Abstract
Background. Pancreatic cancers of the body and tail (BT) appear to have poorer survival compared with head (HD) lesions. We hypothesized that potential disparities in outcome may be related to tumor location. Our objective was to examine the relationship between tumor location and survival. Methods. The Surveillance, Epidemiology, and End Results registry identified 33,752 patients with pancreatic adenocarcinoma and 6443 patients who underwent cancer-directed surgery between 1988 and 2004. Differences in survival and relationships between tumor location and clinical factors were assessed. Multivariate analysis was performed to determine the prognostic significance of tumor location. Results. Median survival for the entire cohort was five months and was significantly lower for BT compared to HD lesions (four vs. six months, p<0.001). Distant metastases (67% vs. 36%, p<0.001) were greater and cancer-directed surgery (16% vs. 30%, p<0.001) was lower for BT tumors. Of 6443 resected patients, HD patients (n=5118) were younger, had a greater number of harvested lymph nodes, were more likely to be lymph node-positive, and had a higher proportion of T3/T4 lesions. Significant univariate predictors of survival included age, T-stage, number of positive and harvested lymph nodes. On multivariate analysis, BT location was a significant prognostic factor for decreased survival (OR 1.11, 95% CI 1.00–1.23, p=0.05). Discussion. Pancreatic BT cancers have a lower rate of resectability and poorer overall survival compared to HD lesions. Prospective large-cohort studies may definitively prove that tumor location is a prognostic factor for survival in patients with pancreatic cancer.
Keywords: SEER, tumor location, pancreatic cancer
Introduction
According to the American Cancer Society, there continues to be an annual increase in the number of patients with pancreatic cancer with approximately 37,170 new cases in 2007 1. Surgical resection remains the only option for potential curative treatment. However, only 15–20% of patients are eligible for surgery 1. Five-year survival rates after curative-intent surgery have improved in recent reports 2,3,4,5 and several clinicopathologic factors for survival have been identified 6,7,8,9.
The anatomic location of pancreatic tumors has been suggested as a potential determinant of survival 10,11,12. Approximately 65% of pancreatic cancers occur in the head (HD) of the pancreas, whereas 15% occur in the body and tail (BT); the remaining lesions diffusely involve the gland 13. A potential survival disparity between HD and BT tumors has been attributed to the relatively late clinical presentation of patients with BT tumors 10,12,14. However, a report by Sohn et al. suggests that BT lesions of the pancreas may be associated with worse survival than HD, neck, and uncinate lesions, independent of stage of presentation and extent of disease 15.
We hypothesized that tumor location predicts survival in pancreatic cancer, independent of established prognostic factors such as T-stage and lymph node status. In this report we present a population-based analysis of patients with pancreatic cancer that describes clinicopathologic and survival patterns associated with HD and BT tumors of the pancreas.
Patients and methods
Cancer database
The Surveillance Epidemiology and End Results (SEER) program of the National Cancer Institute assembles and distributes information on cancer incidence and survival from 18 geographical areas covering approximately 26% of the United States population 16. This registry collects data on patient demographics, year of diagnosis, primary tumor site, tumor morphology, treatment, and follow-up for vital status. Additionally, annual quality control studies verify that accurate information is being collected with standard case ascertainment of at least 98% or greater 16.
Study population
The SEER registry was used to identify 33,752 patients with histologically confirmed pancreatic “adenocarcinoma” or “ductal adenocarcinoma” (SEER histology codes 8140 and 8500, respectively) diagnosed between the years 1988 and 2004. A subset of 6443 patients with non-metastatic disease who underwent cancer-directed surgery was selected for further analysis. Patients were excluded from analysis if they had undergone exploratory surgery without resection, biopsies, nodal dissections alone or unknown/unspecified treatments.
Statistical analysis
The primary outcome measure was overall survival, calculated in months from the date of diagnosis to the date of death. Non-deaths were censored at the time of last follow-up. The main prognostic factor of interest was tumor location as coded in the SEER database. Location was categorized into lesions of the HD, BT and other. HD and BT cases were selected for analysis. Additional prognostic factors which were examined included age, T-stage, node-positivity in patients with ≥1 harvested lymph node, total number of lymph nodes harvested, and histologic grade. Age and total number of harvested lymph nodes were dichotomized at the median value for Kaplan–Meier analysis and coded as continuous variables for descriptive and Cox regression analysis; T-stage was categorized as T1/T2 and T3/T4; and grade was categorized as well-differentiated (I), moderately differentiated (II), poorly differentiated (III) and undifferentiated/anaplastic (IV).
Rates of metastatic disease and cancer-directed surgery for HD and BT lesions were determined and compared for the entire cohort using the chi-square test. Descriptive characteristics of the subset of patients with non-metastatic disease undergoing cancer-directed surgery were compared between HD and BT lesions using independent sample student t tests for continuous variables and chi-square tests for categorical variables. Overall survival was determined for the entire cohort, as well as the non-metastatic surgical subset via the Kaplan–Meier method. Differences in survival by tumor location and other clinical factors were determined using the log-rank test. Univariate and multivariate Cox regression analyses were used to determine the association of tumor location with survival independent of other clinicopathologic factors. The independent variables used in the multivariate analysis were chosen to adjust for factors that were significantly different by tumor location. Age and number of harvested lymph nodes were entered as continuous variables and indicator variables were used for categorical variables. The results were expressed as hazard ratios with p-values and 95% confidence intervals. A p-value of ≤0.05 was considered statistically significant. The statistical analysis was performed using SPSS (version 12.0; SPSS Inc., Chicago, IL).
Results
Analysis of all pancreatic cancer patients
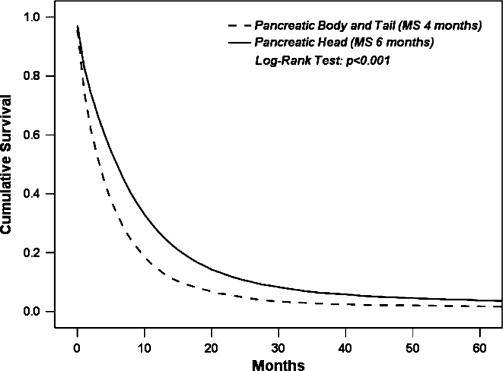
Of 33,752 patients with pancreatic cancer, 18,666 (56%) had tumors that were located in the pancreatic head and 5982 (18%) in the body or tail. The remaining 9104 (26%) patients had pancreatic tumors with locations coded in SEER as “pancreatic duct”, “islet of langerhans”, “overlapping” or “unknown/unspecified”. Table I shows a comparison of surgical resection and metastatic disease rates with HD vs. BT lesions in all patients with pancreatic cancer. BT tumors were associated with a significantly higher rate of metastatic disease (67% vs. 36%, p<0.001) and a significantly lower rate of cancer-directed surgery (16% vs. 30%, p<0.001). The median survival for entire cohort was five months. When comparing survival by anatomic location, patients with BT tumors had a statistically significally lower median survival compared to patients with HD tumors (four vs. six months, respectively, p<0.001) (Figure 1).
Table I. Patients with pancreatic adenocarcinoma.
| Head No. (Valid%)a | Body/Tail No. (Valid%)a | p-value | |
|---|---|---|---|
| Cancer-directed surgery | |||
| No | 13,185 (70.1%) | 1999 (83.9%) | <0.001 |
| Yes | 5637 (29.9%) | 960 (16.1%) | |
| Extent of disease | |||
| Localized | 11,345 (64.5%) | 1882 (32.9%) | <0.001 |
| Metastatic | 6252 (35.5%) | 3841 (67.1%) | |
aExcludes patients with missing data.
Figure 1. .
Comparison of overall survival by tumor location in the entire cohort (MS four vs. six months, BT vs. HD lesions, respectively, p<0.001).
Analysis of surgical patients
From the original cohort of patients with pancreatic cancer, only 6443 patients had cancer-directed surgery for non-metastatic disease. Table II compares clinicopathologic factors with respect to tumor location for non-metastatic patients who underwent surgical resection. There were 5118 patients (79%) and 663 patients (10%) with resected HD and BT tumors, respectively. The remaining patients (n=662; 10%) had tumors in other parts of the pancreas, overlapping regions, or the location was unknown. Patients with resected BT tumors were significantly older than patients with pancreatic HD resections (67 vs. 65 years old, p<0.001). Gender was equally distributed among the two groups. BT lesions were associated with a significantly lower number of harvested lymph nodes (8 vs. 10, p<0.001) and a significantly lower likelihood of lymph node-positive disease when at least one node was harvested (50% vs. 60%, p<0.001) (Table II). Additionally, patients with BT lesions had a lower percentage of stage T3/T4 cancers compared to patients with HD lesions (69.8% vs. 77.2%, p<0.001).
Table II. Comparison of surgical patient characteristics by tumor location.
| Head and neck (n=5118) | Body and tail (n=663) | p-value | |
|---|---|---|---|
| Age (years, mean ±SE) | 65.1±0.15 | 66.7±0.40 | <0.001 |
| Gender | 0.47 | ||
| Female | 2532 (49.5%) | 338 (51.0%) | |
| Male | 2586 (50.5%) | 325 (49.0%) | |
| Number of harvested lymph nodes (mean ±SE) | 9.7±0.11 | 7.7±0.30 | <0.001 |
| Number of positive lymph nodes (mean ±SE) | 1.7±0.04 | 1.2±0.08 | <0.001 |
| Lymph node status | <0.001 | ||
| Negative | 1907 (40.1%) | 278 (50.5%) | |
| Positive | 2845 (59.9%) | 273 (49.5%) | |
| T-Stage | <0.001 | ||
| ½ | 1151 (22.8%) | 197 (30.2%) | |
| 3/4 | 3899 (77.2%) | 456 (69.8%) |
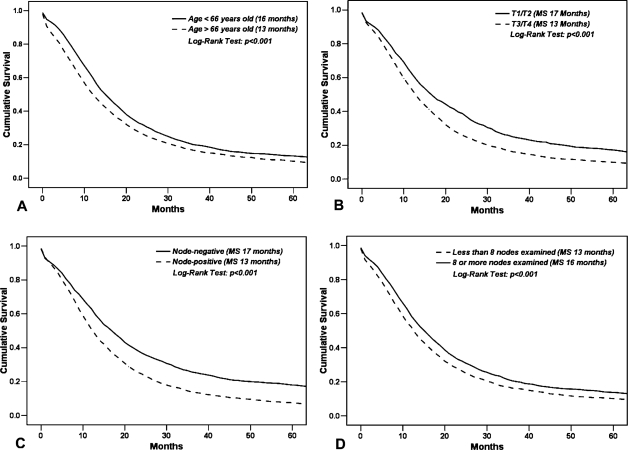
On univariate analysis – Kaplan–Meier analysis (Table III and Figure 2) and Cox regression analysis (Table IV) – increasing age at diagnosis, lymph node-positivity, and T3/T4 stage were all significant predictors of poorer survival. In contrast, a greater number of harvested lymph nodes was associated with improved survival (HR 0.99, CI 0.986–0.994, p<0.001; Table IV) with a median survival of 16 months in patients with ≥8 harvested nodes and 13 months for those with <8 harvested nodes (p<0.001, Table III and Figure 2D). Tumor location (BT vs. HD) was not predictive of survival on univariate analysis. On multivariate Cox regression analysis, however, tumor location was a significant prognostic factor for survival (Table V) with BT location conferring an approximately 11% greater risk of death compared to HD lesions (HR 1.11, CI 1.00–1.23, p=0.05). Age, nodal status, and T-stage were also significantly associated with survival. The addition of harvested lymph nodes as an independent variable in the multivariate model negated the effect of tumor location (data not shown), potentially suggesting that the decreased survival noted in BT tumors may be related to fewer number of harvested lymph nodes associated with surgical resection of BT tumors.
Table III. Kaplan–Meier analysis of overall survival by other clinical factors in resected patients.
| Median survivala (months) | p-valueb | |
|---|---|---|
| Agec (years) | ||
| > 66 (n=3196) | 13 | <0.001 |
| ≤ 66 (n=3247) | 16 | |
| Gender | ||
| Female (n = 3182) | 14 | 0.21 |
| Male (n = 3261) | 14 | |
| Number of harvested lymph nodesc | ||
| = 8 (n = 3109) | 16 | <0.001 |
| < 8 (n = 2866) | 13 | |
| Lymph node status | ||
| Negative (n = 2444) | 17 | <0.001 |
| Positive (n = 3406) | 13 | |
| T-Stage | ||
| 1/2 (n = 1539) | 17 | <0.001 |
| 3/4 (n = 4793) | 13 | |
aPatients with missing values excluded.
bLog-rank test.
cDichotomized at median value.
Figure 2. .
Comparison of overall survival by other clinical factors: (A) age (dichotomized at the median value) (p<0.001); (B) T-stage (p<0.001); (C) lymph node status (p<0.001); and (D) number of examined lymph nodes (p<0.001).
Table IV. Univariate Cox regression analysis for overall survival (n=5781).
| Hazard ratio | 95% Confidence interval | p-value | |
|---|---|---|---|
| Age (years) | 1.01 | 1.008–1.014 | <0.001 |
| Tumor location | |||
| Head | (Reference) | – | – |
| Body/Tail | 1.09 | 0.99–1.19 | 0.09 |
| Number of examined lymph nodes | 0.99 | 0.986–0.994 | <0.001 |
| Lymph node status | |||
| Negative | (Reference) | – | – |
| Positive | 1.41 | 1.33–1.51 | <0.001 |
| T-Stage | |||
| 1/2 | (Reference) | – | – |
| 3/4 | 1.33 | 1.25–1.42 | <0.001 |
Table V. Multivariate Cox regression analysis for overall survival (n=5781).
| Hazard ratio | P-value | |
|---|---|---|
| Age (years) | 1.01 | <0.001 |
| Tumor location | ||
| Head | (Reference) | – |
| Body/Tail | 1.11 | 0.05 |
| Lymph node status | ||
| Negative | (Reference) | – |
| Positive | 1.39 | <0.001 |
| T-Stage | ||
| 1/2 | (Reference) | – |
| 3/4 | 1.24 | <0.001 |
Discussion
Using the SEER cancer registry we demonstrated that patients with BT pancreatic adenocarcinoma present more frequently with advanced, metastatic disease and subsequently have lower rates of cancer-directed surgery compared to patients with HD tumors (Table I). As a result, BT tumors were associated with worse survival compared to HD tumors (Figure 1). However, our results also demonstrated that location of pancreatic cancers in the BT was an independent predictor of survival in patients who had undergone curative resection. BT location was associated with worse survival in patients with non-metastatic, resected disease despite having a lower frequency of advanced T-stage lesions and a lower frequency of lymph node-positivity compared to HD tumors.
Patients with HD tumors were significantly younger at diagnosis than patients with BT tumors; and age was a significant predictor of survival (Table IV). We discovered that the total number of harvested lymph nodes affected survival. Although patients with BT lesions had a lower frequency of T3/T4 stage, they were less likely to have additional harvested lymph nodes compared to HD lesions. Harvested lymph nodes above a minimum threshold could provide more thorough pathologic examination and could potentially affect proper surgical staging 17,18,19,20. This is in contrast to extended lymphadenectomy which has been shown to have little or no clinical benefit 21,22,23,24,25. To further investigate the effect of lymph node number on survival, we added that variable into the multivariate model and discovered that the significance of tumor location on survival was lost, suggesting that decreased survival with BT pancreatic tumors may be potentially related to the extent of lymphadenectomy in BT patients undergoing curative surgery.
Several large single-institution series have examined prognostic factors and surgical resection in BT tumors and noted findings similar to our report. Balcom et al. reported that 18% of their total resections for pancreatic adenocarcinoma were BT resections; however, they did not assess survival outcomes 26. Another report from the Mayo Clinic examined patients who underwent BT resection for ductal adenocarcinoma and noted that stage, tumor size, and age were also significant predictors of survival 27. Goh et al. found that R2 resection, increasing tumor size, and lymph node involvement were all negative predictors of survival 28. Finally, Brennan et al. directly compared HD and BT lesions and similarly found lower resectability rates in BT lesions and decreased survival in BT lesions after resection 10.
An explanation for the difference in survival may also relate to the timing of diagnosis or lead-time bias. Watanabe et al. examined the onset of symptoms with tumor location and discovered that patients with medically prompting symptoms such as jaundice were rare in the BT group 12. Moreover, they noted that jaundice as a presenting complaint was associated with significantly better survival than other symptoms such as back pain or fatigue, thereby concluding that onset of symptoms in relation to tumor location was a significant prognostic factor for survival. Consequently, patients with HD pancreatic tumors may show improved survival due to the additional time gained from earlier diagnosis, rather than from a difference in tumor biology or the impact of intervention between sites.
Admittedly, there are inherent weaknesses in using SEER data. We were unable to control for important prognostic variables, including the status of surgical margins. However, posterior or retroperitoneal margins are rarely reported for BT tumors, whereas positive retroperitoneal or uncinate margins have been reported in up to 28% of pancreaticoduodenectomies for pancreatic cancer 29,30,31. If all clinicopathologic factors were equal, a higher rate of positive surgical margins for HD lesions compared to BT lesions would favor BT tumors in terms of overall survival. However, our data demonstrates the opposite with survival favoring HD lesions, indicating that surgical margin status is potentially not a factor for determining differences in survival. Chemotherapy data are also unavailable through the SEER registry and the use of adjuvant therapy between the two groups could not be compared. The benefits of adjuvant chemotherapy, however, are minimal at best and, therefore, would have little influence on our findings 32.
In summary, tumor location in pancreatic cancer significantly affects overall survival. Patients with BT lesions had decreased median survival, increased frequency of metastatic disease, and were less likely to undergo cancer-directed surgery than their HD lesion counterparts. These findings can be explained, perhaps, by earlier onset of symptoms associated with HD tumors. Consistent with previous studies, statistically significant prognostic indicators included age at diagnosis, T stage, and lymph node-positivity. We also demonstrated that on multivariate analysis, patients with BT cancers had an 11% increased risk of death. Large prospective multiinstitutional studies may better define the role of tumor location as a prognostic factor.
Acknowledgements and disclosures
Presented at the 2008 American Hepato Pancreato Biliary Association Annual Meeting, Fort Lauderdale, FL.
References
- 1.Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun MJ. Cancer statistics, 2007. CA Cancer J Clin. 2007;57:43–66. doi: 10.3322/canjclin.57.1.43. [DOI] [PubMed] [Google Scholar]
- 2.Winter JM, Cameron JL, Campbell KA, Arnold MA, Chang DC, Coleman J, et al. 1423 pancreaticoduodenectomies for pancreatic cancer: a single-institution experience. J Gastrointest Surg. 2006;10:1199–210. doi: 10.1016/j.gassur.2006.08.018. [DOI] [PubMed] [Google Scholar]
- 3.Greer SE, Pipas JM, Sutton JE, Zaki BI, Tsapakos M, Colacchio TA, et al. Effect of neoadjuvant therapy on local recurrence after resection of pancreatic adenocarcinoma. J Am Coll Surg. 2008;206:451–7. doi: 10.1016/j.jamcollsurg.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 4.Picozzi VJ, Kozarek RA, Traverso LW. Interferon-based adjuvant chemoradiation therapy after pancreaticoduodenectomy for pancreatic adenocarcinoma. Am J Surg. 2003;185:476–80. doi: 10.1016/s0002-9610(03)00051-5. [DOI] [PubMed] [Google Scholar]
- 5.Hines OJ, Reber HA. Pancreatic surgery. Curr Opin Gastroenterol. 2006;22:520–6. doi: 10.1097/01.mog.0000239866.81586.f6. [DOI] [PubMed] [Google Scholar]
- 6.Cameron JL, Riall TS, Coleman J, Belcher KA. One thousand consecutive pancreaticoduodenectomies. Ann Surg. 2006;244:10–5. doi: 10.1097/01.sla.0000217673.04165.ea. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Griffin JF, Smalley SR, Jewell W, Paradelo JC, Reymond RD, Hassanein RE, et al. Patterns of failure after curative resection of pancreatic carcinoma. Cancer. 1990;66:56–61. doi: 10.1002/1097-0142(19900701)66:1<56::aid-cncr2820660112>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 8.Geer RJ, Brennan MF. Prognostic indicators for survival after resection of pancreatic adenocarcinoma. Am J Surg. 1993;165:68–72. doi: 10.1016/s0002-9610(05)80406-4. [DOI] [PubMed] [Google Scholar]
- 9.Nitecki SS, Sarr MG, Colby TV, van Heerden JA. Long-term survival after resection for ductal adenocarcinoma of the pancreas. Is it really improving? Ann Surg. 1995;221:59–66. doi: 10.1097/00000658-199501000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brennan MF, Moccia RD, Klimstra D. Management of adenocarcinoma of the body and tail of the pancreas. Ann Surg. 1996;223:506–11. doi: 10.1097/00000658-199605000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wade TP, Virgo KS, Johnson FE. Distal pancreatectomy for cancer: results in US department of Veterans affairs hospitals, –1991. Pancreas ;11. 1987;1995:341–4. [PubMed] [Google Scholar]
- 12.Watanabe I, Sasaki S, Konishi M, Nakagohri T, Inoue K, Oda T, et al. Onset symptoms and tumor locations as prognostic factors of pancreatic cancer. Pancreas. 2004;28:160–5. doi: 10.1097/00006676-200403000-00007. [DOI] [PubMed] [Google Scholar]
- 13.Greenlee RT, Murray T, Bolden S, Wingo PA. Cancer statistics, 2000. CA Cancer J Clin. 2000;50:7–33. doi: 10.3322/canjclin.50.1.7. [DOI] [PubMed] [Google Scholar]
- 14.Kalser MH, Barkin J, MacIntyre JM. Pancreatic cancer. Assessment of prognosis by clinical presentation. Cancer. 1985;56:397–402. doi: 10.1002/1097-0142(19850715)56:2<397::aid-cncr2820560232>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 15.Sohn TA, Yeo CJ, Cameron JL, Koniaris L, Kaushal S, Abrams RA, et al. Resected adenocarcinoma of the pancreas-616 patients: results, outcomes, and prognostic indicators. J Gastrointest Surg. 2000;4:567–79. doi: 10.1016/s1091-255x(00)80105-5. [DOI] [PubMed] [Google Scholar]
- 16.Surveillance, Epidemiology, and End Results (SEER). About SEER. National Cancer Institute. URL:http://www.seer.cancer.gov/about.(accessed 30 May 2007) [Google Scholar]
- 17.Schwarz RE, Smith DD. Extent of lymph node retrieval and pancreatic cancer survival: information from a large US population database. Ann Surg Oncol. 2006;13:1189–200. doi: 10.1245/s10434-006-9016-x. [DOI] [PubMed] [Google Scholar]
- 18.Pawlik TM, Gleisner AL, Cameron JL, Winter JM, Assumpcao L, Lillemoe KD, et al. Prognostic relevance of lymph node ratio following pancreaticoduodenectomy for pancreatic cancer. Surg. 2007;141:610–8. doi: 10.1016/j.surg.2006.12.013. [DOI] [PubMed] [Google Scholar]
- 19.Tomlinson JS, Jain S, Bentrem DJ, Sekeris EG, Maggard MA, Hines OJ, et al. Accuracy of staging node-negative pancreas cancer: a potential quality measure. Arch Surg. 2007;142:767–23. doi: 10.1001/archsurg.142.8.767. [DOI] [PubMed] [Google Scholar]
- 20.Hellan M, Sun CL, Artinyan A, Mojica-Manosa P, Bhatia S, Ellenhorn JDI, et al. The impact of lymph node number on survival in patients with lymph node negative pancreatic cancer. Pancreas. 2008;37:19–24. doi: 10.1097/MPA.0b013e31816074c9. [DOI] [PubMed] [Google Scholar]
- 21.Iacono C, Accordini S, Bortolasi L, Facci E, Zamboni G, Montresor E, et al. Results of pancreaticoduodenectomy for pancreatic cancer: extended versus standard procedure. World J Surg. 2002;26:1309–14. doi: 10.1007/s00268-002-5976-6. [DOI] [PubMed] [Google Scholar]
- 22.Gazzaniga GM, Cappato S, Papadia F, Mori L, Filauro M. D1 versus D2 pancreatoduodenectomy in surgical therapy of pancreatic head cancer. Hepatogastroenterol. 2001;48:1471–8. [PubMed] [Google Scholar]
- 23.Farnell MB, Pearson RK, Sarr MG, DiMagno EP, Burgart LJ, Dahl TR, et al. A prospective randomized trial comparing standard pancreatoduodenectomy with pancreatoduodenectomy with extended lymphadenectomy in resectable pancreatic head adenocarcinoma. Surg. 2005;138:618–28. doi: 10.1016/j.surg.2005.06.044. [DOI] [PubMed] [Google Scholar]
- 24.Pedrazzoli S, DiCarlo V, Dionigi R, Mosca F, Pederzoli P, Pasquali C, et al. Standard versus extended lymphadenectomy associated with pancreatoduodenectomy in the surgical treatment of adenocarcinoma of the head of the pancreas: a multicenter, prospective, randomized study. Lymphadenectomy Study Group. Ann Surg. 1998;228:508–17. doi: 10.1097/00000658-199810000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yeo CJ, Cameron JL, Lillemoe KD, Sohn TA, Campbell KA, Sauter PK, et al. Pancreaticoduodenectomy with or without distal gastrectomy and extended retroperitoneal lymphadenectomy for periampullary adenocarcinoma, part 2: randomized controlled trial evaluating survival, morbidity, and mortality. Ann Surg. 2002;236:355–66. doi: 10.1097/00000658-200209000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Balcom JH, Rattner DW, Warshaw AL, Chang Y, Fernandez-del Castillo C. Ten-year experience with 733 pancreatic resections. Changing indications, older patients, and decreasing length of hospitalization. Arch Surg. 2001;136:391–8. doi: 10.1001/archsurg.136.4.391. [DOI] [PubMed] [Google Scholar]
- 27.Christein JD, Kendrick ML, Iqbal CW, Nagorney DM, Farnell MB. Distal pancreatectomy for resectable adenocarcinoma of the body and tail of the pancreas. J Gastrointest Surg. 2005;9:922–7. doi: 10.1016/j.gassur.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 28.Goh BK, Tan YM, Cheow PC, Chung YF, Chow PK, Wong WK, et al. Outcome of distal pancreatectomy for pancreatic adenocarcinoma. Dig Surg. 2008;25:32–8. doi: 10.1159/000117821. [DOI] [PubMed] [Google Scholar]
- 29.Westgaard A, Tafjord S, Farstad IN, Cvancarova M, Eide TJ, Mathisen O, et al. Resectable adenocarcinomas in the pancreatic head: the retroperitoneal resection margin is an independent prognostic factor. BMC Cancer. 2008;8:5. doi: 10.1186/1471-2407-8-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Winter JM, Cameron JL, Campbell KA, Arnold MA, Chang DC, Coleman J, et al. 1423 pancreaticoduodenectomies for pancreatic cancer: a single-institution experience. J Gastrointest Surg. 2006;10:1199–210. doi: 10.1016/j.gassur.2006.08.018. [DOI] [PubMed] [Google Scholar]
- 31.Neoptolemos JP, Stocken DD, Friess H, Bassi C, Dunn JA, Hickey H, et al. A randomized trial of chemoradiotherapy and chemotherapy after resection of pancreatic cancer. N Engl J Med. 2004;350:1200–10. doi: 10.1056/NEJMoa032295. [DOI] [PubMed] [Google Scholar]
- 32.Stocken DD, Buchler MW, Dervenis C, Bassi C, Jeekel H, Klinkenbijl JH, et al. Meta-analysis of randomised adjuvant therapy trials for pancreatic cancer. Br J Cancer. 2005;92:1372–81. doi: 10.1038/sj.bjc.6602513. [DOI] [PMC free article] [PubMed] [Google Scholar]