Abstract
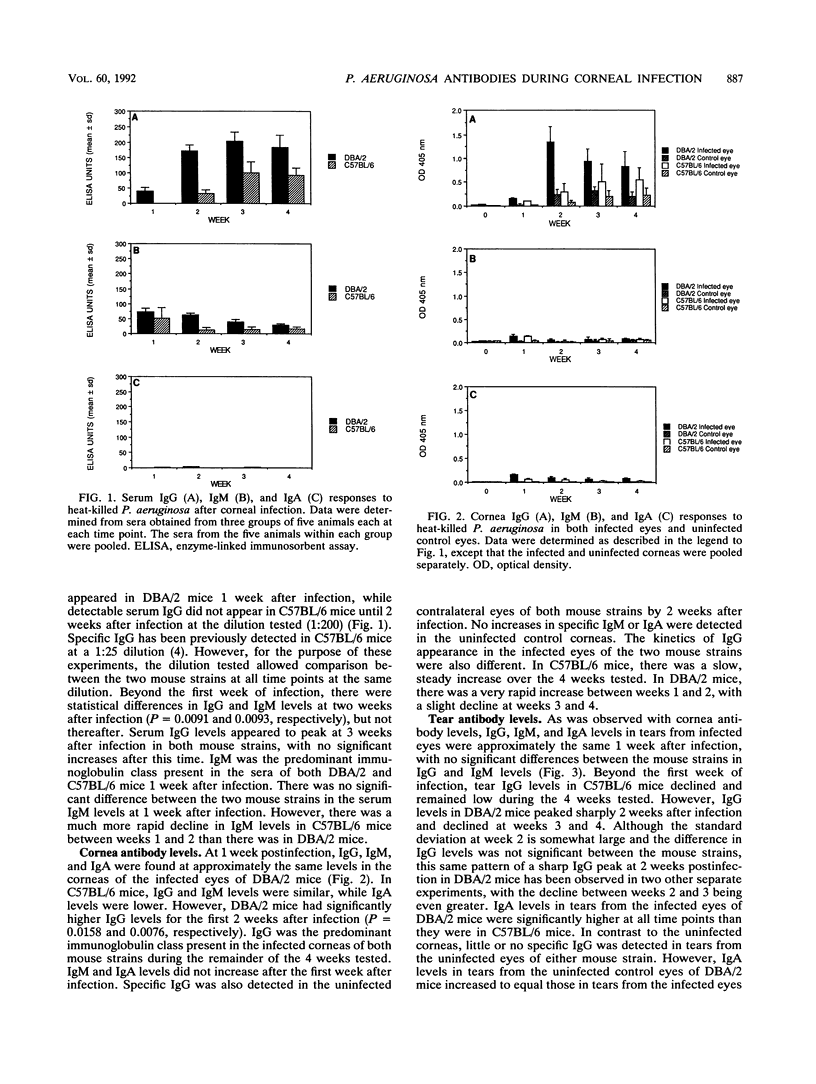
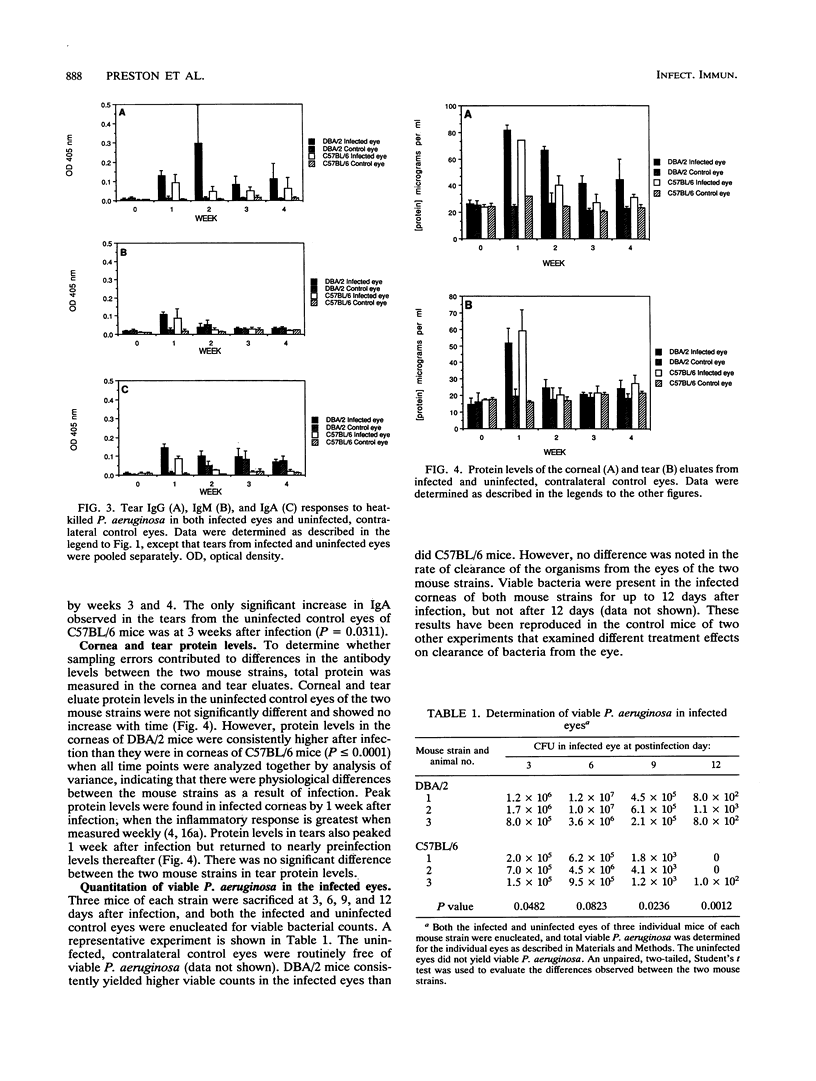
The studies described here are aimed at determining the kinetics of antibody responses specific to Pseudomonas aeruginosa ATCC 19660 in sera, tears, and corneas of naturally resistant DBA/2 mice and susceptible C57BL/6 mice after intracorneal infection. Immunoglobulin (IgG) and IgM responses in sera were significantly greater in DBA/2 mice for the first 2 weeks postinfection. Little or no IgA was detected in the sera of mice from either strain. IgG was the predominant immunoglobulin class present in the corneas of the infected eyes from both mouse strains. However, differences in both the magnitude and the kinetics of the corneal IgG responses were noted between mouse strains. The kinetics of the corneal IgG responses were more similar to those of the serum IgG response than to those of the tear IgG response. Tear antibody responses in DBA/2 mice differed from those of C57BL/6 mice in two ways. First, there was a sharp increase in tear IgG levels 2 weeks after infection in DBA/2 mice that was not present in C57BL/6 mice. Second, IgA levels present in tears from the infected eyes of C57BL/6 mice dropped to nearly preinfection levels after the first week, whereas in DBA/2 mice, IgA levels remained elevated in the infected eyes after the first week. Determination of P. aeruginosa-specific antibody responses in the uninfected, contralateral control eyes revealed that IgA was detectable in the tears but not in the corneas of DBA/2 mice. Very little IgA was detected in the tears of the uninfected eyes of C57BL/6 mice. IgG was the only immunoglobulin class present in the uninfected corneas in both mouse strains tested. These results suggest that ocular IgA was made locally, whereas most ocular IgG may have originated from the serum, with some possible local synthesis. These immunological results indicate that DBA/2 and C57BL/6 mice respond differently to corneal challenge with P. aeruginosa.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allansmith M. R., Whitney C. R., McClellan B. H., Newman L. P. Immunoglobulins in the human eye. Location, type, and amount. Arch Ophthalmol. 1973 Jan;89(1):36–45. doi: 10.1001/archopht.1973.01000040038010. [DOI] [PubMed] [Google Scholar]
- Berk R. S., Hazlett L. D. Further studies on the genetic control of murine corneal response to Pseudomonas aeruginosa. Rev Infect Dis. 1983 Nov-Dec;5 (Suppl 5):S936–S940. doi: 10.1093/clinids/5.supplement_5.s936. [DOI] [PubMed] [Google Scholar]
- Berk R. S., Leon M. A., Hazlett L. D. Genetic control of the murine corneal response to Pseudomonas aeruginosa. Infect Immun. 1979 Dec;26(3):1221–1223. doi: 10.1128/iai.26.3.1221-1223.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berk R. S., Montgomery I. N., Hazlett L. D. Serum antibody and ocular responses to murine corneal infection caused by Pseudomonas aeruginosa. Infect Immun. 1988 Dec;56(12):3076–3080. doi: 10.1128/iai.56.12.3076-3080.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bienenstock J., Befus A. D. Mucosal immunology. Immunology. 1980 Oct;41(2):249–270. [PMC free article] [PubMed] [Google Scholar]
- Chusid M. J., Davis S. D. Experimental bacterial keratitis in neutropenic guinea pigs: polymorphonuclear leukocytes in corneal host defense. Infect Immun. 1979 Jun;24(3):948–952. doi: 10.1128/iai.24.3.948-952.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleveland R. P., Hazlett L. D., Leon M. A., Berk R. S. Role of complement in murine corneal infection caused by Pseudomonas aeruginosa. Invest Ophthalmol Vis Sci. 1983 Feb;24(2):237–242. [PubMed] [Google Scholar]
- Epstein R. J., Hendricks R. L., Stulting R. D. Interleukin-2 induces corneal neovascularization in A/J mice. Cornea. 1990 Oct;9(4):318–323. [PubMed] [Google Scholar]
- Epstein R. J., Stulting R. D. Corneal neovascularization induced by stimulated lymphocytes in inbred mice. Invest Ophthalmol Vis Sci. 1987 Sep;28(9):1505–1513. [PubMed] [Google Scholar]
- Ferreira P., Soares R., Arala-Chaves M. Susceptibility to infection with Mycobacterium avium is paradoxically correlated with increased synthesis of specific anti-bacterial antibodies. Int Immunol. 1991 May;3(5):445–452. doi: 10.1093/intimm/3.5.445. [DOI] [PubMed] [Google Scholar]
- Gerke J. R., Magliocco M. V. Experimental Pseudomonas aeruginosa Infection of the Mouse Cornea. Infect Immun. 1971 Feb;3(2):209–216. doi: 10.1128/iai.3.2.209-216.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazlett L. D., Wells P., Berk R. S. Immunocytochemical localization of IgA in the mouse cornea. Exp Eye Res. 1981 Jan;32(1):97–104. doi: 10.1016/s0014-4835(81)80043-7. [DOI] [PubMed] [Google Scholar]
- Hazlett L. D., Zelt R., Cramer C., Berk R. S. Pseudomonas aeruginosa induced ocular infection. A histological comparison of two bacterial strains of different virulence. Ophthalmic Res. 1985;17(5):289–296. doi: 10.1159/000265388. [DOI] [PubMed] [Google Scholar]
- Kessler E., Mondino B. J., Brown S. I. The corneal response to Pseudomonas aeruginosa: histopathological and enzymatic characterization. Invest Ophthalmol Vis Sci. 1977 Feb;16(2):116–125. [PubMed] [Google Scholar]
- Kielty D., Cousins S. W., Atherton S. S. HSV-1 retinitis and delayed hypersensitivity in DBA/2 and C57BL/6 mice. Invest Ophthalmol Vis Sci. 1987 Dec;28(12):1994–1999. [PubMed] [Google Scholar]
- Kreger A. S., Lyerly D. M., Hazlett L. D., Berk R. S. Immunization against experimental Pseudomonas aeruginosa and Serratia marcescens keratitis. Vaccination with lipopolysaccharide endotoxins and proteases. Invest Ophthalmol Vis Sci. 1986 Jun;27(6):932–939. [PubMed] [Google Scholar]
- Kreger A. S. Pathogenesis of Pseudomonas aeruginosa ocular diseases. Rev Infect Dis. 1983 Nov-Dec;5 (Suppl 5):S931–S935. doi: 10.1093/clinids/5.supplement_5.s931. [DOI] [PubMed] [Google Scholar]
- McDermott M. R., Bienenstock J. Evidence for a common mucosal immunologic system. I. Migration of B immunoblasts into intestinal, respiratory, and genital tissues. J Immunol. 1979 May;122(5):1892–1898. [PubMed] [Google Scholar]
- Mestecky J., McGhee J. R., Michalek S. M., Arnold R. R., Crago S. S., Babb J. L. Concept of the local and common mucosal immune response. Adv Exp Med Biol. 1978;107:185–192. doi: 10.1007/978-1-4684-3369-2_22. [DOI] [PubMed] [Google Scholar]
- Mondino B. J., Laheji A. K., Adamu S. A. Ocular immunity to Staphylococcus aureus. Invest Ophthalmol Vis Sci. 1987 Mar;28(3):560–564. [PubMed] [Google Scholar]
- Montgomery P. C., Rockey J. H., Majumdar A. S., Lemaitre-Coelho I. M., Vaerman J. P., Ayyildiz A. Parameters influencing the expression of IgA antibodies in tears. Invest Ophthalmol Vis Sci. 1984 Mar;25(3):369–373. [PubMed] [Google Scholar]
- Moon M. M., Hazlett L. D., Hancock R. E., Berk R. S., Barrett R. Monoclonal antibodies provide protection against ocular Pseudomonas aeruginosa infection. Invest Ophthalmol Vis Sci. 1988 Aug;29(8):1277–1284. [PubMed] [Google Scholar]
- Peppard J. V., Mann R. V., Montgomery P. C. Antibody production in rats following ocular-topical or gastrointestinal immunization: kinetics of local and systemic antibody production. Curr Eye Res. 1988 May;7(5):471–481. doi: 10.3109/02713688809031800. [DOI] [PubMed] [Google Scholar]
- Pier G. B., Ames P. Mediation of the killing of rough, mucoid isolates of Pseudomonas aeruginosa from patients with cystic fibrosis by the alternative pathway of complement. J Infect Dis. 1984 Aug;150(2):223–228. doi: 10.1093/infdis/150.2.223. [DOI] [PubMed] [Google Scholar]
- Preston M. J., Berk J. M., Hazlett L. D., Berk R. S. Serum antibody response to Pseudomonas aeruginosa antigens during corneal infection. Infect Immun. 1991 Jun;59(6):1984–1990. doi: 10.1128/iai.59.6.1984-1990.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steuhl K. P., Döring G., Henni A., Thiel H. J., Botzenhart K. Relevance of host-derived and bacterial factors in Pseudomonas aeruginosa corneal infections. Invest Ophthalmol Vis Sci. 1987 Sep;28(9):1559–1568. [PubMed] [Google Scholar]
- Streilein J. W., Niederkorn J. Y., Shadduck J. A. Systemic immune unresponsiveness induced in adult mice by anterior chamber presentation of minor histocompatibility antigens. J Exp Med. 1980 Oct 1;152(4):1121–1125. doi: 10.1084/jem.152.4.1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twining S. S., Lohr K. M., Moulder J. E. The immune system in experimental Pseudomonas keratitis. Model and early effects. Invest Ophthalmol Vis Sci. 1986 Apr;27(4):507–515. [PubMed] [Google Scholar]
- Van Horn D. L., Davis S. D., Hyndiuk R. A., Pederson H. J. Experimental Pseudomonas keratitis in the rabbit: bacteriologic, clinical, and microscopic observations. Invest Ophthalmol Vis Sci. 1981 Feb;20(2):213–221. [PubMed] [Google Scholar]
- Verhagen C., Breeboart A. C., Kijlstra A. Diffusion of immunoglobulin G from the vascular compartment into the normal rabbit cornea. Invest Ophthalmol Vis Sci. 1990 Aug;31(8):1519–1525. [PubMed] [Google Scholar]



