unmask the C3 and C22 hydroxyl groups were unsuccessful.
Scheme 9.
Johnson's synthesis of rac-sophoradiol using a biomimetic pentacarbocyclization
4.2 Progesterone Congeners
Johnson was also interested in the total synthesis of these steroidal natural products in chiral nonracemic form. He developed two techniques which led to enantioenrichment during the polyene cyclization. First, he discovered that a chiral center along the polyolefin backbone, specifically at pro-C11, led diastereoselectively to the tetracyclic product. This was applied to the total synthesis of 11α-hydroxyprogesterone (44),81 which is the key intermediate in the commercial production of hydrocortisone acetate (Scheme 10). The final product was produced in a 92:8 ratio of 44 to its C11 diastereomer.
Scheme 10.
Johnson's biomimetic polyolefin electrophilic cyclization towards 11α-hydroxyprogesterone
Johnson also developed the use of chiral acetals as initiators for the polyene cyclization reaction. Starting with optically active acetal 45, low temperature cyclization gave tetracyclic products in over 70% yield with 46a as the major component (38%) (Scheme 11).82 Conversion to d-4β-hydroxyandrostan-17-one (47) was accomplished in 21% overall yield from tetracyclic ether 46a.
Scheme 11.
Johnson's diastereoselective biomimetic polyolefin electrophilic cyclization towards d-4β-hydroxyandrostan-17-one
4.3 (−)-Ambrox
In 1999, Yamamoto developed the first enantioselective biomimetic cyclization of a polyprenoid using a Lewis acid-assisted chiral Brønsted acid (LBA, Figure 13) as an artificial cyclase and used it to synthesize (−)-ambrox (51).83 Ambrox is used as a commericial substitute for ambergris, and is found in such fragrances as Givenchy’s Extravagance d’Amarige, due to its unique olfactory and fixative properties.84 The cyclization of homofarnesol (50) promoted by LBA 48 proceeded with 42% ee (Scheme 12, eq 1). This enantioselective cyclization was further improved in 2002 using LBA 49 as the promoter.85 The ether (−)-51 was obtained in 54% yield with 75% ee and 76% dr from 52 utilizing an enantioselective cyclization, silylation, and diastereoselective cyclization sequence. It should be noted that the first step of this sequence is a complex mixture, and that best results were achieved by use of an achiral Lewis acid in the subsequent silyl ether cyclization.
Figure 13.
Yamamoto's LBA catalysts
Scheme 12.
Yamamoto's enantioselective olefin protonation-initiated polycyclization
Yamamoto applied LBA 49 to the enantioselective cyclization of polyprenoids in which the terminating group is an aromatic ring instead of the more nucleophilic hydroxyl terminator nascent to homofarnesol. LBA 49 promotes the cyclization of 53 to a mixture of 54 and 55 (Scheme 13). The mixture was then treated with an achiral Lewis acid (boron trifluoride etherate) in order to complete the cyclization to tricycle 54. The first two rings are formed in approximately 78% ee. Since (±)-54 had previously been reported by King86 and Ghatak en route to the total synthesis of ferruginol (56),87 this provides the opportunity for the synthesis of enantioenriched ferruginol.
Scheme 13.
Yamamoto's enantioselective synthesis of (5S,10S)-54
Although not all LBA catalyzed total syntheses can be listed here, 88,89,90,91 perhaps Yamamoto’s most impressive enantioselective cyclization to date was on substrate 57 (Scheme 14).85 Again, he utilized his two-step procedure of initial enantioselective formation of the A ring, followed by diastereoselective formation of the B ring. This sequence provided product 61 in a remarkable 89% yield and 75% ee. This compound is easily converted to 62 which is an intermediate in the total syntheses of isophyllocladene, phyllocladene, hibaone, manool, sclareol, manoyl oxide, isoabienol, trans-abienol, and anticopelic acid.92
Scheme 14.
Yamamoto's enantioselective synthesis of (5S,10S)-61
5. Epoxysqualene Cyclase
The polycyclization of epoxysqualene to its terpene natural products would, at first sight, appear a direct parallel to squalene-derived natural products. However, there are key differences in both enzyme structures and their distribution of products. The reactions of squalene cyclases are both enantioselective and diastereoselective, whereas epoxysqualene is formed first from squalene in an enantioselective epoxidation by squalene oxidase prior to its processing by an epoxysqualene cyclase.15 And in the polycyclization, epoxysqualene cyclase enforces a chair-boat-chair conformation in many cyclases, instead of the chair-chair-chair conformation enforced by squalene-hopene cyclase.93 The result is both an enzyme-based increase of product diversity, as well as a challenge currently unmet by total synthesis.
Exceptions to the enzymatic cyclizations of epoxysqualene, which instead proceed through chair-chair-chair arrangements, are found in certain plants.8 Additionally, the C15 methyl of epoxysqualene typically migrates to C14 via a 1,2-methyl shift in the biosynthesis. The reader should therefore be aware that all ‘biomimetic’ polycyclizations from van Tamelen and Johnson to Corey and Overman use a nonnatural epoxysqualene-like substrate bearing a C14 methyl. This tactic favors a chair-chair-chair precyclization conformation, encourages C-ring cyclohexane formation instead of cyclopentane formation, and removes the need to mimic the 1,2-methyl shift in the biosynthesis pathway. Hence, there remains a subtle yet significant disconnect between biosynthesis and biomimetic total synthesis in this area that highlights at present the importance of the peptide superstructure in product stereocontrol.
5.1 Site-Directed Mutagenesis
The high resolution crystallographic data obtained for squalene-hopene cyclase both validated the mutagenesis studies it followed and stimulated further development of models to explain its reactivity and selectivity. However, the long absence of a crystal structure for oxidosqualene cyclase highlighted the importance of mutagenesis studies to the understanding of mechanism for cyclizations of oxidosqualene. Moreover, Corey cast doubt on the prospects for obtaining a crystal structure as late as 1997: “given the hydrophobic character, water insolubility, and instability of the enzyme, the prospects of such a determination in the foreseeable future seem questionable.”94 The product distribution resulting from oxidosqualene cyclases led to a hypothetical active site picture manipulated using site-directed mutagenesis. Only recently has a crystal structure of OSC emerged (vide infra).
In 1997, Corey turned to oxidosqualene cyclase to determine the residues essential to catalytic activity.95 From this work, he developed a hypothesis for how lanosterol cyclase interacted with its substrate, oxidosqualene (Figure 14).94 Using site-directed mutagenesis, Corey successfully identified four residues believed to be essential for catalytic activity. The hydrogen-bond donor that initiates the cascade cyclization, analogous to squalene-hopene cyclase Asp376, was identified as Asp456 in lanosterol cyclase. This residue is activated by a proximal protonated histidine (His146) via a hydrogen-bond to the aspartate, thereby increasing its effective acidity. It was suggested that His146 could also receive the proton in the elimination step to complete the transformation and catalytic cycle. In support of these hypotheses, His146 was mutated to Lys or Arg without loss of activity. However substitution of His146 for Ala results in an inactive mutant.
Figure 14.
Corey's working hypothesis for the enzymatic π-cation cyclization of oxidosqualene to lanosterol
The fourth and final key residue, His234, is believed to be in proximity to the substrate and may stabilize a carbocation intermediate. His234 can be replaced by Phe without loss of activity, but not by Ala, Lys, or Arg. This is consistent with its proposed role of cation stabilization.
Regardless of the nature of subsequent ring-forming steps, it is believed that the epoxide opening to form the A-ring from oxidosqualene is concerted. This was examined by Corey in 199896 using substrate analogs such as 63 and 67 to demonstrate that the initial activation and substrate conformation significantly impacted the rate of cyclization (Scheme 15). The nucleophilic 6,7-olefin enhances the rate of epoxide opening 15-fold relative to the saturated counterpart. The cyclase cavity could increase the population of the chair conformation in which the trisubstituted olefin π-bond donates into the epoxide C-O σ*-orbital, thereby enabling a less Lewis acidic proton (Asp456) to initiate the cyclization. Although the mutagenesis experiments are consistent with a Lewis acidic hydrogen bond on the His146 residue, sequence alignment with squalene-hopene cyclase suggests that His146 is actually in the second domain of the enzyme, and therefore distant from Asp456. Furthermore, in squalene-hopene cyclase Asp374 and Asp377 are believed to balance the positively charged His451 and Asp376 pair. This pair of residues is not conserved in the oxidosqualene cyclases. The Asp456 residue is therefore not likely to be activated in the same way as the corresponding Asp376 residue in squalene-hopene cyclase. The additional activation may not be necessary due to the greater nucleophilicity of the epoxide relative to the 2,3-alkene of squalene. However, any hydrogen bonds that are formed with Asp456 could increase the reaction rate.
Scheme 15.
Models for oxidosqualene cyclase: relative rates for epoxide ring opening
As previously mentioned, oxidosqualene is believed to be arranged by the enzyme active site into a chair-boat-chair conformation.93 B-Ring formation is believed to be concerted with that of the A-ring, as the latter builds positive charge on C6. Analogous to D-ring formation in squalene-hopene cyclase, C-ring formation in oxidosqualene cyclase is believed to be a 5-exo Markovnikov process. Formation of 6-6-5 tricycle 73 from substrate analog 71 supports this theory, thereby necessitating a ring expansion step at some point subsequent to cyclopentannulation (Chart 1).97 It should be noted that 6-6-5 tricycle was formed in only 3% yield whereas the expected product (72) was isolated in 40% yield. Further evidence suggesting a 5-exo C-ring closure can be found in several additional substrate analogs which have been shown to produce 6-6-5 tricyclic products when submitted to oxidosqualene cyclases (Scheme 16).98,99,100,101
Chart 1.
Substrate analogue 20-oxaoxidosqualene and products formed upon cyclization by oxidosqualene cyclase
Scheme 16.
Product formation from oxidosqualene cyclases using nonnatural substrates
Albeit circumstantial as it relates to the cyclization of (natural) oxidosqualene, the argument has been advanced that it is unlikely that each of these diverse substrate analogs follows a cyclization pathway different from that of the natural substrate.9
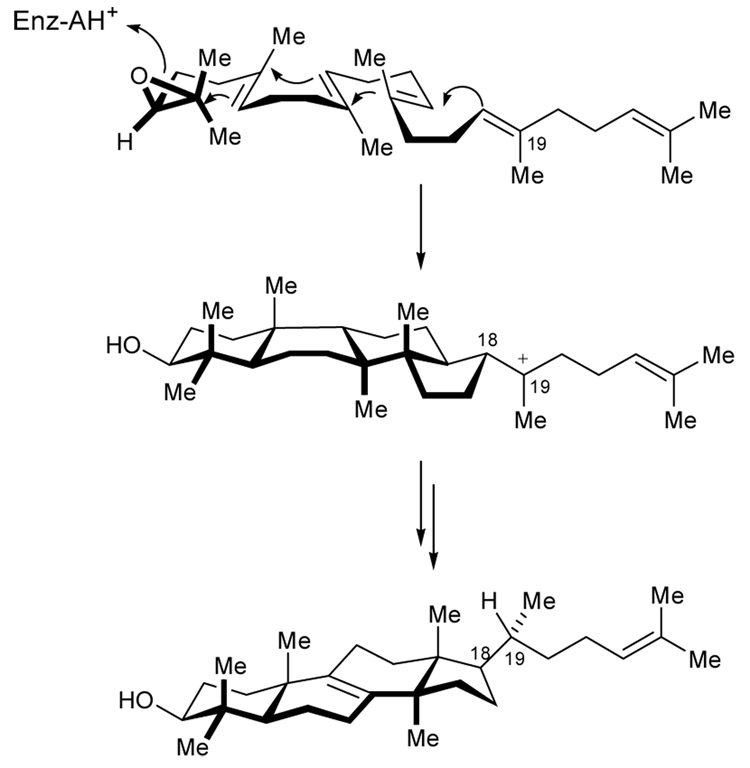
A caveat to the Stork-Eschenmoser hypothesis was Eschenmoser’s prediction that the C18 configuration of lanosterol was α (Scheme 17).24 Corey has since proposed that isolation of 73 from enzyme incubation of 71, suggests a precyclization conformation in which the C18 chain is pseudoaxial, leading to the observed β-configuration (Chart 1).102,103 Cornforth’s rationalization of a carbenium ion conformational change to explain C20-R configuration is therefore no longer necessary.104
Scheme 17.
Eschenmoser's rationale to predict α-C18 configuration
Although methyl and hydride shifts are not observed in the cyclization from squalene to hopene, formation of the protosterol cation by oxidosqualene cyclase is followed by a series of hydride and methyl group migrations that occur to form lanosterol (Scheme 18).61 These migrations are believed to occur with minimal assistance from the natural enzyme. Support for this hypothesis can be found in the 1966 synthetic work of van Tamelen, who showed that cyclization of 5 followed by rearrangement to 86 could be accomplished using a simple (nonenzymatic) Lewis acid (Scheme 19).105 The enzyme is believed to simply protect the intermediates in the cascade to lanosterol from premature quenching, perhaps by proton loss or nucleophilic attack by water.
Scheme 18.
Lanosterol biosynthesis: oxidosqualene cyclization and group migrations
Scheme 19.
van Tamelen's epoxysqualene tricyclization/migration sequence catalyzed by SnCl4
In 2004, the structure of human oxidosqualene cyclase was solved by X-ray diffraction of crystals containing lanosterol. This three dimensional picture compliments those of SHC solved in 1997, 1999, and 2004. Moreover, it provides a timely ruling to some conclusions drawn from mutagenesis studies of the past decade.
Among the SHCs (7) and OSCs (30) cloned and sequenced as of 2001, there is 30% homology in the primary structure. In order for OSC to operate it must accommodate several functional differences: 1) epoxide activation, 2) B-ring boat conformation, 3) control of D-ring size, and 4) migrations to the final product. The Hoffmann-LaRoche team successfully cocrystallized lanosterol (2.1 Å resolution) with human OSC to provide such mechanistic insight. Figure 15 reveals the active site interactions between the oxidosqualene cyclase and its product. A hydrogen bond between the lanosterol 3-hydroxy and Asp455 is the “smoking gun” to mutagenesis studies by Corey that assigned this role to the corresponding aspartic acid residue (Asp456) in S. cerevisiae oxidosqualene cyclase (scOSC). A second key difference is the assignment of Cys456 and Cys533 as hydrogen bond donors that increase the acidity of Asp455.
Figure 15.
X-Ray crystal structure of lanosterol-bound OSC illustrating residues necessary for activation (protonation)
Stabilization of the chair-boat-chair conformation of squalene, as well as the positive charge generated at the rehybridizing carbons during cyclization is achieved by residues Phe444, Tyr503, and Trp581 (positioned near C6 and C10), and Tyr98 (forces C10 methyl downward to encourage B-ring boat conformation) (Figure 16). The comparative SHC/ OSC sequences also that the chair conformation involving the C10 olefin is disfavored in OSC by an additional residue on this face of the folded squalene. Similarly, the boat conformation involving the C10 olefin is disfavored in SHC by an additional residue on this face of squalene en route to hopene in SHC.
Figure 16.
X-Ray crystal structure of lanosterol-bound OSC illustrating residues necessary for charge stabilization during polycyclization
Stabilization of the C14 cation is achieved by the closely positioned His232 and Phe696 (Figure 17). Another fundamental difference between OSC and SHC is the lack of aromatic Trp169 in the former to stabilize the secondary carbenium ion necessary for hopene formation.
Figure 17.
X-Ray crystal structure of lanosterol-bound OSC illustrating residues necessary for charge stabilization during polycyclization
The HLR team also explains recourse of the protosterol ion to rearrangement and facial proton loss by a higher p-electron density near C8/9 (seven aromatic residues). Moreover, water is usable to access these intermediate carbenium ions due to the hydrophobic nature of the active site. His 232 is appropriately positioned for deprotonation leading to the olefin in lanosterol, but Tyr503 was suggested to play a role as well through its hydrogen bond to His 232.
5.2 Antibody Catalyzed Cationic Cyclizations
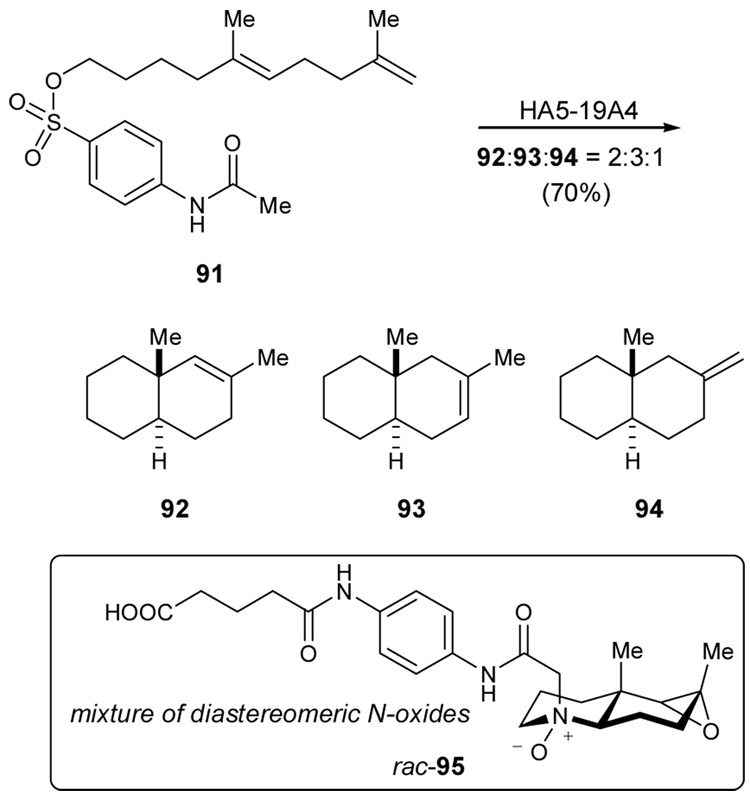
Enzymes and antibodies share the common amino acid building block, and so it is pertinent to wonder whether the overall structural differences might be overcome to find common function. This question has been addressed in the context of oxidosqualene cyclase activity. In 1994, Janda and coworkers elicited an antibody to the charged transition state analog, N-oxide 90, and demonstrated antibody catalysis for the cationic cyclization from 87 to 88 (Scheme 20).106 Selectivity for the vic-silyl alcohol formed by the addition of water to the olefin may indicate a high degree of concertedness across the olefinic bond, reminiscent of the Schinz-Eschenmoser 1,5-diene cyclizations in formic acid. This approach was later extended to the formation of decalin ring systems analogous to the AB ring systems of steroidal natural products.107 Hapten N-oxide 96 was used as a mixture of four stereoisomers – racemic mixtures of diastereomeric N-oxides during the immunization. This procedure led to isolation of an antibody that effected the cyclization of sulfonate 91 to the double carbocyclization products 92–94 with an average 53% enantiomeric excess (Scheme 21). Among these, 94 was formed in 80% ee.
Scheme 20.
Janda's antibody catalyzed cyclization carbocyclization using a sulfonate initiator
Scheme 21.
Janda's antibody catalyzed cyclization of substrate 91 to afford decalin products
In their march toward a catalytic antibody surrogate for oxidosqualene cyclase, Janda and coworkers recently reported their studies involving epoxysqualene substrate mimics such as 96. This represents the first demonstration that an epoxide could be used in combination with the catalytic antibody to trigger a cationic cyclization. N-Oxide hapten 98 was synthesized from lithocholic acid and used to elicit antibody HA8-25A10. In the event, epoxypolyene 96 was cyclized to a mixture of cyclohexenes in 92% yield (Scheme 22). Although accurate determination of the product enantiomeric excess was not possible, analysis of the substrate at 50% conversion indicated an 80% ee.108 Of significance is the failure of the antibody to produce polycyclic products. The authors speculate that the antibody merely lacks the proper peptide cavity for the additional cyclizations, and that epoxysqualene cyclase functions with this distinction. What cannot be excluded is a mechanism in which the substrate binds to the surface of the antibody, and the antibody effects an enantioselective Lewis acid-catalyzed epoxide ring opening. It is reasonable to expect that the field of catalytic antibodies will provide a deeper understanding of squalene/epoxysqualene cyclizations in due course.
Scheme 22.
Cyclization of an oxidosqualene-like substrate by an antibody elicited to a steroidal hapten
6. Biomimetic Total Synthesis Based on Epoxysqualenecyclase
The conversion of 2,3-oxidosqualene to lanosterol is a quintessential cascade reaction in that an epoxide with one stereogenic center is transformed to a tetracyclic steroid containing seven stereogenic centers. Although a great deal has been learned about the enzymatic transformation, the degree of enzymatic assistance remains difficult to determine.109 Through the synthetic work of pioneers that include van Tamelen and Johnson, demonstrations of nonenzymatic, biomimetic total syntheses based on polycarbocyclizations have come into existence. The mere success of these transformations implies that the enzyme’s role is not ‘all important’.
In 1961, van Tamelen used the term biomimetic, or biogenetic-type, as “an organic synthesis designed to follow, in at least its major aspects, biosynthetic pathways proved, or presumed, to be used in the natural construction of the end product.”12 Early work in the epoxysqualene biomimetic carbocyclization area proved difficult in part due to the necessary formation of a 6-membered C-ring closure over a favored 5-exo Markovnikov addition.110 In 1982, van Tamelen utilized an alkyne as the terminating group in his cascade cyclization (99), which allowed for formation of both the 6-membered C-ring and the desired 5-membered D-ring (100) (Scheme 19).111
6.1 Dammarenediol
(S)-2,3-Oxidosqualene is cyclized in some plants to primarily dammarenediol (108). The first total synthesis of this tetracycle using a polyolefin cascade cyclization designed to mirror the biosynthetic transformation was reported by Corey in 1996.112 In contrast to Johnson’s use of fluorine to control the regioselection of C-ring formation (to cyclohexane instead of cyclopentane), a strategy later used in his own synthesis of dammarenediol,113 Corey’s strategy used an enolsilane to direct C-ring formation during a biomimetic tricyclization (Scheme 24). Epoxide 101 was prepared using a regioselective, enantioselective dihydroxylation. Homologation with the silyl imine 102 provided an acylsilane that was olefinated using an addition/Brook rearrangement/elimination sequence to arrive at the key polyolefin 104 in 60% yield.
Scheme 24.
Corey's total synthesis of dammarenediol
Treatment of the epoxide with methyl aluminum dichloride initiated the tricyclization, and after desilylation and thioacetal hydrolysis, diketone 105 could be retrieved in 42% yield. The cascade cyclization proceeds through a chair-chair-chair transition state. The reader should be reminded that plant oxidosqualene cyclases, which are responsible for dammarenediol biosynthesis, are the exceptions rather than the rule in that they favor a chair-chair-chair conformation during the cyclization of oxidosqualene. Aldol condensation, stereoselective enone reduction, and homologation at C17 led to the penultimate product, which furnished the desired dammarenediol in enantioenriched form after carbamate deprotection.
6.2 Scalarenedial
Scalarendial is an unusual squalene-derived natural product in that the relative syn stereochemistry of the C10 and C14 angular methyls does not follow from any precedent in either squalene or epoxysqualene cyclase. This discrepancy notwithstanding, Corey’s strategy to deliver the backbone from a C14-methyl epoxysqualene substrate is quite effective. The development of highly enantioselective olefin dihydroxylations again provided the launch point for Corey’s total synthesis based on Lewis acid-induced epoxide ring-opening/cation cyclization analogous to his strategy for dammarendiol synthesis.114 The polyene 111 in which an acceptor epoxide and donor enolsilane were linked via a triene served as the key intermediate (Scheme 25). Enantioselective dihydroxylation of geranylgeranyl acetate enables synthesis of the epoxide in 95% ee after monomesylation and elimination with base. Conversion of the acetate to bromide 109 and treatment with iminyl silane 102 provided the homologated acylsilane after hydrolysis. The acylsilane functionality serves as the lynchpin to a final coupling that provides an enol ether. Specifically, a sulfone is deprotonated (nBuLi) and added in 1,2-fashion to the acylsilane carbonyl. The intermediate α-silyl alkoxide undergoes Brook rearrangement, followed by elimination of benzene sulfinate.
Scheme 25.
Corey's total synthesis of scalarenedial
The polycyclization is initiated by the action of stoichiometric, Lewis acidic MeAlCl2 on the trisubstituted epoxide 111 (CH2Cl2, −94 °C, 30 minutes). There is at least partial silicon transfer to the aluminum alkoxide, necessitating treatment with aqueous hydrogen fluoride in acetonitrile. Additionally, the D-ring silylmethylene substituent is present as a mixture of epimers and required equilibration with basic methanol to converge to the equatorial diastereomer. Hence, a single tetracycle is formed with a (apparent) high degree of diastereoselection, save the D-ring substituent configuration. The relative configuration dictates a chair-chair-chair-chair transition state, and again, the importance of using a C14 methyl instead of C15 as in epoxysqualene should be noted. Although the overall yield might be critically assessed as low, the sophistication of this cascade tempts the calculation of yield enumerated by rings formed, leading to an average efficiency of 75% yield per ring.
As mentioned above, the target’s direct origin from squalene necessitated removal of the A-ring hydroxyl resulting from the enantiocontrol (Sharpless dihydroxylation) and diastereocontrol (polyolefin cyclization) strategies. The Barton-McCombie reductive protocol was followed by enol triflate formation and oxidative silicon-carbon bond cleavage prior to palladium-mediated carbonylation to the γ-lactone. Reduction of the lactone to the diol was followed by oxidation to the dialdehyde natural product target 117.
6.3 Sedimentary Polyprenoids
Corey’s approach to hexacyclic triterpene 123 and tetracyclic diterpene 129 again employed a diastereoselective epoxide ring-opening polycyclization terminated by an enolsilane.115 A one step sulfone coupling to acylsilane 103 produces enolsilane 119 in 91% yield (Scheme 26). Exposure of this epoxide to MeAlCl2 at −94 °C produced an enolsilane that was deprotected and epimerized with base to give 120 in 32% yield overall.
Scheme 26.
Corey's total synthesis of hexacyclic sedimentary triterpene 123
Similarly, epoxyketone 124 was elaborated to epoxide 125 for treatment with an identical series of reagents as for 119 (Scheme 27). This biscyclization was considerably more efficient at 84% yield, and the hydroxy remnant from the cyclizations was then conveniently removed using the Barton-McCombie protocol. Curiously, the third and final ring was formed by Friedel-Crafts acylation in 85% yield.
Scheme 27.
Corey's total synthesis of tetracyclic sedimentary diterpene 129
6.4 Adociasulfates
The adociasulfates are a family of hexaprenoid hydroquinone sulfates isolated from the sea sponge Haliclona (a.k.a. Adocia) that exhibit micromolar inhibition of motor proteins in the kinesin super family of proteins. Adociasulfates 1 and 7 are also proton pump inhibitors. Given the sterol substructure and the target’s stereochemical complexity, Overman utilized an epoxysqualene-type tetracyclization of arene 140.
Synthesis of the key geranylgeraniol intermediate 140 commenced from bromoarene 131 (Scheme 28). Coupling of two geranylgeranyl subunits using a combination of cuprate reagents and sulfone alkylation/reduction provided 138 in concise fashion. Sharpless epoxidation then provided the epoxide necessary for tetracyclization attempts. In contrast to Corey’s polycyclizations, the terminating group is a more electron rich trialkoxybenzene ring. Both BF3·OEt2 (−50 °C) and FeCl3·6H2O (23 °C) were reported to give desired pentacycle 141 in 10% yield, whereas MeAlCl2 proved to be less effective. Scandium triflate (0 °C) emerged as the most effective Lewis acid, providing 141 in 15% yield. Thirteen additional steps were required to complete the total synthesis.
Scheme 28.
Overman's total synthesis of the adociasulfate from an epoxysqualene-like polycyclization
7. Cyclizations Based on Radical Transformations
Breslow speculated in 1962 that the biosynthesis of sterols from squalene might involve an oxidative free radical pathway.116 Although squalene would not cyclize in the presence of hydroxyl radical, Breslow maintained that the enzyme may exert conformational control to promote the cyclization. Epoxysqualene was ultimately hypothesized117,118 and confirmed as the biosynthetic intermediate, but the radical pathway has nevertheless been exploited successfully in several polycarbocyclizations. These demonstrations have established that carbenium ions are not necessary intermediates for diastereoselective carbocyclizations in the stereocontrolled formation of terpene frameworks. Indeed, Breslow stated that his 1968 model study was “of limited biochemical interest”,119 but it became the foundation for the remarkable transformations we describe here.
 |
(7) |
Breslow used a CuCl-catalyzed thermal decomposition to benzoyloxy radicals, with cupric benzoate added as the terminator. This protocol afforded the cyclized product in 20–30% yield (eq 7). The relative stereochemistry of 152 reflects that of α-onocerin (153), a product of the enzymatic cyclization of squalene.
Julia’s 1973 tricyclization provided a significant advance in which an aromatic D-ring could be formulated.120 And in 1990, Snider developed manganese(III)-based oxidative free-radical cyclizations, again in the presence of copper(II).121 This work led to the formation of a wide variety of fused ring systems. Soon thereafter, Snider extended this methodology to include 1,3-dicarbonyl compounds.122 In 1991, Zoretic was able to transform tetraene 154 to tetracycle 155 in 31% yield using the Mn(III)/Cu(II) combination (eq 8);123 seven stereogenic centers are formed in an apparent diastereoselective reaction.
 |
(8) |
That this homolytic polycyclization is not limited to radical cation intermediates was demonstrated later by Pattenden who used an acyl radical generated from an acyl selenide and Bu3SnH-AIBN to effect a tetracyclization (eq 9).124 This transformation creates six new stereogenic centers with a high level of diastereselection.
 |
(9) |
This strategy was more recently elaborated to a short biomimetic synthesis of a steroidal skelton.126 (−)-Menthone was used as a remote chiral auxiliary on a terminus of the polyalkene (158). Photoinduced electron transfer then initiated the polycyclization by formation of radical cation 161. From the ensuing cascade, eight stereogenic centers were created to form only two of the greater than 200 possible isomers (Scheme 29).
Scheme 29.
Demuth's formation of a steroid skeleton using a diastereoselective PET strategy
This resulting ketal is formed in 10% yield as a 7:1 diastereomeric ratio. Separation of the diastereomers and removal of the chiral auxiliary afforded ester 160 in >99% ee, showcasing a remarkable example of remote asymmetric induction. It is significant to note that the C3 hydroxy results from water in this approach, thereby providing a possible alternative biosynthetic pathway to produce epoxysqualene-derived steroids under nonoxidative conditions.127
8. Summary and Outlook
Insofar as many total syntheses based on biomimetic strategies have indeed been successful, it is important to still measure the value of mimicking biology in a case-by-case manner. And when reflecting on the success of a biomimetic strategy in total synthesis, irrational exuberance must remain in check as van Tamelen12 stated:
It seems hardly necessary to add that the success of a ‘biogenetic-type’synthesis by itself does not constitute evidence for the operation of a particular chemical step in nature (although in a remarkable case, the temptation to draw such a conclusion will be great.)
This review has identified benchmarks in the evolution of terpene biosynthesis and total synthesis. In all cases, biosynthesis inspired a key polycyclization that generated substantial complexity in a single transformation. Similarly, synthetic studies of the polycyclization led to discovery of the need to stabilize developing charge at specific carbons in the squalene backbone during cyclization. This same need was discovered much later during mutagenesis studies, indication that the cyclase had long used this tactic. This is exactly how organic synthesis and biosynthetic studies are synergistic as each evolves in parallel with the other (Figure 18). The complexity of the protein environment, in conjunction with levels of selectivity that were considered (not long ago) unattainable with small molecule catalysts tempt one to believe that selectivity and catalyst complexity are directly related.128 For example, squalene-hopene cyclase requires a set of primary and secondary residues to both activate and orient squalene for cyclization. However, the Yamamoto catalysts clearly lack the functional complexity, much less the steric size, but are still able to furnish polycyclic products with high selectivity. Only further work in biomimetic catalyst development will determine whether both selectivity and efficiency can be controlled simultaneously to approximate that of an enzyme catalyst. Similarly, total synthesis stands to benefit immensely from biosynthetic thinking since it often provides a basis, however tenuous and circumstantial, for taking strategic risk that promises tremendous benefit if successful.
Figure 18.
A few of the landmarks in the stories of terpene biosynthesis and total synthesis
Supplementary Material
Scheme 23.
van Tamelen's tetracyclization favoring 6- and 5-membered C- and D-rings
Acknowledgments
Our research programs in total synthesis and enantioselective catalysis are supported through the generosity of the National Institutes of Health (GM), the National Science Foundation (Division of Chemistry), and Eli Lilly, Boehringer-Ingelheim, Yamanouchi, Astellas, Amgen, and Procter & Gamble. R. A. Y. is grateful for a McCormick Science grant and ACS Division of Organic Chemistry Graduate Fellowship (2005–6). We thank Julie Pigza and Benjamin Nugent for their assistance with the preparation of this manuscript. Finally, the section concerning the ‘Stork-Eschenmoser’ hypothesis was developed in consultation with these individuals (GS, AE), as well as with translations of references 33 and 45 from AE. The first draft of the latter was done by Lucy Stark, and initiated by Prof. Eric Sorensen. We extend our sincere appreciation for their priceless insight and time.
Biographies
Professor Johnston graduated summa cum laude from Xavier University in 1992 with a B.S. Chemistry degree. His graduate work with Leo A. Paquette at The Ohio State University led to a Ph.D. in 1997. Following his work as an NIH Postdoctoral Fellow with David A. Evans at Harvard University, he began his independent career in 1999 at Indiana University where he is presently Associate Professor of Chemistry. His research interests include the development of new reactions and reagents, including nonconventional free radical-mediated addition reactions to azomethines, chiral protic acid catalysis, and the total synthesis of alkaloid natural products. His research program has been recognized by several young investigator awards, including those of Boehringer-Ingelheim and Amgen, the Eli Lilly Grantee Award, the Astellas USA Foundation, and an IU Outstanding Junior Faculty Award.
Ryan Yoder graduated from Indiana University in 2002 with a B.S. Chemistry degree (Honors). He then joined the Johnston research program as a graduate student, and began work that ultimately resulted in the first definitive example of chiral proton catalysis. His interests include the development of a mechanistic understanding of these reagents, and their application to natural product synthesis. He was recognized in 2004 with a McCormick Science Grant and in 2005 with an ACS Division of Organic Chemistry Fellowship.
References
- 1.Li T, Janda KD, Ashley JA, Lerner RA. Science. 1994;264:1289. doi: 10.1126/science.8191282. [DOI] [PubMed] [Google Scholar]; (b) Zhu X, Tanaka F, Hu Y, Heine A, Fuller R, Zhong G, Olson AJ, Lerner RA, Barbas CF, III, Wilson IA. J. Mol. Biol. 2004;343:1269. doi: 10.1016/j.jmb.2004.08.102. [DOI] [PubMed] [Google Scholar]
- 2.(a) Robinson R. J. Chem. Soc. 1917;111:762. [Google Scholar]; (b) Robinson R. J. Chem. Soc. 1917;111:876. [Google Scholar]
- 3.Sorensen EJ. Biorg. Med. Chem. 2003;11:3225. doi: 10.1016/s0968-0896(03)00185-8. [DOI] [PubMed] [Google Scholar]
- 4.Cane DE. Acc. Chem. Res. 1985;18:220. [Google Scholar]
- 5.Sacchettini JC, Poulter CD. Science. 1997;277:1788. doi: 10.1126/science.277.5333.1788. [DOI] [PubMed] [Google Scholar]
- 6.De la Torre M, Sierra MA. Angew. Chem. Int. Ed. Engl. 2004;43:160. doi: 10.1002/anie.200200545. [DOI] [PubMed] [Google Scholar]
- 7.(a) Tietze L. Chem. Rev. 1996;96:115–136. doi: 10.1021/cr950027e. [DOI] [PubMed] [Google Scholar]; (b) Malacria M. Chem. Rev. 1996;96:289. doi: 10.1021/cr9500186. [DOI] [PubMed] [Google Scholar]
- 8.Abe I, Rohmer M, Prestwich GD. Chem. Rev. 1993;93:2189. [Google Scholar]
- 9.Wendt KU, Schulz GE, Corey EJ, Liu DR. Angew. Chem. Int. Ed. 2000;39:2812. [PubMed] [Google Scholar]
- 10.Cattel L, Ceruti M. Crit. Rev. Biochem. Mol. Biol. 1998;33:353. doi: 10.1080/10409239891204378. [DOI] [PubMed] [Google Scholar]
- 11.Johnson WS. Acc. Chem. Res. 1968;1:1. [Google Scholar]
- 12.van Tamelen EE. Fortschr. Chem. Org. Naturst. 1961;19:242. doi: 10.1007/978-3-7091-7156-1_5. [DOI] [PubMed] [Google Scholar]
- 13.Giner J-L. Chem. Rev. 1993;93:1735. [Google Scholar]
- 14.Dewar MJ, Ruiz JM. Tetrahedron. 1987;43:2661. [Google Scholar]
- 15.Jarstfer MB, Blagg BSJ, Rogers DH, Poulter CD. J. Am. Chem. Soc. 1996;118:13089. doi: 10.1021/ja020411a. [DOI] [PubMed] [Google Scholar]
- 16.Langdon RG, Bloch K. J. Am. Chem. Soc. 1952;74:1869. [Google Scholar]
- 17.Woodward RB, Bloch K. J. Am. Chem. Soc. 1953;75:2023. [Google Scholar]
- 18.Langdon RG, Bloch K. J. Biol. Chem. 1953;200:135. [PubMed] [Google Scholar]
- 19.Cornforth JW, Hunter GD, Popják G. Arch. Biochem. Biophys. 1953;42:481. doi: 10.1016/0003-9861(53)90381-6. [DOI] [PubMed] [Google Scholar]
- 20.Voser W, Mijovic MV, Heusser H, Jeger O, Ruzicka L. Helv. Chim. Acta. 1952;35:2414. [Google Scholar]
- 22.Ourisson G, Rohmer M, Poralla K. Ann. Rev. Microbiol. 1987;41:301. doi: 10.1146/annurev.mi.41.100187.001505. [DOI] [PubMed] [Google Scholar]
- 23.Kannenberg EL, Poralla K. Naturwissenschaften. 1999;86:168. [Google Scholar]
- 24.(a) Rohmer M, Bouvier P, Ourisson G. Eur. J. Biochem. 1980;112:557. doi: 10.1111/j.1432-1033.1980.tb06121.x. [DOI] [PubMed] [Google Scholar]; (b) Rohmer M, Anding C, Ourisson G. Eur. J. Biochem. 1980;112:541. doi: 10.1111/j.1432-1033.1980.tb06117.x. [DOI] [PubMed] [Google Scholar]; (c) Bouvier P, Berger Y, Rohmer M, Ourisson G. Eur. J. Biochem. 1980;112:549. doi: 10.1111/j.1432-1033.1980.tb06119.x. [DOI] [PubMed] [Google Scholar]
- 25.Abe I, Rohmer M. J. Chem. Soc. Perkin Trans. 1994;1:783. [Google Scholar]
- 26.Barton DHR, Jarman TR, Watson KC, Widdowson DA, Boar RB, Damps K. J. Chem. Soc., Perkin Trans. 1975;1:1134. doi: 10.1039/p19750001134. [DOI] [PubMed] [Google Scholar]
- 27.Garson MJ. Chem. Rev. 1993;93:1699. [Google Scholar]
- 28.Barton DHR. Experientia. 1950;6:316. doi: 10.1007/BF02170915.See also: Chemistry 1963–1970. Amsterdam: Elsevier; 1972. Nobel Lectures.
- 29.Robinson R. Chem. Ind. (London) 1934;53:1062. [Google Scholar]
- 30.Bloch K, Rittenberg D. J. Biol. Chem. 1945;159:45. [Google Scholar]
- 31.Cornforth JW. Pure Appl. Chem. 1954;4:275. [Google Scholar]
- 32.Stork G, Burgstahler AW. J. Am. Chem. Soc. 1955;77:5068. [Google Scholar]
- 33.Eschenmoser A, Ruzicka L, Jeger O, Arigoni D. Helv. Chim. Acta. 1955;38:1890.Although this paper is written in the German language, an English translation will appear in Helv. Chim. Acta at the end of this year (2005) with a commentary by Professors Eschenmoser and Arigoni (ETH).
- 35.Tiemann F, Krueger P. Berichte Deutsch. Chem. Ges. 1893;26:2675. [Google Scholar]
- 36.Cohen A, Cook JW, Hewett CL, Girard A. J. Chem. Soc. 1934;653 [Google Scholar]
- 37.Barton DHR, Rydon HN, Elvidge JA. Biogr. Mem. Fellows R.. Soc. 1968;14:309. [Google Scholar]
- 38.Hibbit DC, Linstead RP. J. Chem. Soc. 1936;470 [Google Scholar]
- 39.(a) Linstead RP, Wang ABL, Williams JH, Errington KD. J. Chem. Soc. 1937:1136. [Google Scholar]; (b) Linstead RP, Millidge AF, Walpole AL. J. Chem. Soc. 1937:1140. [Google Scholar]
- 40.Stork G. Harvard University Organic Colloquium Abstract. 1950. Mar 14, The Stereochemistry of Polyene Cyclization.see Supporting Information; Burgstahler AW. Ph.D. Thesis. Harvard University: 1952. [Google Scholar]
- 41.(a) Burnop VCE, Linstead RP. J. Chem. Soc. 1940:720. [Google Scholar]; (b) Stork G, Conroy H. J. Am. Chem. Soc. 1951;73:4748. [Google Scholar]
- 44.Stadler PA, Nechvatal A, Frey AJ, Eschenmoser A. Helv. Chim. Acta. 1957;40:1373.Stadler PA, Eschenmoser A, Schinz H, Stork G. Helv. Chim. Acta. 1957;40:2191.For an account of some of this work in English, see Eschenmoser A, Felix D, Gut M, Meier J, Stadler P. CIBA Foundation Symposium on Biosynthesis of Terpenes and Steroids. 1959:217.
- 45.Gamboni G, Schinz H, Eschenmoser A. Helv. Chim. Acta. 1954;37:964.Note: translation of original text provided by Professor Eschenmoser (ETH), private communication.
- 46.Ruzicka L, Eschenmoser A, Heusser H. Experientia. 1953;9:362. [Google Scholar]
- 47.Ruzicka L. Nobel Lectures, Chemistry 1922–1941. Amsterdam: Elsevier; 1966. [Google Scholar]
- 49.Note also that the chair-chair conformational drawing was made in the Burgstahler dissertation (ref. 39b, p. 7).
- 50.For example: Fieser LF, Fieser M. New York: Advanced Organic Chemistry Reinhold; 1961. p. 1012.
- 55.Feil C, Süssmuth R, Jung G, Poralla K. Eur. J. Biochem. 1996;242:51. doi: 10.1111/j.1432-1033.1996.0051r.x. [DOI] [PubMed] [Google Scholar]
- 56.Wendt KU, Poralla K, Schulz GE. Science. 1997;277:1811. doi: 10.1126/science.277.5333.1811. [DOI] [PubMed] [Google Scholar]
- 57.Wendt KU, Lenhart A, Schulz GE. J. Mol. Biol. 1999;286:175. doi: 10.1006/jmbi.1998.2470. [DOI] [PubMed] [Google Scholar]
- 58.Merkofer T, Pale-Grosdemange C, Wendt KU, Rohmer M, Poralla K. Tetrahedron Lett. 1999;40:2121. [Google Scholar]
- 60.Sato T, Hoshino T. Biosci. Biotechnol. Biochem. 2001;65:2233. doi: 10.1271/bbb.65.2233. [DOI] [PubMed] [Google Scholar]
- 61.Füll C, Poralla K. FEMS Microbiol. Lett. 2000;183:221. doi: 10.1111/j.1574-6968.2000.tb08961.x. [DOI] [PubMed] [Google Scholar]
- 62.Reinert DJ, Balliano G, Schulz GE. Chem. Biol. 2004;11:121. doi: 10.1016/j.chembiol.2003.12.013. [DOI] [PubMed] [Google Scholar]
- 63.Hoshino T, Sato T. Chem. Commun. 2002:291. doi: 10.1039/b108995c. [DOI] [PubMed] [Google Scholar]
- 64.Hoshino T, Sato T. Chem. Commun. 1999:2005. [Google Scholar]
- 65.Hoshino T, Kouda M, Abe T, Ohashi S. Biosci. Biotechnol. Biochem. 1999;63:2038. doi: 10.1271/bbb.63.2038. [DOI] [PubMed] [Google Scholar]
- 66.Hoshino T, Kouda M, Abe T, Sato T. Chem. Commun. 2000:1485. [Google Scholar]
- 67.Johnson WS, Telfer SJ, Cheng S, Schubert U. J. Am. Chem. Soc. 1987;109:2517. [Google Scholar]
- 68.Johnson WS, Lindell SD, Steele J. J. Am. Chem. Soc. 1987;109:5852. [Google Scholar]
- 69.Stryer L. Biochemistry. San Francisco: W. H. Freeman and Co.; 1975. pp. 164–168. [Google Scholar]
- 70.Pale-Grosdemange C, Feil C, Rohmer M, Poralla K. Angew. Chem. Int. Ed. 1998;37:2237. doi: 10.1002/(SICI)1521-3773(19980904)37:16<2237::AID-ANIE2237>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 71.Jenson C, Jorgensen WL. J. Am. Chem. Soc. 1997;119:10846. [Google Scholar]
- 72.Gao D, Pan Y-K, Byun K, Gao J. J. Am. Chem. Soc. 1998;120:4045. [Google Scholar]
- 73.Hess BA., Jr J. Am. Chem. Soc. 2002;125:10268. [Google Scholar]
- 74.Hess BA., Jr Org. Lett. 2003;5:165. doi: 10.1021/ol027449c. [DOI] [PubMed] [Google Scholar]
- 75.Hess BA., Jr Eur. J. Org. Chem. 2004;12:2747. [Google Scholar]
- 76.Rajamani R, Gao J. J. Am. Chem. Soc. 2003;125:12768. doi: 10.1021/ja0371799. [DOI] [PubMed] [Google Scholar]
- 77.Hess BA, Jr, Smentek L. Org. Lett. 2004;6:1717. doi: 10.1021/ol0496125. [DOI] [PubMed] [Google Scholar]
- 78.Johnson WS. A Fifty-Year Love Affair With Organic Chemistry. Washington, D.C.: ACS; 1998. [Google Scholar]
- 79.Nicolaou KC, Sorensen EJ. Classics in Total Synthesis. Weinheim: VCH Publishers, Inc.; 1996. p. 83. [Google Scholar]
- 80.Fish PV, Johnson WS. J. Org. Chem. 1994;59:2324. [Google Scholar]
- 81.Johnson WS, Brinkmeyer RS, Kapoor VM, Yarnell TM. J. Am. Chem. Soc. 1977;99:8341. doi: 10.1021/ja00467a048. [DOI] [PubMed] [Google Scholar]
- 82.Johnson WS, Fletcher VR, Chenera B, Bartlett WR, Tham FS, Kullnig RK. J. Am. Chem. Soc. 1993;115:497. [Google Scholar]
- 83.Ishihara K, Nakamura S, Yamamoto H. J. Am. Chem. Soc. 1999;121:4906. [Google Scholar]
- 84.Ohloff G, Winter B, Fehr C. In: Perfumes, Art, Science and Technology. Muller PM, Lamparsky D, editors. New York: Elsevier Applied Science; 1991. p. 289. [Google Scholar]
- 85.Ishihara K, Ishibashi H, Yamamoto H. J. Am. Chem. Soc. 2002;124:3647. doi: 10.1021/ja0124865. [DOI] [PubMed] [Google Scholar]
- 86.King FE, King TJ, Topliss JG. J. Chem. Soc. 1957:573. [Google Scholar]
- 87.Banik BK, Chakraborti AK, Ghatak UR. J. Chem. Res. (S) 1986:3391. [Google Scholar]
- 88.Nakamura S, Ishihara K, Yamamoto H. J. Am. Chem. Soc. 2000;122:8131. [Google Scholar]
- 89.Ishihara K, Ishibashi H, Yamamoto H. J. Am. Chem. Soc. 2001;123:1505. [Google Scholar]
- 90.Ishibashi H, Ishihara K, Yamamoto H. Chem. Rec. 2002;2:177. doi: 10.1002/tcr.10020. [DOI] [PubMed] [Google Scholar]
- 91.Kumazawa K, Ishihara K, Yamamoto H. Org. Lett. 2004;6:2551. doi: 10.1021/ol049126h. [DOI] [PubMed] [Google Scholar]
- 92.Jannsen CG, Godefroi EF. J. Org. Chem. 1982;47:3274. [Google Scholar]
- 93.van Tamelen EE, Willett JD, Clayton RB, Lord KE. J. Am. Chem. Soc. 1982;104:648. doi: 10.1021/ja00972a058. [DOI] [PubMed] [Google Scholar]
- 94.Corey EJ, Cheng H, Baker CH, Matsuda SPT, Li D, Song X. J. Am. Chem. Soc. 1997;119:1289. [Google Scholar]
- 95.Corey EJ, Cheng H, Baker HC, Matsuda SPT, Li D, Song X. J. Am. Chem. Soc. 1997;119:1277. [Google Scholar]
- 96.Corey EJ, Staas DD. J. Am. Chem. Soc. 1998;120:3526. [Google Scholar]
- 97.Corey EJ, Virgil SC, Cheng H, Baker CH, Matsuda SPT, Singh V, Sarhar S. J. Am. Chem. Soc. 1995;117:11819. [Google Scholar]
- 98.Hoshino T, Sakai Y. Chem. Commun. 1998:1591. [Google Scholar]
- 99.van Tamelen EE, Sharpless KB, Hanzlik R, Clayton RB, Burlingame PC, Wszolek PC. J. Am. Chem. Soc. 1967;89:7150. doi: 10.1021/ja01002a077. [DOI] [PubMed] [Google Scholar]
- 100.Corey EJ, Cheng H. Tetrahedron Lett. 1996;37:2709. [Google Scholar]
- 101.Corey EJ, Virgil SC, Liu DR, Sarshar S. J. Am. Chem. Soc. 1992;114:1524. [Google Scholar]
- 102.Corey EJ, Virgil SC, Sarshar S. J. Am. Chem. Soc. 1991;113:8171. [Google Scholar]
- 103.Corey EJ, Virgil SC. J. Am. Chem. Soc. 1991;113:4025. [Google Scholar]
- 104.Cornforth JW. Angew. Chem. Int. Ed. Engl. 1968;7:903. doi: 10.1002/anie.196809031. [DOI] [PubMed] [Google Scholar]
- 105.van Tamelen EE, Willet J, Schwartz M, Nadeau R. J. Am. Chem. Soc. 1966;88:5937. doi: 10.1021/ja00976a048. [DOI] [PubMed] [Google Scholar]
- 106.Li T, Janda KD, Ashley JA, Lerner RA. Science. 1994;264:1289. doi: 10.1126/science.8191282. [DOI] [PubMed] [Google Scholar]
- 107.Hasserodt J, Janda KD, Lerner RA. J. Am. Chem. Soc. 1997;119:121. [Google Scholar]
- 108.Hasserodt J, Janda KD, Lerner RA. J.Am. Chem. Soc. 2000;122:40. [Google Scholar]
- 109.For a review of epoxy-ene and epoxy-arene cyclizations, see: Taylor SK. Org. Prep. Proc. Int. 1992;24:245.
- 110.Nishizawa M, Iwamoto Y, Takao H, Imagawa H, Sugihara T. Org. Lett. 2000;2:1685. doi: 10.1021/ol0057582. [DOI] [PubMed] [Google Scholar]
- 111.van Tamelen EE, Leiden TM. J. Am. Chem. Soc. 1982;104:2061. [Google Scholar]
- 112.Corey EJ, Lin S. J. Am. Chem. Soc. 1996;118:8765–8766. [Google Scholar]
- 113.Johnson WS, Bartlett WR, Czeskis BA, Gautier A, Lee CH, Lemoine R, Leopold EJ, Luedtke GR, Bancroft KJ. J. Org. Chem. 1999;64:9587–9595. [Google Scholar]
- 114.Corey EJ, Luo G, Lin LS. J. Am. Chem. Soc. 1997;119:9927. [Google Scholar]
- 115.Corey EJ, Luo G, Lin LS. Angew. Chem. Int. Ed. Engl. 1998;37:1126. doi: 10.1002/(SICI)1521-3773(19980504)37:8<1126::AID-ANIE1126>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 116.Breslow R, Barrett E, Mohaosi E. Tetrahedron Lett. 1962;3:1207. [Google Scholar]
- 117.Corey EJ, Russey WE, Ortiz de Montellano PR. J. Am. Chem. Soc. 1966;88:4750. doi: 10.1021/ja00972a056. [DOI] [PubMed] [Google Scholar]
- 118.van Tamelen EE, Willett JD, Clayton RB, Lord KE. J. Am. Chem. Soc. 1966;88:4752. doi: 10.1021/ja00972a058. [DOI] [PubMed] [Google Scholar]
- 119.Breslow R, Olin SS, Groves JT. Tetrahedron Lett. 1968;9:1837. [Google Scholar]
- 120.Lallemand JY, Julia M, Mansuy D. Tetrahedron Lett. 1973;14:4461. [Google Scholar]
- 121.Dombroski MA, Kates SA, Snider BB. J. Am. Chem. Soc. 1990;112:2759. [Google Scholar]
- 122.Kates SA, Dombroski MA, Snider BB. J. Org. Chem. 1990;55:2427. [Google Scholar]
- 123.Zoretic PA, Weng X, Caspar ML. Tetrahedron Lett. 1991;32:4819. [Google Scholar]
- 124.Chen L, Gill GB, Pattenden G. Tetrahedron Lett. 1994;35:2593. [Google Scholar]
- 126.Heinemann C, Demuth M. J. Am. Chem. Soc. 1999;121:4894. [Google Scholar]
- 127.For a discussion of anaerobic steroid formation, see: Caspi E. Acc. Chem. Res. 1980;13:97.
- 128.The concept of ‘catalysis by minimal peptides’ addresses this concept directly: Miller SJ. Acc. Chem. Res. 2004;37:601. doi: 10.1021/ar030061c.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.