Abstract
Purpose. Disease-specific survival (DSS) for proximal bile duct cancer has been reported to be worse than for carcinoma of the distal duct. Methods. Review of two prospectively maintained databases identified 204 patients who underwent resection for extrahepatic cholangiocarcinoma (proximal: n=106, 52%; distal: n=98, 48%) between December 1987 and December 2005. Patient, tumor, and treatment-related variables were reviewed. Analyses were performed to compare tumor presentation, treatment, and DSS between patients with resected proximal and distal lesions. Results. Median follow-up for the 204 resected patients was 24 months (range 1–165 months) and 56 months for those alive at last follow-up. Combined liver/bile duct resection was performed in 82% of patients with proximal lesions, and pancreaticoduodenectomy was performed in 92% of patients with distal lesions. Patients experienced similar postoperative length of stay (median: proximal, 13 days vs. distal, 13 days; p=0.64) and operative mortality (30-day: proximal, 4% vs. distal, 3%; p=1.0, Fishers). Margin positive rates were similar (proximal, 23% vs. distal, 15%; p=0.20). Estimated five-year DSS for all patients was 35%. Tumor location (proximal vs. distal) was not associated with five-year estimated DSS (proximal, 29% vs. distal, 43%; p=0.44). Factors associated with five-year DSS included stage at presentation (node negative, 42% vs. node positive, 22%; p=<0.001), differentiation (papillary, 53% vs. non-papillary, 27%; p=0.01), and margin status (margin negative 42% vs. margin positive 27%; p<0.001). Conclusions. These results suggest that patients with resected proximal and distal cholangiocarcinoma will experience similar operative outcomes and DSS.
Keywords: bile ducts, cholangiocarcinoma, survival
Introduction
Primary bile duct cancer is an uncommon cancer in the USA (incidence: 1–2/100,000/year) and may arise anywhere within the biliary tree. Tumors involving the extrahepatic ducts have been considered to be more common than intrahepatic lesions, and reports of selected patients suggest that lesions of the proximal extrahepatic ducts are more common than lesions of the distal duct 1. The division of extrahepatic cholangiocarcinoma into proximal (hilar), distal, and sometimes mid-duct categories has been based primarily on differences in approach to biliary drainage, diagnosis, and operative resection rather than to any identified difference in tumor biology 2,3,4.
Because of differences in approach and management there are currently only a few large studies directly comparing outcome between proximal and distal cholangiocarcinoma 3,4,5,6,7. These studies have in general reported improved survival in patients with distal lesions. The interpretation of these studies is complicated by the fact that many include patients who did not undergo resection, and are from an era when operative mortality was significantly higher for proximal lesions. Tompkins et al. published a review in 1981 of 96 patients with extrahepatic cholangiocarcinoma and the median survival was nine months, 10 months, and 21 months for proximal, mid, and distal lesions, respectively 4. All patients treated at their institution between 1954 and 1978 were included. Fewer than half of the patients (47%) with proximal lesions underwent resection and the operative mortality rate was 23%. Resection was performed in 67% of patients with distal lesions, and the operative mortality in this group was 8%.
The goal of the current study was to evaluate and compare the operative results and long-term disease-specific outcome of patients resected for extrahepatic bile duct cancer at a single institution. Comparisons between proximal and distal lesions were limited to only those patients who underwent resection in an effort to compare outcome in a group of patients with similar stage disease.
Methods
Over an 18-year period from December 1987 to December 2005, 204 patients underwent resection for extrahepatic bile duct carcinoma at Memorial Sloan-Kettering Cancer Center (MSKCC). All patients were identified from one of two prospectively maintained operative databases (liver database, pancreatic database) that contain demographic, clinical, operative, pathologic, and follow-up data. Permission for studying these patients was obtained from the MSKCC Institutional Review and Privacy Board according to institutional policy for protected health information.
Patients were included if they underwent resection of an extrahepatic bile duct carcinoma (cholangiocarcinoma). Patients who were explored but did not undergo resection were excluded. Patients with gallbladder carcinoma were not included. Proximal tumors were defined as those lesions involving the biliary confluence or from the extrahepatic right or left hepatic ducts and typically requiring hepatic resection. Distal tumors were defined as those arising from the distal duct, generally distal to the insertion of the cystic duct, and typically requiring pancreatic resection. Patients with intrahepatic cholangiocarcinoma secondarily involving the extrahepatic ducts were excluded. In patients who did not undergo liver resection (proximal tumors) or pancreatic resection (distal tumors) the classification of proximal or distal was based on the relationship to the cystic duct. In many reports these lesions would be classified as mid-duct tumors, however in an effort to avoid selective exclusion, these were classified as proximal if arising proximal to the cystic duct insertion.
Patient, tumor, and treatment-related variables were retrieved from the database and confirmed by chart review. Patient variables included age, gender, and selected preoperative laboratory values. Treatment-related variables included the nature of the resection (liver and bile duct resection, bile duct resection, pancreatectomy), operative blood loss, and postoperative length of stay. Patients were considered to have died from postoperative complications if death occurred within 30 days of operation. Patients were categorized at the time of last follow-up into the following categories: no evidence of disease (NED), alive with disease (AWD), dead of disease (DOD), postoperative death (POD), and dead of other causes (DOC). The length of follow-up was calculated as the time between the date of operation and the date of last follow-up or death. Death of disease was considered an event in the disease-specific survival (DSS) analysis.
Final pathology reports were reviewed retrospectively to confirm the presence of extrahepatic cholangiocarcinoma. All tumors were staged according to the 6th edition of the American Joint Committee on Cancer Staging Handbook 8. Primary tumor size was recorded as the largest diameter axis through the sectioned specimen. Histologic differentiation was categorized into two groups for analysis: well-differentiated and not well-differentiated (poor and moderate). All lesions were reviewed for the presence of papillary components. The previously published methodology for the classification of the papillary sub-type was utilized 9. The absence of microscopic disease involving any resection margin was considered a margin-negative (R0) resection. The total number of examined lymph nodes and the number of histologically positive metastatic lymph nodes within each surgical specimen were recorded.
Analyses were performed to compare tumor presentation, treatment, and DSS between patients with resected proximal and distal cholangiocarcinoma. Continuous variables were dichotomized at their median values. Differences between proximal and distal groups were tested using chi-square tests for categorical variables and Wilcoxon tests for continuous variables, with the exception of operative mortality where Fisher's exact test was used to account for a small number of events. Univariate Cox proportional hazards regression was used to identify factors individually predictive of DSS. Stepwise multivariate Cox proportional hazards regression was used to identify factors multivariately predictive of DSS. Entry and exit criteria in the stepwise model were set at an alpha level of 0.10. P-values from the univariate and multivariate Cox models were from the Wald test. All tests were two-sided and statistical significance was achieved at p<0.05. Statistical analysis was performed with SAS statistical software version 9.1 (SAS institute Inc, Cary, NC).
Results
Over the 18-year time period 204 patients underwent resection for extrahepatic bile duct carcinoma. Roughly half of these lesions were proximal tumors (n=106, 52%). The patient, tumor, and treatment-related variables for these 204 patients are presented in Table I. Resection required combined liver and bile duct resection for 87 of the 106 tumors classified as proximal lesions (82%), and pancreatic resection was performed in 90 of the 98 tumors classified as distal (90/98, 92%). Bile duct resection with portal node dissection alone was performed in 27 patients and in 19 of these cases the lesions were categorized as proximal. The majority of tumors were pathologically classified as T3 (n=131, 66%) and metastatic spread to regional lymph nodes was identified in 34% of patients. The median postoperative length of stay was 13 days and the operative mortality (30-day mortality) was 4% for proximal lesions and 3% for distal lesions (p=1.0, Fishers exact).
Table I. Patient, tumor, and treatment-related variables for 204 patients resected for extrahepatic cholangiocarcinoma.
| Factor | No. of patients | % |
|---|---|---|
| Gender (male) | 122 | 60 |
| Tumor location | ||
| Proximal bile duct | 106 | 52 |
| Distal bile duct | 98 | 48 |
| Operation | ||
| Liver and bile duct resection | 87 | 43 |
| Pancreatectomy | 90 | 44 |
| Bile duct resection | 27 | 13 |
| Resection margin positive | 39 | 19 |
| T-stage (n=194) | ||
| T1 | 7 | 4 |
| T2 | 56 | 28 |
| T3 | 131 | 66 |
| Node positive | 68 | 33 |
| Postoperative death | 7 | 3 |
| Median | Range | |
| Age at diagnosis (years) | 68 | 34–87 |
| Postoperative length of stay (days) | 13 | 6–82 |
| Length of follow-up (months) | 24 | 1–165 |
A comparison between patient, tumor, and treatment-related variables in patients with proximal and distal cholangiocarcinoma is presented in Table II. Patients with distal lesions had a greater number of regional nodes pathologically assessed (median: distal, 12 vs. proximal, 3; p<0.001), and were more likely to be node positive (47% vs. 21%, p<0.001). Distal lesions were more likely to have a more advanced AJCC stage (see Table II). No patient with a distal lesion underwent resection and was found to have an in situ or T1, N0 tumor. Patients who underwent resection for proximal cholangiocarcinoma were more likely to present with tumors described as well-differentiated and/or papillary in nature. Lesions of the proximal duct were noted to be larger in size (median: proximal, 2.4 cm vs. distal, 2.0 cm; p=0.05).
Table II. Comparison of patient, tumor, and treatment-related variables for patients undergoing resection for proximal and distal extrahepatic cholangiocarcinoma (n=204).
| Variable | Proximal (n=106) n (%) | Distal (n=98) n (%) | P-value* |
|---|---|---|---|
| Gender | |||
| Male | 58 (55%) | 64 (65%) | 0.12 |
| Age | |||
| Years (mean) | 65 | 67 | 0.22 |
| Stage | |||
| 0 (Tis) | 5 (5) | 0 (0) | <0.0001 |
| 1A | 8 (8) | 1 (1) | |
| 1B | 29 (27) | 15 (16) | |
| 2A | 42 (40) | 32 (34) | |
| 2B | 22 (21) | 47 (49) | |
| Nodal status | |||
| Node negative | 84 (79) | 51 (53) | <0.0001 |
| Node positive | 22 (21) | 46 (47) | |
| Margins | |||
| Negative margins | 82 (77) | 82 (85) | 0.20 |
| Positive margins | 24 (23) | 15 (15) | |
| Differentiation | |||
| Not well-differentiated | 68 (66) | 74 (86) | 0.002 |
| Well-differentiated | 35 (34) | 12 (14) | |
| Papillary | |||
| Yes | 25 (24) | 9 (3) | <0.001 |
| No | 81 (76) | 95 (97) | |
| Size | |||
| Median, cm (range) | 2.4 (0.4–10.7) | 2.0 (0.2–8.4) | 0.05 |
| Number nodes assessed | |||
| Median (range) | 3 (0–11) | 12 (2–42) | <0.001 |
| Length of stay | |||
| Median, days (range) | 13 (6–49) | 13 (6–82) | 0.64 |
| Estimated blood loss | |||
| Median, CC (range) | 800 (130–7000) | 800 (100–7500) | 0.23 |
| Postoperative deaths | 4 (3.8) | 3 (3.1) | 1.0 |
*P-values for categorical variables calculated from χ2 test.
Note: P-values for continuous variables are from Wilcoxon test with exception of p-value for postoperative deaths which was calculated from Fishers Exact test.
P-Values < 0.05 highlighted in bold.
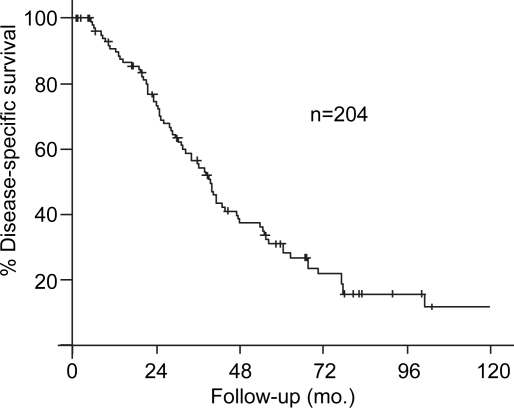
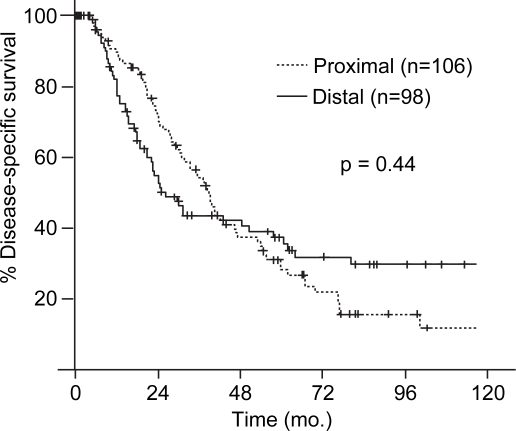
The median follow-up for the 204 resected patients was 24 months (range 1–165 months) and 56 months for those alive at the time of last follow-up. The estimated five-year DSS for the 204 patients in the study was 35% (Figure 1). The univariate Cox model results between patient, tumor, and treatment-related variables and DSS is presented in Table III. The location of the lesion was not associated with estimated five-year DSS (proximal, 29% vs. distal, 43%; p=0.44, Figure 2). Factors associated with five-year DSS included stage at presentation (node negative, 42% vs. node positive, 22%; p= < 0.001), tumor differentiation (papillary, 53% vs. non-papillary, 27%; p=0.01), and the presence of a positive margin (margin negative 42% vs. margin positive 10%; p<0.001). Multivariate analysis identified a negative margin (hazard ratio, 3.68; 95% confidence interval, 2.38–5.69) and well-differentiation (hazard ratio, 0.42; 95% confidence interval, 0.26–0.67) to be predictors of improved survival.
Figure 1. .
Disease-specific survival (DSS) in 204 patients resected for extrahepatic cholangiocarcinoma.
Table III. Univariate Cox proportional hazards results for disease-specific survival (excludes operative deaths).
| Variable | Hazard ratio* and 95% CI | P-value** |
|---|---|---|
| Proximal vs. Distal | 1.16 (0.80–1.69) | 0.44 |
| Node positive vs. Node negative | 2.19 (1.50–3.21) | <0.0001 |
| Positive margins vs. Negative margins | 3.18 (2.09–4.83) | <0.0001 |
| Well-differentiated vs. not Well-differentiated | 0.46 (0.29–0.74) | 0.001 |
| Size (continuous) | 0.97 (0.85–1.11) | 0.65 |
| Size ≥2.5 vs. Size <2.5 | 0.85 (0.56–1.30) | 0.45 |
| Length of stay (continuous) | 0.99 (0.97–1.02) | 0.60 |
| Length of stay ≥13 days vs. Length of stay <13 days | 1.00 (0.69–1.46) | 0.99 |
| Estimated blood loss in 100 cm3 (continuous) | 1.00 (0.98–1.02) | 0.96 |
| Estimate blood loss ≥800 cm3 vs. Estimated blood loss <800 cm3 | 0.98 (0.66–1.44) | 0.90 |
*Hazard ratio determined from univariate Cox proportional hazards model.
**P-value from Wald test.
P-Values < 0.05 highlighted in bold.
Figure 2. .
Comparison of estimated disease-specific survival (DSS) between patients resected for proximal and distal cholangiocarcinoma.
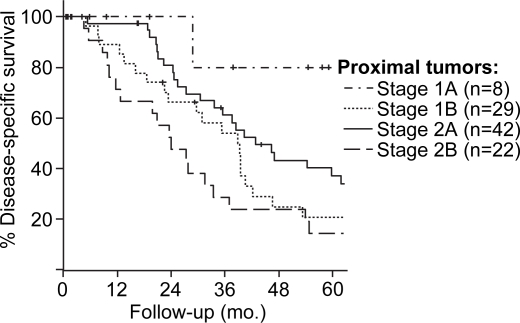
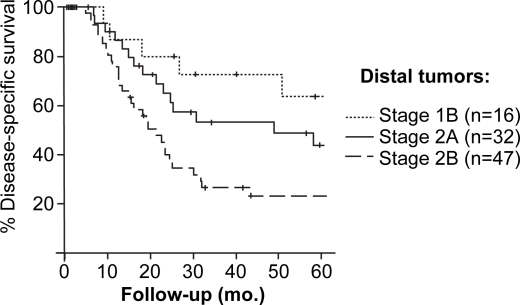
AJCC stage at presentation was associated with DSS for the 204 resected patients. Patients with stage 1B (T2, N0, M0) lesions, however, experienced similar estimated five-year DSS as patients resected with stage 2A lesions (T3, N0, M0). This similarity in DSS between T2 and T3 tumors appeared to be secondary to overlap in the survival of patients with proximal lesions. Within the proximal group the estimated five-year DSS for T2 lesions was lower 21%, although not statistically lower, than five-year DSS for T3 lesions (33%, p=0.23, Figure 3). For patients with distal lesions, the DSS estimates for T2 and T3 tumors (within the node negative group) stratified as would be expected (Figure 4). The five-year DSS for patients with distal lesions who underwent resection for a T2 lesion was 62%, and 40% for those resected for T3 lesions (p=0.08).
Figure 3. .
Stage-specific disease-specific survival for patients resected for extrahepatic cholangiocarcinoma.
Figure 4. .
Stage-specific disease-specific survival for patients resected for proximal extrahepatic cholangiocarcinoma.
Discussion
In the current study 204 patients underwent resection for extrahepatic bile duct cancer over an 18-year time period. Proximal tumors were present in 52% of patients and distal tumors were present in 48% of patients. Patient and operative outcomes were similar between patients with proximal and distal lesions. In both groups the median age was similar (65 years proximal vs. 67 years distal), postoperative length of stay was 13 days, estimated blood loss was similar (median 800 cm3, both groups) and postoperative mortality was similar (30-day mortality: proximal, 4% vs. distal, 3%; p=NS). Estimated five-year DSS was 35% for the 206 resected patients and did not differ between those with proximal and distal tumors (five-year DSS: proximal, 29% vs. distal, 43%; p=0.44).
Previous studies have suggested that patients who present with proximal bile duct cancer have worse disease-specific outcomes than those with distal lesions 4,6. In one of the largest single institution studies of proximal and distal bile duct cancer from Johns Hopkins Medical Center 294 patients with cholangiocarcinoma (intrahepatic in 18 patients) were presented 6. In this study the five-year survival was 11% for patients with proximal tumors and 28% for patients with distal lesions. Similarly, in a report by Tompkins et al. of 96 patients with extrahepatic cholangiocarcinoma the median survival was nine months, 10 months, and 21 months for proximal, mid, and distal lesions, respectively 4. The conclusion from this latter study was that the location of the lesion within the bile duct appeared to bear the most important relationship to prognosis.
The prognostic significance of location within the duct appears to be most associated with the ability to resect the lesion, obtain a negative margin, and with the risk of postoperative mortality rather than with a primary difference in tumor biology. In the study from Johns Hopkins noted above the resection rate for patients with proximal tumors was 56% compared to 91% in patients with distal lesions. The operative mortality rate was higher in patients resected for proximal lesions (3.6% for proximal lesions and 1.3% for distal lesions) and the margin positive rate was 74% in proximal lesions compared to 10% for distal lesions. These studies, which include both resected and unresected patients, demonstrate a better outcome for distal lesions but suggest that this is secondary to a decreased ability to resect proximal lesions because of later stage at diagnosis and the technical challenges of proximal resection.
Reports that have limited comparison to only patients who have undergone resection have found similar results as our study 3,5,10. Nagorney et al. reported on 171 patients with extrahepatic cholangiocarcinoma and found similar survival amongst resected patients with proximal and distal lesions 3. Hernandez et al. reported on 91 patients resected for extrahepatic cholangiocarcinoma at the University of South Florida 10. Operative mortality was similar (postoperative mortality: proximal, 15% vs. distal, 9%; p=0.36) and median survival was similar (median survival: proximal, 22 months vs. distal, 17 months; p=NS).
Factors identified in the current study as predictive of decreased DSS included metastatic disease to regional nodes, poorly differentiated tumors, and positive surgical margins. The latter factor was similar between proximal and distal groups; however, patients with distal lesions were statistically more likely to have positive nodes identified and less likely to have well-differentiated tumors. The majority of well-differentiated lesions in the proximal duct had a papillary component. Papillary lesions in the distal dust were extremely uncommon. Protein expression profiles suggest differences between papillary and non-papillary tumors which are associated with improved prognosis and do suggest a possible biologic difference between papillary and non-papillary cholangiocarcinoma 11.
In the current study, the differences between proximal and distal lesions with respect to tumor differentiation and regional nodal spread did not result in any identifiable difference in survival. It is possible that the observed difference in the rate of regional nodal spread may represent a difference in nodal resection and pathologic assessment. Patients with resected distal lesions had a median of 12 nodes assessed and patients with proximal lesions had a median of two nodes assessed (p<0.001). This difference is almost certainly due the anatomic limitations of portal node dissection for proximal tumors and highlights the importance of both operative dissection and pathologic assessment for accurate staging. As nodal status is a strong predictor of survival many groups have recommended a more extensive nodal dissection for proximal tumors 12,13. This dissection may result in more precise staging, however, a therapeutic effect has never been demonstrated, and thus the potential morbidity of extended lymphadenectomy should be considered.
The current AJCC staging system (6th edition) for bile duct cancer utilizes a similar classification system as for pancreatic adenocarcinoma. The current study, as well as others, has identified limitations of this system within the tumor (T) staging category. In the current study the survival for patients with T2 and T3 lesions overlapped for the entire group and this lack of stratification appeared to be secondary to the proximal group alone. T2 lesions are defined as invading beyond the wall of the bile duct, and T3 lesions are defined as invading another organ or blood vessel. It is possible that this system is more applicable to distal lesions because this segment of the bile duct is within the head of the pancreas. Thus, as lesions grow in the distal duct they invade into the pancreas in a consistent fashion. The proximal duct is not embedded within any organ and therefore invasion into the liver or portal vein may not be consistent, and very dependent on the exact location. This finding has also been reported by others, and should encourage the evaluation of other approaches to primary tumor prognostic stratification 5.
In conclusion, the results from the current study suggest that patients undergoing resection for extrahepatic bile duct cancer will experience similar short-term and long-term survival regardless of location. In this study the histologic factors of tumor differentiation, the stage-related factors such as regional nodal spread, and the ability to obtain a negative margin were predictive of DSS. Operative resection is warranted in patients who present with radiographically resectable extrahepatic cholangiocarcinoma regardless of location within the bile duct.
References
- 1.Jarnagin WR, D'Angelica M, Blumgart LH. Blumgart LH. Saunders Elsevier; Philadelphia: 2007. Intrahepatic and extrahepatic biliary cancer, Surgery of the liver, biliary tract, and pancreas; pp. 783–826. [Google Scholar]
- 2.Fong Y, Blumgart LH, Lin E, Fortner MF, Brennan MF. Outcome of treatment for distal bile duct cancer. Br J Surg. 1996;83:1712–15. doi: 10.1002/bjs.1800831217. [DOI] [PubMed] [Google Scholar]
- 3.Nagorney DM, Donohue JH, Farnell MB, Schleck CD, Ilstrup DM. Outcomes after curative resections of cholangiocarcinoma. Arch Surg. 1993;128:871–7. doi: 10.1001/archsurg.1993.01420200045008. [DOI] [PubMed] [Google Scholar]
- 4.Tompkins RK, Thomas D, Wile A, Longmire WP., Jr Prognostic factors in bile duct carcinoma: analysis of 96 cases. Ann Surg. 1981;194:447–57. doi: 10.1097/00000658-198110000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reding R, Buard JL, Lebeau G, Launois B. Surgical management of 552 carcinomas of the extrahepatic bile ducts (gallbladder and periampullary tumors excluded). Results of the French surgical association survey. Ann Surg. 1991;213:236–41. doi: 10.1097/00000658-199103000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nakeeb A, Pitt HA, Sohn TA, Coleman J, Abrams RA, Piantadosi S, et al. Cholangiocarcinoma. A spectrum of intrahepatic, perihilar, and distal tumors. Ann Surg. 1996;224:463–73. doi: 10.1097/00000658-199610000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Heron DE, Stein DE, Eschelman DJ, Topham AK, Waterman FM, Rosato EL, et al. Cholangiocarcinoma: the impact of tumor location and treatment strategy on outcome. Am J Clin Oncol. 2003;26:422–8. doi: 10.1097/01.COC.0000026833.73428.1F. [DOI] [PubMed] [Google Scholar]
- 8.AJCC Cancer Staging Handbook. 6th ed. New York: Springer; 2002. [Google Scholar]
- 9.Jarnagin WR, Bowne W, Klimstra DS, Ben-Porat L, Cymes K, et al. Papillary phenotype confers improved survival after resection of hilar cholangiocarcinoma. Ann Surg. 2005;241:703–12. doi: 10.1097/01.sla.0000160817.94472.fd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hernandez J, Cowgill SM, Al-Saadi S, Villadolid D, Ross S, Kraemer E, et al. An aggressive approach to extrahepatic cholangiocarcinomas is warranted: margin status does not impact survival after resection. Ann Surg Oncol. 2008;15:807–14. doi: 10.1245/s10434-007-9756-2. [DOI] [PubMed] [Google Scholar]
- 11.Jarnagin WR, Klimstra DS, Hezel M, Gonen M, Fong Y, Roggin K, et al. Differential cell cycle-regulatory protein expression in biliary tract adenocarcinoma: correlation with anatomic site, pathologic variables, and clinical outcome. J Clin Oncol. 2006;24:1152–60. doi: 10.1200/JCO.2005.04.6631. [DOI] [PubMed] [Google Scholar]
- 12.Kitagawa Y, Nagino M, Kamiya J, Uesaka K, Sano T, Yamamoto H, et al. Lymph node metastasis from hilar cholangiocarcinoma: audit of 110 patients who underwent regional and paraaortic node dissection. Ann Surg. 2001;233:385–92. doi: 10.1097/00000658-200103000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Morimoto Y, Tanaka Y, Ito T, Nakahara M, Nakaba H, Nishida T, et al. Long-term survival and prognostic factors in the surgical treatment for intrahepatic cholangiocarcinoma. J Hepatobiliary Pancreat Surg. 2003;10:432–40. doi: 10.1007/s00534-002-0842-3. [DOI] [PubMed] [Google Scholar]